Abstract
In the title compound, C16H12N2S2, the thiophene groups are rotationally disordered over two sets of sites, by approximately 180°, with occupancy ratios of 0.916 (2):0.084 (2) and 0.903 (2):0.097 (2). The major components of the thiophene and methylene substituted thiophene rings are canted by 24.06 (12) and 85.07 (10)°, respectively, from the benzimidazole ring system plane and the dihedral angle between the major component thiophene ring planes is 84.90 (14)°. In the crystal, there is a weak C—H⋯N hydrogen bond which links molecules into chains.
Related literature
For a discussion of the rearrangement of 1,2-diiminobenzene species to form benzimidazoles, see: Smith & Ho (1971 ▶). See Varala et al. (2007 ▶) for examples of proline-catalysed 1,2-disubstituted benzimidazole syntheses. Reich et al. (2004 ▶) provide examples of intermolecular aldimine coupling. For other syntheses of substituted benzimidazoles, see: Grimmett (1997 ▶); Bahrami et al. (2007 ▶); Du & Wang (2007 ▶). For the biological activity of benzimidazole derivatives, see: López-Rodríguez et al. (1999 ▶); Horton et al. (2003 ▶).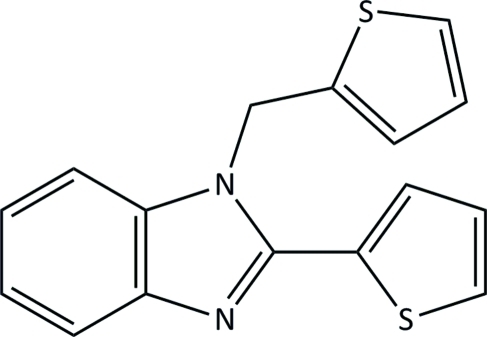
Experimental
Crystal data
C16H12N2S2
M r = 296.40
Monoclinic,
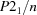
a = 8.9859 (13) Å
b = 9.1601 (11) Å
c = 17.476 (3) Å
β = 93.629 (5)°
V = 1435.6 (3) Å3
Z = 4
Mo Kα radiation
μ = 0.36 mm−1
T = 300 K
0.80 × 0.30 × 0.10 mm
Data collection
Bruker SMART X2S benchtop diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2008 ▶) T min = 0.761, T max = 0.965
8857 measured reflections
2527 independent reflections
1942 reflections with I > 2σ(I)
R int = 0.033
Refinement
R[F 2 > 2σ(F 2)] = 0.040
wR(F 2) = 0.103
S = 1.05
2527 reflections
210 parameters
26 restraints
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.18 e Å−3
Δρmin = −0.25 e Å−3
Data collection: APEX2 (Bruker, 2010 ▶); cell refinement: SAINT (Bruker, 2009 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: XSHELL (Bruker, 2004 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536811055103/lh5359sup1.cif
Supplementary material file. DOI: 10.1107/S1600536811055103/lh5359Isup2.mol
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811055103/lh5359Isup3.hkl
Supplementary material file. DOI: 10.1107/S1600536811055103/lh5359Isup4.mol
Supplementary material file. DOI: 10.1107/S1600536811055103/lh5359Isup5.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C16—H16⋯N2i | 0.93 | 2.54 | 3.445 (4) | 166 |
Symmetry code: (i) 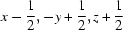 .
.
Acknowledgments
This work was supported by a Congresssionally directed grant from the US Department of Education for the X-ray diffractometer and a grant from the Geneseo Foundation.
supplementary crystallographic information
Comment
Benzimidazole derivatives are of interest because of their pharmacological uses. Examples include inhibitors of serotonin activated neurotransmission (López-Rodríguez et al., 1999) and antiviral agents (Varala et al., 2007). They have also found use as antiarrhythmic, antihistamine, antiulcer, anticancer, fungicidal, anthelmintical drugs (Horton et al., 2003).
Numerous methods are available for the synthesis of substituted benzimidazoles (Grimmett, 1997). One pot procedures for the synthesis of 2-aryl substituted benzimidazoles involving the condensation of 1,2-diaminobenzene with aldehydes employing hydrogen peroxide and ceric ammonium nitrate (Bahrami et al., 2007) or hypervalent iodine (Du & Wang, 2007) as oxidizing agents have been reported. Syntheses of 1-arylmethyl-2-aryl substituted benzimidazoles via the proline catalyzed condensation of 1,2-diaminobenzene derivatives with aryl aldehydes have also been reported (Varala et al., 2007). Attempts to prepare N,N'-dibenzal-o-phenylenediamine via the condensation of 1,2-diaminobenzene and benzaldehyde lead to presumed rearrangement of the initially formed Schiff base yielding 1-benzyl-2-phenylbenzimidazole (Smith & Ho, 1971).
Our efforts have focused on the preparation of benzimidazole analogues which have substituents capable of binding metals. Toward that end, we have prepared 1-(thiophene-2-methyl)-2-(2-thiophene)benzimidazole, TMTB, from a reaction of 1,2-diaminobenzene with 2-thiophenecarboxaldehyde.
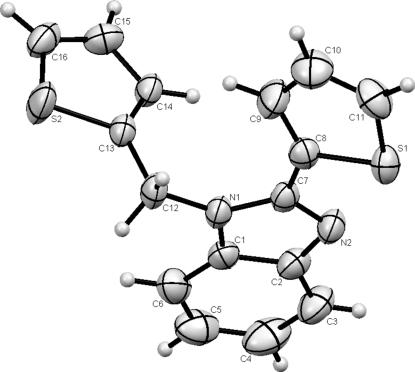
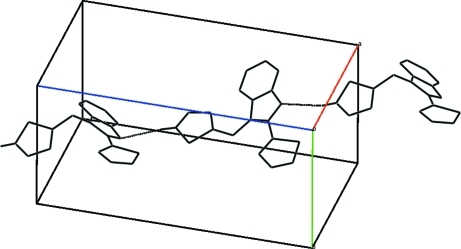
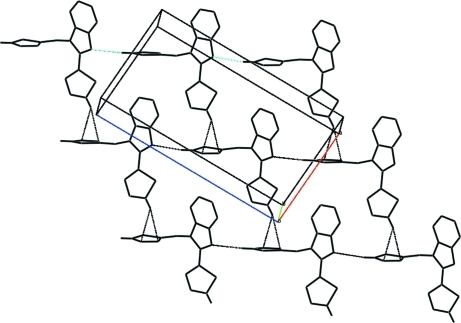
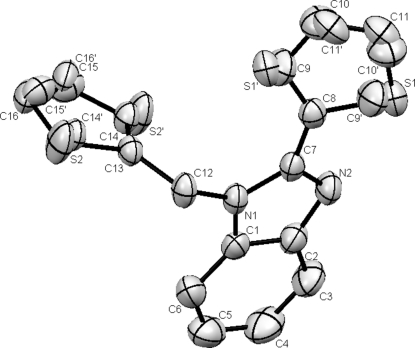
Figure 1 shows a perspective view of TMTB with the atom-labeling scheme. Only the major components of the disordered thiophene groups are displayed. Close C—H16···N2 intermolecular interactions (2.535 Å, 165.8°) result in chains of TMTB as shown in Figure 2. Bifurcated C—H11···C14 and C15 intermolecular contacts (2.843 Å and 2.848 Å) join the chains to form a layer network (see Figure 3). Figure 4 is a perspective view of the compound showing both major and minor components of the disordered thiophene groups.
The structure exhibits the expected planar benzimidazole moiety (maximum deviation 0.0076(0.0014) Å, N1). The major components of the disordered thiophene and methylthiophene rings are canted 24.06 (12) ° and 85.07 (10) °, respectively, from the benzimidazole plane, with a thiophene-methylthiophene dihedral angle of 84.90 (14) °. Together, the thiophene groups provide a structure with the potential to behave as a bidentate ligand employing the sulfur atoms. We are exploring the coordination chemistry of TMTB.
Experimental
The title compound was prepared by the reaction of two equivalents of 2-thiophene with 1,2-diaminobenzene in the presence of a catalytic amount of aluminium trichloride under nitrogen in dichloromethane. The reaction mixture was refluxed for 8 h,filtered and the solvent removed by rotary evaporation. The crude product was purified by column chromatography (silica gel)using 20%(v/v) ethylacetate in hexanes.
The purified product was characterized via1H and 13C NMR spectroscopies. 1H NMR spectrum (DMSO-d6, 400 MHz, p.p.m.): 8.14 (2H, m), 7.98 (1 H, m), 7.50 (2 H, m), 7.44 (1 H, m), 7.39 (1 H, m), 7.10 (1 H, m), 6.94 (1 H, m), 6.05 (2 H, s). 13C NMR spectrum (DMSO-d6, 100 MHz p.p.m.): 177.15, 150.63, 143.52,138.59, 135.33, 133.05, 130.91, 129.49, 128.70, 128.01, 188.14, 117.51, 117.13, 115.37, 22.58.
Single crystals were grown via vapor diffusion of cyclohexane into a concentrated methanolic solution.
Refinement
During the course of the refinement, alternate positions for the thiophene sulfur atoms were apparent in the residual electron density maps. Refinement of the site occupancy factors led to values of about 8% for the minor components. Based on these values, a model was constructed for the minor component of each of the thiophene rings using the metrics of the major components as a guide. The pivot atoms (C8 and C13) were assumed to have full occupancy and so were not included in the disorder model. The minor four-atom components (S1', C11', C10' and C9; S2', C16', C15', and C14') were constrained to planarity using FLAT. Using DFIX, the S—C bond distances were set to 1.70 Å. Corresponding bond distances of the minor component and major component were set equal using SADI and corresponding thermal parameters were held the same using EADP. All atoms were refined anisotropically with hydrogen atoms in calculated positions using a riding model. With these constraints, the site occupancies of the major components of the methylthiophene and the thiophene refined to 91.6% and 90.3%, respectively. Based on this model, the angles between the mean planes of the major and minor components of the methylthiophene and thiophene are 5.7(1.9)° and 6.6 (9)°.
Figures
Fig. 1.
Perspective view of the title compound with displacement ellipsoids of non-hydrogen atoms drawn at the 50% probability level. The disorder is not shown.
Fig. 2.
View of the C—H···N intermolecular interactions leading to chain formation.
Fig. 3.
View of layer formed by joining of chains by bifurcated C—H···C intermolecular contacts.
Fig. 4.
The title molecule with displacement ellipsoids of non-hydrogen atoms drawn at the 50% probability level. Major and minor components of the disordered thiophene groups are shown. Hydrogen atoms have been ommitted for clarity.
Crystal data
| C16H12N2S2 | F(000) = 616 |
| Mr = 296.40 | Dx = 1.371 Mg m−3 |
| Monoclinic, P21/n | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2yn | Cell parameters from 2556 reflections |
| a = 8.9859 (13) Å | θ = 2.3–24.0° |
| b = 9.1601 (11) Å | µ = 0.36 mm−1 |
| c = 17.476 (3) Å | T = 300 K |
| β = 93.629 (5)° | Plate, clear yellow |
| V = 1435.6 (3) Å3 | 0.80 × 0.30 × 0.10 mm |
| Z = 4 |
Data collection
| Bruker SMART X2S benchtop diffractometer | 2527 independent reflections |
| Radiation source: fine-focus sealed tube | 1942 reflections with I > 2σ(I) |
| graphite | Rint = 0.033 |
| ω scans | θmax = 25.0°, θmin = 2.5° |
| Absorption correction: multi-scan (SADABS; Bruker, 2008) | h = −10→10 |
| Tmin = 0.761, Tmax = 0.965 | k = −10→10 |
| 8857 measured reflections | l = −20→20 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.040 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.103 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.05 | w = 1/[σ2(Fo2) + (0.0518P)2 + 0.2347P] where P = (Fo2 + 2Fc2)/3 |
| 2527 reflections | (Δ/σ)max < 0.001 |
| 210 parameters | Δρmax = 0.18 e Å−3 |
| 26 restraints | Δρmin = −0.25 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| S1 | 0.21859 (8) | 0.28769 (8) | 0.08330 (3) | 0.0467 (2) | 0.916 (2) |
| C9 | 0.0913 (5) | 0.3386 (5) | 0.2054 (2) | 0.0620 (12) | 0.916 (2) |
| H9 | 0.0724 | 0.3488 | 0.2568 | 0.074* | 0.916 (2) |
| C10 | −0.0188 (4) | 0.3558 (5) | 0.1464 (2) | 0.0648 (10) | 0.916 (2) |
| H10 | −0.1168 | 0.3819 | 0.1540 | 0.078* | 0.916 (2) |
| C11 | 0.0332 (3) | 0.3301 (5) | 0.07718 (18) | 0.0585 (9) | 0.916 (2) |
| H11 | −0.0251 | 0.3349 | 0.0313 | 0.070* | 0.916 (2) |
| S1' | 0.0789 (14) | 0.3656 (15) | 0.2202 (7) | 0.0467 (2) | 0.084 (2) |
| C9' | 0.208 (3) | 0.300 (4) | 0.1075 (11) | 0.0620 (12) | 0.084 (2) |
| H9' | 0.2835 | 0.2677 | 0.0773 | 0.074* | 0.084 (2) |
| C11' | −0.017 (3) | 0.384 (6) | 0.1320 (15) | 0.0585 (9) | 0.084 (2) |
| H11' | −0.1150 | 0.4159 | 0.1246 | 0.070* | 0.084 (2) |
| C10' | 0.071 (3) | 0.344 (6) | 0.0747 (16) | 0.0648 (10) | 0.084 (2) |
| H10' | 0.0434 | 0.3457 | 0.0225 | 0.078* | 0.084 (2) |
| S2 | 0.18792 (12) | 0.42615 (9) | 0.47929 (5) | 0.0647 (3) | 0.903 (2) |
| C14 | 0.2441 (4) | 0.1896 (4) | 0.41297 (19) | 0.0411 (8) | 0.903 (2) |
| H14 | 0.2767 | 0.1211 | 0.3785 | 0.049* | 0.903 (2) |
| C15 | 0.1692 (5) | 0.1519 (4) | 0.4778 (3) | 0.0510 (8) | 0.903 (2) |
| H15 | 0.1469 | 0.0563 | 0.4907 | 0.061* | 0.903 (2) |
| C16 | 0.1329 (4) | 0.2674 (5) | 0.51922 (17) | 0.0549 (10) | 0.903 (2) |
| H16 | 0.0835 | 0.2618 | 0.5643 | 0.066* | 0.903 (2) |
| S2' | 0.2508 (19) | 0.1572 (12) | 0.3994 (8) | 0.0647 (3) | 0.097 (2) |
| C14' | 0.187 (3) | 0.394 (3) | 0.4642 (13) | 0.0411 (8) | 0.097 (2) |
| H14' | 0.1810 | 0.4923 | 0.4762 | 0.049* | 0.097 (2) |
| C16' | 0.146 (5) | 0.146 (3) | 0.478 (2) | 0.0549 (10) | 0.097 (2) |
| H16' | 0.1140 | 0.0590 | 0.4989 | 0.066* | 0.097 (2) |
| C15' | 0.117 (5) | 0.280 (4) | 0.5038 (19) | 0.0510 (8) | 0.097 (2) |
| H15' | 0.0564 | 0.2969 | 0.5442 | 0.061* | 0.097 (2) |
| N1 | 0.41824 (18) | 0.32345 (17) | 0.29374 (9) | 0.0385 (4) | |
| N2 | 0.47184 (19) | 0.18925 (19) | 0.19147 (9) | 0.0453 (4) | |
| C1 | 0.5604 (2) | 0.2661 (2) | 0.31017 (12) | 0.0405 (5) | |
| C2 | 0.5918 (2) | 0.1839 (2) | 0.24593 (12) | 0.0450 (5) | |
| C3 | 0.7282 (3) | 0.1124 (3) | 0.24430 (15) | 0.0606 (7) | |
| H3 | 0.749 (3) | 0.062 (3) | 0.2051 (14) | 0.073* | |
| C4 | 0.8285 (3) | 0.1262 (3) | 0.30724 (16) | 0.0731 (8) | |
| H4 | 0.9203 | 0.0793 | 0.3071 | 0.088* | |
| C5 | 0.7950 (3) | 0.2087 (3) | 0.37082 (16) | 0.0671 (7) | |
| H5 | 0.8650 | 0.2161 | 0.4122 | 0.081* | |
| C6 | 0.6600 (2) | 0.2799 (3) | 0.37371 (13) | 0.0536 (6) | |
| H6 | 0.6369 | 0.3345 | 0.4162 | 0.064* | |
| C7 | 0.3719 (2) | 0.2722 (2) | 0.22212 (11) | 0.0380 (5) | |
| C8 | 0.2290 (2) | 0.3058 (2) | 0.18189 (11) | 0.0398 (5) | |
| C12 | 0.3420 (2) | 0.4205 (2) | 0.34473 (11) | 0.0422 (5) | |
| H12A | 0.2690 | 0.4782 | 0.3148 | 0.051* | |
| H12B | 0.4139 | 0.4870 | 0.3695 | 0.051* | |
| C13 | 0.2649 (2) | 0.3368 (2) | 0.40501 (10) | 0.0374 (5) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| S1 | 0.0584 (4) | 0.0489 (4) | 0.0324 (4) | 0.0037 (3) | 0.0007 (3) | −0.0022 (3) |
| C9 | 0.0552 (18) | 0.079 (3) | 0.053 (2) | 0.0069 (17) | 0.0129 (16) | −0.0182 (18) |
| C10 | 0.0447 (14) | 0.078 (3) | 0.071 (2) | 0.0102 (15) | −0.0007 (15) | −0.0031 (18) |
| C11 | 0.0576 (19) | 0.0612 (18) | 0.0542 (16) | 0.0000 (18) | −0.0167 (15) | −0.0041 (14) |
| S1' | 0.0584 (4) | 0.0489 (4) | 0.0324 (4) | 0.0037 (3) | 0.0007 (3) | −0.0022 (3) |
| C9' | 0.0552 (18) | 0.079 (3) | 0.053 (2) | 0.0069 (17) | 0.0129 (16) | −0.0182 (18) |
| C11' | 0.0576 (19) | 0.0612 (18) | 0.0542 (16) | 0.0000 (18) | −0.0167 (15) | −0.0041 (14) |
| C10' | 0.0447 (14) | 0.078 (3) | 0.071 (2) | 0.0102 (15) | −0.0007 (15) | −0.0031 (18) |
| S2 | 0.0900 (6) | 0.0564 (6) | 0.0515 (5) | −0.0007 (4) | 0.0340 (4) | −0.0160 (3) |
| C14 | 0.0445 (14) | 0.044 (2) | 0.0355 (19) | 0.0040 (14) | 0.0106 (13) | −0.0070 (13) |
| C15 | 0.045 (2) | 0.0523 (16) | 0.0560 (16) | −0.0033 (12) | 0.0097 (14) | 0.0101 (13) |
| C16 | 0.0478 (17) | 0.083 (2) | 0.0345 (17) | 0.0014 (15) | 0.0113 (15) | 0.0023 (15) |
| S2' | 0.0900 (6) | 0.0564 (6) | 0.0515 (5) | −0.0007 (4) | 0.0340 (4) | −0.0160 (3) |
| C14' | 0.0445 (14) | 0.044 (2) | 0.0355 (19) | 0.0040 (14) | 0.0106 (13) | −0.0070 (13) |
| C16' | 0.0478 (17) | 0.083 (2) | 0.0345 (17) | 0.0014 (15) | 0.0113 (15) | 0.0023 (15) |
| C15' | 0.045 (2) | 0.0523 (16) | 0.0560 (16) | −0.0033 (12) | 0.0097 (14) | 0.0101 (13) |
| N1 | 0.0465 (9) | 0.0396 (9) | 0.0300 (9) | −0.0002 (8) | 0.0080 (7) | −0.0035 (7) |
| N2 | 0.0478 (10) | 0.0536 (11) | 0.0354 (10) | 0.0049 (8) | 0.0092 (8) | −0.0070 (8) |
| C1 | 0.0421 (11) | 0.0424 (12) | 0.0375 (12) | −0.0069 (9) | 0.0068 (9) | 0.0027 (9) |
| C2 | 0.0428 (11) | 0.0526 (13) | 0.0405 (12) | 0.0011 (10) | 0.0106 (10) | 0.0035 (10) |
| C3 | 0.0491 (14) | 0.0785 (18) | 0.0558 (16) | 0.0117 (13) | 0.0157 (12) | −0.0021 (13) |
| C4 | 0.0437 (13) | 0.100 (2) | 0.076 (2) | 0.0125 (14) | 0.0076 (14) | 0.0171 (17) |
| C5 | 0.0483 (14) | 0.090 (2) | 0.0617 (17) | −0.0087 (13) | −0.0084 (12) | 0.0143 (15) |
| C6 | 0.0548 (14) | 0.0645 (15) | 0.0411 (13) | −0.0096 (11) | −0.0009 (11) | 0.0010 (11) |
| C7 | 0.0458 (11) | 0.0380 (11) | 0.0309 (11) | −0.0025 (9) | 0.0088 (9) | 0.0011 (9) |
| C8 | 0.0463 (11) | 0.0373 (11) | 0.0360 (11) | −0.0022 (9) | 0.0036 (9) | 0.0019 (9) |
| C12 | 0.0575 (12) | 0.0360 (11) | 0.0341 (11) | 0.0022 (9) | 0.0108 (10) | −0.0061 (9) |
| C13 | 0.0398 (10) | 0.0423 (12) | 0.0303 (10) | 0.0048 (9) | 0.0046 (9) | −0.0055 (9) |
Geometric parameters (Å, °)
| S1—C11 | 1.708 (3) | C14'—C13 | 1.388 (17) |
| S1—C8 | 1.728 (2) | C14'—C15' | 1.42 (2) |
| C9—C8 | 1.362 (4) | C14'—H14' | 0.9300 |
| C9—C10 | 1.392 (5) | C16'—C15' | 1.345 (19) |
| C9—H9 | 0.9300 | C16'—H16' | 0.9300 |
| C10—C11 | 1.345 (4) | C15'—H15' | 0.9300 |
| C10—H10 | 0.9300 | N1—C7 | 1.377 (2) |
| C11—H11 | 0.9300 | N1—C1 | 1.394 (3) |
| S1'—C8 | 1.638 (10) | N1—C12 | 1.460 (2) |
| S1'—C11' | 1.726 (14) | N2—C7 | 1.316 (2) |
| C9'—C8 | 1.303 (19) | N2—C2 | 1.393 (3) |
| C9'—C10' | 1.39 (2) | C1—C6 | 1.387 (3) |
| C9'—H9' | 0.9300 | C1—C2 | 1.395 (3) |
| C11'—C10' | 1.366 (19) | C2—C3 | 1.392 (3) |
| C11'—H11' | 0.9300 | C3—C4 | 1.383 (4) |
| C10'—H10' | 0.9300 | C3—H3 | 0.86 (2) |
| S2—C16 | 1.700 (4) | C4—C5 | 1.393 (4) |
| S2—C13 | 1.7166 (18) | C4—H4 | 0.9300 |
| C14—C13 | 1.370 (4) | C5—C6 | 1.381 (3) |
| C14—C15 | 1.397 (4) | C5—H5 | 0.9300 |
| C14—H14 | 0.9300 | C6—H6 | 0.9300 |
| C15—C16 | 1.334 (4) | C7—C8 | 1.457 (3) |
| C15—H15 | 0.9300 | C12—C13 | 1.507 (3) |
| C16—H16 | 0.9300 | C12—H12A | 0.9700 |
| S2'—C13 | 1.653 (10) | C12—H12B | 0.9700 |
| S2'—C16' | 1.711 (15) | ||
| C11—S1—C8 | 91.82 (13) | N1—C1—C2 | 105.49 (18) |
| C8—C9—C10 | 114.7 (3) | C3—C2—N2 | 130.3 (2) |
| C8—C9—H9 | 122.6 | C3—C2—C1 | 119.6 (2) |
| C10—C9—H9 | 122.6 | N2—C2—C1 | 110.09 (17) |
| C11—C10—C9 | 112.0 (3) | C4—C3—C2 | 118.1 (2) |
| C11—C10—H10 | 124.0 | C4—C3—H3 | 121.3 (18) |
| C9—C10—H10 | 124.0 | C2—C3—H3 | 120.6 (18) |
| C10—C11—S1 | 112.1 (2) | C3—C4—C5 | 121.4 (2) |
| C10—C11—H11 | 123.9 | C3—C4—H4 | 119.3 |
| S1—C11—H11 | 123.9 | C5—C4—H4 | 119.3 |
| C8—S1'—C11' | 92.6 (11) | C6—C5—C4 | 121.3 (2) |
| C8—C9'—C10' | 118 (2) | C6—C5—H5 | 119.3 |
| C8—C9'—H9' | 121.1 | C4—C5—H5 | 119.3 |
| C10'—C9'—H9' | 121.1 | C5—C6—C1 | 116.8 (2) |
| C10'—C11'—S1' | 110.5 (16) | C5—C6—H6 | 121.6 |
| C10'—C11'—H11' | 124.7 | C1—C6—H6 | 121.6 |
| S1'—C11'—H11' | 124.7 | N2—C7—N1 | 113.07 (18) |
| C11'—C10'—C9' | 108 (2) | N2—C7—C8 | 121.92 (18) |
| C11'—C10'—H10' | 125.8 | N1—C7—C8 | 125.00 (17) |
| C9'—C10'—H10' | 125.8 | C9'—C8—C9 | 103.6 (14) |
| C16—S2—C13 | 92.54 (14) | C9'—C8—C7 | 122.6 (13) |
| C13—C14—C15 | 113.6 (3) | C9—C8—C7 | 133.7 (2) |
| C13—C14—H14 | 123.2 | C9'—C8—S1' | 110.7 (13) |
| C15—C14—H14 | 123.2 | C9—C8—S1' | 10.0 (6) |
| C16—C15—C14 | 113.1 (3) | C7—C8—S1' | 126.6 (5) |
| C16—C15—H15 | 123.5 | C9'—C8—S1 | 6.0 (13) |
| C14—C15—H15 | 123.5 | C9—C8—S1 | 109.2 (2) |
| C15—C16—S2 | 111.6 (2) | C7—C8—S1 | 116.84 (14) |
| C15—C16—H16 | 124.2 | S1'—C8—S1 | 116.5 (5) |
| S2—C16—H16 | 124.2 | N1—C12—C13 | 111.81 (16) |
| C13—S2'—C16' | 93.3 (11) | N1—C12—H12A | 109.3 |
| C13—C14'—C15' | 110.4 (19) | C13—C12—H12A | 109.3 |
| C13—C14'—H14' | 124.8 | N1—C12—H12B | 109.3 |
| C15'—C14'—H14' | 124.8 | C13—C12—H12B | 109.3 |
| C15'—C16'—S2' | 110.1 (16) | H12A—C12—H12B | 107.9 |
| C15'—C16'—H16' | 124.9 | C14—C13—C14' | 102.4 (12) |
| S2'—C16'—H16' | 124.9 | C14—C13—C12 | 130.06 (19) |
| C16'—C15'—C14' | 114 (2) | C14'—C13—C12 | 127.4 (11) |
| C16'—C15'—H15' | 123.1 | C14—C13—S2' | 10.1 (5) |
| C14'—C15'—H15' | 123.1 | C14'—C13—S2' | 112.2 (12) |
| C7—N1—C1 | 106.23 (16) | C12—C13—S2' | 120.1 (4) |
| C7—N1—C12 | 129.49 (17) | C14—C13—S2 | 109.18 (17) |
| C1—N1—C12 | 124.27 (17) | C14'—C13—S2 | 8.3 (11) |
| C7—N2—C2 | 105.12 (16) | C12—C13—S2 | 120.76 (15) |
| C6—C1—N1 | 131.82 (19) | S2'—C13—S2 | 119.1 (4) |
| C6—C1—C2 | 122.7 (2) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C16—H16···N2i | 0.93 | 2.54 | 3.445 (4) | 166 |
Symmetry codes: (i) x−1/2, −y+1/2, z+1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: LH5359).
References
- Bahrami, K., Khodaei, M. M. & Naali, F. (2007). J. Org. Chem. 73, 6835–6837. [DOI] [PubMed]
- Bruker (2004). XSHELL Bruker AXS Inc., Madison, Wisconsin, USA.
- Bruker (2008). SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
- Bruker (2009). SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Bruker (2010). APEX2 Bruker AXS Inc., Madison, Wisconsin, USA.
- Du, L.-H. & Wang, Y.-G. (2007). Synthesis, pp. 675–678.
- Grimmett, M. R. (1997). In Imidazole and Benzidmidazole Synthesis San Diego: Academic Press.
- Horton, D. A., Bourne, G. T. & Smythe, M. L. (2003). Chem. Rev. 103, 893–930. [DOI] [PubMed]
- López-Rodríguez, M. L., Benhamú, B., Morcillo, M. J., Tejeda, I. D., Orensanz, L., Alfaro, M. J. & Martín, M. I. (1999). J. Med. Chem. 42, 5020–5028. [DOI] [PubMed]
- Reich, B. J. E., Justice, A. K., Beckstead, B. T., Reibenspies, J. H. & Miller, S. A. (2004). J. Org. Chem. 69, 1357–1359. [DOI] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Smith, J. G. & Ho, I. (1971). Tetrahedron Lett. 38, 3541–3544.
- Varala, R., Nasreen, A., Enugala, R. & Adapa, S. R. (2007). Tetrahedron Lett. 48, 69–72.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536811055103/lh5359sup1.cif
Supplementary material file. DOI: 10.1107/S1600536811055103/lh5359Isup2.mol
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811055103/lh5359Isup3.hkl
Supplementary material file. DOI: 10.1107/S1600536811055103/lh5359Isup4.mol
Supplementary material file. DOI: 10.1107/S1600536811055103/lh5359Isup5.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report