Abstract
Farnesoid X receptor (FXR) is known to play important regulatory roles in bile acid, lipid, and carbohydrate metabolism. Aged (>12 months old) Fxr−/− mice also develop spontaneous liver carcinomas. In this report, we used three mouse models to investigate the role of FXR deficiency in obesity. As compared with low-density lipoprotein receptor (Ldlr) knockout (Ldlr−/−) mice, the Ldlr−/−Fxr−/− double-knockout mice were highly resistant to diet-induced obesity, which was associated with increased expression of genes involved in energy metabolism in the skeletal muscle and brown adipose tissue. Such a striking effect of FXR deficiency on obesity on an Ldlr−/− background led us to investigate whether FXR deficiency alone is sufficient to affect obesity. As compared with wild-type mice, Fxr−/− mice showed resistance to diet-induced weight gain. Interestingly, only female Fxr−/− mice showed significant resistance to diet-induced obesity, which was accompanied by increased energy expenditure in these mice. Finally, we determined the effect of FXR deficiency on obesity in a genetically obese and diabetic mouse model. We generated ob−/−Fxr−/− mice that were deficient in both Leptin and Fxr. On a chow diet, ob−/−Fxr−/− mice gained less body weight and had reduced body fat mass as compared with ob/ob mice. In addition, we observed liver carcinomas in 43% of young (<11 months old) Ob−/−Fxr−/− mice. Together these data indicate that loss of FXR prevents diet-induced or genetic obesity and accelerates liver carcinogenesis under diabetic conditions.
Nuclear receptors are ligand-activated transcription factors that play important roles in reproduction and adult physiology. Farnesoid X receptor (FXR) is a nuclear hormone receptor that is important in regulating bile acid, lipid, and glucose homeostasis. Activation of FXR induces many beneficial effects; activated FXR, for instance, lowers blood triglyceride and cholesterol levels, improves insulin sensitivity in diabetic mouse models (reviewed in Refs. 1 and 2), and protects against atherosclerosis in Ldlr−/− or Apoe−/− mice (3–5) and nonalcoholic fatty liver disease in animal models (6, 7).
Consistent with the role of FXR activation in lipid metabolism, Fxr−/− mice have increased plasma and hepatic lipid levels (8, 9). In addition, Fxr−/− mice developed spontaneous hepatocellular carcinomas when they are older than 12 months (10, 11). The spontaneous development of liver carcinomas in Fxr−/− mice is thought to result from elevated bile acid levels, increased activation of the Wnt/β-catenin pathway, and increased inflammation in the liver (10–12).
Bile acids are the endogenous ligands for FXR. However, bile acids may also activate a number of pathways independent of FXR (13). Watanabe et al. (14) showed that bile acids prevent diet-induced obesity by activating a G protein-coupled bile acid receptor (TGR5) to induce energy expenditure in brown adipose tissue and skeletal muscle. Although Fxr−/− mice have increased bile acid pool size (15) and plasma bile acid levels (8, 15), the role of FXR deficiency in obesity remains to be determined.
In this report, we show that loss of FXR in low-density lipoprotein receptor (Ldlr) knockout (Ldlr−/−) mice prevents diet-induced obesity. We further show that loss of FXR alone is sufficient to induce energy expenditure and provide resistance to diet-induced obesity. Finally, we show that loss of FXR in ob/ob mice prevents weight gain and promotes liver carcinogenesis in both young (<11 months old) and aged (>12 months old) ob/ob mice. Together these data demonstrate that FXR deficiency prevents diet-induced or genetic obesity and accelerates liver carcinogenesis under diabetic conditions.
Materials and Methods
Mice and diets
C57BL/6J mice, Ldlr−/− mice, ob/ob mice, and Fxr−/− mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Fxr−/− mice have been backcrossed to C57BL/6 mice for seven generations. Western diet [no. D12108, 40% kcal from fat, 1.25% cholesterol (wt/wt)] and a high fat diet (no. D12492, 60% kcal from fat, 0.03% cholesterol) were purchased from Research Diets (New Brunswick, NJ). ob−/−Fxr−/− mice were generated by crossing ob/ob mice with Fxr−/− mice. All experiments were approved by the Institutional Animal Care and Use Committee at the Northeast Ohio Medical University, the University of California at Los Angeles, or Kent State University.
Real-time PCR
RNA was isolated using TRIzol reagent (Invitrogen, CA) and mRNA levels determined by quantitative RT-PCR (qRT-PCR) using SYBR Green Supermix and a real-time PCR machine from Applied Biosystems (Foster City, CA). Results were normalized to 36b4 mRNA.
Lipid analysis
Approximately 100 mg liver was homogenized in methanol and lipids were extracted in chloroform/methanol [2:1 (vol/vol)] as described (33). Triglyceride and cholesterol levels were then quantified using kits from Wako Chemicals (Richmond, VA).
Primary hepatocytes
Primary hepatocytes were isolated as described (34) and cultured in DMEM containing 10% fetal bovine serum (FBS) and 25 mm glucose for 6 h, followed by culture in DMEM containing 10% FBS and 0 mm glucose for 18 h. These cells were then incubated in DMEM containing 10% FBS/0 mm glucose or 10% FBS/27.5 mm glucose for 24 h before RNA isolation.
Intestinal fat absorption
Mice were injected iv with 500 mg of Triton WR1339 (Tyloxapol) per kilogram of body weight as a 10% solution in 0.9% NaCl. Immediately after the Tyloxapol injection, mice were given an intragastric 200 μl olive oil containing 7 μCi of [14C]triolein. Blood samples were drawn at 60, 120, 180, and 240 min after the administration of [14C]triolein. The amount of 3H radioactivity in plasma was determined using a liquid scintillation counter.
Glucose tolerance test
A glucose tolerance test was performed after an overnight fast as described (35).
Liver histology
Mouse tissues were fixed in 10% neutral buffered formalin and embedded in paraffin. Sections were subjected to standard hematoxylin/eosin staining.
cAMP measurement
cAMP levels in brown adipose tissue were determined using a cAMP Biotrak assay kit from GE Healthcare (Piscataway, NJ).
Fat content
Mouse fat content was quantified using an EchoMRI-700 (Echo Medical Systems, Houston, TX) or a minispec nuclear magnetic resonance analyzer (Bruker Optics, Billerica, MA).
Twenty-four-hour measurement of energy expenditure and activity
An Oxymax Fast System with attached activity monitors (Columbus Instruments, Columbus, OH) was used to measure oxygen consumption (VO2), CO2 production (VCO2), and physical activity (horizontal, vertical, and ambulatory). Detailed methods are shown in Supplemental Information, published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org.
Maximal VO2 treadmill exercise test
The detailed method is shown in Supplemental Information.
Statistical analysis
Statistical significance was analyzed using an unpaired Student t test, one-way or repeated-measures ANOVA, or χ2 test (for tumor incidence analysis) (GraphPad InStat3 and PASW software; GraphPad Inc., San Diego, CA). Because of the fundamental influence of body weight and composition on energy expenditure and to avoid the bias and overcorrection inherent in mass-based allometric corrections for energy expenditure in animals of different body weights, we used analyses of covariance to compare energy expenditure between groups; repeated-measures ANOVA were used to compare hourly VO2, VCO2, and respiratory quotient over the 24-h measurement periods. All values are expressed as mean ± sem. Differences were considered statistically significant at P < 0.05.
Results
Loss of FXR in Ldlr−/− mice prevents diet-induced obesity
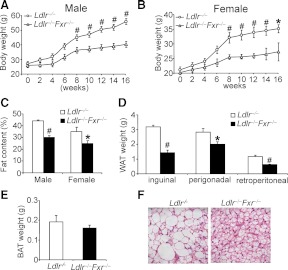
In an effort to study the role of FXR deficiency in atherosclerosis, we generated Ldlr−/−Fxr−/− double-knockout mice as described (9). Interestingly, as compared with Ldlr−/− mice, both male and female Ldlr−/−Fxr−/− mice were highly resistant to diet-induced weight gain (Fig. 1, A and B) with no change in food intake (Supplemental Fig. 1). Both male and female Ldlr−/−Fxr−/− mice had approximately 30% reduction in body fat content (percent body fat) (Fig. 1C). The data of Fig. 1D show that the mass of white adipose tissue (WAT), including inguinal, perigonadal, and retroperitoneal adipose tissue, was significantly reduced in Ldlr−/−Fxr−/− mice. There was no change in the mass of brown adipose tissue (BAT) (Fig. 1E). However, the size of brown adipocytes of Ldlr−/−Fxr−/− mice was smaller than those in Ldlr−/− mice (Fig. 1F). In contrast, there was no change in the size of white adipocytes (Supplemental Fig. 2A). Consistent with the latter observation, there was no change in glucose tolerance between the Ldlr−/−Fxr−/− mice and Ldlr−/− mice (Supplemental Fig. 2B). Together the data of Fig. 1 show that loss of FXR in Ldlr−/− mice leads to resistance to diet-induced obesity.
Fig. 1.
FXR deficiency in Ldlr−/− mice prevents Western diet-induced obesity. Ldlr−/− mice or Ldlr−/−Fxr−/− mice were fed a Western diet for 16 wk (n = 8 mice per group). A and B, The body weight of males (A) or females (B) from each genotype was measured. C, Fat content was measured using nuclear magnetic resonance. D and E, The weight of WAT (D) and BAT (E) in each genotype was measured. F, Representative hematoxylin and eosin staining from each genotype in the BAT (original magnification, ×400) was shown. *, P < 0.05; #, P < 0.01.
Genes involved in energy expenditure are induced in Ldlr−/−Fxr−/− mice
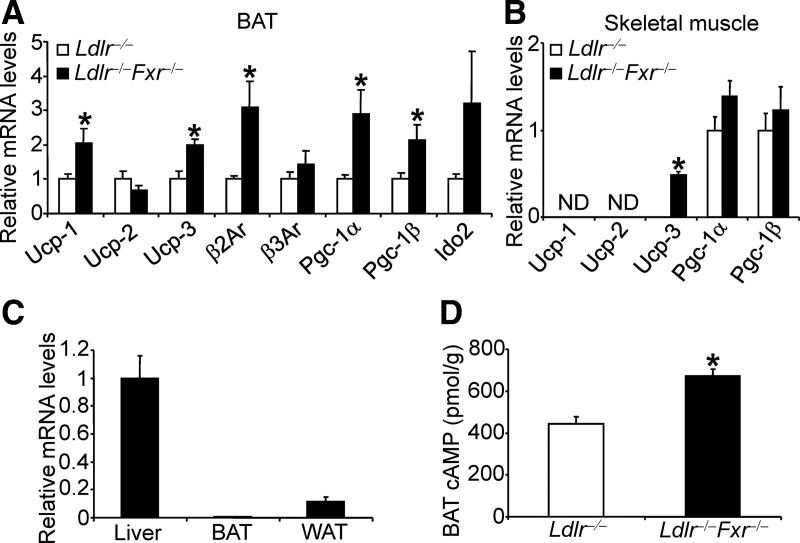
Consistent with the reduced size in brown adipocyte (Fig. 1F), qRT-PCR data show that uncoupled protein (Ucp)-1, Ucp3, β2-adrenergic receptor (β2Ar), and peroxisome proliferator-activated receptor-γ coactivator-1 (Pgc-1)-α and -β were induced in the BAT (Fig. 2A). Interestingly, Ucp3 was also induced in the skeletal muscle of Ldlr−/− −/−Fxr−/− mice (Fig. 2B).
Fig. 2.
FXR deficiency in Ldlr−/− mice resulted in increased expression of genes involved in energy metabolism in BAT and skeletal muscle. A and B, The mRNA levels of genes in BAT (A) or skeletal muscle (B) were determined by qRT-PCR (n = 8 per group). C, FXR mRNA levels were determined by qRT-PCR in wild-type mice (n = 5 per group). D, cAMP levels were determined (n = 8 per group). Ido2, Type 2 iodothyronine deiodinase. *, P < 0.05.
FXR is not expressed in BAT (Fig. 2C) or muscle (16). However, FXR deficiency is known to increase plasma levels of bile acids (8), which may in turn activate the TGR5 pathway in BAT and skeletal muscle to induce energy expenditure (14). Consistent with the latter hypothesis, plasma bile acid levels (Supplemental Fig. 2C) and cAMP levels were significantly increased in the BAT of Ldlr−/−Fxr−/− mice (Fig. 2D). Thus, the data of Fig. 2 show that loss of FXR in Ldlr−/− mice induces expression of genes involved in energy expenditure in BAT and skeletal muscle.
Fxr−/− mice are resistant to diet-induced obesity
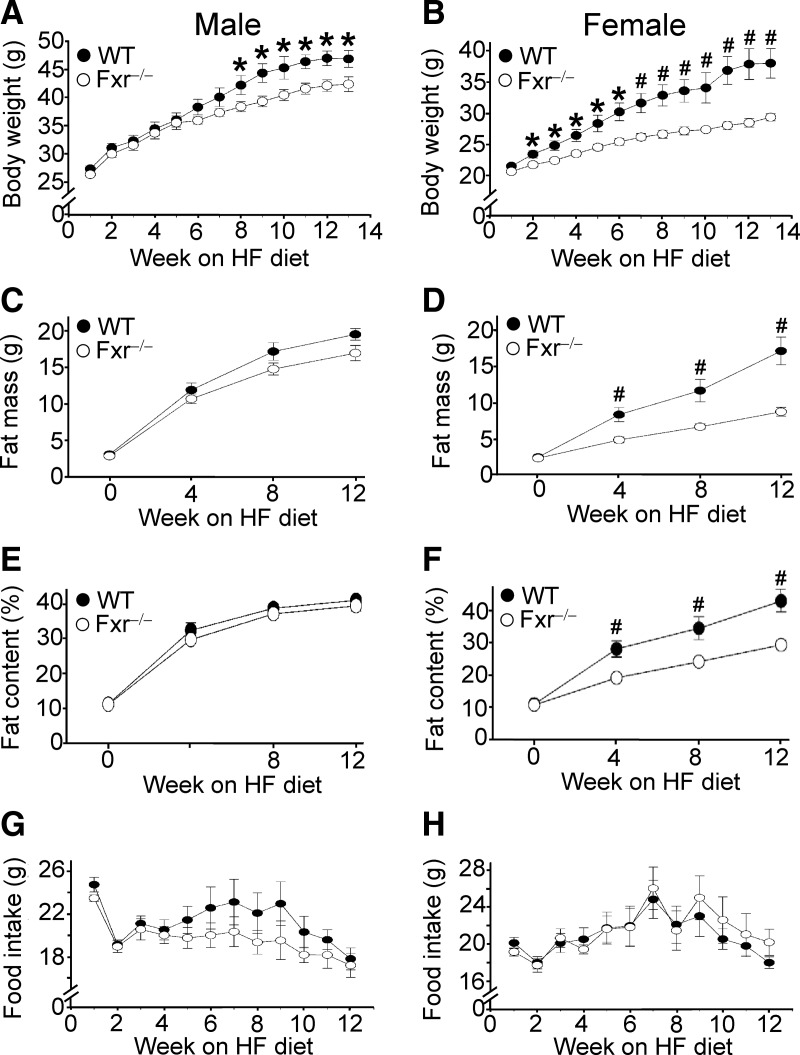
The data of Figs. 1 and 2 show that FXR deficiency in Ldlr−/− mice causes resistance to diet-induced obesity. Such data led us to investigate whether loss of FXR alone is sufficient to prevent diet-induced obesity. Consequently, we fed wild-type mice and Fxr−/− mice a high-fat diet and characterized the role of FXR in diet-induced obesity in detail. Compared with wild-type mice, both male and female Fxr−/− mice had reduced body weight gain, with female mice being more resistant to diet-induced weight gain (Fig. 3, A and B). Female Fxr−/− mice displayed reduced body fat mass (Fig. 3D) and fat content after 4, 8, or 12 wk of high-fat feeding (Fig. 3F), whereas these changes were not observed in male Fxr−/− mice (Fig. 3, C and E). Importantly, there was no change in food intake associated with genotype in either males (Fig. 3G) or females (Fig. 3H) (P > 0.05). Thus, the data of Fig. 3 show that female Fxr−/− mice are highly resistant to diet-induced obesity.
Fig. 3.
FXR deficiency alone is sufficient to cause resistance to high-fat diet-induced obesity. Wild-type (WT) and Fxr hematoxylin and eosin mice were fed a high-fat diet for 14 wk (n = 8 mice per group). A and B, Body weight of male (A) or female (B) mice was measured. C–H, Fat mass (C and D), fat content (E and F), and food intake (G and H; weekly food intake per mouse) of male mice (C, E, and G) or female mice (D, F, and H) was determined. *, P < 0.05; #, P < 0.01.
Female Fxr−/− mice have increased energy expenditure
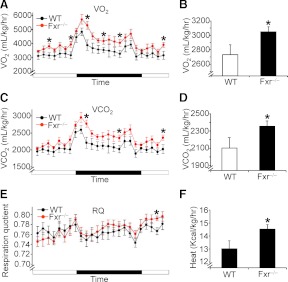
The finding that female Fxr−/− mice have reduced obesity but unchanged food intake (Fig. 3) suggests that female Fxr−/− mice may have increased energy expenditure. Consequently, we determined oxygen consumption and CO2 production using indirect calorimetry. Female Fxr−/− mice had higher VO2 (Fig. 4, A and B) and VCO2 (Fig. 4, C and D), unchanged respiration quotient (RQ) (Fig. 4E), and increased heat production (Fig. 4F); significant differences were detected in only 6 hourly periods during the 24-h measurement period (fourth, seventh, and 11th hours after lights-on, and second, fifth, and eighth hours after lights-off for VO2). The finding that RQ was within the range of 0.74–0.79 (Fig. 4E) indicates that fatty acids were the major fuel consumed in these mice. In contrast with the findings from female wild-type and Fxr−/− mice, there was no change in VO2, VCO2, or energy expenditure in male wild-type vs. Fxr−/− mice (Supplemental Fig. 3).
Fig. 4.
FXR deficiency increases energy expenditure. Female wild-type (WT) and Fxr hematoxylin and eosin mice were fed a high-fat diet for 14 wk (n = 8 mice per group). Oxygen (O2) consumption (A and B), CO2 production (C and D), RQ (E), and heat production (F) were determined. In A, C and E, the dark bar indicates the dark cycle (1900 h to 0700 h). *, P < 0.05.
To determine whether FXR deficiency affects activity, we measured the horizontal, vertical, and ambulatory (nonstationary counts) physical activity of both wild-type and Fxr−/− mice. We did not see any change in activity between these two genotypes (Supplemental Table 1). Together these data suggest that female Fxr−/− mice have increased energy expenditure, which may account, at least in part, for the reduced obesity in these mice.
Fxr−/− mice have increased expression of genes involved in energy expenditure in skeletal muscle and increased intestinal fat absorption
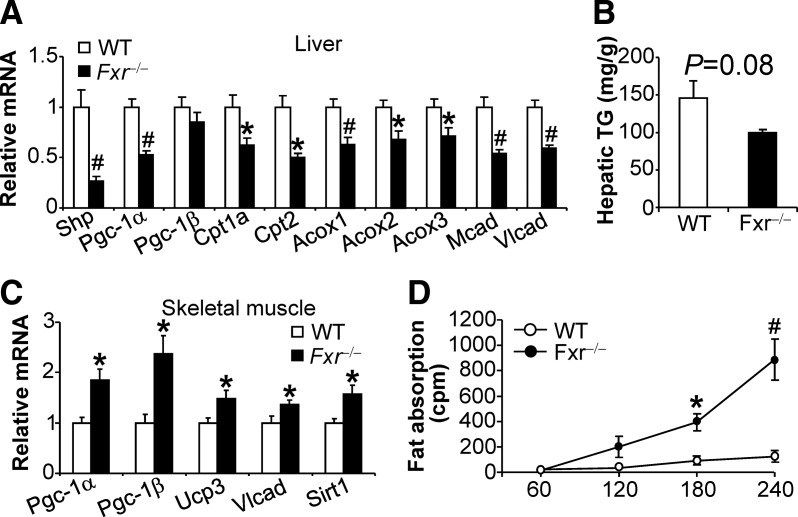
To determine the underlying mechanism by which female Fxr−/− mice may have increased energy expenditure, we analyzed gene expression in the liver, BAT, and skeletal muscle. In the liver, the FXR target gene Shp (small heterodimer partner), and many genes involved in fatty acid oxidation, such as Pgc-1α, Cpt1a, Cpt2, Acox1 (acyl-coenzyme A oxidase 1), Acox2, Acox3, Mcad (medium chain acyl-CoA dehydrogenase), and Vlcad (very long chain acyl-CoA dehydrogenase) were significantly reduced in Fxr−/− mice (Fig. 5A). Despite the reduced expression of genes involved in fatty acid oxidation in the liver, hepatic triglyceride levels tended to decrease in Fxr−/− mice when fed a high-fat diet (P = 0.08) (Fig. 5B). The resistance to high-fat diet-induced fatty liver in Fxr−/− mice may be partly due to a reduction in hepatic expression of sterol regulatory element-binding protein 1c (Srebp-1c) and fatty acid synthase (Fas) (Supplemental Fig. 4A). Another possibility is that hepatic fatty acids in Fxr−/− mice are diverted for oxidation in other organ(s).
Fig. 5.
Fxr hematoxylin and eosin mice have increased expression of genes involved in energy expenditure in skeletal muscle and increased fat absorption. Female wild-type (WT) and Fxr hematoxylin and eosin mice were fed a high-fat diet for 14 wk (n = 8 mice per group). A and C, The mRNA levels of genes in the liver (A) and skeletal muscle (C) were determined by qRT-PCR. B, Hepatic triglyceride levels were quantified. D, Intestinal fat absorption was determined after injection of [14C]triolein and tyloxapol. *, P < 0.05; #, P < 0.01.
In the skeletal muscle, several genes involved in fatty acid oxidation or energy uncoupling, such as Pgc-1α, Pgc-1β, Ucp3, Vlcad, and Sirt1, were significantly induced (Fig. 5C). Interestingly, the mRNA levels of Pgc-1α, Pgc-1β, Ucp1, Ucp2, Ucp3, β2-Ar, β3-Ar, or Ido2 were unchanged in the BAT of wil-type vs. Fxr−/− mice (Supplemental Fig. 4B).
To exclude the possibility that fat absorption may be changed in Fxr−/− mice, we determined intestinal fat absorption in mice fed a high-fat diet. As shown in Fig. 5D, Fxr−/− mice had significantly increased fat absorption at 60, 120, 180, or 240 min after intragastric administration of 14C-triolein. These data are consistent with the enlarged bile acid pool size in Fxr−/− mice (15) and a role of bile acids in facilitating fat absorption.
Collectively the data of Fig. 5 suggest that Fxr−/− mice have increased energy expenditure likely as a result of increased expression of genes involved in energy metabolism in the skeletal muscle.
Loss of FXR in ob/ob mice protects against weight gain and reduces fat mass
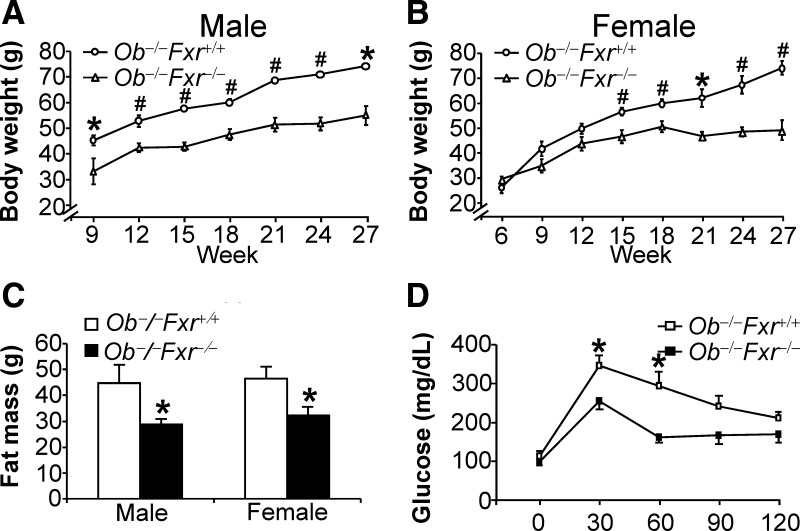
Ob/ob mice, as a result of deficiency in the Leptin gene, are obese and develop metabolic syndrome. To determine whether FXR deficiency affects obesity associated with metabolic syndrome, we generated Leptin−/−Fxr−/− (or ob−/−Fxr−/−) mice. On a chow diet, both male and female ob−/−Fxr−/− mice had significantly reduced body weight gain (Fig. 6, A and B) and fat mass (Fig. 6C), as compared with ob−/−Fxr+/+ mice. No difference in food intake was noted (data not shown). Consistent with the reduced body weight, ob−/−Fxr−/− mice had increased glucose tolerance (Fig. 6D).
Fig. 6.
FXR deficiency in ob/ob mice reduces body weight and fat mass and increases glucose tolerance. The double-knockout (ob−/−Fxr−/−) mice and their controls (ob−/−Fxr+/+) were fed a chow diet (n = 7–8 mice per group). A–C, The body weight of male (A) and female (B) mice as well as fat mass (C) was measured. D, Glucose tolerance test was performed after an overnight fast (n = 5 per group). *, P < 0.05; #, P < 0.01.
On a chow diet, we did not see any difference in fat content, oxygen consumption, or CO2 production (Supplemental Fig. 5, A–C). The finding that the RQ was close to 1 (Supplemental Fig. 5D) suggests that these mutant mice use glucose as the major fuel. In addition, there was no change in activity between these two genotypes (Supplemental Table 2).
Because ob−/−Fxr−/− or ob−/−Fxr+/+ mice were already superobese, we did not challenge these mice with a high-fat diet to further induce obesity. Nonetheless, the data of Fig. 6 indicate that loss of FXR also prevents weight gain and reduces fat mass under diabetic conditions.
Loss of FXR in ob/ob mice promotes liver carcinogeneisis
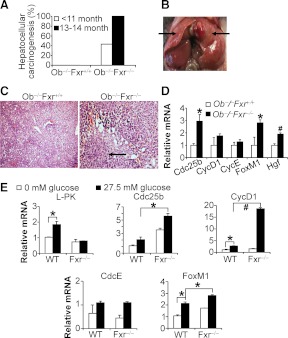
Fxr−/− mice develop spontaneous liver carcinomas when they are more than 12 months old (10, 11). Diabetes is often associated with increased risk of cancinogenesis, including bladder, breast, colorectal, endometrial, kidney, liver, and pancreatic cancer (17). However, the causative relationship between diabetes and cancer has not been established. Here we studied the effect of FXR deficiency on liver carcinogenesis in the setting of diabetes. As expected, 100% ob−/−Fxr−/− mice developed liver carcinogenesis when they were 13–14 months old (Fig. 7A). Interestingly, 43% of the young (<11 months old) ob−/−Fxr−/− mice also developed liver carcinomas (Fig. 7, A and B) (P < 0.05).
Fig. 7.
FXR deficiency accelerates liver carcinogenesis in ob/ob mice. The double-knockout (ob−/−Fxr−/−) mice and their controls (ob−/−Fxr+/+) were fed a chow diet (n = 7–9 mice per group). A, The incidence of liver carcinomas in both genotypes is shown. Forty-three percent of the young ob−/−Fxr−/− mice developed liver carcinomas. B, Representative image of liver carcinoma from an Ob−/−Fxr−/−mouse is shown. C, Hematoxylin and eosin staining of liver sections from ob−/−Fxr+/+ mice (left panel) or ob−/−Fxr−/− mice (right panel) was shown (original magnification, ×100). D, Hepatic mRNA levels were determined by qRT-PCR. Arrows indicate the carcinoma in the liver (B) or microscopic lesion (C). E, Primary hepatocytes were isolated from wild-type or Fxr−/− mice and cultured in DMEM containing 0 mm glucose or 27.5 mm glucose for 24 h. mRNA levels were determined by qRT-PCR. The results represent two independent studies. L-PK, Liver-type pyruvate kinase (positive control). *, P < 0.05; #, P < 0.01.
As shown in Fig. 7C, the normal architecture of liver parenchyma in ob−/−Fxr−/− mice was altered, with inflammatory cell infiltration. Consistent with the formation of liver tumors, hepatic genes involved in proliferation, such as Cdc25b, FoxM1, and hepatocyte growth factor (Hgf), were significantly induced in ob−/−Fxr−/− mice (Fig. 7D). Thus, the data of Fig. 7, A–D, suggest that FXR deficiency causes liver carcinogenesis in aged mice and this process is accelerated under diabetic conditions.
To understand why loss of FXR accelerates liver carcinogenesis in ob/ob mice, we treated primary hepatocytes isolated from wild-type mice and Fxr−/− mice with high or low concentrations of glucose or insulin because ob/ob mice are known to have high blood glucose and insulin levels. Interestingly, glucose but not insulin significantly induces Cdc25b, Cyclin D1 (CycD1), and FoxM1 in wild-type hepatocytes, and such inductions were further potentiated when FXR was inactivated (Fig. 7E and data not shown). Because Cdc25b, Cyclin D1, and FoxM1 play an important role in proliferation (18–20), the data of Fig. 7E suggest that glucose and loss of FXR function may synergistically promote liver carcinogenesis.
Discussion
Obesity has reached epidemic proportions worldwide. Activation of FXR has been considered as a therapeutic approach for treatment of diseases, such as fatty liver disease, atherosclerosis, and insulin resistance. Here we used several mouse models to show that loss of FXR prevents diet-induced or genetic obesity, suggesting that long-term activation of FXR may increase obesity. Consistent with our data, a very recent report by Watanabe et al (21) showed that long-term feeding of a synthetic FXR agonist increased diet-induced obesity. Together these loss- and gain-of function data indicate that FXR plays an important role in diet-induced obesity (DIO) or genetic obesity.
The effect of loss of FXR on obesity appears to be more striking on an Ldlr−/− background (Fig. 1) than on a C57BL/6 background alone (Fig. 3). The Ldlr−/−Fxr−/− double-knockout mice had higher plasma bile acid levels than Fxr−/− mice (340 vs. 153 μm; P < 0.01) (Supplemental Fig. 2C and data not shown). Bile acids are known to increase energy expenditure and prevent DIO through activating the G protein-coupled receptor TGR5 in the BAT (14). Consistent with the latter report (14), we observed elevated bile acid levels in the plasma (Supplemental Fig. 2C), increased cAMP levels, and increased expression of genes involved in energy expenditure in the BAT of Ldlr−/−Fxr−/− mice (Fig. 2). As compared with the Ldlr−/−Fxr−/− mice, we did not see any change in the BAT of Fxr−/− mice (Supplemental Fig. 4 and data not shown). Thus, it is likely that it is the higher plasma bile acid levels that activate the bile acid-TGR5 signaling in the BAT of Ldlr−/−Fxr−/− mice but not Fxr−/− mice.
In contrast with the Ldlr−/−Fxr−/− mice, the reduced obesity in Fxr−/− mice or ob−/−Fxr−/− mice is unlikely to result from elevated plasma bile acid levels (bile acid-TGR5 pathway) in these mice. First, genes involved in energy expenditure in the BAT are not up-regulated in Fxr−/− mice or ob−/−Fxr−/− mice (Supplemental Fig. 4B and data not shown). Second, the induction of genes involved in energy expenditure in the skeletal muscle of Fxr−/− mice (Fig. 5C) is not caused by elevated plasma bile acids because cholic acid feeding does not induce these genes in skeletal muscle (14). Because FXR is not expressed in skeletal muscle or BAT (Fig. 2C) (16) and genes involved in energy expenditure are not induced in the liver (Fig. 5C and data not shown), FXR deficiency-induced resistance to DIO must be secondary to the change(s) in other signaling pathway(s). One of our future directions will be to determine how FXR deficiency results in induction of genes involved in energy expenditure in the skeletal muscle.
High-fat-fed Fxr−/− mice have reduced hepatic Srebp-1c and Fas expression (Supplemental Fig. 4A). These mice also tend to have reduced hepatic triglyceride levels (Fig. 5B). In addition, hepatic cholesterol levels were unchanged, whereas plasma triglyceride and cholesterol levels were significantly increased (Supplemental Fig. 4, C–E). These data suggest that hepatic lipogenesis likely does not contribute to the resistance to DIO in Fxr−/− mice.
Interestingly, only female Fxr−/− mice show striking resistance to DIO, which may be partially a result of increased energy expenditure (Figs. 3–5); differences in VO2 between genotypes were detected in some hours of the day, but not others, suggesting that heightened VO2 seen in female Fxr−/− mice may not have been solely secondary to the difference in body weight, which would be expected to result in increased VO2 at all times of day. Male Fxr−/− mice are resistant to diet-induced increase in weight gain and fat mass, but the fat content remains similar between male wild-type and male Fxr−/− mice (Fig. 3). Analysis of gene expression profile in the liver, BAT, and skeletal muscle of male Fxr−/− mice vs. male wild-type mice show that many genes that are induced in the skeletal muscle of female Fxr−/− vs. female wild-type mice are not induced in male Fxr−/− mice (Supplemental Fig. 6, A and B, and data not shown). The differential expression of genes involved in energy expenditure in the skeletal muscle between male and female mice may explain why only female Fxr−/− mice show striking resistance to DIO.
Ob/ob mice are known to have increased bile acid pool size (22). On a leptin-deficient background, loss of FXR prevents weight gain and reduces fat mass in chow-fed males and females and increases glucose tolerance (Fig. 6). The improved glucose homeostasis in the ob−/−Fxr−/− mice may partly contribute to the lean phenotype. Interestingly, we did not see any change in whole-body glucose tolerance or insulin sensitivity in Fxr−/− mice vs. wild-type mice or Ldlr−/−Fxr−/− mice vs. Ldlr−/− mice (Supplemental Fig. 2B and data not shown). It is possible that the improved glucose homeostasis in ob−/−Fxr−/− mice is secondary to other metabolic change(s) or body weight loss in these mice.
After we completed our studies, Prawitt et al. (23) recently reported that loss of FXR protected against body weight gain and improved glucose tolerance in ob/ob mice, and improved glucose homeostasis in high-fat-fed male Fxr−/− mice. Although their observations with ob−/−Fxr−/− mice are similar to ours, we did not see any change in glucose homeostasis in Fxr−/− mice vs. wild-type mice (data not shown). One possibility for this latter difference is that Fxr−/− mice used in these studies may have different genetic background. In addition, Prawitt et al. (23) studied only male Fxr−/− mice vs. male wild-type mice and did not observe any change in body fat content. This latter observation is consistent with our finding that only female Fxr−/− mice are resistant to DIO. Thus, it is important to study both males and females because some phenotypes are gender dependent.
High expression of FXR is limited to the liver, intestine, kidney, and adrenal gland (24). The expression of FXR in WAT is very low (Fig. 2C) or negligible (16). In addition, we did not see any FXR protein in WAT (Supplemental Fig. 7A). On a chow diet, Fxr−/− mice and wild-type mice have similar fat content and similar adipocyte size/number (Supplemental Fig. 7B and data not shown). These data raise the question regarding whether FXR in adipose tissue is functional in vivo. Although Prawitt et al. (23) observed increased insulin signaling in WAT of ob−/−Fxr−/− mice vs. ob/ob mice, it remains unclear whether such a change is secondary to reduced body fat.
Previous data have clearly demonstrated that Fxr−/− mice that are younger than 12 months do not develop liver tumors (10–12). Small heterodimer partner (SHP) is a known FXR target gene. Fxr−/− mice have significantly reduced Shp expression in the liver (Fig. 5A and Supplemental Fig. 6A). Shp−/− mice spontaneously develop liver carcinomas (25), suggesting that the reduced Shp expression in the liver may play a role in promoting liver carcinogenesis in Fxr−/− mice. Although diabetes is often found to be associated with carcinogenesis, there is no evidence that diabetes is the causative factor for carcinogenesis. The findings that young (<11 months old) ob−/−Fxr−/− mice also develop liver carcinomas and that glucose induces proliferative genes in wild-type hepatocytes and this induction is potentiated when FXR is inactivated (Fig. 7), suggests that high blood glucose levels in diabetes may promote liver carcinogenesis, and this process will be aggravated by FXR inactivation. Glucose is known to regulate gene expression via carbohydrate response element-binding protein (26–28) or epigenetic regulation of acetylation or methylation (29–32). One of our future directions will be directed toward understanding how glucose and FXR inactivation cooperate to regulate proliferative genes and whether such regulations have functional consequences.
In summary, by using three different mouse models, we demonstrate that loss of FXR protects against DIO or weight gain. These data, together with two very recent reports (21, 23), indicate that FXR plays an important role in DIO or genetic obesity. Although activation of FXR has been shown to display many beneficial effects and FXR agonists are being used in various phases of clinical trials, it will be interesting to see whether FXR agonists affect obesity in humans. Finally, our data suggest that loss of FXR accelerates liver carcinogenesis under diabetic conditions via a mechanism likely involving glucose-mediated proliferation.
Supplementary Material
Acknowledgments
This work was supported by Grants 1R01HL103227 and 1R15DK088733 from the National Institutes of Health (NIH) and a Scientist Development Grant 0830255N from the American Heart Association (to Y.Z.), NIH Grants HL30568 and HL68445 (to P.A.E.), and NIH Grant NINDS55859 (to C.M.N.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BAT
- Brown adipose tissue
- DIO
- diet-induced obesity
- FBS
- fetal bovine serum
- FXR
- farnesoid X receptor
- qRT-PCR
- quantitative RT-PCR
- RQ
- respiration quotient
- SHP
- small heterodimer partner
- TGR5
- G protein-coupled bile acid receptor
- VCO2
- CO2 production
- VO2
- oxygen consumption
- WAT
- white adipose tissue.
References
- 1. Zhang Y, Edwards PA. 2008. FXR signaling in metabolic disease. FEBS Lett 582:10–18 [DOI] [PubMed] [Google Scholar]
- 2. Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. 2009. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev 89:147–191 [DOI] [PubMed] [Google Scholar]
- 3. Mencarelli A, Renga B, Distrutti E, Fiorucci S. 2009. Antiatherosclerotic effect of farnesoid X receptor. Am J Physiol Heart Circ Physiol 296:H272–H281 [DOI] [PubMed] [Google Scholar]
- 4. Hartman HB, Gardell SJ, Petucci CJ, Wang S, Krueger JA, Evans MJ. 2009. Activation of farnesoid X receptor prevents atherosclerotic lesion formation in LDLR−/− and apoE−/− mice. J Lipid Res 50:1090–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Flatt B, Martin R, Wang TL, Mahaney P, Murphy B, Gu XH, Foster P, Li J, Pircher P, Petrowski M, Schulman I, Westin S, Wrobel J, Yan G, Bischoff E, Daige C, Mohan R. 2009. Discovery of XL335 (WAY-362450), a highly potent, selective, and orally active agonist of the farnesoid X receptor (FXR). J Med Chem 52:904–907 [DOI] [PubMed] [Google Scholar]
- 6. Zhang S, Wang J, Liu Q, Harnish DC. 2009. Farnesoid X receptor agonist WAY-362450 attenuates liver inflammation and fibrosis in murine model of non-alcoholic steatohepatitis. J Hepatol 51:380–388 [DOI] [PubMed] [Google Scholar]
- 7. Fiorucci S, Rizzo G, Antonelli E, Renga B, Mencarelli A, Riccardi L, Orlandi S, Pruzanski M, Morelli A, Pellicciari R. 2005. A farnesoid x receptor-small heterodimer partner regulatory cascade modulates tissue metalloproteinase inhibitor-1 and matrix metalloprotease expression in hepatic stellate cells and promotes resolution of liver fibrosis. J Pharmacol Exp Ther 314:584–595 [DOI] [PubMed] [Google Scholar]
- 8. Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. 2000. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell 102:731–744 [DOI] [PubMed] [Google Scholar]
- 9. Zhang Y, Wang X, Vales C, Lee FY, Lee H, Lusis AJ, Edwards PA. 2006. FXR deficiency causes reduced atherosclerosis in Ldlr−/− mice. Arterioscler Thromb Vasc Biol 26:2316–2321 [DOI] [PubMed] [Google Scholar]
- 10. Yang F, Huang X, Yi T, Yen Y, Moore DD, Huang W. 2007. Spontaneous development of liver tumors in the absence of the bile acid receptor farnesoid X receptor. Cancer Res 67:863–867 [DOI] [PubMed] [Google Scholar]
- 11. Kim I, Morimura K, Shah Y, Yang Q, Ward JM, Gonzalez FJ. 2007. Spontaneous hepatocarcinogenesis in farnesoid X receptor-null mice. Carcinogenesis 28:940–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wolfe A, Thomas A, Edwards G, Jaseja R, Guo GL, Apte U. 2011. Increased activation of Wnt/β-catenin pathway in spontaneous hepatocellular carcinoma observed in farnesoid X receptor knockout mice. J Pharmacol Exp Ther 338:12–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee FY, Lee H, Hubbert ML, Edwards PA, Zhang Y. 2006. FXR, a multipurpose nuclear receptor. Trends Biochem Sci 31:572–580 [DOI] [PubMed] [Google Scholar]
- 14. Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O, Kodama T, Schoonjans K, Bianco AC, Auwerx J. 2006. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 439:484–489 [DOI] [PubMed] [Google Scholar]
- 15. Kok T, Hulzebos CV, Wolters H, Havinga R, Agellon LB, Stellaard F, Shan B, Schwarz M, Kuipers F. 2003. Enterohepatic circulation of bile salts in farnesoid X receptor-deficient mice: efficient intestinal bile salt absorption in the absence of ileal bile acid-binding protein. J Biol Chem 278:41930–41937 [DOI] [PubMed] [Google Scholar]
- 16. Bookout AL, Jeong Y, Downes M, Yu RT, Evans RM, Mangelsdorf DJ. 2006. Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell 126:789–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vigneri R. 2009. Diabetes: diabetes therapy and cancer risk. Nat Rev Endocrinol 5:651–652 [DOI] [PubMed] [Google Scholar]
- 18. Lavecchia A, Coluccia A, Di Giovanni C, Novellino E. 2008. Cdc25B phosphatase inhibitors in cancer therapy: latest developments, trends and medicinal chemistry perspective. Anticancer Agents Med Chem 8:843–856 [DOI] [PubMed] [Google Scholar]
- 19. Musgrove EA, Caldon CE, Barraclough J, Stone A, Sutherland RL. 2011. Cyclin D as a therapeutic target in cancer. Nat Rev Cancer 11:558–572 [DOI] [PubMed] [Google Scholar]
- 20. Koo CY, Muir KW, Lam EW. 2012. FOXM1: from cancer initiation to progression and treatment. Biochim Biophys Acta 1819:28–37 [DOI] [PubMed] [Google Scholar]
- 21. Watanabe M, Horai Y, Houten SM, Morimoto K, Sugizaki T, Arita E, Mataki C, Sato H, Tanigawara Y, Schoonjans K, Itoh H, Auwerx J. 2011. Lowering bile acid pool size with a synthetic farnesoid X receptor (FXR) agonist induces obesity and diabetes through reduced energy expenditure. J Biol Chem 286:26913–26920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hyogo H, Roy S, Paigen B, Cohen DE. 2002. Leptin promotes biliary cholesterol elimination during weight loss in ob/ob mice by regulating the enterohepatic circulation of bile salts. J Biol Chem 277:34117–34124 [DOI] [PubMed] [Google Scholar]
- 23. Prawitt J, Abdelkarim M, Stroeve JH, Popescu I, Duez H, Velagapudi VR, Dumont J, Bouchaert E, van Dijk TH, Lucas A, Dorchies E, Daoudi M, Lestavel S, Gonzalez FJ, Oresic M, Cariou B, Kuipers F, Caron S, Staels B. 2011. Farnesoid X receptor deficiency improves glucose homeostasis in mouse models of obesity. Diabetes 60:1861–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang Y, Kast-Woelbern HR, Edwards PA. 2003. Natural structural variants of the nuclear receptor farnesoid X receptor affect transcriptional activation. J Biol Chem 278:104–110 [DOI] [PubMed] [Google Scholar]
- 25. Zhang Y, Xu P, Park K, Choi Y, Moore DD, Wang L. 2008. Orphan receptor small heterodimer partner suppresses tumorigenesis by modulating cyclin D1 expression and cellular proliferation. Hepatology 48:289–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ma L, Robinson LN, Towle HC. 2006. ChREBP*Mlx is the principal mediator of glucose-induced gene expression in the liver. J Biol Chem 281:28721–28730 [DOI] [PubMed] [Google Scholar]
- 27. Uyeda K, Repa JJ. 2006. Carbohydrate response element binding protein, ChREBP, a transcription factor coupling hepatic glucose utilization and lipid synthesis. Cell Metab 4:107–110 [DOI] [PubMed] [Google Scholar]
- 28. Postic C, Dentin R, Denechaud PD, Girard J. 2007. ChREBP, a transcriptional regulator of glucose and lipid metabolism. Annu Rev Nutr 27:179–192 [DOI] [PubMed] [Google Scholar]
- 29. Wellen KE, Hatzivassiliou G, Sachdeva UM, Bui TV, Cross JR, Thompson CB. 2009. ATP-citrate lyase links cellular metabolism to histone acetylation. Science 324:1076–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. El-Osta A, Brasacchio D, Yao D, Pocai A, Jones PL, Roeder RG, Cooper ME, Brownlee M. 2008. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J Exp Med 205:2409–2417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brasacchio D, Okabe J, Tikellis C, Balcerczyk A, George P, Baker EK, Calkin AC, Brownlee M, Cooper ME, El-Osta A. 2009. Hyperglycemia induces a dynamic cooperativity of histone methylase and demethylase enzymes associated with gene-activating epigenetic marks that coexist on the lysine tail. Diabetes 58:1229–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Villeneuve LM, Reddy MA, Lanting LL, Wang M, Meng L, Natarajan R. 2008. Epigenetic histone H3 lysine 9 methylation in metabolic memory and inflammatory phenotype of vascular smooth muscle cells in diabetes. Proc Natl Acad Sci USA 105:9047–9052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bligh EG, Dyer WJ. 1959. A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917 [DOI] [PubMed] [Google Scholar]
- 34. Zhang Y, Castellani LW, Sinal CJ, Gonzalez FJ, Edwards PA. 2004. Peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α) regulates triglyceride metabolism by activation of the nuclear receptor FXR. Genes Dev 18:157–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ge X, Yin L, Ma H, Li T, Chiang JY, Zhang Y. 2011. Aldo-keto reductase 1B7 is a target gene of FXR and regulates lipid and glucose homeostasis. J Lipid Res 52:1561–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.