Abstract
Lin-11, Isl-1, and Mec-3 (LIM)-homeodomain (HD)-class transcription factors are critical for many aspects of mammalian organogenesis. Of these, LHX3 is essential for pituitary gland and nervous system development. Pediatric patients with mutations in coding regions of the LHX3 gene have complex syndromes, including combined pituitary hormone deficiency and nervous system defects resulting in symptoms such as dwarfism, thyroid insufficiency, infertility, and developmental delay. The pathways underlying early pituitary development are poorly understood, and the mechanisms by which the LHX3 gene is regulated in vivo are not known. Using bioinformatic and transgenic mouse approaches, we show that multiple conserved enhancers downstream of the human LHX3 gene direct expression to the developing pituitary and spinal cord in a pattern consistent with endogenous LHX3 expression. Several transferable cis elements can individually guide nervous system expression. However, a single 180-bp minimal enhancer is sufficient to confer specific expression in the developing pituitary. Within this sequence, tandem binding sites recognized by the islet-1 (ISL1) LIM-HD protein are essential for enhancer activity in the pituitary and spine, and a pituitary homeobox 1 (PITX1) bicoid class HD element is required for spatial patterning in the developing pituitary. This study establishes ISL1 as a novel transcriptional regulator of LHX3 and describes a potential mechanism for regulation by PITX1. Moreover, these studies suggest models for analyses of the transcriptional pathways coordinating the expression of other LIM-HD genes and provide tools for the molecular analysis and genetic counseling of pediatric patients with combined pituitary hormone deficiency.
Studies of animal models and human patients have established that Lin-11, Isl-1, Mec-3 (LIM)-homeodomain (HD) genes encode regulatory transcription factors that are critical for the development of specialized cells in many tissues and organs, including the nervous system, skeletal muscle, the heart, the kidneys, and endocrine organs, such as the pancreas and the pituitary gland (1). The pituitary secretes hormones that control key developmental and physiological processes, including the stress response, reproduction, metabolism, growth, and lactation. The gland has dual origins with the posterior pituitary lobe originating from the neuroectoderm or diencephalon and the anterior and intermediate lobes (IL) developing from the oral ectoderm. Signaling gradients between the diencephalon and oral ectoderm result in invagination of the oral ectoderm, forming Rathke's pouch, the primordium of the anterior/IL (2). Subsequent cellular determination, differentiation, and proliferation events are controlled in part by multiple transcription factors to establish the specialized hormone-secreting cell types of the pituitary (reviewed in Ref. 3). The five main hormone-secreting cell types found in the anterior pituitary are the corticotropes, gonadotropes, thyrotropes, somatotropes, and lactotropes secreting ACTH, FSH and LH, thyroid-stimulating hormone (thyrotropin), GH, and prolactin, respectively.
The LHX3 LIM-HD transcription factor is required for the formation of the anterior pituitary gland, including specification of somatotropes, thyrotropes, lactotropes, and gonadotropes, and has additional roles in the development of spinal motoneurons (reviewed in Ref.4). The defects displayed by Lhx3 knockout mice and patients with coding region mutations in the LHX3 gene emphasize the importance of LHX3 proteins in pituitary development. Lhx3 knockout mice die shortly after birth. In these animals, Rathke's pouch forms but fails to effectively develop, lacking four of the five differentiated cell types, with only a residual population of corticotropes being observed (5). To date, eleven homozygous mutations in the protein-coding regions of human LHX3 have been identified that result in combined pituitary hormone deficiency (CPHD) syndrome. Although some patients with LHX3 mutations survive, they are short in stature and have hypothyroidism and hypogonadism. These deficiencies are similar to the abnormal pituitary development and hormone losses seen in the Lhx3 knockout mice (5). Development of the nervous system also is affected, causing loss of normal neck rotation, developmental delay, and deafness in some cases (6–11).
The mammalian LHX3 genes have seven coding exons and six introns and produce two major mRNA, called LHX3a and LHX3b. These RNA are transcribed from two TATA-less, GC-rich promoters located upstream of exons Ia and Ib that are recognized by specificity protein-1 and nuclear factor I (12). Several upstream factors, including fibroblast growth factor 8, pituitary homeobox (PITX)1, PITX2, sex-determining region Y-box (SOX)2, LHX4, and forkhead box P1 have been implicated in the regulation of LHX3 transcription in pituitary and neural tissues. The expression of fibroblast growth factor 8 in the adjacent diencephalon and Rathke's pouch is required for the activation of mouse Lhx3 and Lhx4 (2). Pitx1/Pitx2 double knockout mice fail to express Lhx3 and have a similar phenotype to Lhx3-null mice (13). LHX3 protein expression is maintained in Pitx1-null and Pitx2-null mice, suggesting an overlapping function of PITX1 and PITX2 with expression of either sufficient to activate Lhx3 during pituitary development (14, 15). SOX2 has also been shown to bind and activate the human LHX3a promoter in vitro (9). In Lhx4 knockout mice, LHX3 protein expression is delayed but returns to normal by embryonic day (e)14.5, indicating that LHX4 is required for timely activation of Lhx3 (16). The forkhead box P1 forkhead transcription factor represses LHX3 expression in cultured neuroendocrine cell lines and occupies the proximal Lhx3a promoter in cell lines and e13.5 spinal cords, suggesting a possible role in Lhx3 gene transcription during spinal cord development (17).
The key regulatory elements necessary for expression of LHX3 in vivo are unknown. In this study, we use transgenic approaches to show that multiple, conserved enhancers located downstream of the LHX3 gene guide temporal and spatial transcription in patterns consistent with the endogenous gene expression. Within this cluster of regulatory elements, several sequences confer specific expression in the nervous system, and one 180-bp minimal enhancer targets expression to both the developing pituitary and spinal cord. This modular pituitary enhancer is a transcriptional target of PITX and islet (ISL) proteins.
Results
Conserved distal enhancers downstream of the human LHX3 gene direct pituitary and nervous system expression
We previously characterized two promoters of the human LHX3 gene (the LHX3a and LHX3b promoters) with basal activity in vitro (12). To test the function of the promoters in vivo, a β-galactosidase reporter gene transgenic mouse model was used. Transgenes were constructed by placing the promoters (−3.24 kb of the upstream ‘a’ promoter or the entire −1.8 kb ‘b’ promoter) in a vector containing β-galactosidase with a nuclear localization signal followed by an untranslated region (UTR) and a polyadenylation signal from the human EF1α gene flanked by murine H19 insulator regions (Fig. 1B). Transient transgenics were analyzed at e14.5, a time point when mouse Lhx3 gene expression is high in both the developing pituitary and spinal cord (18–20). The activity of the combined a+b promoter region was also examined in the mouse model to test the hypothesis that interactions between the two upstream promoter regions were required to guide gene expression in vivo. Neither the individual nor the combined promoter regions were able to drive reporter gene expression (Fig. 1, B and C, transgenes I–III). A minimum of six independent founders was analyzed for each promoter transgene. Together, these results suggested that elements outside of the basal promoters are required to direct LHX3 gene expression in vivo.
Fig. 1.
Conserved distal downstream regions of the human LHX3 gene direct expression to the developing pituitary and spinal cord. A, Comparative genomic analysis. The ECR and VISTA enhancer browser computational datasets were used to compare sequences surrounding human LHX3 and a far 3′ region to frog, chicken, opossum, rat, mouse, dog, and cow sequences. CNE were defined as regions more than or equal to 70% identity and more than or equal to 100 bp length. CNE are shown in red color, coding exons in blue, conserved intronic regions in salmon, UTR in yellow, and transposable elements or simple repeats in green. B, Reporter gene constructs (I–VI) used to generate transgenic mice. nLacZ, Nuclear localized β-galactosidase gene; HSP68, mouse Hsp68 promoter. C, Reporter genes containing the 7.9-kb 3′ region (IV and V) direct consistent expression in the pituitary or spinal cord. Sagittal cryosections of e14.5 founder embryos stained for β-galactosidase activity. The fraction of transgenic embryos expressing β-galactosidase in the pituitary or spinal cord is shown below each respective image. An asterisk indicates broad ectopic expression. P, Posterior lobe; I, IL; A, anterior lobe; D, dorsal; V, ventral.
Conserved noncoding elements (CNE) are associated with gene enhancer activity in multiple tissues, including neural tissues and the developing heart (21, 22). To find conserved regulatory regions, the ECR browser was used to perform bioinformatic searches of sequences ±10 kb surrounding the human LHX3 gene (23). Multiple CNE within a 7.9-kb region directly 3′ of the gene were discovered with more than or equal to 70% conservation between humans and multiple other vertebrates (Fig. 1A). Because of the lack of in vivo activity of the promoter transgenic mice, which included 3.24-kb 5′ of the LHX3 start codon (Fig. 1C, transgenes I and III) and the close location of the upstream quiescin Q6 sulfhydryl oxidase 2 gene 5′ of LHX3, we focused on analysis of the 3′ CNE. To examine the function of these CNE in vivo, we tested the combined human promoter region plus the 3′ 7.9-kb region in the transgenic mouse model. Unlike the promoter constructs, this construct was able to drive tissue-specific expression in the developing pituitary and spinal cord at e14.5 (Fig. 1C, IV). This suggests that the 3′ region contains one or more enhancers required for spatial and temporal expression of the LHX3 gene.
One recognized characteristic of an enhancer is the ability to act independent of position to guide expression from a heterologous basal promoter (24). Transgene IV containing the LHX3 promoters and the 3′ region in its native position exhibits tissue-specific expression. To determine whether the 3′ region acts as an independent enhancer, we tested a transgene containing the 3′ region upstream of the Hsp68 promoter, a basal promoter that lacks activity in the absence of an enhancer in transgenic mice (Fig. 1B, construct V) (25, 26). The observed transgene V expression pattern recapitulates that seen with construct IV (Fig. 1C, transgenes IV and V); therefore, the identified 3′ enhancer region has transferrable activity independent of its native promoters and genomic context.
An additional CNE located far downstream (Far 3′ element) was also identified from the VISTA enhancer browser computational dataset (21) and was similarly tested in vivo. This element was able to direct some expression to the developing spinal cord but not to the pituitary gland at e14.5 (Fig. 1C, transgene VI). The Far 3′ CNE lies 63 kb 3′ of LHX3 and 31 kb 5′ of the nucleus accumbens-associated protein 2 (NACC2) gene. NACC2 is expressed in the developing nervous system but not the pituitary (27). It is therefore possible the Far 3′ is an enhancer of NACC2 expression. In mouse, Lhx3 expression begins at approximately e9.5. By e11.5, robust Lhx3 expression is detected in both the developing spinal cord and pituitary (18–20). Additional Far 3′ transient transgenic founders were evaluated at e11.5, and no β-galactosidase activity was detected (data not shown). This result, and the fact that the nearest gene, NACC2, is expressed in neural tissues, makes the role of the Far 3′ CNE in LHX3 gene regulation difficult to interpret.
Endogenous LHX3 expression correlates with the expression pattern guided by the 3′ enhancer region during development
To more completely dissect the spatial and temporal expression patterns of the enhancer region, stable transgenic lines were generated using the 3′ enhancer region upstream of the Hsp68 promoter (Fig. 1B, construct V). In previous studies, it has been shown that mouse Lhx3 is activated by approximately e9.5 in both the pituitary and spinal cord (18–20). To examine whether activation directed by the 3′ enhancer region coincided with endogenous Lhx3 activation, serial sections from the same embryo were stained for either β-galactosidase activity or immunohistochemistry using antibodies against mouse LHX3 protein. LHX3 expression and the spatial pattern of enhancer-directed transgene activity were consistent with a role for the 3′ enhancer region in LHX3 gene activation (Fig. 2A). Whole-mount β-galactosidase activity staining of the embryos showed that the enhancer element was activated at about e9.5 in Rathke's pouch, the primordium of the pituitary. Spinal cord expression slightly preceded pituitary expression and was readily detectable at e9.0–e9.5 (Fig. 2B and data not shown).
Fig. 2.
Expression patterns guided by the 7.9-kb 3′ enhancer region during development correlate with endogenous LHX3 expression. A, Antibody staining of endogenous mLHX3 protein and β-galactosidase activity staining of serial sections at e9.5 in a stable transgenic line containing transgenic construct V. The onset of β-galactosidase activity is consistent with the onset of mLHX3 protein expression detected by antibody staining in Rathke's pouch (RP) and in the developing spinal cord. B, X-gal-stained embryos at e9.5 and e12.5 show strong staining in the developing pituitary (arrow and circle) and spinal cord consistent with known mLhx3 expression patterns. C, Representative X-gal staining images of sagittal cryosections of the developing pituitary at e10.5, e12.5, e14.5, and e17.5 and coronal cryosections at P1. The 3′ enhancer directs expression throughout the developing anterior pituitary at e10.5 and e12.5. At e14.5, e17.5 and P1 expression is restricted to the anterior lobe and is absent from the intermediate and posterior lobes. IF, Infundibulum; H, hypothalamus; P, posterior lobe; I, IL; A, anterior lobe.
Whole-mount staining at e12.5 revealed high levels of transgene expression in the developing spinal cord and pituitary in a pattern consistent with the expression pattern of endogenous mouse LHX3 protein (Fig. 2B). Some ectopic expression was found in the epidermis and the nasal cavity (Fig. 2B). LHX3 has not been reported to be expressed in these areas to date. This activity may reflect endogenous low level expression patterns or human/mouse differences. It may also be attributed to effects from the site of transgene integration or because other regulatory elements not contained within the transgene are needed to more tightly regulate LHX3 expression.
Expression of the transgene in the pituitary at later time points was consistent with a subset of endogenous LHX3 expression. The transgene was expressed throughout the unclosed Rathke's pouch, recapitulating LHX3 expression patterns, but became more restricted to the ventral portion of the pituitary by e14.5 (Fig. 2C). Although LHX3 protein is found in both the dorsal and ventral pituitary, it is more highly expressed dorsally by e12.5 (16). At postnatal day (P)1, transgene expression was present in the anterior lobe but absent from the IL (Fig. 2C).
The 3′ region contains several nervous system enhancers and a pituitary enhancer
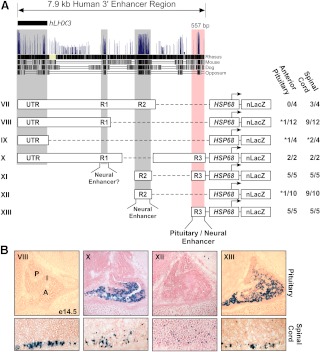
The identified 3′ region contains several strongly conserved sequences that were designated regions R1 (355 bp), R2 (680 bp), and R3 (557 bp) (Fig. 3A). To evaluate the importance of these sequences, a series of systematic deletions was generated and tested in transgenic mice. Expression in the developing spinal cord was observed in constructs containing R1, R2, or R3 but not transgenes containing only the UTR (Fig. 3, A and B).
Fig. 3.
Deletion analysis of the 3′ region reveals several nervous system enhancers and a pituitary enhancer. A, Alignment of 3′ region sequences across the indicated species from the UCSC Genome Browser. Deletion constructs (VII–XIII) used to generate transgenic mice were designed considering the three major areas of sequence conservation (R1, R2, and R3). The fraction of transgenic embryos expressing β-galactosidase in the pituitary or spinal cord is shown beside each construct. Asterisks indicate nonspecific ectopic expression likely due to effects from the site integration of the transgene. B, Representative sagittal sections of e14.5 embryos harboring constructs VIII (UTR R1), X (ΔR2), XII (R2), and XIII (R3) stained for β-galactosidase activity. Only constructs containing the R3 region (557 bp)-targeted expression to the pituitary (X and XIII). R1, R2, and R3 directed expression in the developing spinal cord (VIII, XII, X, and XIII). P, Posterior lobe; I, IL; A, anterior lobe.
Only transgenic founders generated using reporter genes containing the R3 element directed expression to both the developing spinal cord and pituitary (Fig. 3, A and B, transgenes X, XI, and XIII). Expression in the spinal cord was similar to the pattern seen with the full 7.9-kb region. However, pituitary expression was expanded to include the dorsal portion of the developing anterior pituitary more closely matching endogenous LHX3 expression (Fig. 3B, transgene XIII). Intriguingly, the pattern of pituitary expression in a construct with a deleted R2 subregion (transgene X) shows repression in the dorsal anterior pituitary similar to the expression pattern for the full enhancer element (Fig. 3B). This result suggests that a silencer or repressor element may be contained within either the UTR R1 region or in the region between R2 and R3.
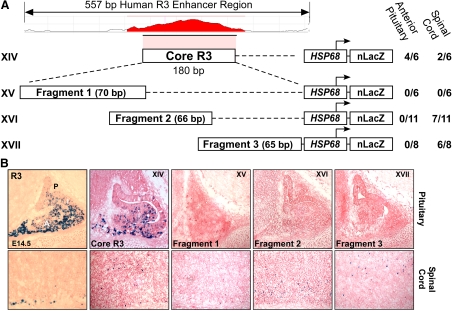
A 180-bp minimal region is sufficient to direct expression to the developing pituitary
Sequence alignment of the R3 pituitary enhancer region from multiple species using the ECR browser identified a highly conserved subregion of 180 bp (Fig. 4A, designated Core R3). Transgenic reporter gene mice containing the Core R3 region directed expression to the developing pituitary (Fig. 4, transgene XIV). Additional transgenic mice were generated expressing reporter genes containing overlapping fragments of the Core R3 enhancer. In isolation, these small fragments were unable to direct pituitary expression (Fig. 4, transgenes XV–XVII). Overall, these data indicate that key elements required to direct pituitary expression are contained in the 180-bp Core R3 enhancer.
Fig. 4.
A highly conserved 180-bp minimal region (Core R3) is sufficient to direct expression to the developing pituitary. A, Within the conserved R3 region, a highly CNE was designated Core R3 and is shown in red color. To test the properties of the Core R3 region, constructs XIV through XVII were used to generate transgenic mice, and the fraction of transgenic embryos expressing β-galactosidase in the pituitary or spinal cord is shown beside each construct diagram. B, Representative sagittal cryosections of e14.5 embryos stained for β-galactosidase activity. P, Posterior lobe; I, IL; A, anterior lobe.
Core R3 enhancer sequences for multiple species were aligned, and conserved predicted trans-acting factor binding sites were identified (Fig. 5). The Core R3 enhancer contained four conserved A/T-rich sequences containing the TAAT/ATTA motifs that are characteristic of some HD protein recognition sites (Fig. 5, sites AT1–AT4). These sites were compared with the consensus binding sites of factors with suggested roles in pituitary development and LHX3 gene regulation. AT1 matches the PITX bicoid-class protein consensus binding site. PITX1 and PITX2 are required for activation of mouse Lhx3 in vitro and in vivo (13, 28). AT2 was identified as a possible binding site for LHX3 and LHX4 proteins (29). This sequence could potentially be important for autoregulation by LHX3 or regulation by LHX4. In vivo studies have shown that Lhx4 is required for proper activation of Lhx3. For example, in the Lhx4 knockout mice, LHX3 expression is delayed but returns to normal by e14.5 (16). The AT3 and AT4 sequences were predicted to contain a tandem binding site for ISL1 (30). Isl1 expression precedes and then overlaps Lhx3 expression in the developing spine and pituitary (31, 32) in a pattern consistent with a possible role in Lhx3 regulation. The pituitary defect in the Isl1 knockout is similar to that observed in the Lhx3 null mice, with Lhx3 expression absent from the pituitary. However, ISL1 was suggested to block differentiation at an early stage rather than acting directly upstream of Lhx3 (2). Conditional motoneuron knockouts of Isl1 do not display any markers of motoneuron development, including Lhx3 (32). In developing bipolar interneurons of the eye, Lhx3 and Lhx4 are expressed at P9 and colocalize partially with ISL1. Further, in conditional neural retina Isl1 null mice, Lhx4 expression is maintained, whereas Lhx3 expression is lost (33).
Fig. 5.
Alignment of Core R3 enhancer sequences reveals conserved transcription factor binding consensus sequences. Putative SOX sites (yellow), potential A/T-rich HD factor recognition sites (AT1-AT4, green), and a possible CEBP site (gray) are indicated. Analyses were performed with ClustalW2.0 and rVISTA.
Additional transcription factor binding sites within the Core R3 included putative elements for CCAAT enhancer binding protein (CEBP)α and SOX2 sites (Fig. 5). CEBPα has not been implicated in LHX3 regulation but is important in differentiation and proliferation events. Mouse Cebpα is expressed in the developing pituitary and has been shown to regulate the prolactin pituitary promoter with Pit-1 (34). SOX2 can bind to the human LHX3a promoter in vitro (9), and both human patients and mouse models heterozygous for SOX2 gene mutations have anterior pituitary hormone deficiencies (35).
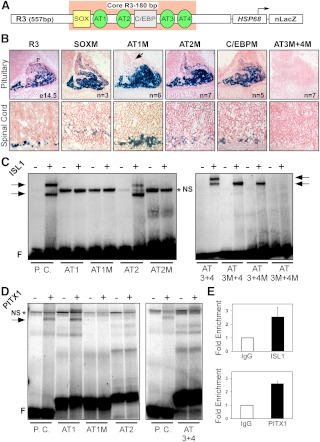
To determine whether these elements are required to direct LHX3 expression in vivo, transgenic mice were generated with individual mutations of the putative SOX, AT1, AT2, and CEBPα binding sites or a combined mutation of the tandem AT3 and AT4 sites in the context of the 557-bp R3 enhancer. Founders with mutations in AT1 lost expression in the dorsal portion of the pituitary in comparison with the wild-type R3 expression pattern (Fig. 6B). This is similar to the expression pattern of the full 7.9-kb 3′ enhancer (see Fig. 1C, transgenes IV and V). This suggests a role for the AT1 element in spatial control of pituitary LHX3 expression. Mutation of the tandem AT3 and AT4 sites abolished expression in both the pituitary and the developing spinal cord (Fig. 6B), demonstrating that AT3 and AT4 are required for enhancer-directed expression. However, because fragment 3 of the Core R3, which contains AT3 and AT4, did not direct pituitary expression (see Fig. 4, transgene XVII), the AT3 and AT4 elements are required, but not sufficient, for enhancer function. Mutation of the putative SOX, AT2, or CEBP sites did not alter the expression pattern of the R3 enhancer at e14.5 (Fig. 6B). These sites are therefore not required to direct expression at e14.5; however, it is possible that they may have functions at other developmental time points.
Fig. 6.
ISL and PITX binding sites in the Core R3 enhancer are critical for expression in the developing pituitary and spinal cord. A, Potential transcription factor binding sites in the R3 region are shown in the context of the R3-HSP68-LacZ (XI) transgenic reporter gene. B, Mutation analyses in transgenic mice demonstrate that sites AT3 and AT4 are required for pituitary and spinal cord expression in vivo and that site AT1 is required for dorsal pituitary expression (arrow indicates loss of expression). C, ISL1 binds specifically to AT2, AT3, and AT4 in EMSA. The A3/A4 element from the enhancer of the rat insulin promoter was used as the positive control (30). P.C., Positive control; −, empty vector programmed lysate; +, PITX1 or ISL1 expression vector-programmed lysate; arrows, specific bound complex; *NS, nonspecific band; F, free probe. D, PITX1 binds specifically to AT1 sites in EMSA. A Bcd2x5n bicoid element was used as the positive control (51). E, ChIP experiments show occupancy of the Core R3 enhancer by ISL1 and PITX1 proteins in αT3 pituitary cells. Pull-down and input DNA were characterized for the presence of the Core R3 enhancer element by quantitative PCR. Immunoprecipitation with nonimmune species-appropriate IgG was carried out as a control, and relative enrichment was calculated as the fold difference above the 2−ΔΔCt for the normal immunoglobulin samples. Values are mean ± sem for three independent experiments.
To test whether candidate factors could bind to the A/T-rich elements in the Core R3 enhancer, EMSA and chromatin immunoprecipitation (ChIP) experiments were performed. In EMSA, ISL1 protein was able to bind probes containing sites AT2, AT3, and AT4, and the mutation of these sites abolished binding (Fig. 6C). Additionally, PITX1 and PITX2 proteins bound to probes containing the element AT1 but did not bind to the mutated AT1 probe or other AT sites (Fig. 6D and Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org). Further, both LHX3 and LHX4 proteins were able to bind probes containing AT2 (Supplemental Fig. 1). In parallel negative controls, lysates programmed with empty vector did not bind to the probes specifically (Fig. 6, C and D). In luciferase reporter gene assays in either αT3 pregonadotrope or LβT2 gonadotrope cell lines, addition of the R3 enhancer upstream of the LHX3 promoters or the minimal prolactin promoter failed to increase gene activation above basal levels. Consistent with this lack of R3-mediated reporter gene activation in vitro, cotransfection of PITX1 and ISL1 proteins did not synergistically activate the constructs (data not shown). It is possible that, although the transgenic experiments show enhancer activity that well reflects endogenous LHX3 expression, the in vitro environment of transfection experiments is insufficient to recapitulate an appropriate context for gene activity requiring complex proximal and distal elements. To examine in vivo occupancy of the ISL1 and PITX1 proteins at the Core R3 enhancer element, ChIP experiments were performed using αT3 cells. LHX3 protein is detected by Western blotting in αT3 cells (36 and data not shown). Similar to the EMSA results, ChIP experiments using αT3 cells showed occupancy of PITX1 and ISL1 proteins in a cellular context. Quantitative ChIP at the Core R3 enhancer with PITX1 antibody showed a 2.5-fold increase in enrichment relative to mock IgG controls (Fig. 6E). ISL1 ChIP experiments showed a similar enrichment with 2.6-fold increased enrichment over IgG controls (Fig. 6E).
Discussion
The critical roles that LIM-HD genes play in the establishment of organs and systems underlines the importance of understanding the mechanisms that mediate their precise transcription regulation during development. The pituitary gland is essential for mammalian development and physiology, and studies of patients and animal models have demonstrated that LHX3 is required for pituitary development and function (e.g. Refs. 6–11, 37, 38). Although much is known about the actions of the LHX3 gene and its encoded proteins, this study is the first characterization of the elements that coordinate in vivo regulation of the LHX3 gene. Multiple enhancers downstream of the LHX3 gene control pituitary and spinal cord expression. The R1, R2, and R3 enhancers direct nervous system expression in the developing mouse embryo, whereas the Core R3 180 enhancer guides specific expression in both the pituitary and spinal cord. Elements within the Core R3 bind the ISL1 and PITX1 transcriptional regulators, playing essential roles in the function of the Core R3 element (summarized in Fig. 7).
Fig. 7.
A model for regulation of LHX3 transcription by the 3′ enhancer region that lies downstream of exon VII and the UTR. CNS, Central nervous system; R1, R2, and R3, modular, conserved regulatory elements.
Although the PITX1 and PITX2 genes have been shown to act either directly or indirectly upstream of LHX3 in the cascade of gene regulation that controls pituitary development (13, 28), no mechanism has been described. Our data show that the PITX proteins are capable of binding to the AT1 element in vitro and occupy the core enhancer in pituitary cells. Furthermore, mutation of the AT1 element affects the spatial pattern guided by the LHX3 enhancer in vivo. This study therefore suggests a mechanism whereby PITX1 regulates the spatial pattern of LHX3 expression in the pituitary.
The LHX3 enhancer-directed expression patterns in the developing pituitary and spinal cord depend on a short sequence containing the tandem AT-rich sites (AT3/AT4). However, the 65-bp sequence surrounding this element is insufficient to direct pituitary expression of a heterologous promoter (see Fig. 4B, transgene XV), indicating that it works in combination with other factors in the context of the 180-bp minimal functional enhancer. The ISL1 LIM-HD transcription factor binds to the AT3 and AT4 elements and occupies the Core R3 enhancer in vivo, implicating for the first time ISL1 as a possible regulator of LHX3 gene expression in the anterior pituitary and the spinal cord.
Evidence from previous studies is consistent with this role for ISL1 in the regulation of LHX3 gene expression. For example, ISL1 protein expression precedes LHX3 expression in Rathke's pouch, and at the time of Lhx3 activation, ISL1 is colocalized with Lhx3 in the mouse Rathke's pouch (31, 39). The Is11−/− mice display a similar pituitary phenotype to Lhx3−/− mice and lack LHX3 expression (2). Tissue-specific knockouts of Isl1 in motoneurons and in the neural retina also lack LHX3 expression (32, 33). ISL1 has not previously been identified as a potential upstream factor of LHX3 mainly because, after overlap of expression in early development, the two proteins segregate into a largely inverse expression patterns with ISL1 restricted to the most ventral region and LHX3 expressed more dorsally. This led to the interpretation that the phenotype of the Isl1 knockout in both the pituitary and motoneurons blocked development at an early stage and was not the result of LHX3 loss (2, 32, 33). Taken together, our data indicate that, among its many early roles in development, ISL1 may also function as an activator of LHX3.
The full 3′ LHX3 enhancer directs expression throughout Rathke's pouch at e9.5, but by e12.5, it is expressed more ventrally, and by e14.5, the enhancer directs strongly ventral transcription with little dorsal activity (Fig. 2). This is consistent with endogenous LHX3 expression at e9.5 and a subset of LHX3 expression patterns later in development. LHX3 is similarly expressed at e9.5, but unlike enhancer-directed expression, by e12.5, it is also enriched in the dorsal region of the developing pituitary (18–20). Because this study uses the human enhancer region, it is possible that the differences in observed pituitary expression represent differences between mouse and human expression patterns or differences in control mechanisms between the two species. Equally plausible is that this enhancer is not responsible for all aspects of LHX3 pituitary expression. It is possible that pituitary expression of LHX3 is regulated by multiple enhancers, and the identified 3′ enhancer region serves as an activating enhancer with later roles in the ventral anterior pituitary.
The quiescin Q6 sulfhydryl oxidase 2 gene is very close upstream (5′) of LHX3. The closest gene downstream (3′) of the LHX3 gene/enhancer complex, NACC2, lies approximately 94 kb downstream and is expressed in the developing nervous system but absent from the pituitary (27). We cannot exclude the possibility that the enhancer elements described here act on this or other neighboring genes. However, the large distance from other genes and the close correlation of enhancer-driven activity with LHX3 expression patterns in both the pituitary and developing spinal cord strongly suggest that these enhancers located just 3′ of the LHX3 gene are regulatory elements of the LHX3 gene.
Although distinct in the mouse, the IL in humans is rudimentary in adults. Interestingly, the human LHX3 enhancer identified in this study shows little activity in the IL, but both the developing human and mouse IL express LHX3 (18–20, 40). The expression pattern directed by the characterized human enhancer may be an indication of the level of human LHX3 expression in the IL. The difference could possibly represent morphological or species differences between the human IL and that of mice. If LHX3 levels are reduced in the human IL as a result of reduced activity of the human enhancer in the IL, it is possible this could affect IL development. It is conceivable that apoptosis results from low LHX3 expression in the IL, which would result in loss of all but just a few cells of the adult human IL. Reductions in LHX3 action are known to increase apoptosis in the ventral portion of Rathke's pouch during development and the IL of the Lhx3−/− mice and Lhx3Cre/Cre hypomorph mice are reduced in size at e15.5 (13, 38, 41).
Another interesting finding from this study is the presence of potential silencing elements. Our results suggest that repressors blocking expression in the dorsal pituitary may be contained within either the UTR R1 region or in the region between R2 and R3. Potential CCCTC-binding factor (CTCF) binding sites were identified using TRANSFAC in R1, but these did not strongly match the known consensus sequence of CTCF. Numerous studies have shown CTCF is found at many repressors but how CTCF functions in enhancer insulating and blocking is unknown (42). Additional experiments are needed to determine whether the R1 element described here is a repressor element, what trans-acting factors are needed for its functions, and whether CTCF binding is required for R1 repressor function.
The AT1 PITX binding site defined here was shown by in vivo mutational analysis as important in spatial control of the enhancer-directed pituitary expression. This pattern could indicate a function of this site in dorsal pituitary repression of the larger full 3′ enhancer. A potential mechanism for this observed activity is that an unknown dorsal factor bound to the repressor element facilitates chromatin looping between the R3 enhancer and the repressor. This activity could be mediated by PITX protein bound to the AT1 element and would isolate the R3 enhancer away from the proximal promoter. In the context of the R3-Hsp68 transgene, without the repressor regions, the enhancer is not sequestered away from the proximal promoter and is able to direct dorsal expression in the pituitary. A similar mechanism has been identified for the H19ICR insulator (43).
This study describes and characterizes the first known pituitary and spinal cord enhancers of LHX3 and is the first step in uncovering the mechanisms required for proper spatial and temporal expression of the LHX3 gene. However, other regulatory elements outside of this enhancer complex also likely contribute to full transcriptional control of the LHX3 gene. In addition to identifying other important regulatory regions, future research will need to explore the interactions between the LHX3 proximal promoters, insulator regions, and enhancers. Uncovering how these elements interact to direct tissue-specific gene expression will be essential to understanding the mechanisms behind LHX3 gene regulation and understanding pituitary development on a molecular level. Further work will also be needed to fully describe the role of the identified enhancer sequences in guiding cell-specific expression in the developing spinal cord and nervous system. The information presented here about LHX3 could have implications in the regulation of other LIM-HD genes and provide insight into general mechanisms of gene regulation. Further, the cause of CPHD is unknown in the majority of patients. The LHX3 gene regulatory regions identified in this study will permit the identification of possible novel genetic defects responsible for CPHD and facilitate patient treatment and genetic counseling.
Materials and Methods
Bioinformatic analyses
NCBI (www.ncbi.nlm.nih.gov/genome) and the Ensembl genome browser (www.ensembl.org) were used to retrieve human LHX3 gene and putative enhancer sequences. The ECR (http://ecrbrowser.dcode.org), UCSC (http://genome.ucsc.edu/), and VISTA (http://genome.lbl.gov/vista/) genome browsers were used to identify CNE of the LHX3 gene as described previously (23, 44, 45). Putative transcription factor binding sites were predicted with TRANSFAC and rVISTA (46, 47).
Transgene constructs
The −3.24 kb LHX3a, −1.8 kb LHX3b, and −3.24 kb LHX3a promoter-LHX3 Exon Ia-LHX3b human promoter regions (12) were subcloned into the pWHERE vector (InvivoGen, San Diego, CA). Additional human LHX3 sequences were amplified from BAC clone RP11–83N9/ALI38781 using Pfu Ultra II HS DNA polymerase (Stratagene, La Jolla, CA). The Hsp68 promoter was removed from Hsp68-Hand2-LacZ pSK-Bluescript (a kind gift from Simon Conway, Indiana University School of Medicine). Enhancer deletions were generated by using endogenous restriction sites or were individually amplified by PCR. Site-directed mutagenesis was performed using the QuikChange II system (Stratagene). Constructs were verified by sequence analysis (Biochemistry Biotechnology Facility, Indiana University School of Medicine). For primer sequences, see Supplemental Table 1.
Transgenic mouse generation and breeding
The Indiana University Committee on Use and Care of Animals approved all procedures, and experiments were performed in agreement with the principles and procedures outlined in the National Institutes of Health Guidelines for the Care and Use of Experimental Animals. Transgene plasmids were linearized by Pac I digestion and submitted either to the Purdue Transgenic Mouse Core Facility (West Lafayette, IN) or the Indiana University Transgenic and Knock-out Mouse Core (Indianapolis, IN) for microinjection into F2 zygotes from FVB/N or C3H parents. After microinjection, two-cell stage embryos were transferred to 0.5 postcoitum pseudopregnant females. Founder transgenic mice were either harvested at e12.5 or e14.5 for transient transgenic studies or were bred as adults to generate stable transgenic lines. Harvested embryos were designated e0.5 on the day after microinjection of the transgene. For stable transgenic lines, founder animals and progeny were bred to C3H mice (Harlan Laboratories, Indianapolis, IN) to generate heterozygotes. The morning after copulation was considered e0.5, and the day of birth was P1. Genomic DNA was purified from mouse tail snips taken between 14 and 21 d of age using the genomic DNA solution set (Gerard Biotech, Oxford, OH). Genotyping for transgenic mouse lines was performed using a multiplex PCR amplifying the transgenic region and wild-type control region. For primer sequences, see Supplemental Table 1.
β-Galactosidase enzyme detection
Whole embryos were fixed on ice in 2% paraformaldehyde and 0.2% glutaraldehyde, washed in PBS (pH 7.2), then incubated overnight at room temperature in X-gal solution (35 mm potassium ferrocyanide, 35 mm potassium ferricyanide, 2 mm MgCl2, 0.2% each of Triton X, Nonidet P-40 and Tween 20, and 1 mg/ml X-gal diluted in dimethylformamide, in PBS). After dehydration in ethanol, stained embryos were cleared in methyl salicylate and imaged immediately. Wild-type litter mate embryos served as negative controls. For cryosections, fixed and washed embryos were cryoprotected in 20% sucrose overnight then embedded in O.C.T. compound (Sakura Finetek, Torrance, CA) and sectioned at a thickness of 7 μm. Sections were air dried and fixed for 10 min with 0.5% glutaraldehyde then washed in PBS followed by staining in X-gal solution as described above. After staining, slides were washed, dehydrated, and eosin counterstained.
Immunohistochemistry
Immunostaining was performed with rabbit polyclonal antibodies against mouse LHX3 (1:100) (Anti-LIM-3 from Chemicon, Temecula, CA) as described (48).
Protein preparation and EMSA
ISL1 and PITX1 proteins used in EMSA were synthesized in vitro from 1 μg of expression vector substrates (pCS2 rat ISL-1-Myc plasmid, a kind gift from Samuel Pfaff, Salk Institute, La Jolla, CA) and pcDNA3 hPITX1 plasmid (kind gift from Marie-Hélène Quentien, Département de Neuroendocrinologie & Neuroimmunologie, Université de la Méditerranée, Marseille, France) using a TnT T7 Quick Coupled Transcription/Translation reaction kit (Promega, Madison, WI). Parallel negative controls were programmed with empty vector. EMSA conditions for ISL1 were as described (29). PITX1 EMSA was performed using conditions modified from Amendt et al. (49). Briefly, 10 μl of in vitro-translated protein lysate and 32P-labeled oligonucleotides were incubated in 20 mm HEPES (pH 7.5), 5% glycerol, 50 mm NaCl, 1 mm EDTA, 1 mm dithiothreitol, and 1.0 μg of poly(dI·dC) on ice for 30 min. The samples were separated for 2.5 h at 250 V in an 8% polyacrylamide gel with 0.25× Tris-borate EDTA at 4 C after preelectrophoresis of the gels for 15 min at 200 V. For oligonucleotide sequences, see Supplemental Table 1.
Chromatin immunoprecipitation
Chromatin cross-linking and ChIP analyses were performed using mouse αT3 pituitary cells with the EZ Chip Chromatin Immunoprecipitation kit (Millipore, Billerica, MA). Protein DNA chromatin complexes were fragmented by sonication with conditions optimized to obtain the majority of DNA fragments within the range of 200-1000 bp. One million cells were used for each immunoprecipitation. Precleared protein-DNA chromatin complexes were incubated overnight at 4 C with either 5 μg of anti-PITX1 rabbit polyclonal antibody (Abnova Corp., Walnut, CA) or a cocktail of anti-ISL1 monoclonal antibodies (3 μg each; Developmental Studies Hybridoma Bank 39.4D5, 39.3F7, 40.3A4, 40.2D6) as used previously (50). Parallel negative controls were incubated with normal mouse immunoglobulin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) or normal rabbit immunoglobulin (Sigma, St. Louis, MO) as appropriate. Quantitative real-time PCR was performed on 5 μl of immunoprecipitated or input DNA using SYBR Green PCR master mix (Applied Biosystems, Carlsbad, CA) and an ABI Prism 7900 instrument. The 2−ΔΔCt, where ΔΔCt = ΔCt,input − ΔCt,sample, was calculated for each sample. Relative enrichment was calculated as the fold difference above the 2−ΔΔCt for the control mouse or rabbit normal immunoglobulin samples. For oligonucleotide sequences, see Supplemental Table 1.
Supplementary Material
Acknowledgments
We thank the Indiana Transgenic and Knock-Out Mouse Core for expert technical assistance. We also thank Dr. S. Conway, Dr. R. Day, Dr. T. Footz, Dr. P. Herring, Dr. C. Hunter, Dr. S. Konieczny, Dr. S. Pfaff, Dr. M.-H. Quentien, Dr. D. Skalnik, and Dr. M. Walter for reagents and advice.
This work was supported by National Institutes of Health Grant HD42024 (to S.J.R.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CEBP
- CCAAT enhancer binding protein
- ChIP
- chromatin immunoprecipitation
- CNE
- conserved noncoding elements
- CPHD
- combined pituitary hormone deficiency
- e
- embryonic day
- HD
- homeodomain
- IL
- intermediate lobe
- ISL
- islet
- LIM
- Lin-11, Isl-1, Mec-3
- NACC2
- nucleus accumbens-associated protein 2
- PITX
- pituitary homeobox
- SOX
- sex-determining region Y box
- UTR
- intranslated region.
References
- 1. Hunter CS, Rhodes SJ. 2005. LIM-homeodomain genes in mammalian development and human disease. Mol Biol Rep 32:67–77 [DOI] [PubMed] [Google Scholar]
- 2. Takuma N, Sheng HZ, Furuta Y, Ward JM, Sharma K, Hogan BL, Pfaff SL, Westphal H, Kimura S, Mahon KA. 1998. Formation of Rathke's pouch requires dual induction from the diencephalon. Development 125:4835–4840 [DOI] [PubMed] [Google Scholar]
- 3. Kelberman D, Rizzoti K, Lovell-Badge R, Robinson IC, Dattani MT. 2009. Genetic regulation of pituitary gland development in human and mouse. Endocr Rev 30:790–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Colvin SC, Mullen RD, Pfaeffle RW, Rhodes SJ. 2009. LHX3 and LHX4 transcription factors in pituitary development and disease. Pediatr Endocrinol Rev 6(Suppl 2):283–290 [PubMed] [Google Scholar]
- 5. Sheng HZ, Moriyama K, Yamashita T, Li H, Potter SS, Mahon KA, Westphal H. 1997. Multistep control of pituitary organogenesis. Science 278:1809–1812 [DOI] [PubMed] [Google Scholar]
- 6. Netchine I, Sobrier ML, Krude H, Schnabel D, Maghnie M, Marcos E, Duriez B, Cacheux V, Moers A, Goossens M, Grüters A, Amselem S. 2000. Mutations in LHX3 result in a new syndrome revealed by combined pituitary hormone deficiency. Nat Genet 25:182–186 [DOI] [PubMed] [Google Scholar]
- 7. Bhangoo AP, Hunter CS, Savage JJ, Anhalt H, Pavlakis S, Walvoord EC, Ten S, Rhodes SJ. 2006. Clinical case seminar: a novel LHX3 mutation presenting as combined pituitary hormonal deficiency. J Clin Endocrinol Metab 91:747–753 [DOI] [PubMed] [Google Scholar]
- 8. Pfaeffle RW, Savage JJ, Hunter CS, Palme C, Ahlmann M, Kumar P, Bellone J, Schoenau E, Korsch E, Brämswig JH, Stobbe HM, Blum WF, Rhodes SJ. 2007. Four novel mutations of the LHX3 gene cause combined pituitary hormone deficiencies with or without limited neck rotation. J Clin Endocrinol Metab 92:1909–1919 [DOI] [PubMed] [Google Scholar]
- 9. Rajab A, Kelberman D, de Castro SC, Biebermann H, Shaikh H, Pearce K, Hall CM, Shaikh G, Gerrelli D, Grueters A, Krude H, Dattani MT. 2008. Novel mutations in LHX3 are associated with hypopituitarism and sensorineural hearing loss. Hum Mol Genet 17:2150–2159 [DOI] [PubMed] [Google Scholar]
- 10. Kriström B, Zdunek AM, Rydh A, Jonsson H, Sehlin P, Escher SA. 2009. A novel mutation in the LIM homeobox 3 gene is responsible for combined pituitary hormone deficiency, hearing impairment, and vertebral malformations. J Clin Endocrinol Metab 94:1154–1161 [DOI] [PubMed] [Google Scholar]
- 11. Bonfig W, Krude H, Schmidt H. 2011. A novel mutation of LHX3 is associated with combined pituitary hormone deficiency including ACTH deficiency, sensorineural hearing loss, and short neck-a case report and review of the literature. Eur J Pediatr 170:1017–1021 [DOI] [PubMed] [Google Scholar]
- 12. Yaden BC, Garcia M, 3rd, Smith TP, Rhodes SJ. 2006. Two promoters mediate transcription from the human LHX3 gene: involvement of nuclear factor I and specificity protein 1. Endocrinology 147:324–337 [DOI] [PubMed] [Google Scholar]
- 13. Charles MA, Suh H, Hjalt TA, Drouin J, Camper SA, Gage PJ. 2005. PITX genes are required for cell survival and Lhx3 activation. Mol Endocrinol 19:1893–1903 [DOI] [PubMed] [Google Scholar]
- 14. Lanctôt C, Moreau A, Chamberland M, Tremblay ML, Drouin J. 1999. Hindlimb patterning and mandible development require the Ptx1 gene. Development 126:1805–1810 [DOI] [PubMed] [Google Scholar]
- 15. Szeto DP, Rodriguez-Esteban C, Ryan AK, O'Connell SM, Liu F, Kioussi C, Gleiberman AS, Izpisúa-Belmonte JC, Rosenfeld MG. 1999. Role of the Bicoid-related homeodomain factor Pitx1 in specifying hindlimb morphogenesis and pituitary development. Genes Dev 13:484–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Raetzman LT, Ward R, Camper SA. 2002. Lhx4 and Prop1 are required for cell survival and expansion of the pituitary primordia. Development 129:4229–4239 [DOI] [PubMed] [Google Scholar]
- 17. Morikawa Y, Komori T, Hisaoka T, Senba E. 2009. Detailed expression pattern of Foxp1 and its possible roles in neurons of the spinal cord during embryogenesis. Dev Neurosci 31:511–522 [DOI] [PubMed] [Google Scholar]
- 18. Zhadanov AB, Bertuzzi S, Taira M, Dawid IB, Westphal H. 1995. Expression pattern of the murine LIM class homeobox gene Lhx3 in subsets of neural and neuroendocrine tissues. Dev Dyn 202:354–364 [DOI] [PubMed] [Google Scholar]
- 19. Bach I, Rhodes SJ, Pearse RV, 2nd, Heinzel T, Gloss B, Scully KM, Sawchenko PE, Rosenfeld MG. 1995. P-Lim, a LIM homeodomain factor, is expressed during pituitary organ and cell commitment and synergizes with Pit-1. Proc Natl Acad Sci USA 92:2720–2724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Seidah NG, Barale JC, Marcinkiewicz M, Mattei MG, Day R, Chrétien M. 1994. The mouse homeoprotein mLIM-3 is expressed early in cells derived from the neuroepithelium and persists in adult pituitary. DNA Cell Biol 13:1163–1180 [DOI] [PubMed] [Google Scholar]
- 21. Visel A, Minovitsky S, Dubchak I, Pennacchio LA. 2007. VISTA enhancer browser—a database of tissue-specific human enhancers. Nucleic Acids Res 35:D88–D92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Blow MJ, McCulley DJ, Li Z, Zhang T, Akiyama JA, Holt A, Plajzer-Frick I, Shoukry M, Wright C, Chen F, Afzal V, Bristow J, Ren B, Black BL, Rubin EM, Visel A, Pennacchio LA. 2010. ChIP-Seq identification of weakly conserved heart enhancers. Nat Genet 42:806–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Couronne O, Poliakov A, Bray N, Ishkhanov T, Ryaboy D, Rubin E, Pachter L, Dubchak I. 2003. Strategies and tools for whole-genome alignments. Genome Res 13:73–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Blackwood EM, Kadonaga JT. 1998. Going the distance: a current view of enhancer action. Science 281:60–63 [DOI] [PubMed] [Google Scholar]
- 25. Kothary R, Clapoff S, Darling S, Perry MD, Moran LA, Rossant J. 1989. Inducible expression of an hsp68-lacZ hybrid gene in transgenic mice. Development 105:707–714 [DOI] [PubMed] [Google Scholar]
- 26. Pennacchio LA, Loots GG, Nobrega MA, Ovcharenko I. 2007. Predicting tissue-specific enhancers in the human genome. Genome Res 17:201–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Visel A, Thaller C, Eichele G. 2004. GenePaint.org: an atlas of gene expression patterns in the mouse embryo. Nucleic Acids Res 32:D552–D556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tremblay JJ, Lanctôt C, Drouin J. 1998. The pan-pituitary activator of transcription, Ptx1 (pituitary homeobox 1), acts in synergy with SF-1 and Pit1 and is an upstream regulator of the Lim-homeodomain gene Lim3/Lhx3. Mol Endocrinol 12:428–441 [DOI] [PubMed] [Google Scholar]
- 29. Bridwell JA, Price JR, Parker GE, McCutchan Schiller A, Sloop KW, Rhodes SJ. 2001. Role of the LIM domains in DNA recognition by the Lhx3 neuroendocrine transcription factor. Gene 277:239–250 [DOI] [PubMed] [Google Scholar]
- 30. Karlsson O, Thor S, Norberg T, Ohlsson H, Edlund T. 1990. Insulin gene enhancer binding protein Isl-1 is a member of a novel class of proteins containing both a homeo- and a Cys-His domain. Nature 344:879–882 [DOI] [PubMed] [Google Scholar]
- 31. Ericson J, Norlin S, Jessell TM, Edlund T. 1998. Integrated FGF and BMP signaling controls the progression of progenitor cell differentiation and the emergence of pattern in the embryonic anterior pituitary. Development 125:1005–1015 [DOI] [PubMed] [Google Scholar]
- 32. Pfaff SL, Mendelsohn M, Stewart CL, Edlund T, Jessell TM. 1996. Requirement for LIM homeobox gene Isl1 in motor neuron generation reveals a motor neuron-dependent step in interneuron differentiation. Cell 84:309–320 [DOI] [PubMed] [Google Scholar]
- 33. Elshatory Y, Everhart D, Deng M, Xie X, Barlow RB, Gan L. 2007. Islet-1 controls the differentiation of retinal bipolar and cholinergic amacrine cells. J Neurosci 27:12707–12720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Enwright JF, 3rd, Kawecki-Crook MA, Voss TC, Schaufele F, Day RN. 2003. A PIT-1 homeodomain mutant blocks the intranuclear recruitment of the CCAAT/enhancer binding protein α required for prolactin gene transcription. Mol Endocrinol 17:209–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kelberman D, Rizzoti K, Avilion A, Bitner-Glindzicz M, Cianfarani S, Collins J, Chong WK, Kirk JM, Achermann JC, Ross R, Carmignac D, Lovell-Badge R, Robinson IC, Dattani MT. 2006. Mutations within Sox2/SOX2 are associated with abnormalities in the hypothalamo-pituitary-gonadal axis in mice and humans. J Clin Invest 116:2442–2455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Granger A, Bleux C, Kottler ML, Rhodes SJ, Counis R, Laverrière JN. 2006. The LIM-homeodomain proteins Isl-1 and Lhx3 act with steroidogenic factor 1 to enhance gonadotrope-specific activity of the gonadotropin-releasing hormone receptor gene promoter. Mol Endocrinol 20:2093–2108 [DOI] [PubMed] [Google Scholar]
- 37. Colvin SC, Malik RE, Showalter AD, Sloop KW, Rhodes SJ. 2011. Model of pediatric pituitary hormone deficiency separates the endocrine and neural functions of the LHX3 transcription factor in vivo. Proc Natl Acad Sci USA 108:173–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sheng HZ, Zhadanov AB, Mosinger B, Jr, Fujii T, Bertuzzi S, Grinberg A, Lee EJ, Huang SP, Mahon KA, Westphal H. 1996. Specification of pituitary cell lineages by the LIM homeobox gene Lhx3. Science 272:1004–1007 [DOI] [PubMed] [Google Scholar]
- 39. Ellsworth BS, Butts DL, Camper SA. 2008. Mechanisms underlying pituitary hypoplasia and failed cell specification in Lhx3-deficient mice. Dev Biol 313:118–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sobrier ML, Attié-Bitach T, Netchine I, Encha-Razavi F, Vekemans M, Amselem S. 2004. Pathophysiology of syndromic combined pituitary hormone deficiency due to a LHX3 defect in light of LHX3 and LHX4 expression during early human development. Gene Expr Patterns 5:279–284 [DOI] [PubMed] [Google Scholar]
- 41. Zhao Y, Morales DC, Hermesz E, Lee WK, Pfaff SL, Westphal H. 2006. Reduced expression of the LIM-homeobox gene Lhx3 impairs growth and differentiation of Rathke's pouch and increases cell apoptosis during mouse pituitary development. Mech Dev 123:605–613 [DOI] [PubMed] [Google Scholar]
- 42. Noonan JP, McCallion AS. 2010. Genomics of long-range regulatory elements. Annu Rev Genomics Hum Genet 11:1–23 [DOI] [PubMed] [Google Scholar]
- 43. Yoon YS, Jeong S, Rong Q, Park KY, Chung JH, Pfeifer K. 2007. Analysis of the H19ICR insulator. Mol Cell Biol 27:3499–3510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ovcharenko I, Nobrega MA, Loots GG, Stubbs L. 2004. ECR Browser: a tool for visualizing and accessing data from comparisons of multiple vertebrate genomes. Nucleic Acids Res 32:W280–W286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, Haussler D. 2002. The human genome browser at UCSC. Genome Res 12:996–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Loots GG, Ovcharenko I, Pachter L, Dubchak I, Rubin EM. 2002. rVista for comparative sequence-based discovery of functional transcription factor binding sites. Genome Res 12:832–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Matys V, Fricke E, Geffers R, Gössling E, Haubrock M, Hehl R, Hornischer K, Karas D, Kel AE, Kel-Margoulis OV, Kloos DU, Land S, Lewicki-Potapov B, Michael H, Münch R, Reuter I, Rotert S, Saxel H, Scheer M, Thiele S, Wingender E. 2003. TRANSFAC: transcriptional regulation, from patterns to profiles. Nucleic Acids Res 31:374–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Savage JJ, Mullen RD, Sloop KW, Colvin SC, Camper SA, Franklin CL, Rhodes SJ. 2007. Transgenic mice expressing LHX3 transcription factor isoforms in the pituitary: effects on the gonadotrope axis and sex-specific reproductive disease. J Cell Physiol 212:105–117 [DOI] [PubMed] [Google Scholar]
- 49. Amendt BA, Sutherland LB, Russo AF. 1999. Multifunctional role of the Pitx2 homeodomain protein C-terminal tail. Mol Cell Biol 19:7001–7010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Du A, Hunter CS, Murray J, Noble D, Cai CL, Evans SM, Stein R, May CL. 2009. Islet-1 is required for the maturation, proliferation, and survival of the endocrine pancreas. Diabetes 58:2059–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Saadi I, Kuburas A, Engle JJ, Russo AF. 2003. Dominant negative dimerization of a mutant homeodomain protein in Axenfeld-Rieger syndrome. Mol Cell Biol 23:1968–1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.