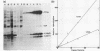
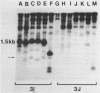
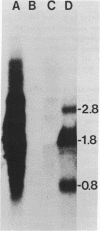
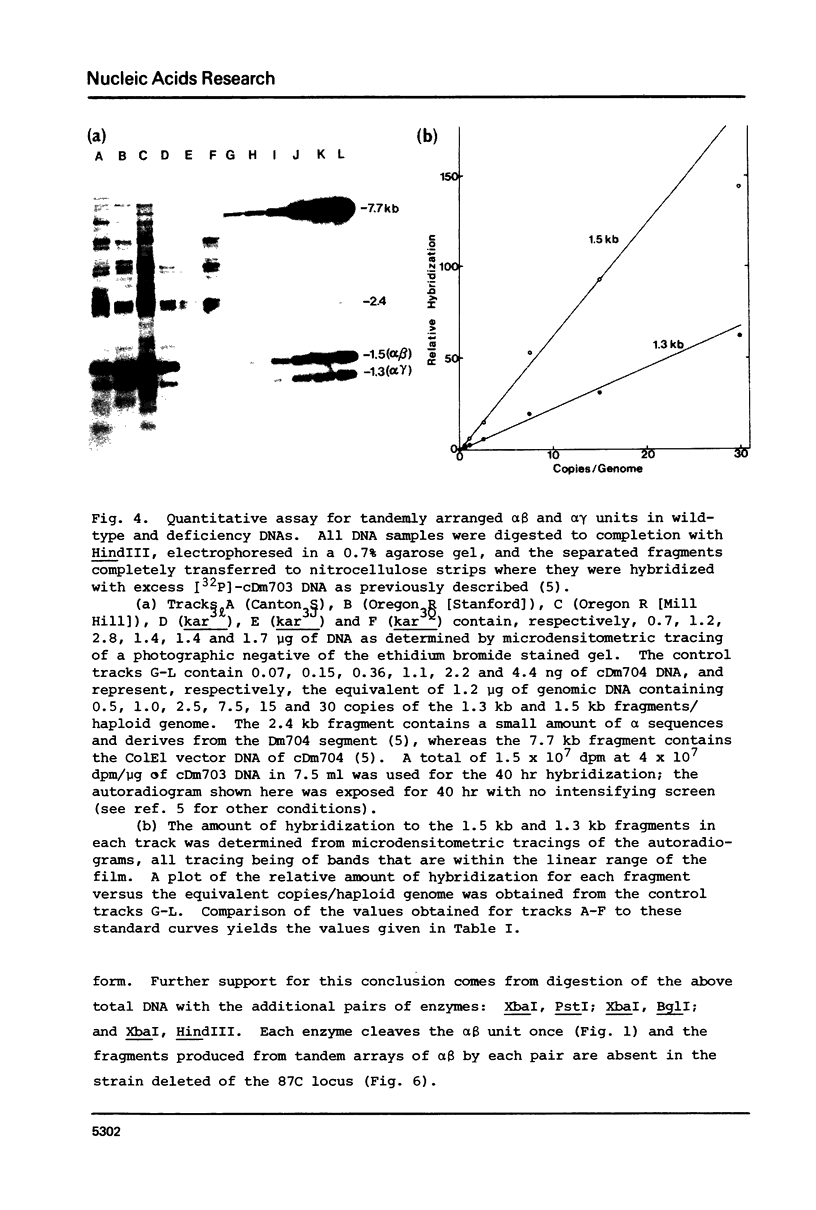
Abstract
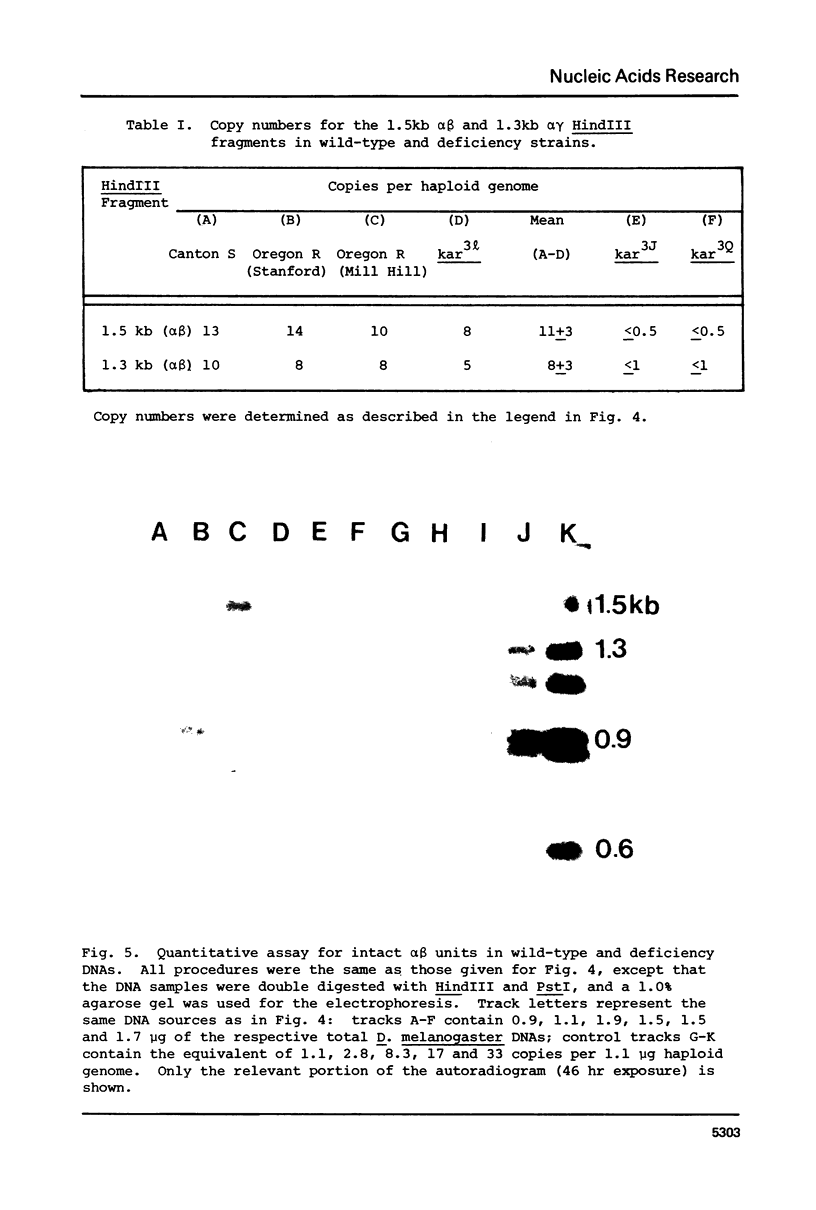
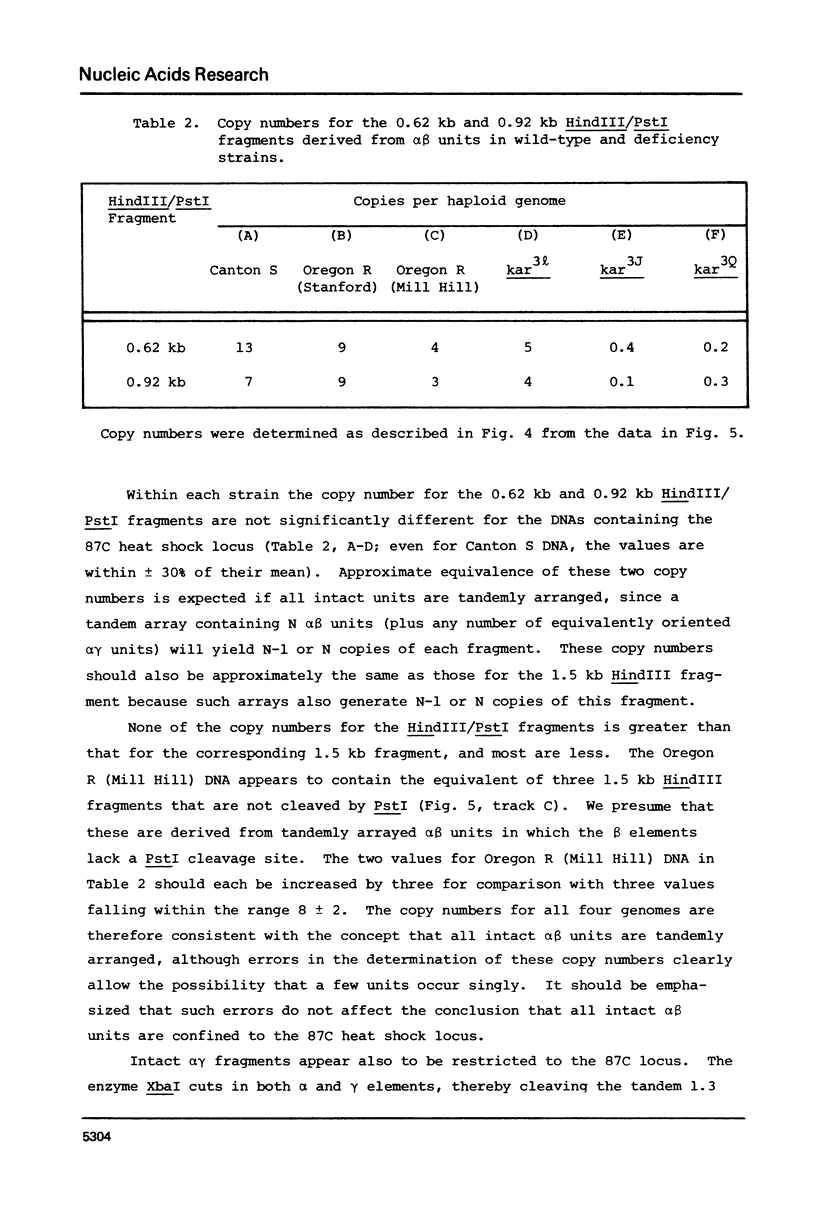
Previous studies have shown that (i) several RNAs induced by heat shock of Drosophila melanogaster cells are homologous to tandemly repeated alpha beta units found in cloned segments of D. melanogaster DNA, and (ii) the alpha beta sequences are present both at a major heat shock locus, 87Cl, and the chromocenter of polytene chromosomes (Lis, J.T., Prestidge, L. and Hogness, D.S. [1978] Cell 14, 901-919). We have used deficiencies that delete DNA from the 87C region to examine the arrangement of alpha beta sequences at this locus and in the centromeric heterochromatin that comprises the chromocenter, and also to determine the chromosomal location of the induced transcription. The tandemly repeated alpha beta units are restricted to the 87C locus. In contrast, the chromocentral alpha beta sequences do not form intact alpha beta units, and are dispersed at heterochromatic sites in some other form. Although only half of the alpha beta DNA is at the 87C locus, essentially all alpha beta transcripts (greater than 99.5%) are derived from this locus.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S., Schedl P., Mirault M. E., Moran L., Lis J. Genes for the 70,000 dalton heat shock protein in two cloned D. melanogaster DNA segments. Cell. 1979 May;17(1):9–18. doi: 10.1016/0092-8674(79)90290-3. [DOI] [PubMed] [Google Scholar]
- Ashburner M., Bonner J. J. The induction of gene activity in drosophilia by heat shock. Cell. 1979 Jun;17(2):241–254. doi: 10.1016/0092-8674(79)90150-8. [DOI] [PubMed] [Google Scholar]
- Hennig W., Meer B. Reduced polyteny of ribosomal RNA cistrons in giant chromosomes of Drosophila hydei. Nat New Biol. 1971 Sep 15;233(37):70–72. doi: 10.1038/newbio233070a0. [DOI] [PubMed] [Google Scholar]
- Ilyin Y. V., Tchurikov N. A., Ananiev E. V., Ryskov A. P., Yenikolopov G. N., Limborska S. A., Maleeva N. E., Gvozdev V. A., Georgiev G. P. Studies on the DNA fragments of mammals and Drosophila containing structural genes and adjacent sequences. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):959–969. doi: 10.1101/sqb.1978.042.01.097. [DOI] [PubMed] [Google Scholar]
- Ish-Horowicz D., Holden J. J., Gehring W. J. Deletions of two heat-activated loci in Drosophila melanogaster and their effects on heat-induced protein synthesis. Cell. 1977 Nov;12(3):643–652. doi: 10.1016/0092-8674(77)90264-1. [DOI] [PubMed] [Google Scholar]
- Ish-Horowicz D., Pinchin S. M., Gausz J., Gyurkovics H., Bencze G., Goldschmidt-Clermont M., Holden J. J. Deletion mapping of two D. melanogaster loci that code for the 70,000 dalton heat-induced protein. Cell. 1979 Jul;17(3):565–571. doi: 10.1016/0092-8674(79)90264-2. [DOI] [PubMed] [Google Scholar]
- Ish-Horowicz D., Pinchin S. M. Genomic organization of the 87A7 and 87Cl heat-induced loci of Drosophila melanogaster. J Mol Biol. 1980 Sep 15;142(2):231–245. doi: 10.1016/0022-2836(80)90047-9. [DOI] [PubMed] [Google Scholar]
- Ish-Horowicz D., Pinchin S. M., Schedl P., Artavanis-Tsakonas S., Mirault M. E. Genetic and molecular analysis of the 87A7 and 87C1 heat-inducible loci of D. melanogaster. Cell. 1979 Dec;18(4):1351–1358. doi: 10.1016/0092-8674(79)90245-9. [DOI] [PubMed] [Google Scholar]
- Leigh Brown A. J., Ish-Horowicz D. Evolution of the 87A and 87C heat-shock loci in Drosophila. Nature. 1981 Apr 23;290(5808):677–682. doi: 10.1038/290677a0. [DOI] [PubMed] [Google Scholar]
- Lis J. T., Prestidge L., Hogness D. S. A novel arrangement of tandemly repeated genes at a major heat shock site in D. melanogaster. Cell. 1978 Aug;14(4):901–919. doi: 10.1016/0092-8674(78)90345-8. [DOI] [PubMed] [Google Scholar]
- Lis J., Neckameyer W., Mirault M. E., Artavanis-Tsakonas S., Lall P., Martin G., Schedl P. DNA sequences flanking the starts of the hsp 70 and alpha beta heat shock genes are homologous. Dev Biol. 1981 Apr 30;83(2):291–300. doi: 10.1016/0012-1606(81)90475-9. [DOI] [PubMed] [Google Scholar]
- Livak K. J., Freund R., Schweber M., Wensink P. C., Meselson M. Sequence organization and transcription at two heat shock loci in Drosophila. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5613–5617. doi: 10.1073/pnas.75.11.5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter S. S., Brorein W. J., Jr, Dunsmuir P., Rubin G. M. Transposition of elements of the 412, copia and 297 dispersed repeated gene families in Drosophila. Cell. 1979 Jun;17(2):415–427. doi: 10.1016/0092-8674(79)90168-5. [DOI] [PubMed] [Google Scholar]
- Rudkin G. T. Replication in polytene chromosomes. Results Probl Cell Differ. 1972;4:59–85. doi: 10.1007/978-3-540-37164-9_3. [DOI] [PubMed] [Google Scholar]
- Schedl P., Artavanis-Tsakonas S., Steward R., Gehring W. J., Mirault M. E., Goldschmidt-Clermont M., Moran L., Tissières A. Two hybrid plasmids with D. melanogaster DNA sequences complementary to mRNA coding for the major heat shock protein. Cell. 1978 Aug;14(4):921–929. doi: 10.1016/0092-8674(78)90346-x. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spear B. B., Gall J. G. Independent control of ribosomal gene replication in polytene chromosomes of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1973 May;70(5):1359–1363. doi: 10.1073/pnas.70.5.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel E., Dunsmuir P., Rubin G. M. Polymorphisms in the chromosomal locations of elements of the 412, copia and 297 dispersed repeated gene families in Drosophila. Cell. 1979 Jun;17(2):429–439. doi: 10.1016/0092-8674(79)90169-7. [DOI] [PubMed] [Google Scholar]
- Young M. W. Middle repetitive DNA: a fluid component of the Drosophila genome. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6274–6278. doi: 10.1073/pnas.76.12.6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zain B. S., Roberts R. J. A new specific endonuclease from Xanthomonas badrii. J Mol Biol. 1977 Sep 15;115(2):249–255. doi: 10.1016/0022-2836(77)90101-2. [DOI] [PubMed] [Google Scholar]