Abstract
Osteoclast-mediated bone resorption plays an essential role in calcium homeostasis and lactation. The cytokine receptor activator of nuclear factor κB ligand (RANKL) is one of a number of factors that controls the production, survival, and activity of osteoclasts. Calciotropic hormones, such as PTH, control RANKL transcription in part via an enhancer known as the distal control region (DCR), and mice lacking this enhancer have fewer osteoclasts under normal physiological conditions. Here, we have addressed the role of the DCR in situations in which activation of the PTH receptor is thought to stimulate bone resorption via elevation of RANKL expression. Dietary calcium deficiency stimulated RANKL expression in the bone of young (1 month old) wild-type, but not DCR knockout (KO), mice. Consistent with this, the cancellous bone loss and the increase in osteoclasts caused by dietary calcium deficiency were blunted in young KO mice. DCR deletion also prevented the increase in RANKL expression caused by dietary calcium deficiency in 6-month-old mice. However, the diet-induced bone loss was similar in wild-type and KO mice at this age. The increase in RANKL expression caused by lactation was also blunted in DCR KO mice, but lactation-induced bone loss was similar in both genotypes. These results demonstrate that, even though the DCR is required for the increase in RANKL expression associated with hyperparathyroidism or lactation, this increase is not required for the bone loss caused by these conditions in adult mice, suggesting that changes in other factors, such as osteoprotegerin or estrogen levels, play a dominant role.
Bone resorption is essential for mineral homeostasis, lactation, adaptation of the skeleton to changes in load, and fracture repair (1–4) and is performed by osteoclasts, highly specialized cells derived from monocyte/macrophage precursors (5). Increased resorption that is not balanced by formation of new bone by osteoblasts leads to loss of bone mass in conditions such as sex steroid deficiency and hyperparathyroidism (6). The TNF-family cytokine receptor activator of nuclear factor κB ligand (RANKL) is essential for osteoclast differentiation, function, and survival (7). RANKL expression in stromal cells, osteocytes, and chondrocytes is controlled by hormones that stimulate bone resorption such as PTH and 1,25-dihydroxyvitamin D3 (8–12). The same hormones also control the availability of RANKL by suppressing expression of the RANKL decoy receptor osteoprotegerin (OPG) (10, 13).
Previous studies have shown that PTH and 1,25-dihydroxyvitamin D3 control RANKL transcription, in part, via a distant enhancer designated the distal control region (DCR) (14–16). This enhancer is 76 kb upstream of the RANKL transcription start site and contributes to hormonal regulation of RANKL in vitro and in vivo (14, 15). Deletion of the DCR from the mouse genome decreases basal RANKL expression and leads to a high bone mass phenotype associated with low bone turnover (15). However, the physiological role of this enhancer in the response of the skeleton to elevated calciotropic hormone levels is unknown.
Dietary calcium deficiency leads to an increase in PTH, which in turn stimulates bone resorption either directly or indirectly by increasing the synthesis of 1,25-dihydroxyvitamin D3 (17). One mechanism by which PTH and 1,25-dihydroxyvitamin D3 may increase bone resorption is stimulation of RANKL expression. Lactation involves transfer of calcium from the mother's skeleton to offspring via breast milk. Lactation-induced bone loss is associated with increased production of PTH-related peptide (PTHrP) and decreased estrogen levels (2, 18). Moreover, RANKL increases during lactation and decreases upon weaning, suggesting that increases in RANKL may be one of the mechanisms by which resorption is stimulated during lactation (19).
In both of these conditions, activation of the PTH receptor, which binds both PTH and PTHrP, is thought to drive the increase in RANKL expression (20). However, whether this increase requires the DCR, and whether an increase in RANKL expression is required for the increase in bone resorption, is unknown. Here, we used mice lacking the DCR to demonstrate that this enhancer is required for the increase in RANKL expression associated with either dietary calcium deficiency or lactation. However, the bone loss caused by these conditions did not depend on an increase in RANKL expression in adult mice.
Results
The RANKL DCR contributes to cancellous bone loss due to dietary calcium deficiency in growing but not adult mice
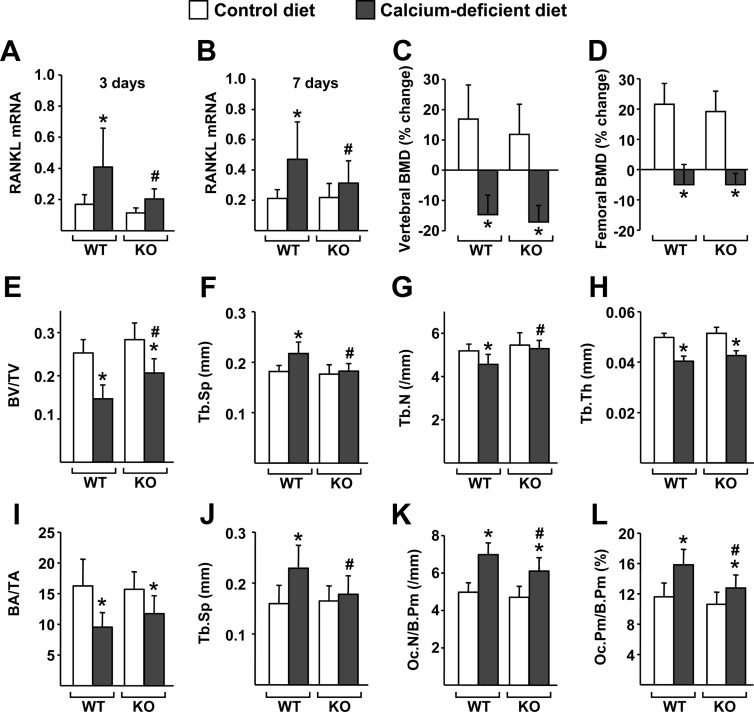
We have shown previously that 7 d of dietary calcium deficiency increased RANKL mRNA in the bones of 1-month-old wild-type (WT), but not DCR knockout (KO), mice (14). To determine whether this failure to increase RANKL expression in the DCR KO mice influenced the loss of bone that occurs with dietary calcium deficiency, we have now monitored changes in bone mass by dual energy x-ray absorptiometry (DEXA) and microcomputed tomography (μCT) in repeat experiments. Consistent with our previous results, dietary calcium deficiency elevated RANKL mRNA in the bones of WT but not KO mice, measured after either 3 or 7 d (Fig. 1, A and B). However, bone loss, as measured by DEXA, was equivalent in both genotypes (Fig. 1, C and D). Because of our previous demonstration that the DCR controls turnover in cancellous but not cortical bone, we investigated the possibility that the loss of cancellous bone may have been blunted in the KO mice by performing μCT analysis of the L4 vertebra. In contrast to what we had observed in older mice (15), there was no difference in cancellous bone volume of 1-month-old WT and DCR KO mice fed a normal diet (Fig. 1E). However, after 7 d of dietary calcium deficiency, the KO mice had a higher bone volume than WT mice, suggesting partial protection from cancellous bone loss. Consistent with this, the increase in trabecular spacing and the decrease in trabecular number that occurred in WT mice with dietary calcium deficiency did not occur in mice lacking the DCR (Fig. 1, F and G). Trabecular thickness decreased to a similar extent in both genotypes (Fig. 1H). Histomorphometric analysis of the lumbar vertebra revealed changes in trabecular architecture that were similar to the results observed by μCT except that bone area in the WT and KO mice on the calcium-deficient diet was not significantly different (Fig. 1, I and J). Moreover, consistent with blunting of the bone loss and of the increase in RANKL expression, dietary calcium deficiency increased osteoclast perimeter and osteoclast number to a lesser extent in KO mice compared with WT controls (Fig. 1, K and L). Taken together, these results demonstrate that the DCR transcriptional enhancer mediates the increase in RANKL associated with secondary hyperparathyroidism. In addition, the increase in RANKL contributes to the increase in osteoclasts and the decrease in cancellous bone caused by this condition in growing mice.
Fig. 1.
Deletion of the DCR blunts cancellous bone loss induced by dietary calcium deficiency in growing mice. A–L, One-month-old WT and DCR KO mice were fed control or calcium-deficient diet for 3 (A) or 7 d (B–L). A and B, Quantitative RT-PCR of RANKL mRNA in L5 vertebrae of WT and KO mice after 3 (A) or 7 d (B) of experimental diet. C and D, Percent change in BMD of the spine (C) and femur (D) of WT or DCR KO mice, determined by DEXA. E–H, μCT analysis of bone volume over tissue volume (BV/TV) (E), trabecular spacing (Tb.Sp) (F), trabecular number (Tb.N) (G), and trabecular thickness (Tb.Th) (H) in L4 vertebrae. I–L, Histomorphometric analysis of bone area over tissue area (BA/TA) (I), trabecular spacing (Tb.Sp) (J), osteoclast number per bone perimeter (Oc.N/B.Pm) (K), and osteoclast perimeter per bone perimeter (Oc.Pm/B.Pm) (L) in L1–L3 vertebra. All quantitative RT-PCR values were normalized to the housekeeping gene ribosomal protein S2. BMD percent change and gene expression values represent the mean of 10–12 animals per group (both sexes), and μCT and histomorphometry values represent the mean of five to seven animals per group (females). All statistical comparisons were done with two-way ANOVA. *, P < 0.05 effect of diet within genotype. #, P < 0.05 effect of genotype within diet.
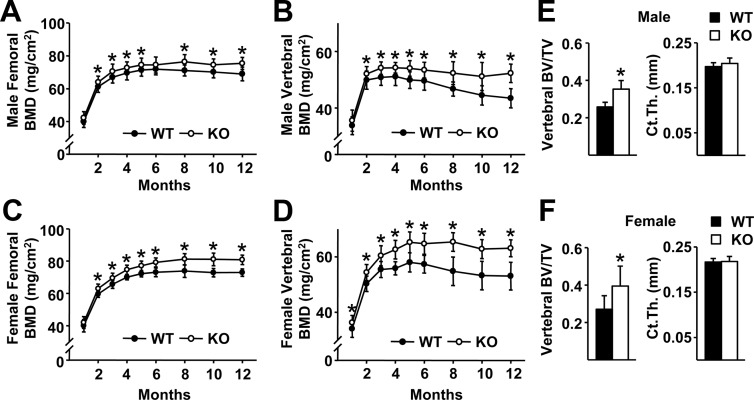
Analysis of DCR KO mice up to 5 months of age in our previous work had suggested that the skeletal phenotype becomes more pronounced in adult animals (15). To confirm this observation, we analyzed cohorts of WT and DCR KO mice using DEXA up to 12 months of age and found that indeed the difference in bone mass becomes greater after 2 months of age (Fig. 2, A–D). μCT analysis of L4 vertebra and femur at 12 months of age revealed that the DCR null mice had higher cancellous bone volume, but no difference in cortical thickness, compared with WT controls (Fig. 2, E and F). These latter results are consistent with our earlier finding of increased bone area, but no change in cortical width, in 5-month-old mice as measured by histomorphometry (15). Because the impact of DCR deletion becomes more pronounced in adult mice, we examined whether loss of the DCR might have a greater impact on bone loss induced by dietary calcium deficiency at 6 months of age.
Fig. 2.
The difference in bone mass between WT and DCR KO increases with age. A–D, Femoral and vertebral BMD of male (A and B) and female (C and D) WT and DCR KO mice were measured serially from 1 to 12 months of age. The same cohort of animals was used for each time point. E and F, Bone volume per tissue volume (BV/TV) of L4 vertebrae and femoral cortical thickness (Ct.Th) were determined by μCT analysis in male (E) and female (F) mice. BMD values represent the mean of 12–19 mice per group, and μCT values represent the mean of four to eight animals per group. *, P < 0.05 vs. WT by Student's t test.
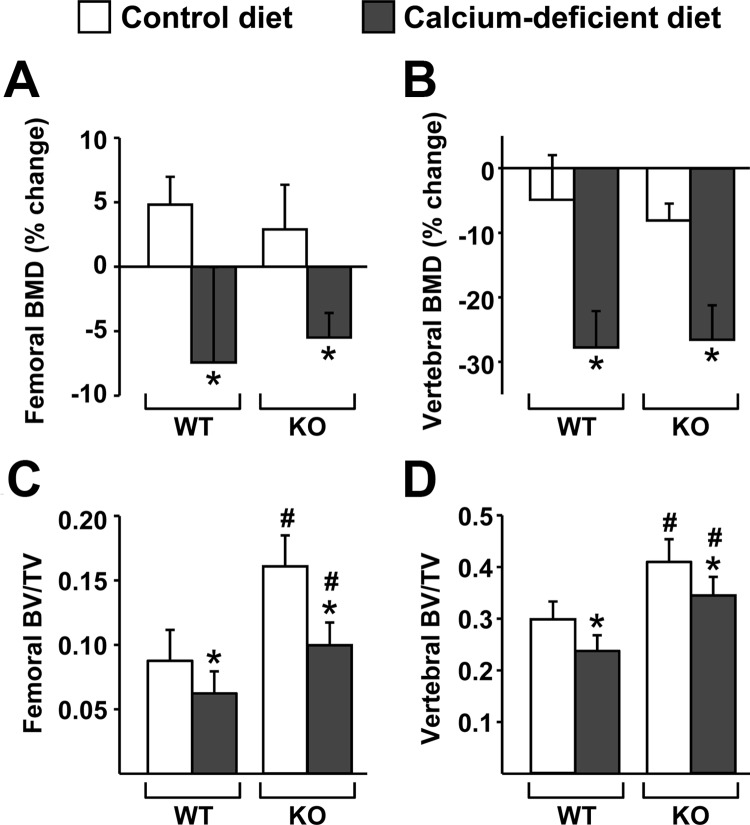
Similar to our findings in 1-month-old mice, dietary calcium deficiency caused a comparable amount of bone loss in WT and DCR KO mice, as determined by DEXA (Fig. 3, A and B). Thirty days of dietary calcium deficiency was used at this age, as opposed to 7 d in the younger mice, because pilot experiments demonstrated that longer times are required to produce consistent bone loss in adult mice (data not shown). In contrast to our findings in younger mice, μCT analysis revealed that the changes in cancellous bone volume caused by dietary calcium deficiency were similar in 6-month-old WT and KO mice, whether analyzed in the femur or the spine (Fig. 3, C and D). In these adult mice, dietary calcium deficiency did not significantly increase trabecular spacing or decrease trabecular number in either genotype (data not shown).
Fig. 3.
DCR deletion did not alter bone loss induced by dietary calcium deficiency in adult mice. Six-month-old WT and DCR KO female mice were fed control or calcium-deficient diet for 30 d. A and B, Percent change in BMD in the femur (A) and spine (B) was determined by comparison of DEXA at the start and end of 30 d of experimental diet. C and D, Bone volume over tissue volume (BV/TV) ratios of cancellous bone were determined by μCT analysis of femur (C) and L4 vertebrae (D). Values represent the mean of 10–12 animals per group. All statistical comparisons were done using two-way ANOVA. *, P < 0.05 effect of diet within genotype. #, P < 0.05 effect of genotype within diet.
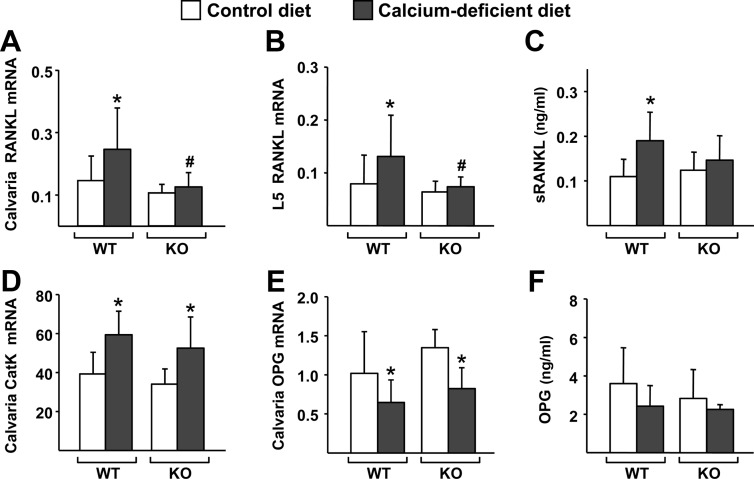
In spite of the similar skeletal responses to dietary calcium deficiency, but consistent with our studies in 1-month-old animals, the diet-induced increase in RANKL mRNA that occurred in the calvaria and lumbar vertebrae of WT mice did not occur in DCR KO mice (Fig. 4, A and B). Also consistent with the changes in mRNA, dietary calcium deficiency increased soluble RANKL (sRANKL) protein in the circulation in WT but not DCR KO mice (Fig. 4C). The lack of change in RANKL expression in DCR KO mice notwithstanding, cathepsin K gene expression, which reflects the number of osteoclasts in bone, was elevated by dietary calcium deficiency in both genotypes (Fig. 4D), suggesting that factors other than RANKL might be responsible for the changes in bone resorption. Because changes in OPG, the decoy receptor for RANKL, can alter osteoclast formation even in the absence of changes in RANKL expression (21, 22), we analyzed OPG mRNA and found that expression was reduced by dietary calcium deficiency to a similar extent in both WT and DCR KO mice (Fig. 4E). Despite reduced mRNA levels, dietary calcium deficiency did not alter circulating OPG levels in either genotype (Fig. 4F). Similar to the results that we obtained after 30 d, 14 d of dietary calcium deficiency also caused similar bone loss in both genotypes and elevation of RANKL mRNA in the bone of WT but not DCR KO mice (Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org). Based on these results, we conclude that the DCR contributes to the increase in RANKL expression that occurs in response to dietary calcium deficiency but that this increase is not required for bone loss in adult mice.
Fig. 4.
The DCR is required for the increase in RANKL caused by dietary calcium deficiency. A and B, Quantitative RT-PCR analysis of RANKL mRNA levels in calvaria (A) and L5 vertebrae (B) of WT and DCR KO mice. C, sRANKL levels measured from blood plasma of WT and KO mice. D and E, Quantitative RT-PCR analysis of cathepsin K (CatK) (D) and OPG (E) mRNA levels in calvaria of WT and DCR KO mice. F, OPG protein levels measured from blood plasma of WT and KO mice. All quantitative RT-PCR values were normalized to ribosomal protein S2 mRNA levels and are the mean of 10–11 animals per group. Blood plasma protein values are the mean of 9–10 samples per group. All statistical comparisons were done using two-way ANOVA. *, P < 0.05 effect of diet within genotype; #, P < 0.05 effect of genotype within diet.
The RANKL DCR is required for the elevation of RANKL mRNA but not the bone loss caused by lactation
Lactation is another situation in which activation of the PTH receptor is thought to stimulate bone resorption via elevation of RANKL expression, although in this situation, PTHrP rather than PTH is the ligand for the PTH receptor (18, 19). Six-month-old WT and DCR KO female mice were mated with WT males, allowed to give birth, and then to lactate for 12 d. Bone mineral density (BMD) measurements were performed at d 4 and 12 of lactation.
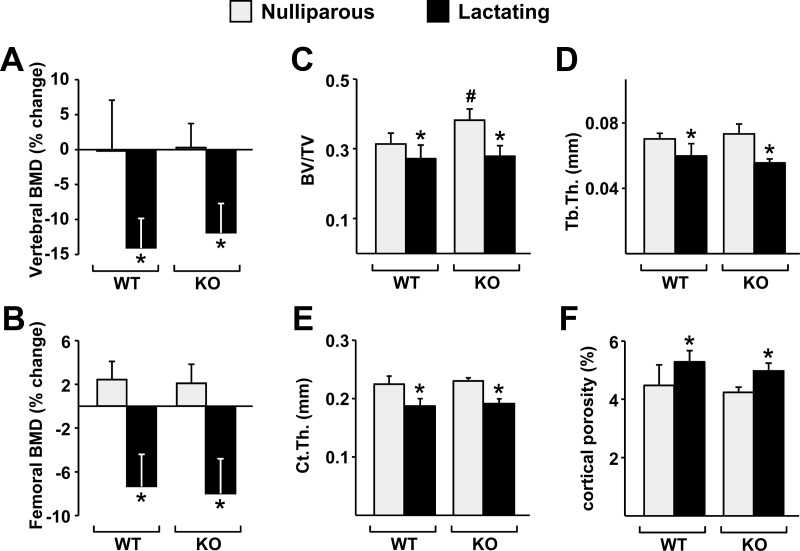
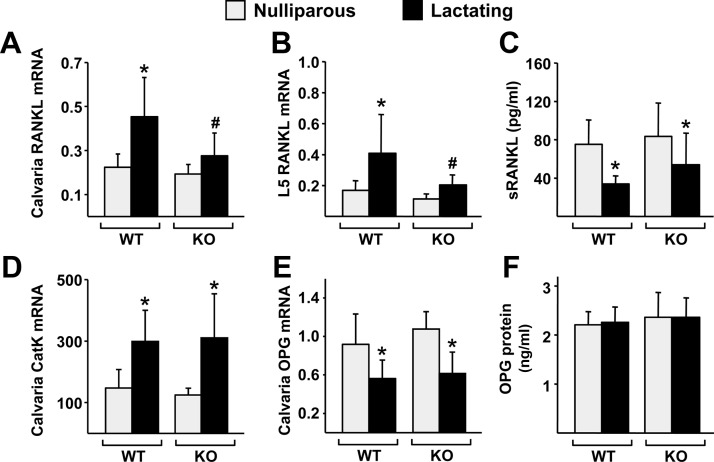
Similar to the results obtained with dietary calcium deficiency in adult mice, bone loss induced by lactation, as measured by DEXA, was not affected by the lack of the DCR (Fig. 5, A and B). In agreement with this finding, lactation caused a similar decline in cancellous bone volume and trabecular thickness in both genotypes (Fig. 5, C and D). Moreover, lactation caused a decrease in cortical thickness and an increase in cortical porosity that was indistinguishable between WT and DCR KO mice (Fig. 5, E and F). Nonetheless, the increase in RANKL expression that occurred in the bones of WT mice did not occur in DCR KO mice in two separate experiments (Fig. 6, A and B, and Supplemental Fig. 2, A and B). In contrast to the mRNA levels, lactation decreased circulating sRANKL protein to a similar extent in both genotypes (Fig. 6C). However, in spite of the lack of RANKL elevation, cathepsin K mRNA was increased by lactation in both genotypes, and this was associated with a suppression of OPG mRNA in bone (Fig. 6, D and E). Circulating OPG protein was not affected by lactation (Fig. 6F). Estrogen levels are reduced during lactation, and this reduction, like changes in OPG expression, can increase osteoclast number without changes in RANKL levels (23). Similar decreases in uterine weight confirmed that estrogen was reduced in both WT and DCR KO mice during lactation (Supplemental Fig. 2C).
Fig. 5.
DCR deletion does not alter bone loss caused by lactation. Six-month-old WT and DCR KO females were mated with C57BL/6 males and then allowed to give birth and lactate for 12 d. A and B, Percent change in the BMD in the spine (A) and femur (B) of WT or DCR KO mice, determined by DEXA at d 4 and 12 of lactation. C and D, Bone volume over tissue volume (BV/TV) (C) and trabecular thickness (Tb.Th) (D) determined by μCT analysis of L4 vertebrae. E and F, Cortical thickness (Ct.Th) (E) and cortical porosity (F) determined by μCT analysis of the femur. BMD values represent the mean of 9–12 animals per group, and the μCT values are the mean of five to nine animals per group. All statistical comparisons were done using two-way ANOVA. *, P < 0.05 lactation vs. nulliparity within genotype; #, P < 0.05 WT vs. KO within lactation status.
Fig. 6.
DCR deletion blunts the lactation-induced increase in RANKL expression. A and B, Quantitative RT-PCR analysis of RANKL mRNA levels in calvaria (A) and L5 vertebrae (B) of WT and DCR KO mice. C, sRANKL levels measured from blood plasma of WT and KO mice. D and E, Quantitative RT-PCR analysis of cathepsin K (CatK) (D) and OPG (E) mRNA levels in calvaria of WT and KO mice. F, OPG protein levels measured from blood plasma of WT and KO mice. All mRNA levels are normalized to ribosomal protein S2 mRNA levels, and the values are the mean of five to nine animals per group. All statistical comparisons were done using two-way ANOVA. *, P < 0.05 lactation vs. nulliparity within genotype; #, P < 0.05 effect of genotype within lactation status.
Discussion
The skeletal reservoir of calcium is essential for survival of amphibians, reptiles, birds, and mammals. Osteoclasts are necessary for access to this reservoir, and thus osteoclast formation, function, and survival are highly regulated. RANKL directly controls each of these processes, and in line with this, RANKL expression and availability are regulated by hormones that control calcium homeostasis. Here, we have examined the consequences of blunting the ability of the RANKL gene to respond to such hormones and found that this results in only a modest decrease in the skeletal response to hormonal changes. Specifically, bone loss due to dietary calcium deficiency was blunted only mildly in growing DCR KO mice and not at all in adult DCR KO mice. DCR deletion also had no effect on the bone loss caused by lactation. Nonetheless, the increase in RANKL mRNA levels in both of these conditions was blunted by loss of the DCR. Thus, these results suggest that the hormone-induced increase in RANKL expression is not essential for retrieval of calcium from the adult skeleton, at least in the two conditions studied here.
Changes in RANKL levels are clearly sufficient to alter osteoclast formation and function (24). Nonetheless, the increase in RANKL caused by dietary calcium deficiency or lactation may represent only one of a number of redundant mechanisms that can stimulate bone resorption in these situations. We observed a decrease in OPG expression in bone in both conditions. Mice lacking OPG have low bone mass due to increased osteoclast number without changes in RANKL mRNA levels (21, 22), and hormones that stimulate RANKL expression, such as PTH and 1,25-dihyroxyvitamin D3, simultaneously suppress OPG expression (10, 13). Because reduced levels of OPG increase the availability of existing RANKL protein, the reduced OPG levels that we observed in hyperparathyroidism and lactation would be expected to increase osteoclast differentiation and function even without changes in RANKL expression.
Reduced estrogen levels may also have contributed to changes in bone resorption in our lactation studies. Estrogen stimulates osteoclast apoptosis in vitro and in vivo via a nongenotropic pathway, and this effect plays a significant role in the bone loss caused by estrogen deficiency (25). Consistent with this, deletion of estrogen receptor α from osteoclasts increases osteoclast number and decreases bone mass without changes in circulating RANKL levels (25, 26). Estrogen levels are reduced during lactation, and this is associated with increased osteoclast surface and bone resorption markers (23). Moreover, estrogen administration to lactating mice blunts lactation-induced bone loss, suggesting that the decrease in estrogen levels is an important mechanism underlying the bone loss in this condition (23). Thus, decreased estrogen levels in our studies may have contributed to bone loss independent of changes in RANKL expression.
Considering the importance of calcium mobilization for mineral homeostasis and lactation, the presence of redundant mechanisms is not surprising. Indeed, a striking example of such redundancy was revealed in mice lacking the PTH gene. These mice display high bone mass and hypocalcemia due to reduced osteoclast formation (27). However, when PTH KO mice are placed on a calcium-deficient diet similar to the one used in our studies, bone resorption is elevated and bone loss occurs to an even greater extent than in WT controls (27). In PTH KO mice, extremely low circulating calcium levels are able to stimulate expression of renal 25-hydroxyvitamin D-1-α-hydroxylase independent of PTH (27). Thus, dietary calcium deficiency is able to induce striking bone loss even in the absence of PTH, the dominant regulator of calcium homeostasis under normal physiological circumstances (17).
It is also possible that the increase in RANKL that occurs in dietary calcium deficiency or lactation may be an inconsequential side effect of the hormonal changes that occur in these conditions. Hormone-dependent changes in factors that are known stimulators of bone remodeling do not always have physiological significance. For example, recent studies have shown that PTHrP expression in osteoblasts increases 5-fold upon weaning, providing a potential explanation for the dramatic increase in bone formation that occurs after lactation ends (28). However, deletion of the PTHrP gene from osteoblasts does not affect the recovery of bone mass after weaning (28).
It remains possible that both dietary calcium deficiency and lactation increased RANKL protein levels in the bones of DCR KO mice without changes in mRNA abundance. Although we did not measure RANKL protein in bone extracts, we did find that dietary calcium deficiency increased circulating sRANKL protein in WT but not DCR KO mice, reflecting the RANKL mRNA changes. In contrast, lactation caused a decrease in circulating sRANKL in both genotypes, even though RANKL mRNA levels increased in bone, at least in WT mice. The reason for the lactation-induced change in circulating sRANKL is unclear, but one interesting possibility is that lactation reduces the amount of RANKL shedding from the cell membrane, which would be expected to increase osteoclast formation even without increased production of RANKL mRNA or protein. Be that as it may, our results demonstrate that both dietary calcium deficiency and lactation do increase RANKL mRNA production in bone, that this increase requires the DCR, and that this increase is not required for the bone loss that occurs in these conditions.
Although DCR deletion did not affect the loss of bone induced by dietary calcium deficiency in adult mice, there was some protection of cancellous bone mass and architecture in growing mice. The reasons for these differences are unclear but may be due to the difference in the duration of dietary calcium deficiency: 1 wk in growing mice vs. 4 wk in adults. It is also possible that the demand for calcium is greater in growing animals, and thus, all available mechanisms are needed to maximally increase bone resorption, at least in cancellous bone.
In conclusion, our results demonstrate that the RANKL DCR is required for increasing RANKL mRNA production in vivo in response to conditions that activate the PTH receptor in bone. However, the failure to increase RANKL mRNA did not alter the increase in bone resorption or the decrease in bone mass caused by dietary calcium deficiency or lactation, most likely because of alternative and redundant mechanisms that have evolved to ensure retrieval of calcium from the skeleton.
Materials and Methods
Animal studies
Generation of DCR−/− mice has been described previously (14). The 1-month-old mice used in the dietary calcium deficient study were littermates in a mixed genetic background of approximately equal parts 129/Sv and C57BL/6. To obtain mice for the remainder of the studies, the DCR-null allele was crossed into the C57BL/6 genetic background for 13 generations, and the resulting DCR+/− mice were crossed to produce DCR+/+ (WT) and DCR−/− (KO) offspring. To induce secondary hyperparathyroidism, 1- or 5-month-old WT and DCR KO female mice were fed either calcium-deficient (0.01% calcium,) or a control diet (0.516% calcium), both from MP Biomedicals (Solon, OH), for 3 or 7 d (1-month-old mice), or for 14 or 30 d (5-month-old mice). For the lactation study, 4.5-month-old WT and DCR KO female mice were mated with C57BL/6 males and then allowed to give birth and lactate for 12 d. Histomorphometric analysis of lumbar vertebrae (L1–L3) was performed as previously described (11) using terminology recommended by the Histomorphometry Nomenclature Committee of the American Society for Bone and Mineral Research (29). All studies involving mice were approved by the Institutional Animal Care and Use Committees of the University of Arkansas for Medical Sciences and the Central Arkansas Veterans Healthcare System.
Gene expression analysis
Total RNA was purified from tissues using Ultraspec reagent (Biotecx Laboratories, Houston, TX), according to the manufacturer's directions. cDNA was made using the High-Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA) according to manufacturer's directions. TaqMan quantitative RT-PCR was performed as previously described (30) using the following primer probe sets from Applied Biosystems: RANKL (Mm0041908-m1), OPG (Mm00435452-m1), cathepsin K (Mm00484036-m1), and ribosomal protein S2 (forward, 5′-CCCAGGATGGCGACGAT-3′; reverse, 5′-CCGAATGCTGTAATGGCGTAT-3′; probe, 5′-FAM-TCCAGAGCAGGATCC-NFQ-3′). Relative mRNA levels were calculated using the ΔCt method (31).
BMD determinations
BMD was measured in live mice by DEXA with a PIXImus mouse densitometer (GE-Lunar Corp., Madison, WI) using the manufacturer's software as previously described (30). Percent change in BMD due to dietary calcium deficiency was assessed by comparison of BMD measurements at the start of the diet vs. 7 or 30 d of diet. Percent BMD change induced by lactation was determined by comparison of DEXA measurements taken on d 4 vs. 12 of lactation. Using a proprietary skeletal phantom, measurements of the total body BMD performed over the past 4 yr had a mean coefficient of variation of 3.1% (n = 285).
Microcomputed tomography
Soft tissue was removed from femurs or L4 vertebra, which were then fixed in 10% Millonig's formalin for 24 h and transferred to 100% ethanol. Bones were loaded into a 12.3-mm diameter scanning tube and imaged using a μCT (model μCT40; Scanco Biomedical, Bruttisellen, Switzerland). Scans were integrated into three-dimensional voxel images (1024 × 1024 pixels), and a Gaussian filter (σ = 0.8, support = 1) was used to reduce signal noise. A threshold of 200 was applied to all scans, at medium resolution (E = 55 kVp, I = 145 μA, integration time = 200 msec). The entire vertebral body was scanned with a transverse orientation. In the distal femur, 151 transverse slices were taken from the epicondyles and extending toward the proximal end of the femur. The cortical bone and the primary spongiosa were manually excluded from the analyses. All trabecular measurements were made by drawing contours every 10–20 slices and using voxel counting for bone volume per tissue volume and sphere-filling distance transformation indices, without preassumptions about the bone shape as a rod or plate for trabecular microarchitecture. Cortical thickness was measured at the femoral mid-diaphysis. Calibration and quality control were performed weekly using five density standards, and spatial resolution was verified monthly using a tungsten wire rod. Beam-hardening correction was based on the calibration records. Corrections were made for 200-mg hydroxyapatite (HA) for all energies. Over the past 3 yr, the coefficient of variation for the fifth density standard (mean five) was 1.28 (781 ± 10 sd mg HA/cm3) and for rod volume was 3.16 (0.0633 ± 0.002 sd cm3).
Blood chemistry
Blood was collected from either the retroorbital sinus or the tail in heparinized tubes at the time of killing. Collected blood was centrifuged at 1500 × g for 10 min to separate plasma from cells. Soluble RANKL and OPG in blood plasma were measured using R&D Systems Quantikine mouse RANKL (catalog no. MTR00) and mouse OPG (catalog no. MOP00) kits according to the manual provided by the manufacturer (R&D Systems, Minneapolis, MN).
Statistics
Data were analyzed using SigmaStat (SPSS Science, Chicago, IL). All values are reported as the mean ± sd, and differences between group means were evaluated using two-way ANOVA or Student's t test.
Supplementary Material
Acknowledgments
We thank P. E. Cazer, S. B. Berryhill, J. J. Goellner, and R. Shelton for technical support and R. L. Jilka for advice on the manuscript. We also thank the staff of the University of Arkansas for Medical Sciences Department of Laboratory Animal Medicine.
This work was supported by the National Institutes of Health Grants AR049794 (to C.A.O.) and AG13918 (to S.C.M.), by the Central Arkansas Veteran's Healthcare System (Merit Reviews to C.A.O., R.S.W., and S.C.M.), by the University of Arkansas for Medical Sciences (UAMS) Translational Research Institute Grant UL1 RR029884, and by UAMS tobacco settlement funds.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMD
- Bone mineral density
- μCT
- microcomputed tomography
- DCR
- distal control region
- DEXA
- dual energy x-ray absorptiometry
- KO
- knockout
- OPG
- osteoprotegerin
- PTHrP
- PTH-related peptide
- RANKL
- receptor activator of nuclear factor κB ligand
- sRANKL
- soluble RANKL
- WT
- wild type.
References
- 1. Parfitt AM. 1987. Bone and plasma calcium homeostasis. Bone 8(Suppl 1):S1–S8 [PubMed] [Google Scholar]
- 2. Kovacs CS. 2005. Calcium and bone metabolism during pregnancy and lactation. J Mammary Gland Biol Neoplasia 10:105–118 [DOI] [PubMed] [Google Scholar]
- 3. Robling AG, Castillo AB, Turner CH. 2006. Biomechanical and molecular regulation of bone remodeling. Annu Rev Biomed Eng 8:455–498 [DOI] [PubMed] [Google Scholar]
- 4. Schindeler A, McDonald MM, Bokko P, Little DG. 2008. Bone remodeling during fracture repair: the cellular picture. Semin Cell Dev Biol 19:459–466 [DOI] [PubMed] [Google Scholar]
- 5. Teitelbaum SL, Ross FP. 2003. Genetic regulation of osteoclast development and function. Nat Rev Genet 4:638–649 [DOI] [PubMed] [Google Scholar]
- 6. Manolagas SC. 2000. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev 21:115–137 (Review) [DOI] [PubMed] [Google Scholar]
- 7. Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G, Itie A, Khoo W, Wakeham A, Dunstan CR, Lacey DL, Mak TW, Boyle WJ, Penninger JM. 1999. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 397:315–323 [DOI] [PubMed] [Google Scholar]
- 8. Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T. 1998. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA 95:3597–3602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. O'Brien CA, Gubrij I, Lin SC, Saylors RL, Manolagas SC. 1999. STAT3 activation in stromal osteoblastic cells is required for induction of the receptor activator of NF-κB ligand and stimulation of osteoclastogenesis by gp130-utilizing cytokines or interleukin-1 but not 1,25-dihydroxyvitamin D-3 or parathyroid hormone. J Biol Chem 274:19301–19308 [DOI] [PubMed] [Google Scholar]
- 10. Lee SK, Lorenzo JA. 1999. Parathyroid hormone stimulates TRANCE and inhibits osteoprotegerin messenger ribonucleic acid expression in murine bone marrow cultures: correlation with osteoclast-like cell formation. Endocrinology 140:3552–3561 [DOI] [PubMed] [Google Scholar]
- 11. Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O'Brien CA. 2011. Matrix-embedded cells control osteoclast formation. Nat Med 17:1235–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Masuyama R, Stockmans I, Torrekens S, Van Looveren R, Maes C, Carmeliet P, Bouillon R, Carmeliet G. 2006. Vitamin D receptor in chondrocytes promotes osteoclastogenesis and regulates FGF23 production in osteoblasts. J Clin Invest 116:3150–3159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fu Q, Jilka RL, Manolagas SC, O'Brien CA. 2002. Parathyroid hormone stimulates receptor activator of NFκB ligand and inhibits osteoprotegerin expression via protein kinase A activation of cAMP-response element-binding protein. J Biol Chem 277:48868–48875 [DOI] [PubMed] [Google Scholar]
- 14. Fu Q, Manolagas SC, O'Brien CA. 2006. Parathyroid hormone controls receptor activator of NF-κB ligand gene expression via a distant transcriptional enhancer. Mol Cell Biol 26:6453–6468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Galli C, Zella LA, Fretz JA, Fu Q, Pike JW, Weinstein RS, Manolagas SC, O'Brien CA. 2008. Targeted deletion of a distant transcriptional enhancer of the receptor activator of nuclear factor-κB ligand gene reduces bone remodeling and increases bone mass. Endocrinology 149:146–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim S, Yamazaki M, Zella LA, Shevde NK, Pike JW. 2006. Activation of receptor activator of NF-κB ligand gene expression by 1,25-dihydroxyvitamin D3 is mediated through multiple long-range enhancers. Mol Cell Biol 26:6469–6486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brown EM, Juppner H. 2006. Parathyroid hormone: synthesis, secretion, and action. In: Favus MJ, ed. Primer on the metabolic bone diseases and disorders of mineral metabolism. 6th ed Washington, DC: American Society for Bone and Mineral Research; 90–99 [Google Scholar]
- 18. Wysolmerski JJ. 2010. Interactions between breast, bone, and brain regulate mineral and skeletal metabolism during lactation. Ann NY Acad Sci 1192:161–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ardeshirpour L, Dann P, Adams DJ, Nelson T, VanHouten J, Horowitz MC, Wysolmerski JJ. 2007. Weaning triggers a decrease in receptor activator of nuclear factor-κB ligand expression, widespread osteoclast apoptosis, and rapid recovery of bone mass after lactation in mice. Endocrinology 148:3875–3886 [DOI] [PubMed] [Google Scholar]
- 20. O'Brien CA. 2010. Control of RANKL gene expression. Bone 46:911–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nakamichi Y, Udagawa N, Kobayashi Y, Nakamura M, Yamamoto Y, Yamashita T, Mizoguchi T, Sato M, Mogi M, Penninger JM, Takahashi N. 2007. Osteoprotegerin reduces the serum level of receptor activator of NF-κB ligand derived from osteoblasts. J Immunol 178:192–200 [DOI] [PubMed] [Google Scholar]
- 22. Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, Scully S, Tan HL, Xu W, Lacey DL, Boyle WJ, Simonet WS. 1998. osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev 12:1260–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. VanHouten JN, Wysolmerski JJ. 2003. Low estrogen and high parathyroid hormone-related peptide levels contribute to accelerated bone resorption and bone loss in lactating mice. Endocrinology 144:5521–5529 [DOI] [PubMed] [Google Scholar]
- 24. Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ. 1998. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93:165–176 [DOI] [PubMed] [Google Scholar]
- 25. Martin-Millan M, Almeida M, Ambrogini E, Han L, Zhao H, Weinstein RS, Jilka RL, O'Brien CA, Manolagas SC. 2010. The estrogen receptor-α in osteoclasts mediates the protective effects of estrogens on cancellous but not cortical bone. Mol Endocrinol 24:323–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nakamura T, Imai Y, Matsumoto T, Sato S, Takeuchi K, Igarashi K, Harada Y, Azuma Y, Krust A, Yamamoto Y, Nishina H, Takeda S, Takayanagi H, Metzger D, Kanno J, Takaoka K, Martin TJ, Chambon P, Kato S. 2007. Estrogen prevents bone loss via estrogen receptor α and induction of Fas ligand in osteoclasts. Cell 130:811–823 [DOI] [PubMed] [Google Scholar]
- 27. Miao D, He B, Lanske B, Bai XY, Tong XK, Hendy GN, Goltzman D, Karaplis AC. 2004. Skeletal abnormalities in Pth-null mice are influenced by dietary calcium. Endocrinology 145:2046–2053 [DOI] [PubMed] [Google Scholar]
- 28. Kirby BJ, Ardeshirpour L, Woodrow JP, Wysolmerski JJ, Sims NA, Karaplis AC, Kovacs CS. 2011. Skeletal recovery after weaning does not require PTHrP. J Bone Miner Res 26:1242–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. 1987. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 2:595–610 [DOI] [PubMed] [Google Scholar]
- 30. O'Brien CA, Jilka RL, Fu Q, Stewart S, Weinstein RS, Manolagas SC. 2005. IL-6 is not required for parathyroid hormone stimulation of RANKL expression, osteoclast formation, and bone loss in mice. Am J Physiol Endocrinol Metab 289:E784–E793 [DOI] [PubMed] [Google Scholar]
- 31. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.