Abstract
Glucocorticoids and mineralocorticoids are steroid hormones classically thought to be secreted exclusively by the adrenal glands. However, recent evidence has shown that corticosteroids can also be locally synthesized in various other tissues, including primary lymphoid organs, intestine, skin, brain, and possibly heart. Evidence for local synthesis includes detection of steroidogenic enzymes and high local corticosteroid levels, even after adrenalectomy. Local synthesis creates high corticosteroid concentrations in extra-adrenal organs, sometimes much higher than circulating concentrations. Interestingly, local corticosteroid synthesis can be regulated via locally expressed mediators of the hypothalamic-pituitary-adrenal (HPA) axis or renin-angiotensin system (RAS). In some tissues (e.g., skin), these local control pathways might form miniature analogs of the pathways that regulate adrenal corticosteroid production. Locally synthesized glucocorticoids regulate activation of immune cells, while locally synthesized mineralocorticoids regulate blood volume and pressure. The physiological importance of extra-adrenal glucocorticoids and mineralocorticoids has been shown, because inhibition of local synthesis has major effects even in adrenal-intact subjects. In sum, while adrenal secretion of glucocorticoids and mineralocorticoids into the blood coordinates multiple organ systems, local synthesis of corticosteroids results in high spatial specificity of steroid action. Taken together, studies of these five major organ systems challenge the conventional understanding of corticosteroid biosynthesis and function.
Keywords: aldosterone, brain, bursa of Fabricius, corticosterone, cortisol, heart, immunosteroids, intestine, neurosteroids, skin, stress, thymus
corticosteroids are steroid hormones produced in the adrenal cortex and are of two types, glucocorticoids and mineralocorticoids. Glucocorticoids, such as corticosterone and cortisol, have numerous effects and can act on nearly all cells in the body. For example, glucocorticoids regulate metabolic activity, immune function, and behavior (84). Circulating glucocorticoid levels increase in response to a variety of stressors under control of the hypothalamic-pituitary-adrenal (HPA) axis. Hypothalamic release of corticotropin-releasing hormone (CRH) triggers pituitary release of adrenocorticotropic hormone (ACTH), which stimulates glucocorticoid production by the zona fasciculata of the adrenals. The adrenals can secrete cortisol, corticosterone, or both, depending on the species.
Mineralocorticoids, such as aldosterone, promote sodium reabsorption in transporting epithelia of the kidneys, salivary glands, and large intestine. Sodium reabsorption is followed by passive reabsorption of water. Circulating aldosterone concentrations rise in response to low blood volume or sodium depletion under control of the renin-angiotensin system (RAS). The kidneys release renin, which converts angiotensinogen to angiotensin I. Angiotensin I is then cleaved by angiotensin-converting enzyme (ACE) to active angiotensin II. Angiotensin II stimulates mineralocorticoid production by the zona glomerulosa of the adrenals.
The classical corticosteroid biosynthetic pathway is shown in Fig. 1. Traditionally, it was thought that glucocorticoids and mineralocorticoids were synthesized solely in the adrenal cortex, and research has been focused overwhelmingly on measuring circulating levels of these steroids in plasma or serum. However, a growing body of evidence has demonstrated de novo synthesis of glucocorticoids and mineralocorticoids in other organs, such as primary lymphoid organs, intestine, skin, brain, and possibly the heart and vasculature (15, 87). Here, we review evidence of local de novo corticosteroid production, regulation of local corticosteroid production, and the potential functions of locally produced corticosteroids.
Fig. 1.
Simplified diagram of the classical corticosteroid synthetic pathway. Enzyme gene names are in italics and gray. StAR (steroidogenic acute regulatory protein) is required for translocation of cholesterol from the outer to the inner mitochondrial membrane, where CYP11A1 (P450 side-chain cleavage, or P450scc) catalyzes the conversion of cholesterol to pregnenolone. Further steroid conversions are performed by 3β-HSD (3β-hydroxysteroid dehydrogenase/Δ5–Δ4 isomerase), CYP17 (17α-hydroxylase/17,20-lyase, or P450c17), CYP21 (21-hydroxylase, or P450c21), CYP11B1 (11β-hydroxylase or P450c11β), CYP11B2 (aldosterone synthase or P450c11AS), 11β-HSD types 1 and 2.
PRIMARY LYMPHOID ORGANS
Primary lymphoid organs are the sites of T and B cell (lymphocyte) development. In mammals, both cell lineages originate from the same early precursors in the bone marrow. T cell precursors migrate to and mature in the thymus, while B cell precursors remain and mature in the bone marrow. The thymus consists of inner medullary and outer cortical epithelial cells, through which immature T cells (thymocytes) migrate over the course of development (40). During development, thymocyte selection ensures the ability of the T cell receptor (TCR) to recognize antigens presented by self MHC molecules (positive selection) and prevents T cell autoreactivity (negative selection). Only thymocytes expressing a TCR with intermediate affinity for antigen:MHC develop into mature T cells; the other thymocytes (∼98%) undergo apoptosis (40). In the bone marrow, a similar process results in removal of autoreactive B cells.
Glucocorticoids can induce apoptosis of lymphocytes, and this effect is especially pronounced in immature lymphocytes. However, glucocorticoids can also inhibit TCR-mediated apoptosis and promote survival (39). This mutual antagonism of glucocorticoid receptor (GR) signaling and TCR signaling suggested a role for glucocorticoids in thymocyte selection (39). Since circulating glucocorticoids are very low in early postnatal life (when lymphocyte production is high), local synthesis might provide a source of glucocorticoids.
Evidence for Local Synthesis
Steroidogenic enzymes.
The first demonstration of thymic steroid production was in the mouse, and using fetal thymic organ culture, Vacchio et al. (110) demonstrated conversion of a cholesterol analog into pregnenolone and 11-deoxycorticosterone. Steroidogenic capability was high in thymic epithelial cells and low in thymocytes (110). Murine thymic epithelial cells have since been shown to have mRNA, protein, and activities of the enzymes required for de novo glucocorticoid synthesis (47, 47, 69, 75, 110) (Fig. 1 and Table 1). The absence of CYP17 activity results in the formation of corticosterone (47), which is also the major adrenal glucocorticoid in mice. Thymic epithelial cells also activate GR-mediated transcription in cocultured cells (69). In addition to thymic epithelial cells, thymocytes themselves also express StAR, CYP11A1, 3β-HSD, CYP17, CYP21, and CYP11B1 mRNA and synthesize corticosterone (10, 75, 76). Moreover, the chicken thymus contains functional CYP11A1, 3β-HSD, CYP21, and CYP11B1 enzymes for glucocorticoid synthesis, but the additional presence of CYP17 activity directs synthesis toward cortisol rather than corticosterone, in contrast to the chicken adrenals (46). All the enzymes found in the chicken thymus are also present in the bursa of Fabricius (hereafter bursa) (46). The bursa is the site of avian B cell development, analogous to mammalian bone marrow. Together, these studies of corticosteroidogenic enzymes demonstrate that the murine and avian thymus, and also the avian bursa, contain the machinery for de novo glucocorticoid synthesis.
Table 1.
Evidence for and against local synthesis of glucocorticoids and mineralocorticoids
| Lymphoid |
Intestine |
Skin |
Brain |
Cardiovascular |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| + | – | + | – | + | – | + | – | + | – | ||
| Steroidogenic enzymes | |||||||||||
| StAR | mRNA | 10, 75, 76 | 17, 78 | 8, 9, 120 | |||||||
| Protein | 99 | 17 | |||||||||
| Activity | 17, 78 | ||||||||||
| CYP11A1 | mRNA | 10, 47, 69, 75, 76 | 3, 11, 13, 42, 57, 58, 61, 62 | 94, 99, 106 | 12, 17, 78 | 9, 41, 103, 120 | 35, 47 | ||||
| Protein | 110 | 11 | 99, 106 | 12, 17 | |||||||
| Activity | 46, 47, 110 | 99, 106 | 12, 17, 78 | 46, 47 | |||||||
| 3β-HSD | mRNA | 10, 75 | 11, 42 | 19 | 12, 17, 78 | 35, 41, 103, 120 | 9 | ||||
| Protein | 19 | 12, 17 | |||||||||
| Activity | 46, 47, 110 | 19, 99 | 12, 17, 78 | 46, 47 | |||||||
| CYP17 | mRNA | 75 | 47 | 94 | 12, 17, 78 | 17 | 9 | 41, 47, 120 | |||
| Protein | 12, 17 | 17 | |||||||||
| Activity | 46 | 47 | 17, 78 | 46, 47 | |||||||
| CYP21 | mRNA | 10, 69, 75 | 11 | 94 | 53, 81 | 5, 101, 121 | 45, 53 | 9, 35, 41 | 53 | ||
| Protein | 45 | ||||||||||
| Activity | 46, 47, 110 | 81, 95 | 45 | 103 | 46 | ||||||
| CYP11B1 | mRNA | 10, 47, 69, 75, 76 | 3, 11, 13, 57, 58, 61, 62 | 27, 51, 54, 101, 118, 121 | 101 | 1, 9, 27, 41, 64, 91, 102, 103, 120 | 29, 34, 35, 47, 51, 117, 120 | ||||
| Protein | 69, 110 | 51 | |||||||||
| Activity | 46, 47 | 11, 42 | 38, 95–98 | 27 | 1, 91 | 46, 47, 64 | |||||
| CYP11B2 | mRNA | 26, 51, 101, 118, 121 | 54, 101 | 1, 26, 34, 35, 41, 91, 102, 103, 120 | 1, 9, 29, 51, 64, 117, 120 | ||||||
| Protein | 51 | ||||||||||
| Activity | 95 | 26 | 91, 102, 103 | 64 | |||||||
| Local steroid levels | |||||||||||
| Glucocorticoids | |||||||||||
| Tissue > plasma | 85, 88 | 14, 48, 88 | 30 | 29 | |||||||
| Present postadrenalectomy | 14 | 30 | 29 | ||||||||
| Synthesis in vitro | 10, 69, 75, 76 | 3, 11, 13, 57, 58, 61, 62 | 38, 95, 97, 98 | 90, 91, 102, 103 | 64 | ||||||
| Mineralocorticoids | |||||||||||
| Tissue > plasma | 30 | 29 | |||||||||
| Present postadrenalectomy | 30 | 29 | |||||||||
| Synthesis in vitro | 95 | 26 | 34, 90, 91, 102 | 1, 64 | |||||||
Nos. are references. +, detected; –, not detected. Note: this table does not account for important factors such as species, age, sex, season, etc.
Local steroid levels.
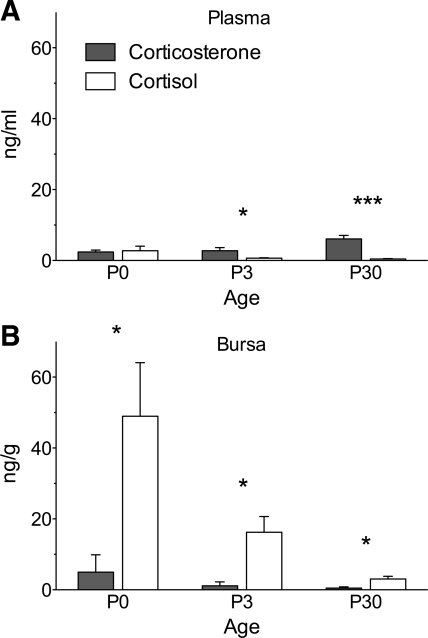
Endogenous glucocorticoid levels have not been measured in rodent thymus but have been measured in the avian thymus and bursa (Fig. 2 and Table 1). The major circulating glucocorticoid in birds is corticosterone, but in zebra finch (Taeniopygia guttata) thymus and bursa, cortisol levels are higher than corticosterone levels. Also, cortisol (but not corticosterone) levels are higher in lymphoid tissue than in plasma, which provides evidence that cortisol is a locally synthesized “immunosteroid” (85, 88). High local levels could also results from accumulation of circulating glucocorticoids, but the local presence of cortisol-synthetic machinery and the low circulating levels of cortisol suggest local origin. Local synthesis of cortisol, rather than corticosterone, offers an opportunity to parse the regulation and functions of lymphoid glucocorticoids from those of adrenal glucocorticoids. Furthermore, glucocorticoid production in the avian bursa raises the possibility that similar production occurs in the mammalian bone marrow. The very high lymphoid cortisol concentrations after hatching show that local glucocorticoid synthesis can result in levels far in excess of those in the circulation.
Fig. 2.
Baseline endogenous glucocorticoid levels in plasma (A) and bursa of Fabricius (bursa; B) of developing zebra finches at ages 0, 3, and 30 days posthatch. The bursa is the site of B lymphocyte development, similar to mammalian bone marrow. Adapted with permission from Ref. 88.
Regulation
In the mouse, in vitro steroidogenesis by thymic epithelium is high at birth and decreases with age (75, 110). A similar age-related decrease is seen in zebra finch bursa; cortisol levels are high at hatching and decrease rapidly with age (88) (Fig. 2). In the first 1–2 wk of life, mice (89), zebra finches (M. D. Taves and K. K. Soma, unpublished data, and Ref. 113), and other altricial species undergo a period of minimal adrenal glucocorticoid production (the stress-hyporesponsive period), which results in low circulating levels. A stress-hyporesponsive period occurs in humans (also altricial) (33). Since the stress-hyporesponsive period coincides with high local synthesis in lymphoid organs, local production may serve to maintain high local glucocorticoid levels in lymphoid organs while systemic levels are low. In contrast to thymic epithelial cells, thymocyte production of glucocorticoids increases at puberty (∼4 wk in mice) (75) and is stimulated by testosterone in males (10).
Glucocorticoid synthesis in primary lymphoid organs is regulated by HPA axis mediators (Fig. 3A). ACTH increases in vitro steroid production by murine thymic epithelial cells (110) (Table 2), although a separate study found little effect on glucocorticoid response element activity (69). In thymocytes, ACTH and cAMP decrease steroidogenic enzyme expression and glucocorticoid response element activity (76). The divergent effects of ACTH on glucocorticoid production by thymic epithelial cells and thymocytes could help to maintain local glucocorticoid levels. Proopiomelanocortin (POMC) mRNA is present in rat thymus (79), and ACTH immunoreactivity has been detected in rat, bird, and human thymi (4, 65). CRH mRNA is also present in rat thymus (2,79), and CRH immunoreactivity has been detected in rat and bird thymi (66). Thus, an exciting possibility is that a local analog of the HPA axis regulates local glucocorticoid production in the thymus. In bone marrow and bursa, less is known about regulatory factors. The avian bursa contains immunoreactive ACTH (22), but CRH has not been reported. The bursa also contains bursal antisteroidogenic peptide (BASP), which suppresses adrenal glucocorticoid production in vitro (7).
Fig. 3.
Regulation and functions of local glucocorticoid synthesis. A: in the thymus, glucocorticoids and T cell receptor (TCR) signaling each function independently as proapoptotic stimuli in developing thymocytes. Glucocorticoids also antagonize TCR signaling and alter the threshold of TCR affinity for self peptide:MHC that results in survival and proliferation vs. apoptosis. B: in the intestine, activated macrophages and TH1 cells secrete the proinflammatory cytokine TNFα, which upregulates the transcription factor LRH-1 in epithelial cells of the intestinal crypt. LRH-1 induces local production of glucocorticoids, which downregulate immune cell activation and resulting inflammation. C: activation of keratinocytes (and other types of skin cells) results in production of CRH, which stimulates production of ACTH and proinflammatory cytokines such as IL-1β and TNFα. ACTH then stimulates local synthesis of glucocorticoids, which inhibit further production of CRH and proinflammatory cytokines, thus downregulating inflammation. ACTH, adrenocorticotropic hormone; CRH, corticotropin-releasing hormone; GCs, glucocorticoids; IFNγ, interferon-γ; IGF-1, insulin-like growth factor I; IL, interleukin; LRH-1, liver receptor homolog-1; MHC, major histocompatibility complex; TH1, type I helper T cell; TNFα, tumor necrosis factor-α.
Table 2.
Regulation of local glucocorticoid and mineralocorticoid synthesis
| Lymphoid | Intestine | Skin | Brain | Cardiovascular | |
|---|---|---|---|---|---|
| Stimulatory Signals | Glucocorticoids | Glucocorticoids | Glucocorticoids | Glucocorticoids | Mineralocorticoids |
| ·ACTH (acting on thymic epithelial cells) | ·T cell activation (TH1) | ·UV radiation | ·Alcohol withdrawal | ·Heart failure | |
| ·Testosterone (acting on thymocytes) | ·Macrophage activation | ·CRH | ·Social defeat | ·Angiotensin II | |
| ·TNFα | ·ACTH | ·Restraint | ·ACTH | ||
| ·LRH-1 | ·Saline perfusion | ||||
| ·Kinase activation | ·ACTH | ||||
| ·LPS | Mineralocorticoids | ||||
| ·Sodium depletion | |||||
| ·ACTH | |||||
| Inhibitory Signals | ·ACTH (acting on thymocytes) | ·ACTH | ·Glucocorticoids (negative feedback) | Glucocorticoids | ·unknown |
| ·cAMP (acting on thymocytes) | ·cAMP | ·IGF-1 | ·unknown | ||
| ·SF-1 | Mineralocorticoids | ||||
| ·unknown adrenal factor | |||||
| References | 10, 69, 75, 76, 88, 110 | 3, 11, 13, 57, 58, 61, 62 | 38, 82, 94, 96, 97, 105, 112 | 14, 48, 52, 59, 88, 104, 118, 119 | 18, 20, 52, 67, 90, 91, 111 |
Function
Locally produced glucocorticoids have major effects on thymocyte development (Fig. 3A and Table 3). In general, thymocyte selection is thought to be driven largely by TCR affinity for antigen presented by self MHC molecules. Low affinity (weak or absent TCR signal) results in death; intermediate affinity (moderate signal) results in positive selection and survival; and high affinity (strong signal) results in negative selection and death. The discovery of thymic glucocorticoid synthesis has suggested an alternative model (“mutual antagonism”), in which TCR signaling induces apoptotic signals in thymocytes with intermediate- or high-affinity TCR, and glucocorticoids antagonize these proapoptotic signals to allow survival of thymocytes with intermediate-affinity TCR (110). The mutual antagonism model is supported by in vitro observations that TCR signaling decreases glucocorticoid-induced thymocyte apoptosis (39) and that endogenous thymic glucocorticoids decrease TCR-dependent thymocyte apoptosis (109, 110). Furthermore, in thymi of fetal mice, thymocyte-specific underexpression of the GR (via antisense transgene) reduces thymocyte numbers, suggesting that GR signaling promotes survival (44).
Table 3.
Possible functions of locally synthesized glucocorticoids and mineralocorticoids
| Lymphoid | Intestine | Skin | Brain | Cardiovascular | |
|---|---|---|---|---|---|
| Local function | Glucocorticoids | Glucocorticoids | Glucocorticoids | Glucocorticoids | Mineralocorticoids |
| ·Antagonism of T cell receptor signal | ·Downregulate TH1 immune response and inflammation | ·Local stress response to different environmental stimuli | ·Collagen deposition | ||
| ·Promote thymocyte apoptosis | ·Allow tissue repair | ·Regulation of inflammatory response | ·Drug-seeking behavior? | ·Regulation of blood pressure and systemic sodium balance? | |
| ·B cell development? | ·Prevent induction of autoimmune response to commensal gut bacteria? | ·Regulation of skin immune response? | ·Submissive behavior? | ||
| ·Anti-predator behavior? | |||||
| Mineralocorticoids | |||||
| ·Regulation of blood pressure, systemic sodium balance | |||||
| References | 24, 44, 60, 68, 70, 86, 109, 110 | 13, 61–63 | 16, 93, 112 | 25, 28, 31, 32, 36, 37, 52, 83, 84 | 52, 71 |
The effects of glucocorticoids on thymocytes, however, change with age. At puberty, GR underexpression (as above) increases thymocyte numbers, whereas overexpression decreases thymocyte numbers (70). Thus, at puberty, endogenous glucocorticoids promote thymocyte apoptosis rather than survival. GR overexpression also delays thymus growth and involution (68). The necessity of GR function for thymocyte development has also been tested in knockout mice. Mutants with partial (74) or complete (6) abrogation of GR function retain functional thymocyte maturation. GR signaling is therefore not necessary for thymocyte maturation. Nonetheless, in intact adult mice, inhibition of thymic glucocorticoid production by low-dose metyrapone treatment (which did not significantly affect plasma glucocorticoid levels in this study) increased thymocyte numbers, showing that locally produced glucocorticoids have physiological effects even in the presence of functional adrenal glands (76).
In sum, GR signaling promotes thymocyte survival in fetal thymus, promotes apoptosis in postnatal (pubertal) thymus, but is not critical for thymocyte maturation. How glucocorticoids interact with TCR signaling is unclear, but this might involve membrane-associated receptors rather than cytosolic receptors. The unbound GR has been shown to associate at the cell membrane with TCR kinases and facilitate TCR signaling, while glucocorticoid binding causes dissociation of this complex and inhibition of TCR signaling (49, 50).
Far less work has been done on locally synthesized glucocorticoids and B cell development. Systemic glucocorticoid treatment depletes B lineage cells in murine bone marrow (24) and decreases bursa size in the chicken (60). In the zebra finch, lymphoid cortisol may act on different receptors than adrenal corticosterone, because cortisol (but not corticosterone) shows specific binding to bursa membranes (86). The accessibility of the avian bursa and the ability to remove it intact make this a useful model for investigating the effects of local glucocorticoid synthesis in B cell development.
INTESTINE
The intestine is a critical barrier between the internal environment (within the organism) and external environment (the lumen; outside the organism). The intestinal mucosa contains the largest number of immune cells in the body and protects the epithelial surface from pathogens as well as commensal bacteria. Intestinal immune cells are concentrated in distinct lymphoid tissues (Peyer's patches, mesenteric lymph nodes, and appendix) and are also present as individual cells throughout the epithelium (40). Tight regulation of immune activation is necessary to maintain intestinal homeostasis.
Evidence for Local Synthesis
Steroidogenic enzymes.
The de novo steroidogenic capacity of the gut was first suggested in a study using in situ hybridization, which detected CYP11A1 and 3β-HSD mRNA in the mouse gut during embryonic development (42) (Table 1). Subsequent studies have demonstrated expression of mRNA and protein for several glucocorticoid-synthetic enzymes in murine small intestine and colon (11, 13). CYP11A1 mRNA is highest in the proximal small intestine, intermediate in the middle and distal small intestine, and lowest in the colon (11). The mRNAs for steroidogenic enzymes are restricted to the proliferating epithelial cells of the crypts (3, 11), and differentiation of immature intestinal epithelial cells into mature nonproliferating cells results in a decrease of steroidogenic enzyme mRNA (3). Also, CYP11A1 and CYP11B1 mRNAs are detectable in a murine intestinal epithelial cell line (57). Murine small intestine contains CYP11A1 protein, and the activity of this and other steroidogenic enzymes is demonstrated by ex vivo synthesis of corticosterone (11, 13). Corticosterone synthesis is blocked by metyrapone (11, 13). In humans, CYP11A1 and CYP11B2 mRNAs are present in colon biopsies (13), suggesting that the human intestine, like that of the mouse, is capable of glucocorticoid synthesis.
Local steroid levels.
To our knowledge, local corticosteroid levels within the intestinal epithelium have not been measured in vivo.
Regulation
In the murine intestine, expression of specific steroidogenic enzymes increases in response to immune activation and inflammation (Fig. 3B). In vivo treatment of mice with anti-CD3 (to activate T cells) profoundly increases ex vivo corticosterone production by the small intestine (11) (Table 2). Mouse small intestine and colon constitutively express mRNA of most enzymes required for corticosterone synthesis (11, 13), but CYP11A1 and CYP11B1 expression reach high levels only after T cell activation (11) or inflammation (61, 62). The inflammation-induced increases in enzyme expression and corticosterone synthesis require the secretion of tumor necrosis factor-α (TNFα), a proinflammatory cytokine (61, 62). Immune activation, possibly via TNFα induction of NF-κB (nuclear factor-κ light-chain enhancer of activated B cells, a proinflammatory transcription factor) (61), increases intestinal expression of the transcription factor liver receptor homolog-1 (LRH-1) (58). LRH-1 is closely related to steroidogenic factor-1 (SF-1), which regulates the expression of most steroidogenic enzymes in the adrenal (57). LRH-1 is important in cell cycle progression, and LRH-1 expression in intestine is limited to proliferating crypt cells (3). In a murine intestinal epithelial cell line, overexpression of LRH-1 increases CYP11A1 and CYP11B1 mRNA levels and corticosterone production (58). In vivo, LRH-1 haploinsufficiency prevents T cell activation-induced upregulation of CYP11A1 mRNA and corticosterone production in small intestine and attenuates upregulation of CYP11A1 and CYP11B1 mRNA levels in large intestine (58). The requirement for both TNFα and LRH-1 suggests that during immune activation TNFα might (in addition to its proinflammatory effects) induce LRH-1 and subsequently increase glucocorticoid production and epithelial cell proliferation (63).
The expression of steroidogenic enzymes is differentially regulated in adrenal and intestinal cells. For example, cAMP increases CYP11A1 and CYP11B1 mRNA levels in an adrenal cell line but has the opposite effects in an intestinal epithelial cell line (57). Also, cAMP increases adrenal corticosterone release but decreases intestinal corticosterone release in culture. Similarly, ACTH administration increases adrenal-derived serum corticosterone levels but has no effect on intestine corticosterone production (57). Local production of ACTH in the intestine is unlikely, as POMC mRNA is undetectable in the gut (21). In contrast to the effects of ACTH and cAMP, protein kinase activation has no effect on enzyme transcription in adrenal cells but increases CYP11B1 transcription in intestinal epithelial cells (57). These findings show that regulatory signals have differing or opposite effects on adrenal and intestinal glucocorticoid production.
Function
Stimulation of T cells or macrophages results in secretion of TNFα and inflammation-mediated damage to the intestinal epithelium (Fig. 3B). TNFα also induces expression of LRH-1, which results in increased glucocorticoid production by and increased proliferation of epithelial cells (61, 62). Together, these processes may downregulate the inflammatory immune response and mediate repair of inflammatory damage (Table 3). Consistent with this hypothesis, intestinal glucocorticoids decrease damage resulting from inflammatory bowel disease in both mice and humans, and glucocorticoid-synthetic enzyme expression in the colon is decreased in patients with Crohn's disease and inflammatory bowel disease (13). Interestingly, endogenous glucocorticoid synthesis may regulate only cell-mediated (Th1-polarized) and not humoral (Th2-polarized) immune responses, as the latter does not stimulate glucocorticoid production in mouse intestine (61). Thus, glucocorticoid synthesis by the intestine is specifically stimulated by activation of a cell-mediated Th1 immune response, and acts to suppress and repair the damaging effects of this response. A cell-mediated Th17 inflammatory response also seems likely to stimulate local glucocorticoid synthesis, but this possibility remains to be tested.
SKIN
The skin, like the intestine, provides a boundary between the internal and external environments and is critical as a physical and immunological barrier. The epidermis is the outermost skin layer, which consists of keratinocytes that are continuously produced. Underneath the epidermis is the dermis, which contains connective tissues, nerve endings, sweat glands, hair follicles, and sebaceous glands. Under the dermis is the subcutaneous layer, which is composed of adipose tissue. The skin is continuously exposed to solar, thermal, mechanical, and immune stressors and responds rapidly to varying stressors to maintain its physical and functional integrity. The discovery of CRH and ACTH expression in skin, along with the presence of steroid-metabolizing enzymes, suggested that the skin might also synthesize glucocorticoids (93).
Evidence for Local Synthesis
Steroidogenic enzymes.
The de novo steroidogenic capacity of human skin was first demonstrated by the conversion of cholesterol into pregnenolone and by the expression of CYP11A1 mRNA and protein (106). Human skin expresses a functional homolog of StAR, StAR-related lipid transfer protein (MLN64, or STARD3) (99), and steroidogenic enzyme mRNA, protein, and activities needed for glucocorticoid synthesis have been shown (19, 81, 94, 112) (Table 1). Localization of steroidogenic enzyme mRNA and protein suggests glucocorticoid production in sebaceous cells (106), keratinocytes (112), fibroblasts (99) and potentially melanocytes (97). Cortisol appears to be the major corticosteroid product in human skin (98).
Mouse skin expresses CYP11A1, but further metabolism from pregnenolone into other steroids has not been reported (99). Glucocorticoid synthesis is likely to occur in rat skin, as most glucocorticoid-synthetic enzymes have been detected by measurements of mRNA (92) or activity (95), but CYP11A1 has not specifically been shown in rat skin.
Local steroid levels.
To our knowledge, local corticosteroid levels within the skin have not been measured.
Regulation
Human skin expresses CRH and ACTH proteins (38, 93, 97, 105). Incubation of melanocytes with CRH increases ACTH and cAMP levels, and incubation with ACTH increases cortisol levels in conditioned media (97) (Fig. 3C and Table 2). Remarkably, these data suggest that melanocytes may contain a miniature “analog” of the HPA axis. A similar local HPA axis also regulates glucocorticoid production in human hair follicles and fibroblasts (38, 96), complete with negative feedback of cortisol on CRH expression (38).
Mouse skin also contains CRH protein (82). The absence of CRH mRNA in mouse skin suggests that this CRH protein originates elsewhere (82). CRH levels in murine skin correspond with hair growth: CRH levels are high during the growth phase and low during the regression and resting phases (82).
Glucocorticoid synthesis in skin is induced by inflammation. Insults such as tissue damage, UV radiation, or pathogens result in local production of CRH (93, 94), which promotes inflammation. CRH-induced proinflammatory cytokines IL-1β and TNFα then increase ACTH and glucocorticoid production in the skin (105, 112).
Function
Glucocorticoid synthesis in the skin functions as a rapid and localized stress-response system (93) (Table 3). It is well known that topical treatment with high doses of glucocorticoids is anti-inflammatory and immunosuppressive. Locally produced glucocorticoids play a similar role, inhibiting production of proinflammatory signal molecules such as CRH and IL-1β (38, 112) (Fig. 3C). In contrast, adrenal glucocorticoids have rapid, short-term enhancing effects on cutaneous immunity by stimulating immune cell migration from circulating blood into skin tissues (16). Localized synthesis of glucocorticoids, by downregulating production of CRH and inflammatory cytokines, aids in preventing subsequent overshoot of the inflammatory immune response and further tissue damage. The physiological functions of skin-derived glucocorticoids, especially in vivo, require further study.
CENTRAL NERVOUS SYSTEM
Steroids play numerous important roles in the development and function of the central nervous system. Robel and Baulieu (80) first observed that steroids (e.g., pregnenolone, dehydroepiandrosterone) were present in the rat brain at high concentrations long after castration and adrenalectomy. Since then, these brain-derived steroids (“neurosteroids”) have been widely studied, but with an overwhelming focus on progestins, androgens, and estrogens (17). As the synthesis of sex steroids in the central nervous system has been extensively reviewed (12, 17, 78, 108), we focus here on the final steps of glucocorticoid or mineralocorticoid production.
Evidence for Local Synthesis
Steroidogenic enzymes.
The rat brain expresses the mRNAs of all the steroidogenic enzymes required for de novo synthesis of glucocorticoids and mineralocorticoids from cholesterol (26, 27, 45, 51, 54, 101) (Table 1). CYP11B1 and CYP11B2 proteins and activities have also been detected, and activity can be blocked by specific enzyme inhibitors (26, 51). The mouse brain contains mRNAs for most corticosteroid-synthetic enzymes, but mRNA levels for CYP11B1 and CYP11B2 are minimal (101). In the human brain, several regions also express enzymes for glucocorticoid and mineralocorticoid synthesis (5, 121). Interestingly, in both rat and human brain, CYP21 mRNA is very low or nondetectable (53, 101), but the same 21-hydroxylase function is performed by an alternate enzyme, CYP2D (CYP2D4 in the rat and CYP2D6 in the human) (45). This example highlights the possibility that corticosteroid synthesis in the brain (and other organs) can differ from corticosteroid synthesis in the adrenals.
Local steroid levels.
In intact rats, corticosterone and aldosterone levels are lower in brain than in plasma, but in adrenalectomized rats, aldosterone (but not corticosterone) levels are higher in brain than in plasma (30). These data suggest that aldosterone is synthesized in the rat brain. In contrast, in intact mice, corticosterone levels are similar or lower in brain compared with plasma, but in adrenalectomized mice, corticosterone levels are higher in brain than in plasma (14). This suggests that corticosterone is synthesized in the mouse brain (aldosterone was not quantified). There is also evidence for glucocorticoid synthesis in the developing and adult songbird brain. In newly hatched zebra finches, cortisol levels are higher in caudal telencephalon than in plasma (88), and in adult song sparrows (Melospiza melodia), corticosterone levels can be higher in plasma from the jugular vein (exiting the brain) than in plasma from the brachial vein (59).
Regulation
Expression of steroidogenic enzymes in the brain during early life is tightly controlled, as sex steroids are critical in neural development (43). Although glucocorticoids are also important in neural development, little is known regarding ontogenetic patterns of corticosteroid synthesis in brain. In the zebra finch, brain glucocorticoid levels during development are low except in the caudal telencephalon immediately after hatching (88). Glucocorticoids have potentially harmful effects during neural development, and altricial species such as finches, rodents, and humans may avoid such effects by maintaining low brain and circulating glucocorticoid levels.
In adult rats and mice, brain corticosterone production is triggered by an acute injection of alcohol (14) or by withdrawal from chronic alcohol consumption (48) (Table 2). Alcohol withdrawal causes dramatic and region-specific increases in brain corticosterone levels, with no change in plasma corticosterone levels. In some cases, corticosterone concentrations are much higher in brain than in plasma (48). Similar effects are seen with a social stressor, social defeat (14). The effects of ethanol and social defeat could be mediated by ACTH, which increases brain CYP11B1 mRNA (119).
In the adult song sparrow, data suggest that acute restraint stimulates brain corticosterone synthesis and secretion during the molt, a life history stage in which corticosterone production by the adrenals is dramatically reduced (to allow feather growth) (59). In the zebra finch, saline perfusion was used to remove blood contamination from the brain prior to measurement of brain steroid levels. Surprisingly, saline perfusion caused a rapid and region-specific increase in corticosterone levels in the brain, suggesting that hypoxia or ischemia could stimulate brain glucocorticoid synthesis (104).
In adult rats, the regulation of brain mineralocorticoid synthesis is well studied. Aldosterone synthesis in the rat brain is regulated by sodium intake. Low sodium intake increases expression of CYP11B2 (but not CYP11B1) mRNA in brain and in adrenals (118). However, high sodium intake or systemic angiotensin II administration does not affect CYP11B2 expression in brain (118). The lack of a response to systemic angiotensin II could be due to its limited crossing of the blood-brain barrier. Interestingly, the brain contains all the components of the RAS, including production of angiotensin II (52). These components could thus function as a miniature analog of the classical RAS.
Function
Systemic glucocorticoids influence behavior and neurophysiology (84). Locally produced brain glucocorticoids could have similar, and possibly additive, effects. In birds, during the molt, when adrenal glucocorticoid production is low, local synthesis in the brain during capture and restraint might facilitate escape behavior or learning (e.g., to avoid capture in the future). Alterations in behavior are also important after social defeat (e.g., to prevent further physical aggression) (Table 3). In contrast, chronic elevation of brain glucocorticoid levels may contribute to cognitive and memory deficits that can result from alcohol withdrawal (48).
Aldosterone acts on mineralocorticoid receptors in the brain to regulate blood pressure and salt consumption (28, 83). Central production of aldosterone is involved in the development of hypertension in the Dahl salt-sensitive rat model, in which hypothalamic aldosterone synthesis is increased relative to a control rat strain (31, 37). Brain-derived aldosterone is critical in driving sodium-induced hypertension. Infusion of a 3β-HSD inhibitor into the lateral ventricle prevents development of systemic hypertension in the adrenal-intact Dahl salt-sensitive rat (32). In addition, infusion of a CYP11B2 inhibitor into the lateral ventricle of adrenal-intact rats dramatically decreases blood pressure induced by salt consumption (31, 36). This is not due to an effect on adrenal aldosterone synthesis, as no decrease in blood pressure is seen with systemic administration of the same dose (Fig. 4). The effect of CYP11B2 inhibition is also reversible, as replacement with control vehicle results in blood pressure elevation (31). These studies demonstrate that, even in the presence of adrenal aldosterone synthesis, brain-derived aldosterone is critical for the regulation of blood pressure and the development of hypertension.
Fig. 4.
Effects of FAD286 (FAD, an inhibitor of CYP11B2 activity and aldosterone synthesis) on systolic blood pressure. Intracerebroventricular (icv) but not subcutaneous (sc) FAD administration decreases systolic blood pressure in hypertensive Dahl salt-sensitive rats. Crossover treatment demonstrates the reversibility of this effect. Importantly, all subjects were adrenal intact, and circulating aldosterone levels were similar in all groups at the conclusion of the experiment. Reprinted with permission from Ref. 31.
Even low levels of corticosteroid synthesis in the brain could have physiological significance, as the blood-brain barrier excludes several corticosteroids. The uptake of cortisol and aldosterone from the systemic circulation into the brain is especially low due to active removal by the transporter mdr1 (p-glycoprotein) (25).
CARDIOVASCULAR SYSTEM
Aldosterone plays an important role in the physiopathology of congestive heart failure, which prompted researchers to examine local synthesis of aldosterone in the heart and vasculature. The possibility of cardiovascular synthesis of aldosterone took on added importance after it was found that mineralocorticoid receptor blockade had beneficial effects in heart failure patients, even when plasma aldosterone levels were normal or low (71).
Evidence for Local Synthesis
Steroidogenic enzymes.
Cardiovascular corticosteroid production was first investigated in human blood vessels and later in the heart itself. In human vascular cells or tissue, local synthesis was suggested by PCR detection of CYP11A1, 3β-HSD, and CYP21 mRNA (35, 41, 120) (Table 1). However, reports are divided on whether CYP11B1 mRNA is detectable (41) or not (35, 120) and whether CYP11B2 mRNA is detectable (34, 35, 41) or not (1, 120). Similarly, in the adult human heart, steroidogenic enzyme mRNAs have been detected (9, 41, 120). Reports are again divided on whether CYP11B1 mRNA is present, but CYP11B2 mRNA is not detectable (9, 41, 120) except in subjects with heart failure (120).
In rats, CYP11A1, CYP11B1, and CYP11B2 mRNAs have been detected in blood vessels (102, 103), and StAR, CYP11B1, and CYP11B2 have been detected in the heart (8, 90). However, subsequent studies found expression of CYP11B1 and CYP11B2 mRNA to be extremely low or nondetectable in the heart of various rat strains (29, 64, 117). Ex vivo perfused rat blood vessels convert labeled pregnenolone to labeled corticosterone (103), and perfused blood vessels from adrenalectomized rats release corticosterone and aldosterone into the perfusate (102). Ex vivo perfused rat heart also contains corticosterone and aldosterone and releases them into the perfusate (90). Studies incubating rat heart homogenate with radiolabeled deoxycorticosterone disagree on the presence of CYP11B1 and CYP11B2 activity (64, 90). In mice, the heart contains the mRNAs for some steroidogenic enzymes, but not the mRNAs for CYP11B1 and CYP11B2, arguing against corticosteroid production (120). The chicken heart appears not to have steroidogenic capacity, because several steroidogenic enzyme activities are not detectable (46).
Local steroid levels.
To measure cardiac aldosterone production in humans, studies have compared blood collected from the cardiac vein or coronary sinus (draining from heart muscle) vs. blood collected from the aorta. In healthy subjects, plasma aldosterone levels do not differ between these locations, but in subjects with heart failure or hypertension, plasma aldosterone levels are higher in the cardiac vein than in the aorta (56, 116). These data suggest that the heart produces aldosterone during cardiovascular disease. Other investigators have found a different pattern: plasma aldosterone levels in the coronary sinus are lower than those in the aorta in healthy subjects, whereas plasma aldosterone levels are similar in the coronary sinus and aorta in subjects with heart failure (107). This pattern is more difficult to interpret but suggests differential aldosterone synthesis, metabolism, or uptake with heart failure.
In adrenal-intact rats, corticosterone and aldosterone levels in the heart tissue closely parallel those in plasma under varying conditions of salt intake (29). In adrenalectomized rats, aldosterone (but not corticosterone) is detectable in heart tissue from 30% of subjects but is not detectable in plasma, suggesting local production (29). However, aldosterone levels in the rat heart are not increased by systemic treatment with 11-deoxycorticosterone (the precursor of aldosterone) (29), indicating that precursor availability is not rate limiting. Taken together, these studies suggest that mammalian blood vessels and possibly heart produce aldosterone under certain conditions. Importantly, however, the variability in results among laboratories clearly suggests that this production is minimal and may occur only in specific contexts, subjects, or anatomic locations. This issue remains controversial (23).
Regulation
Cardiovascular aldosterone production appears to be minimal under normal conditions but may increase under pathological conditions such as heart failure. Under pathological conditions, the regulators of adrenal aldosterone production can also be produced locally in the cardiovascular system and possibly regulate local aldosterone synthesis (Table 2). For example, the neonatal and adult rat heart expresses the mRNA for renin and angiotensinogen (18, 67), and levels of these transcripts in the adult rat heart increase after experimental myocardial infarction (67). Angiotensin II protein and aldosterone levels also increase in the rat heart after experimental myocardial infarction (91). Taken together, these data raise the possibility that renin and angiotensin II of local origin regulate, at least in part, local aldosterone synthesis. Moreover, in pigs, the majority of angiotensin I and II in the heart is made locally rather than taken up from the blood (111). In humans, ACE activity has been detected in the heart, particularly during heart failure (20). Thus, mediators of the classical RAS are expressed locally in the cardiovascular system and may contribute to regulation of local aldosterone production (52).
Function
Aldosterone synthesis by the heart is minimal or absent in healthy individuals. Under normal conditions, these low levels of local aldosterone synthesis are probably insufficient to affect sodium and water reabsorption by the kidney and thus would not affect blood volume and blood pressure by this mechanism. In cases of pathophysiology, when local aldosterone synthesis might increase, chronic production of local aldosterone could paradoxically exacerbate heart problems (Table 3). Aldosterone acts directly upon the heart to stimulate fibrosis and left ventricular hypertrophy (52), which increase the risk of heart failure. This effect is independent of any change in blood pressure. A pathological role of local aldosterone synthesis is consistent with the observation that mineralocorticoid receptor antagonist treatment dramatically lowers human mortality from heart failure, even when circulating aldosterone levels are normal (71). Other mechanisms can explain this finding without the need to invoke local aldosterone synthesis, such as retention of circulating adrenal-derived aldosterone in cardiac tissue (23), but this possibility is unlikely (9).
COMMON THEMES
Locally Synthesized Glucocorticoids
One emerging theme is that local glucocorticoid synthesis occurs in immunologically important tissues. The thymus and bursa are critical sites of lymphocyte development, and the intestine and skin contain large numbers of immune cells. All of these are sites of exposure to antigen and lymphocyte activation. Locally produced glucocorticoids in lymphoid organs, intestine, and skin antagonize signals that promote lymphocyte activation or proliferation, thus acting to prevent lymphocyte overresponsiveness. This role of locally synthesized glucocorticoids is similar to a key role of circulating glucocorticoids in response to an immune challenge (16, 84). The lung, another immunologically important barrier, may also synthesize glucocorticoids (69, 73).
Local glucocorticoid synthesis appears to be independent of, or even in contrast to, patterns of adrenal glucocorticoid synthesis. For example, lymphoid glucocorticoid synthesis is high in early development and decreases over time, whereas adrenal glucocorticoid synthesis is low in early development and increases over time (75, 88, 110). Similarly, in the intestine, ACTH suppresses local glucocorticoid synthesis, although ACTH stimulates adrenal glucocorticoid synthesis (57). In the skin, local glucocorticoid synthesis is regulated by a local HPA axis analog (38) and is induced by local tissue damage (112).
Local glucocorticoid synthesis in primary lymphoid organs, intestine, and skin might have evolved as an adaptive mechanism to allow for localized action of glucocorticoids where and when they are needed, without incurring the costs of exposing all tissues to high glucocorticoid levels. Similar compartmentalization, or “Balkanization,” of steroid synthesis is seen in seasonally breeding birds (87). Breeding male song sparrows (in spring) have high systemic testosterone levels, while nonbreeding males (in winter) have low systemic testosterone levels (114). Both breeding and nonbreeding males must aggressively defend a territory, which is critical for survival and reproduction. In nonbreeding males, local synthesis of sex steroids in the brain is upregulated to support the expression of aggression while avoiding the costs of high systemic testosterone levels during this season (72, 100).
Locally Synthesized Mineralocorticoids
Local synthesis of mineralocorticoids occurs in the brain and may occur in the heart, although cardiac production of aldosterone remains controversial. Studies in lymphoid organs and intestine have not examined aldosterone or its synthetic enzyme CYP11B2, and this would be a useful goal for future research. Lymphoid organs contain few mineralocorticoid receptors, making aldosterone an unlikely product (55, 86), but the possibility has not been ruled out.
In the brain and heart, local aldosterone synthesis may be regulated by local expression of upstream RAS mediators. In brain, aldosterone synthesis is increased in response to low salt intake, in parallel with adrenal aldosterone synthesis. However, systemic treatment with angiotensin II has no effect on brain aldosterone synthesis, suggesting that brain aldosterone synthesis is independent of systemic RAS mediators and may depend on local expression of RAS mediators. Brain aldosterone synthesis plays an important role in systemic blood pressure regulation, as brain-specific inhibition of aldosterone synthesis reversibly decreases blood pressure (31).
While locally synthesized glucocorticoids have anti-inflammatory functions and serve to minimize tissue damage, local aldosterone synthesis in the brain and heart drives hypertension and heart failure, exacerbating tissue damage. Local aldosterone synthesis in healthy individuals may occur at low levels that allow beneficial effects of locally elevated mineralocorticoid levels while avoiding the costs of high systemic aldosterone levels (e.g., high systemic blood pressure). For the brain in particular, the blood-brain barrier allows only minimal uptake of circulating aldosterone (25), suggesting that it is important to minimize the effects of systemic aldosterone on the brain or that it is important for brain aldosterone levels to be regulated independently of systemic aldosterone levels.
CONCLUSIONS
The accumulated mass of evidence (Table 1) demonstrates that de novo corticosteroid synthesis occurs outside the adrenal cortex. The evidence is heavily weighted toward PCR studies of steroidogenic enzyme mRNA and corticosteroid synthesis in vitro. However, some studies have also measured local endogenous corticosteroid levels in adrenal-intact as well as adrenalectomized subjects (14, 29, 30, 48, 85, 88). Together, these different lines of evidence show that locally elevated steroid concentrations cannot simply be accounted for by the sequestration of circulating adrenal steroids. Furthermore, recent studies have used steroidogenic enzyme inhibitors in vivo to decrease local steroid synthesis in adrenal-intact subjects (31, 36, 76). These studies have convincingly demonstrated that local corticosteroid synthesis has functional consequences and is physiologically relevant. The effects of locally synthesized steroids likely depend on high local steroid concentrations either within an entire target organ (Fig. 2) or on a smaller scale (such as at the neuronal synapse). Thus, at level of the receptors, local corticosteroid concentrations can be far higher than those of systemic corticosteroids synthesized by distant adrenal cortices. Furthermore, most circulating glucocorticoids (90–95%) are bound with high affinity to corticosteroid-binding globulin and are unavailable to bind receptors (84).
In addition to the tissues discussed above, corticosteroid synthesis de novo from cholesterol might also occur in the lung (69, 73), retina (122), and kidney (115). In addition to de novo synthesis, bones, joints, liver, muscle, and fat express 11β-HSD type I and can convert circulating inactive glucocorticoid metabolites (e.g., cortisone) into active glucocorticoids (77) (Fig. 1). Also, tissues expressing only CYP11B1 or CYP11B2 can convert circulating precursors (e.g., 11-deoxycorticosterone) into glucocorticoids or mineralocorticoids, respectively. Overall, it is clear that the circulating systemic levels of cortisol, corticosterone, and aldosterone need not (and very often do not) reflect the local levels of these steroids at crucial target tissues.
Clinically, the local administration of glucocorticoids (e.g., via inhaler, topical application, injection into joints) can be advantageous over systemic glucocorticoid administration, which has numerous side effects throughout the body. This practice mimics the endogenous local synthesis of corticosteroids by various organs. Knowledge about these natural physiological processes may inform therapies that target corticosteroids to specific tissues or stimulate endogenous local corticosteroid synthesis at specific sites.
Compared with adrenal corticosteroids, locally produced corticosteroids can be synthesized by different enzymes (45), can differ in identity (46, 88), can bind differentially to receptors (86), can be differentially regulated (57), and have greater spatial specificity. These differences allow locally synthesized corticosteroids to complement the functions of systemic, adrenal-synthesized corticosteroids.
GRANTS
This work was supported by grants from the Canadian Institutes of Health Research (CIHR) and Michael Smith Foundation for Health Research to K. K. Soma, Department of Veterans Affairs Medical Research funds and NIH Grant HL-27255 to C. E. Gomez-Sanchez, and a CIHR Canada Graduate Scholarship to M. D. Taves.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Adam W. Plumb and Kim L. Schmidt for comments on the manuscript.
REFERENCES
- 1. Ahmad N, Romero DG, Gomez-Sanchez EP, Gomez-Sanchez CE. Do human vascular endothelial cells produce aldosterone? Endocrinology 145: 3626–3629, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Aird F, Clevenger CV, Prystowsky MB, Redei EVA. Corticotropin-releasing factor mRNA in rat thymus and spleen. Proc Natl Acad Sci USA 90: 7104–7108, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Atanasov AG, Leiser D, Roesselet C, Noti M, Corazza N, Schoonjans K, Brunner T. Cell cycle-dependent regulation of extra-adrenal glucocorticoid synthesis in murine intestinal epithelial cells. FASEB J 22: 4117–4125, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Batanero E, de Leeuw FE, Jansen GH, van Wichen DF, Huber J, Schuurman HJ. The neural and neuro-endocrine component of the human thymus. II. Hormone immunoreactivity. Brain Behav Immun 6: 249–264, 1992 [DOI] [PubMed] [Google Scholar]
- 5. Beyenburg S, Watzka M, Clusmann H, Blumcke I, Bidlingmaier F, Elger CE, Stoffel-Wagner B. Messenger RNA of steroid 21-hydroxylase (CYP21) is expressed in the human hippocampus. Neurosci Lett 308: 111–114, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Brewer JA, Kanagawa O, Sleckman BP, Muglia LJ. Thymocyte apoptosis induced by T cell activation is mediated by glucocorticoids in vivo. J Immunol 169: 1837–1843, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Byrd J. The effect of the humoral immune system-derived bursal anti-steroidogenic peptide (BASP) on corticosteroid biosynthesis in avian, porcine and canine adrenal cortical cells. Comp Biochem Physiol 108: 221–227, 1994 [DOI] [PubMed] [Google Scholar]
- 8. Casal AJ, Silvestre JS, Delcayre C, Capponi AM. Expression and modulation of steroidogenic acute regulatory protein messenger ribonucleic acid in rat cardiocytes and after myocardial infarction. Endocrinology 144: 1861–1868, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Chai W, Hofland J, Jansen PM, Garrelds IM, de Vries R, van den Bogaerdt AJ, Feelders RA, de Jong FH, Danser AHJ. Steroidogenesis vs. steroid uptake in the heart: do corticosteroids mediate effects via cardiac mineralocorticoid receptors? J Hypertens 28: 1044–1053, 2010 [DOI] [PubMed] [Google Scholar]
- 10. Chen Y, Qiao S, Tuckermann J, Okret S, Jondal M. Thymus-derived glucocorticoids mediate androgen effects on thymocyte homeostasis. FASEB J 24: 5043–5051, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cima I, Corazza N, Dick B, Fuhrer A, Herren S, Jakob S, Ayuni E, Mueller C, Brunner T. Intestinal epithelial cells synthesize glucocorticoids and regulate T cell activation. J Exp Med 200: 1635–1646, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Compagnone NA, Mellon SH. Neurosteroids: biosynthesis and function of these novel neuromodulators. Front Neuroendocrinol 56: 1–56, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Coste A, Dubuquoy L, Barnouin R, Annicotte JS, Magnier B, Notti M, Corazza N, Antal MC, Metzger D, Desreumaux P, Brunner T, Auwerx J, Schoonjans K. LRH-1-mediated glucocorticoid synthesis in enterocytes protects against inflammatory bowel disease. Proc Natl Acad Sci USA 104: 13098–13103, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Croft AP, O'Callaghan MJ, Shaw SG, Connolly G, Jacquot C, Little HJ. Effects of minor laboratory procedures, adrenalectomy, social defeat or acute alcohol on regional brain concentrations of corticosterone. Brain Res 1238: 12–22, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Davies E, Mackenzie SM. Extra-adrenal production of corticosteroids. Clin Exp Pharmacol Physiol 30: 437–445, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Dhabhar FS. Enhancing versus suppressive effects of stress on immune function: implications for immunoprotection and immunopathology. Neuroimmunomodulation 16: 300–317, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Do Rego JL, Seong JY, Burel D, Leprince J, Luu-The V, Tsutsui K, Tonon MC, Pelletier G, Vaudry H. Neurosteroid biosynthesis: enzymatic pathways and neuroendocrine regulation by neurotransmitters and neuropeptides. Front Neuroendocrinol 30: 259–301, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Dostal DE, Rothblum KN, Chernin MI, Cooper GR, Baker KM. Intracardiac detection of angiotensinogen and renin: a localized renin-angiotensin system in neonatal rat heart. Am J Physiol Cell Physiol 263: C838–C850, 1992 [DOI] [PubMed] [Google Scholar]
- 19. Dumont M, Luu-The V, Dupont E, Pelletier G, Labrie F. Characterization, expression, and immunohistochemical localization of 3beta-hydroxysteroid dehydrogenas/Δ5-Δ4 isomerase in human skin. J Invest Dermatol 99: 415–421, 1992 [DOI] [PubMed] [Google Scholar]
- 20. Falkenhahn M, Franke F, Bohle RM, Zhu YC, Stauss HM, Bachmann S, Danilov S, Unger T. Cellular distribution of angiotensin-converting enzyme after myocardial infarction. Hypertension 25: 219–226, 1995 [DOI] [PubMed] [Google Scholar]
- 21. Fink T, Schafer MKH, di Sebastiano, P Frieß H, Buchler M, Weihe E. Differential opioid gene expression in neurons and neuroendocrine cells of rat and human gastrointestinal tract. Regul Pept 53: S149–S150, 1994 [Google Scholar]
- 22. Franchini A, Ottaviani E. Immunoreactive POMC-derived peptides and cytokines in the chicken thymus and bursa of Fabricius microenvironments: age-related changes. J Neuroendocrinol 11: 685–692, 1999 [DOI] [PubMed] [Google Scholar]
- 23. Funder JW. Cardiac synthesis of aldosterone: going, going, gone? Endocrinology 145: 4793–4795, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Garvy BA, King LE, Telford WG, Morford LA, Fraker PJ. Chronic elevation of plasma corticosterone causes reductions in the number of cycling cells of the B lineage in murine bone marrow and induces apoptosis. Immunology 80: 587–592, 1993 [PMC free article] [PubMed] [Google Scholar]
- 25. Geerling JC, Loewy AD. Aldosterone in the brain. Am J Physiol Renal Physiol 297: F559–F576, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gomez-Sanchez CE, Zhou MY, Cozza EN, Morita H, Foecking MF, Gomez-Sanchez EP. Aldosterone biosynthesis in the rat brain. Endocrinology 138: 3369–3373, 1997 [DOI] [PubMed] [Google Scholar]
- 27. Gomez-Sanchez CE, Zhou MY, Cozza EN, Morita H, Eddleman FC, Gomez-Sanchez EP. Corticosteroid synthesis in the central nervous system. Endocr Res 22: 463–470, 1996 [DOI] [PubMed] [Google Scholar]
- 28. Gomez-Sanchez EP. Brain mineralocorticoid receptors: orchestrators of hypertension and end-organ disease. Curr Opin Nephrol Hypertens 13: 191–196, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Gomez-Sanchez EP, Ahmad N, Romero DG, Gomez-Sanchez CE. Origin of aldosterone in the rat heart. Endocrinology 145: 4796–4802, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Gomez-Sanchez EP, Ahmad N, Romero DG, Gomez-Sanchez CE. Is aldosterone synthesized within the rat brain? Am J Physiol Endocrinol Metab 288: E342–E346, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Gomez-Sanchez EP, Gomez-Sanchez CM, Plonczynski M, Gomez-Sanchez CE. Aldosterone synthesis in the brain contributes to Dahl salt-sensitive rat hypertension. Exp Physiol 95: 120–130, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gomez-Sanchez EP, Samuel J, Vergara G, Ahmad N. Effect of 3β-hydroxysteroid dehydrogenase inhibition by trilostane on blood pressure in the Dahl salt-sensitive rat. Am J Physiol Regul Integr Comp Physiol 288: R389–R393, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Gunnar MR, Cheatham CL. Brain and behavior interface: stress and the developing brain. Infant Ment Health J 24: 195- 211, 2003 [Google Scholar]
- 34. Hatakeyama H, Miyamori I, Fujita T, Takeda Y, Takeda R, Yamamoto H. Vascular aldosterone: biosynthesis and a link to angiotensin II-induced hypertrophy of vascular smooth muscle cells. J Biol Chem 269: 24316–24320, 1994 [PubMed] [Google Scholar]
- 35. Hatakeyama H, Miyamori I, Takeda Y, Yamamoto H, Mabuchi H. The expression of steroidogenic enzyme genes in human vascular cells. Biochem Mol Biol Int 40: 639–645, 1996 [DOI] [PubMed] [Google Scholar]
- 36. Huang BS, White RA, Ahmad M, Jeng AY, Leenen FHH. Central infusion of aldosterone synthase inhibitor prevents sympathetic hyperactivity and hypertension by central Na+ in Wistar rats. Am J Physiol Regul Integr Comp Physiol 295: R166–R172, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Huang BS, White RA, Jeng AY, Leenen FHH. Role of central nervous system aldosterone synthase and mineralocorticoid receptors in salt-induced hypertension in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 296: R994–R1000, 2009 [DOI] [PubMed] [Google Scholar]
- 38. Ito N, Ito T, Kromminga A, Bettermann A, Takigawa M, Kees F, Straub RH, Paus R. Human hair follicles display a functional equivalent of the hypothalamic-pituitary-adrenal axis and synthesize cortisol. FASEB J 19: 1332–1334, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Iwata M, Hanaoka S, Sato K. Rescue of thymocytes and T cell hybridomas from glucocorticoid-induced apoptosis by stimulation via the T cell receptor/CD3 complex: a possible in vitro model for positive selection of the T cell repertoire. Eur J Immunol 21: 643–648, 1991 [DOI] [PubMed] [Google Scholar]
- 40. Janeway CA, Travers P, Walport M, Shlomchik MJ. Immunobiology: the Immune System in Health and Disease. New York: Garland, 2005 [Google Scholar]
- 41. Kayes-Wandover KM, White PC. Steroidogenic enzyme gene expression in the human heart. J Clin Endocrinol Metab 85: 2519–2525, 2000 [DOI] [PubMed] [Google Scholar]
- 42. Keeney DS, Ikeda Y, Waterman MR, Parker KL. Cholesterol side-chain cleavage cytochrome P450 gene expression in the primitive gut of the mouse embryo does not require steroidogenic factor 1. Mol Endocrinol 9: 1091–1098, 1995 [DOI] [PubMed] [Google Scholar]
- 43. Kimoto T, Ishii H, Higo S, Hojo Y, Kawato S. Semicomprehensive analysis of the postnatal age-related changes in the mRNA expression of sex steroidogenic enzymes and sex steroid receptors in the male rat hippocampus. Endocrinology 151: 5795–5806, 2010 [DOI] [PubMed] [Google Scholar]
- 44. King LB, Vacchio MS, Dixon K, Hunziker R, Margulies DH, Ashwell JD. A targeted glucocorticoid receptor antisense transgene increases thymocyte apoptosis and alters thymocyte development. Immunity 3: 647–656, 1995 [DOI] [PubMed] [Google Scholar]
- 45. Kishimoto W, Hiroi T, Shiraishi M, Osada M, Imaoka S, Kominami S, Igarashi T, Funae Y. Cytochrome P450 2D catalyze steroid 21-hydroxylation in the brain. Endocrinology 145: 699–705, 2004 [DOI] [PubMed] [Google Scholar]
- 46. Lechner O, Dietrich H, Wiegers GJ, Vacchio MS, Wick G. Glucocorticoid production in the chicken bursa and thymus. Int Immunol 13: 769–776, 2001 [DOI] [PubMed] [Google Scholar]
- 47. Lechner O, Wiegers GJ, Oliveira-Dos-Santos AJ, Dietrich H, Recheis H, Waterman M, Boyd R, Wick G. Glucocorticoid production in the murine thymus. Eur J Immunol 30: 337–346, 2000 [DOI] [PubMed] [Google Scholar]
- 48. Little HJ, Croft AP, O'Callaghan MJ, Brooks SP, Wang G, Shaw SG. Selective increases in regional brain glucocorticoid: a novel effect of chronic alcohol. Neuroscience 156: 1017–1027, 2008 [DOI] [PubMed] [Google Scholar]
- 49. Lowenberg M, Tuynman J, Bilderbeek J, Gaber T, Buttgereit F, van Deventer S, Peppelenbosch M, Hommes D. Rapid immunosuppressive effects of glucocorticoids mediated through Lck and Fyn. Blood 106: 1703–1710, 2005 [DOI] [PubMed] [Google Scholar]
- 50. Lowenberg M, Verhaar AP, Bilderbeek J, van Marle J, Buttgereit F, Peppelenbosch MP, van Deventer SJ, Hommes DW. Glucocorticoids cause rapid dissociation of a T-cell-receptor-associated protein complex containing LCK and FYN. EMBO Rep 7: 1023–1029, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. MacKenzie SM, Clark CJ, Fraser R, Gomez-Sanchez CE, Connell JM, Davies E. Expression of 11beta-hydroxylase and aldosterone synthase genes in the rat brain. J Mol Endocrinol 24: 321–328, 2000 [DOI] [PubMed] [Google Scholar]
- 52. MacKenzie SM, Fraser R, Connell JMC, Davies E. Local renin-angiotensin systems and their interactions with extra-adrenal corticosteroid production. J Renin Angiotensin Aldosterone Syst 3: 214–221, 2002 [DOI] [PubMed] [Google Scholar]
- 53. Mellon SH, Miller WL. Extraadrenal steroid 21-hydroxylation is not mediated by P450c21. J Clin Invest 84: 1497–1502, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mellon SH, Deschepper CF. Neurosteroid biosynthesis: genes for adrenal steroidogenic enzymes are expressed in the brain. Brain Res 629: 283–292, 1993 [DOI] [PubMed] [Google Scholar]
- 55. Miller AH, Spencer RL, Stein M, McEwen BS. Adrenal steroid receptor binding in spleen and thymus after stress or dexamethasone. Am J Physiol Endocrinol Metab 259: E405–E412, 1990 [DOI] [PubMed] [Google Scholar]
- 56. Mizuno Y, Yoshimura M, Yasue H, Sakamoto T, Ogawa H, Kugiyama K, Harada E, Nakayama M, Nakamura S, Ito T, Shimasaki Y, Saito Y, Nakao K. Aldosterone production is activated in failing ventricle in humans. Circulation 103: 72–77, 2001 [DOI] [PubMed] [Google Scholar]
- 57. Mueller M, Atanasov A, Cima I, Corazza N, Schoonjans K, Brunner T. Differential regulation of glucocorticoid synthesis in murine intestinal epithelial versus adrenocortical cell lines. Endocrinology 148: 1445–1453, 2007 [DOI] [PubMed] [Google Scholar]
- 58. Mueller M, Cima I, Noti M, Fuhrer A, Jakob S, Dubuquoy L, Schoonjans K, Brunner T. The nuclear receptor LRH-1 critically regulates extra-adrenal glucocorticoid synthesis in the intestine. J Exp Med 203: 2057–2062, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Newman AEM, Pradhan DS, Soma KK. Dehydroepiandrosterone and corticosterone are regulated by season and acute stress in a wild songbird: jugular versus brachial plasma. Endocrinology 149: 2537–2545, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Norton JM, Wira CR. Dose-related effects of the sex hormones and cortisol on the growth of the bursa of Fabricius in chick embryos. J Steroid Biochem 8: 985–987, 1977 [DOI] [PubMed] [Google Scholar]
- 61. Noti M, Corazza N, Mueller C, Berger B, Brunner T. TNF suppresses acute intestinal inflammation by inducing local glucocorticoid synthesis. J Exp Med 207: 1057–1066, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Noti M, Corazza N, Tuffin G, Schoonjans K, Brunner T. Lipopolysaccharide induces intestinal glucocorticoid synthesis in a TNFalpha-dependent manner. FASEB J 24: 1340–1346, 2010 [DOI] [PubMed] [Google Scholar]
- 63. Noti M, Sidler D, Brunner T. Extra-adrenal glucocorticoid synthesis in the intestinal epithelium: more than a drop in the ocean? Semin Immunopathol 31: 237–248, 2009 [DOI] [PubMed] [Google Scholar]
- 64. Ohtani T, Ohta M, Yamamoto K, Mano T, Sakata Y, Nishio M, Takeda Y, Yoshida J, Miwa T, Okamoto M, Masuyama T, Nonaka Y, Hori M. Elevated cardiac tissue level of aldosterone and mineralocorticoid receptor in diastolic heart failure: beneficial effects of mineralocorticoid receptor blocker. Am J Physiol Regul Integr Comp Physiol 292: R946–R954, 2007 [DOI] [PubMed] [Google Scholar]
- 65. Ottaviani E, Franchini A, Franceschi C. Evolution of neuroendocrine thymus: studies on POMC-derived peptides, cytokines and apoptosis in lower and higher vertebrates. J Neuroimmunol 72: 67–74, 1997 [DOI] [PubMed] [Google Scholar]
- 66. Ottaviani E, Franchini A, Franceschi C. Presence of immunoreactive corticotropin-releasing hormone and cortisol molecules in invertebrate haemocytes and lower and higher vertebrate thymus. Histochem J 30: 61–67, 1998 [DOI] [PubMed] [Google Scholar]
- 67. Passier RC, Smits JF, Verluyten MJ, Daemen MJ. Expression and localization of renin and angiotensinogen in rat heart after myocardial infarction. Am J Physiol Heart Circ Physiol 271: H1040–H1048, 1996 [DOI] [PubMed] [Google Scholar]
- 68. Pazirandeh A, Jondal M, Okret S. Glucocorticoids delay age-associated thymic involution through directly affecting the thymocytes. Endocrinology 145: 2392–2401, 2004 [DOI] [PubMed] [Google Scholar]
- 69. Pazirandeh A, Xue Y, Rafter I, Sjovall J, Jondal M, Okret S. Paracrine glucocorticoid activity produced by mouse thymic epithelial cells. FASEB J 13: 893–901, 1999 [DOI] [PubMed] [Google Scholar]
- 70. Pazirandeh A, Xue Y, Prestegaard T, Jondal M, Okret S. Effects of altered glucocorticoid sensitivity in the T cell lineage on thymocyte and T cell homeostasis. FASEB J 16: 727–729, 2002 [DOI] [PubMed] [Google Scholar]
- 71. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 341: 709–717, 1999 [DOI] [PubMed] [Google Scholar]
- 72. Pradhan DS, Newman AEM, Wacker DW, Wingfield JC, Schlinger BA, Soma KK. Aggressive interactions rapidly increase androgen synthesis in the brain during the non-breeding season. Horm Behav 57: 381–389, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Provost PR, Tremblay Y. Genes involved in the adrenal pathway of glucocorticoid synthesis are transiently expressed in the developing lung. Endocrinology 146: 2239–2245, 2005 [DOI] [PubMed] [Google Scholar]
- 74. Purton JF, Boyd RL, Cole TJ, Godfrey DI. Intrathymic T-cell development and selection proceeds normally in the absence of glucocorticoid receptor signaling. Immunity 13: 179–186, 2000 [DOI] [PubMed] [Google Scholar]
- 75. Qiao S, Chen L, Okret S, Jondal M. Age-related synthesis of glucocorticoids in thymocytes. Exp Cell Res 314: 3027–3035, 2008 [DOI] [PubMed] [Google Scholar]
- 76. Qiao S, Okret S, Jondal M. Thymocyte-synthesized glucocorticoids play a role in thymocyte homeostasis and are down-regulated by adrenocorticotropic hormone. Endocrinology 150: 4163–4169, 2009 [DOI] [PubMed] [Google Scholar]
- 77. Raza K, Hardy R, Cooper MS. The 11beta-hydroxysteroid dehydrogenase enzymes—arbiters of the effects of glucocorticoids in synovium and bone. Rheumatology 49: 2016–2023, 2010 [DOI] [PubMed] [Google Scholar]
- 78. Remage-Healey L, London SE, Schlinger BA. Birdsong and the neural production of steroids. J Chem Neuroanat 39: 72–81, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Revskoy S, Halasz I, Redei E. Corticotropin-releasing hormone and proopiomelanocortin gene expression is altered selectively in the male rat fetal thymus by maternal alcohol consumption. Endocrinology 138: 389–396, 1997 [DOI] [PubMed] [Google Scholar]
- 80. Robel P, Baulieu EE. Neurosteroids: biosynthesis and function. Trends Endocrinol Metab 5: 1–8, 1994 [DOI] [PubMed] [Google Scholar]
- 81. Rogoff D, Gomez-Sanchez CE, Foecking MF, Wortsman J, Slominski A. Steroidogenesis in the human skin: 21-hydroxylation in cultured keratinocytes. J Steroid Biochem Mol Biol 78: 77–81, 2001 [DOI] [PubMed] [Google Scholar]
- 82. Roloff B, Fechner K, Slominski A, Furkert J, Botchkarev VA, Bulfone-Paus S, Zipper J, Krause E, Paus R. Hair cycle-dependent expression of corticotropin-releasing factor (CRF) and CRF receptors in murine skin. FASEB J 12: 287–297, 1998 [DOI] [PubMed] [Google Scholar]
- 83. Sakai RR, Nicolaidis S, Epstein AN. Salt appetite is suppressed by interference with angiotensin II and aldosterone. Am J Physiol Regul Integr Comp Physiol 251: R762–R768, 1986 [DOI] [PubMed] [Google Scholar]
- 84. Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 21: 55–89, 2000 [DOI] [PubMed] [Google Scholar]
- 85. Schmidt KL, Chin EH, Shah AH, Soma KK. Cortisol and corticosterone in immune organs and brain of European starlings: developmental changes, effects of restraint stress, comparison with zebra finches. Am J Physiol Regul Integr Comp Physiol 297: R42–R51, 2009 [DOI] [PubMed] [Google Scholar]
- 86. Schmidt KL, Malisch JL, Breuner CW, Soma KK. Corticosterone and cortisol binding sites in plasma, immune organs and brain of developing zebra finches: intracellular and membrane-associated receptors. Brain Behav Immun 24: 908–918, 2010 [DOI] [PubMed] [Google Scholar]
- 87. Schmidt KL, Pradhan DS, Shah AH, Charlier TD, Chin EH, Soma KK. Neurosteroids, immunosteroids, and the Balkanization of endocrinology. Gen Comp Endocrinol 157: 266–274, 2008 [DOI] [PubMed] [Google Scholar]
- 88. Schmidt KL, Soma KK. Cortisol and corticosterone in the songbird immune and nervous systems: local vs. systemic levels during development. Am J Physiol Regul Integr Comp Physiol 295: R103–R110, 2008 [DOI] [PubMed] [Google Scholar]
- 89. Schmidt M, Enthoven L, van der Mark M, Levine S, de Kloet ER, Oitzl MS. The postnatal development of the hypothalamic-pituitary-adrenal axis in the mouse. Int J Dev Neurosci 21: 125–132, 2003 [DOI] [PubMed] [Google Scholar]
- 90. Silvestre JS, Robert V, Heymes C, Aupetit-Faisant B, Mouas C, Moalic JM, Swynghedauw B, Delcayre C. Myocardial production of aldosterone and corticosterone in the rat. J Biol Chem 273: 4883–4891, 1998 [DOI] [PubMed] [Google Scholar]
- 91. Silvestre JS, Heymes C, Oubenaissa A, Robert V, Aupetit-Faisant B, Carayon A, Swynghedauw B, Delcayre C. Activation of cardiac aldosterone production in rat myocardial infarction: effect of angiotensin II receptor blockade and role in cardiac fibrosis. Circulation 99: 2694–2701, 1999 [DOI] [PubMed] [Google Scholar]
- 92. Simard J, Couet J, Durocher F, Labrie Y, Sanchez R, Breton N, Turgeon C, Labrie F. Structure and tissue-specific expression of a novel member of the rat 3β-hydroxysteroid dehydrogenase/Δ5–Δ4 isomerase (3β-HSD) family. J Biol Chem 268: 19659–19668, 1993 [PubMed] [Google Scholar]
- 93. Slominski A, Wortsman J. Neuroendocrinology of the skin. Endocr Rev 21: 457–487, 2000 [DOI] [PubMed] [Google Scholar]
- 94. Slominski A, Ermak G, Mihm M. ACTH receptor, CYP11A1, CYP17 and CYP21A2 genes are expressed in the skin. J Clin Endocrinol Metab 81: 2746–2749, 1996 [DOI] [PubMed] [Google Scholar]
- 95. Slominski A, Gomez-Sanchez CE, Foecking MF, Wortsman J. Active steroidogenesis in the normal rat skin. Biochim Biophys Acta 1474: 1–4, 2000 [DOI] [PubMed] [Google Scholar]
- 96. Slominski A, Zbytek B, Semak I, Sweatman T, Wortsman J. CRH stimulates POMC activity and corticosterone production in dermal fibroblasts. J Neuroimmunol 162: 97–102, 2005 [DOI] [PubMed] [Google Scholar]
- 97. Slominski A, Zbytek B, Szczesniewski A, Semak I, Kaminski J, Sweatman T, Wortsman J. CRH stimulation of corticosteroids production in melanocytes is mediated by ACTH. Am J Physiol Endocrinol Metab 288: E701–E706, 2005 [DOI] [PubMed] [Google Scholar]
- 98. Slominski A, Zbytek B, Szczesniewski A, Wortsman J. Cultured human dermal fibroblasts do produce cortisol. J Invest Dermatol 126: 1177–1178, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Slominski A, Zjawiony J, Wortsman J, Semak I, Stewart J, Pisarchik A, Sweatman T, Marcos J, Dunbar C, Tuckey RC. A novel pathway for sequential transformation of 7-dehydrocholesterol and the expression of the P450scc system in mammalian skin. Eur J Biochem 271: 4178–4188, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Soma KK. Testosterone and aggression: Berthold, birds and beyond. J Neuroendocrinol 18: 543–551, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Stromstedt M, Waterman MR. Messenger RNAs encoding steroidogenic enzymes are expressed in rodent brain. Mol Brain Res 34: 75–88, 1995 [DOI] [PubMed] [Google Scholar]
- 102. Takeda R, Hatakeyama H, Takeda Y, Iki K, Miyamori I, Sheng WP, Yamamoto H, Blair IA. Aldosterone biosynthesis and action in vascular cells. Steroids 60: 120–124, 1995 [DOI] [PubMed] [Google Scholar]
- 103. Takeda Y, Miyamori I, Yoneda T, Iki K, Hatakeyama H, Blair IA, Hsieh FY, Takeda R. Synthesis of corticosterone in the vascular wall. Endocrinology 135: 2283–2286, 1994 [DOI] [PubMed] [Google Scholar]
- 104. Taves MD, Schmidt KL, Ruhr IM, Kapusta K, Prior NH, Soma KK. Steroid concentrations in plasma, whole blood and brain: effects of saline perfusion to remove blood contamination from brain. PLoS ONE 5: e15727, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Teofoli P, Frezzolini A, Puddu P, De Pita O, Mauviel A, Lotti T. The role of proopiomelanocortin-derived peptides in skin fibroblast and mast cell functions. Ann NY Acad Sci 885: 268–276, 1999 [DOI] [PubMed] [Google Scholar]
- 106. Thiboutot D, Jabara S, McAllister JM, Sivarajah A, Gilliland K, Cong Z, Clawson G. Human skin is a steroidogenic tissue: steroidogenic enzymes and cofactors are expressed in epidermis, normal sebocytes, and an immortalized sebocyte cell line (SEB-1). J Invest Dermatol 120: 905–914, 2003 [DOI] [PubMed] [Google Scholar]
- 107. Tsutamoto T, Wada A, Maeda K, Mabuchi N, Hayashi M, Tsutsui T, Ohnishi M, Sawaki M, Fujii M, Matsumoto T, Horie H, Sugimoto Y, Kinoshita M. Spironolactone inhibits the transcardiac extraction of aldosterone in patients with congestive heart failure. J Am Coll Cardiol 36: 838–844, 2000 [DOI] [PubMed] [Google Scholar]
- 108. Tsutsui K, Ukena K, Usui M, Sakamoto H, Takase M. Novel brain function: biosynthesis and actions of neurosteroids in neurons. Neurosci Res 36: 261–273, 2000 [DOI] [PubMed] [Google Scholar]
- 109. Vacchio MS, Ashwell JD. Thymus-derived glucocorticoids regulate antigen-specific positive selection. J Exp Med 185: 2033–2038, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Vacchio MS, Papadopoulos V, Ashwell JD. Steroid production in the thymus: implications for thymocyte selection. J Exp Med 179: 1835–1846, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. van Kats JP, Danser AH, van Meegen JR, Sassen LM, Verdouw PD, Schalekamp MA. Angiotensin production by the heart: a quantitative study in pigs with the use of radiolabeled angiotensin infusions. Circulation 98: 73–81, 1998 [DOI] [PubMed] [Google Scholar]
- 112. Vukelic S, Stojadinovic O, Pastar I, Rabach M, Krzyzanowska A, Lebrun E, Davis SC, Resnik S, Brem H, Tomic-Canic M. Cortisol synthesis in epidermis is induced by IL-1 and tissue injury. J Biol Chem 286: 10265–10275, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Wada H, Salvante KG, Wagner E, Williams TD, Breuner CW. Ontogeny and individual variation in the adrenocortical response of zebra finch (Taeniopygia guttata) nestlings. Physiol Biochem Zool 82: 325–331, 2009 [DOI] [PubMed] [Google Scholar]
- 114. Wingfield JC, Hahn TP. Testosterone and territorial behaviour in sedentary and migratory sparrows. Anim Behav 47: 77–89, 1994 [Google Scholar]
- 115. Xue C, Siragy HM. Local renal aldosterone system and its regulation by salt, diabetes, and angiotensin II type 1 receptor. Hypertension 46: 584–590, 2005 [DOI] [PubMed] [Google Scholar]
- 116. Yamamoto N, Yasue H, Mizuno Y, Yoshimura M, Fujii H, Nakayama M, Harada E, Nakamura S, Ito T, Ogawa H. Aldosterone is produced from ventricles in patients with essential hypertension. Hypertension 39: 958–962, 2002 [DOI] [PubMed] [Google Scholar]
- 117. Ye P, Kenyon CJ, MacKenzie SM, Jong AS, Miller C, Gray GA, Wallace A, Ryding AS, Mullins JJ, McBride MW, Graham D, Fraser R, Connell JMC, Davies E. The aldosterone synthase (CYP11B2) and 11beta-hydroxylase (CYP11B1) genes are not expressed in the rat heart. Endocrinology 146: 5287–5293, 2005 [DOI] [PubMed] [Google Scholar]
- 118. Ye P, Kenyon CJ, MacKenzie SM, Seckl JR, Fraser R, Connell JMC, Davies E. Regulation of aldosterone synthase gene expression in the rat adrenal gland and central nervous system by sodium and angiotensin II. Endocrinology 144: 3321–3328, 2003 [DOI] [PubMed] [Google Scholar]
- 119. Ye P, Kenyon CJ, Mackenzie SM, Nichol K, Seckl JR, Fraser R, Connell JMC, Davies E. Effects of ACTH, dexamethasone, and adrenalectomy on 11beta-hydroxylase (CYP11B1) and aldosterone synthase (CYP11B2) gene expression in the rat central nervous system. J Endocrinol 196: 305–311, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Young MJ, Clyne CD, Cole TJ, Funder JW. Cardiac steroidogenesis in the normal and failing heart. J Clin Endocrinol Metab 86: 5121–5126, 2001 [DOI] [PubMed] [Google Scholar]
- 121. Yu L, Romero DG, Gomez-Sanchez CE, Gomez-Sanchez EP. Steroidogenic enzyme gene expression in the human brain. Mol Cell Endocrinol 190: 9–17, 2002 [DOI] [PubMed] [Google Scholar]
- 122. Zmijewski MA, Sharma RK, Slominski A. Expression of molecular equivalent of hypothalamic-pituitary-adrenal axis in adult retinal pigment epithelium. J Endocrinol 193: 157–169, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]