Abstract
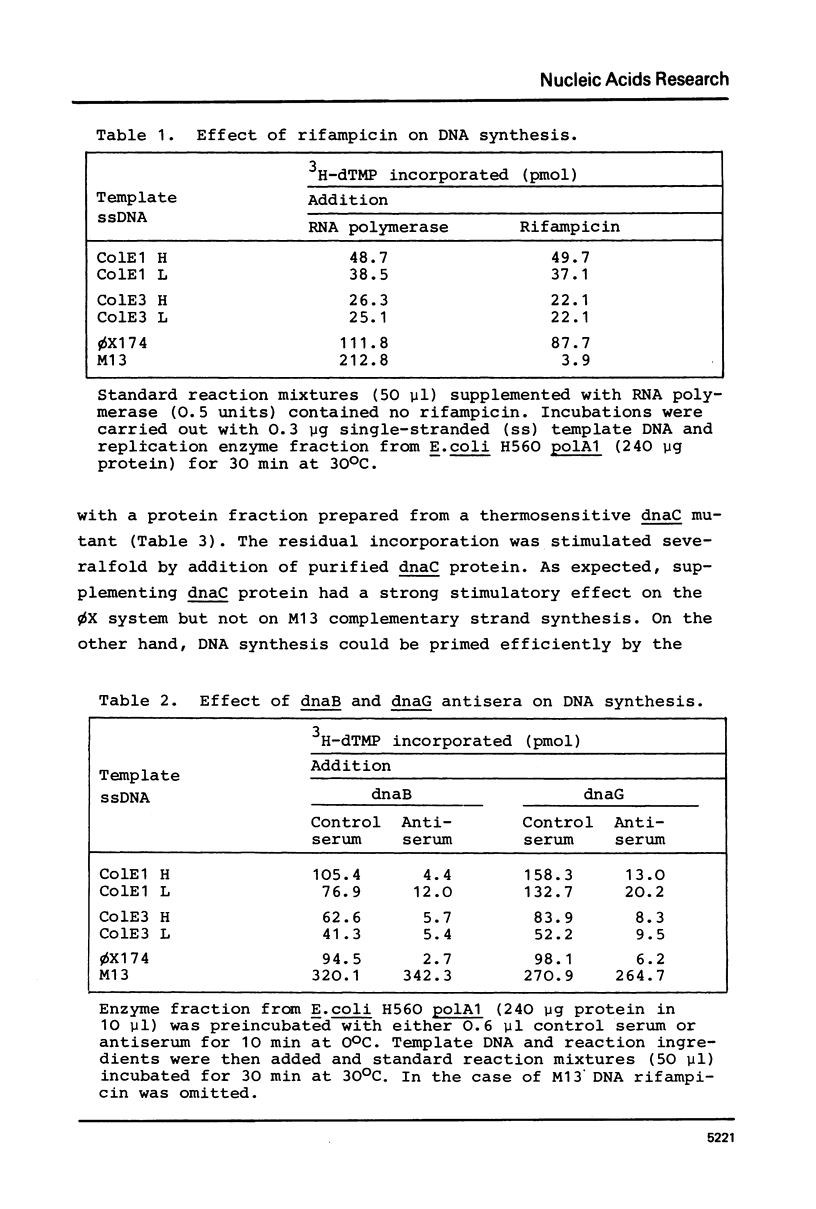
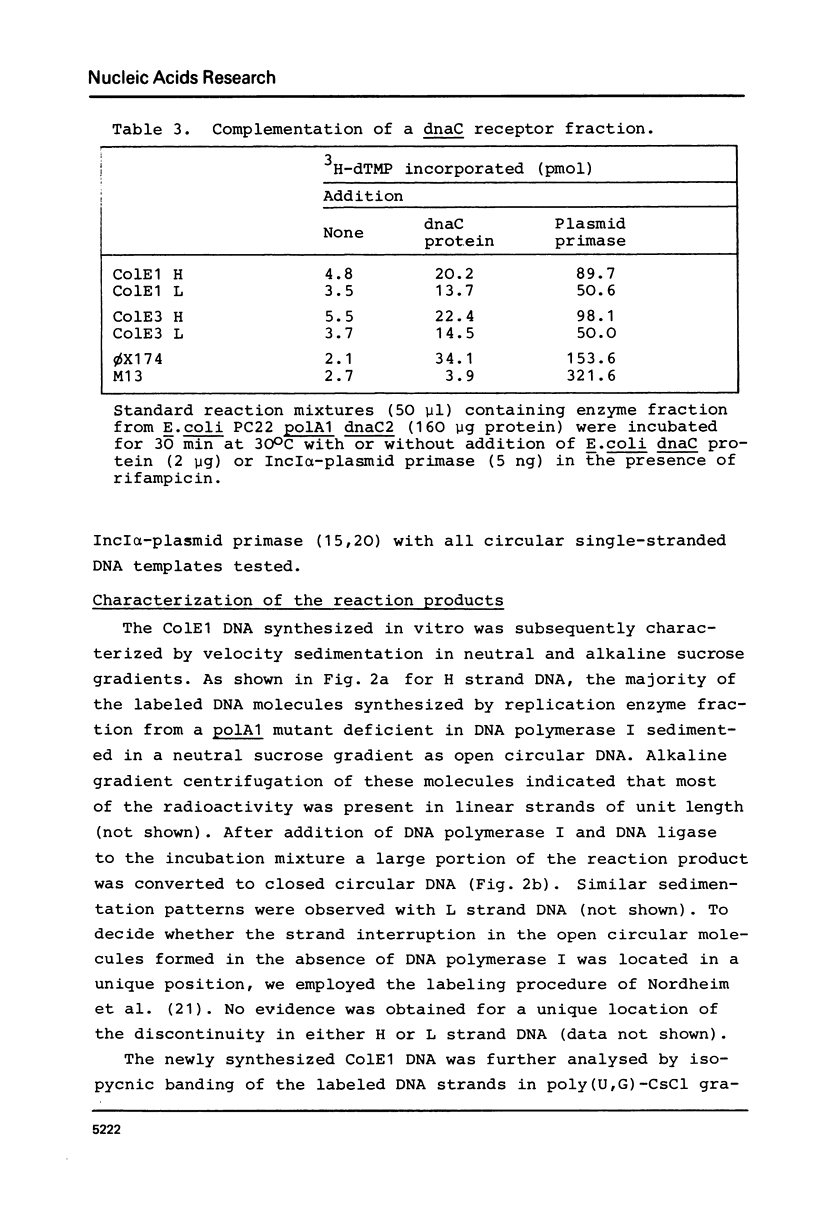
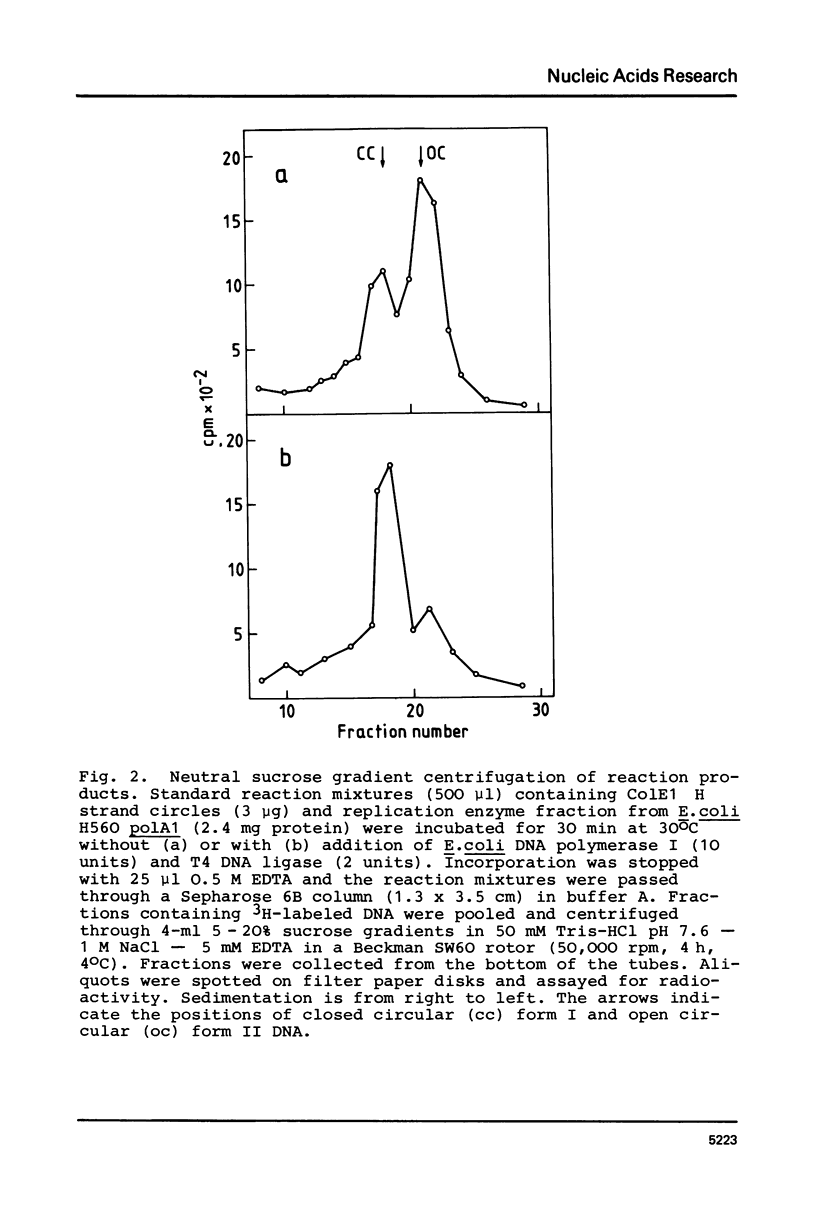
The opposite strands of the ColE1 and ColE3 plasmids were isolated as circular single-stranded DNA molecules. These molecules were compared with M13 and phi X174 viral DNA with respect to their capacity to function as templates for in vitro DNA synthesis by a replication enzyme fraction from Escherichia coli. It was found for both ColE plasmids that the conversion of H as well as L strands to duplex DNA molecules closely resembles phi X174 complementary strand synthesis and occurs by a rifampicin-resistant priming mechanism involving the dnaB, dnaC, and dnaG gene products. Restriction analysis of partially double-stranded intermediates indicates that preferred start sites for DNA synthesis are present on both strands of the ColE1 HaeII-C fragment. Inspection of the nucleotide sequence of this region reveals structural similarities with the origin of phi X174 complementary strand synthesis. We propose that the rifampicin-resistant initiation site (rri) in the ColE1 L strand is required for the priming of discontinuous lagging strand synthesis during vegetative replication and that the rri site in the H strand is involved in the initiation of L strand synthesis during conjugative transfer.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai K., Kornberg A. Unique primed start of phage phi X174 DNA replication and mobility of the primosome in a direction opposite chain synthesis. Proc Natl Acad Sci U S A. 1981 Jan;78(1):69–73. doi: 10.1073/pnas.78.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai K., Low R., Kobori J., Shlomai J., Kornberg A. Mechanism of dnaB protein action. V. Association of dnaB protein, protein n', and other repriming proteins in the primosome of DNA replication. J Biol Chem. 1981 May 25;256(10):5273–5280. [PubMed] [Google Scholar]
- Bartok K., Denhardt D. T. Site of cleavage of superhelical phiX174 replicative form DNA by the single strand-specific Neurospora crassa endonuclease. J Biol Chem. 1976 Jan 25;251(2):530–535. [PubMed] [Google Scholar]
- Bastia D. Determination of restriction sites and the nucleotide sequence surrounding the relaxation site of ColE1. J Mol Biol. 1978 Oct 5;124(4):601–639. doi: 10.1016/0022-2836(78)90174-2. [DOI] [PubMed] [Google Scholar]
- Bastia D. The nucleotide sequence surrounding the origin of DNA replication of Col E1. Nucleic Acids Res. 1977 Sep;4(9):3123–3142. doi: 10.1093/nar/4.9.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayne M. L., Dumas L. B. Isolation of circular viral and complementary strand DNA from bacteriophage f1 duplex replicative-form DNA. Anal Biochem. 1978 Dec;91(2):432–440. doi: 10.1016/0003-2697(78)90528-6. [DOI] [PubMed] [Google Scholar]
- Goldbach R. W., Evers R. F., Borst P. Electrophoretic strand separation of long DNAs with poly (U,G) in agarose gels. Nucleic Acids Res. 1978 Aug;5(8):2743–2754. doi: 10.1093/nar/5.8.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys G. O., Willshaw G. A., Anderson E. S. A simple method for the preparation of large quantities of pure plasmid DNA. Biochim Biophys Acta. 1975 Apr 2;383(4):457–463. doi: 10.1016/0005-2787(75)90318-4. [DOI] [PubMed] [Google Scholar]
- Inselburg J. Colicin factor DNA: a single non-homologous region in Col E2-E3 heteroduplex molecules. Nat New Biol. 1973 Feb 21;241(112):234–237. doi: 10.1038/newbio241234a0. [DOI] [PubMed] [Google Scholar]
- Lanka E., Geschke B., Schuster H. Escherichia coli dnaB mutant defective in DNA initiation: isolation and properties of the dnaB protein. Proc Natl Acad Sci U S A. 1978 Feb;75(2):799–803. doi: 10.1073/pnas.75.2.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanka E., Scherzinger E., Günther E., Schuster H. A DNA primase specified by I-like plasmids. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3632–3636. doi: 10.1073/pnas.76.8.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura N., Ray D. S. Expression of a DNA strand initiation sequence of ColE1 plasmid in a single-stranded DNA phage. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6566–6570. doi: 10.1073/pnas.77.11.6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordheim A., Hashimoto-Gotoh T., Timmis K. N. Location of two relaxation nick sites in R6K and single sites in pSC101 and RSF1010 close to origins of vegetative replication: implication for conjugal transfer of plasmid deoxyribonucleic acid. J Bacteriol. 1980 Dec;144(3):923–932. doi: 10.1128/jb.144.3.923-932.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka A., Nomura N., Morita M., Sugisaki H., Sugimoto K., Takanami M. Nucleotide sequence of small ColE1 derivatives: structure of the regions essential for autonomous replication and colicin E1 immunity. Mol Gen Genet. 1979 May 4;172(2):151–159. doi: 10.1007/BF00268276. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Friedmann T., Air G. M., Barrell B. G., Brown N. L., Fiddes J. C., Hutchison C. A., 3rd, Slocombe P. M., Smith M. The nucleotide sequence of bacteriophage phiX174. J Mol Biol. 1978 Oct 25;125(2):225–246. doi: 10.1016/0022-2836(78)90346-7. [DOI] [PubMed] [Google Scholar]
- Schekman R., Weiner J. H., Weiner A., Kornberg A. Ten proteins required for conversion of phiX174 single-stranded DNA to duplex form in vitro. Resolution and reconstitution. J Biol Chem. 1975 Aug 10;250(15):5859–5865. [PubMed] [Google Scholar]
- Schuster H., Mikolajczyk M., Rohrschneider J., Geschke B. phiX174 DNA-dependent DNA synthesis in vitro: requirement for P1 ban protein in dnaB mutant extracts of Escherichia coli. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3907–3911. doi: 10.1073/pnas.72.10.3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlomai J., Kornberg A. An Escherichia coli replication protein that recognizes a unique sequence within a hairpin region in phi X174 DNA. Proc Natl Acad Sci U S A. 1980 Feb;77(2):799–803. doi: 10.1073/pnas.77.2.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudenbauer W. L., Lanka E., Schuster H. Replication of small plasmids in extracts of Escherichia coli: involvement of the dnaB and dnaC protein in the replication of early replicative intermediates. Mol Gen Genet. 1978 Jul 4;162(3):243–249. doi: 10.1007/BF00268849. [DOI] [PubMed] [Google Scholar]
- Staudenbauer W. L., Scherzinger E., Lanka E. Replication of the colicin E1 plasmid in extracts of Escherichia coli: uncoupling of leading strand from lagging strand synthesis. Mol Gen Genet. 1979;177(1):113–120. doi: 10.1007/BF00267260. [DOI] [PubMed] [Google Scholar]
- Staudenbauer W. L. Structure and replication of the colicin E1 plasmid. Curr Top Microbiol Immunol. 1978;83:93–156. doi: 10.1007/978-3-642-67087-9_3. [DOI] [PubMed] [Google Scholar]
- Sternbach H., Engelhardt R., Lezius A. G. Rapid isolation of highly active RNA polymerase from Escherichia coli and its subunits by matrix-bound heparin. Eur J Biochem. 1975 Dec 1;60(1):51–55. doi: 10.1111/j.1432-1033.1975.tb20974.x. [DOI] [PubMed] [Google Scholar]
- Suggs S. V., Ray D. S. Nucleotide sequence of the origin for bacteriophage M13 DNA replication. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):379–388. doi: 10.1101/sqb.1979.043.01.044. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):77–90. doi: 10.1101/sqb.1979.043.01.013. [DOI] [PubMed] [Google Scholar]
- Tomizawa J. I., Ohmori H., Bird R. E. Origin of replication of colicin E1 plasmid DNA. Proc Natl Acad Sci U S A. 1977 May;74(5):1865–1869. doi: 10.1073/pnas.74.5.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren G. J., Twigg A. J., Sherratt D. J. ColE1 plasmid mobility and relaxation complex. Nature. 1978 Jul 20;274(5668):259–261. doi: 10.1038/274259a0. [DOI] [PubMed] [Google Scholar]
- Wickner S. H. DNA replication proteins of Escherichia coli. Annu Rev Biochem. 1978;47:1163–1191. doi: 10.1146/annurev.bi.47.070178.005503. [DOI] [PubMed] [Google Scholar]
- Wilkins B. M., Boulnois G. J., Lanka E. A plasmid DNA primase active in discontinuous bacterial DNA replication. Nature. 1981 Mar 19;290(5803):217–221. doi: 10.1038/290217a0. [DOI] [PubMed] [Google Scholar]
- Zipursky S. L., Marians K. J. Identification of two Escherichia coli factor Y effector sites near the origins of replication of the plasmids (ColE1 and pBR322. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6521–6525. doi: 10.1073/pnas.77.11.6521. [DOI] [PMC free article] [PubMed] [Google Scholar]