Abstract
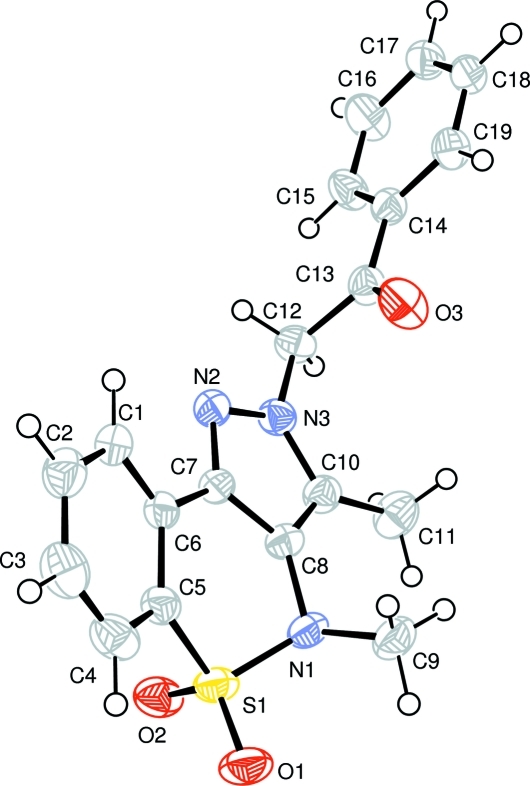
In the title molecule, C19H17N3O3S, the heterocyclic thiazine ring adopts a half-chair conformation with the S and N atoms displaced by 0.530 (5) and 0.229 (6) Å, respectively, on opposite sides of the mean plane formed by the remaining ring atoms. The ethanone group lies at an angle of 3.8 (3)° with respect to the benzene ring, which lies almost perendicular to the pyrazole ring, with a dihedral between the two planes of 89.22 (11)°. Weak intermolecular C—H⋯O hydrogen-bonding interactions are present.
Related literature
For the biological activity of pyrazoles, see: Farag et al. (2008 ▶); Ciciani et al. (2008 ▶); Cunico et al. (2006 ▶); Ahmad et al. (2010 ▶). For related structures, see: Siddiqui et al. (2008 ▶).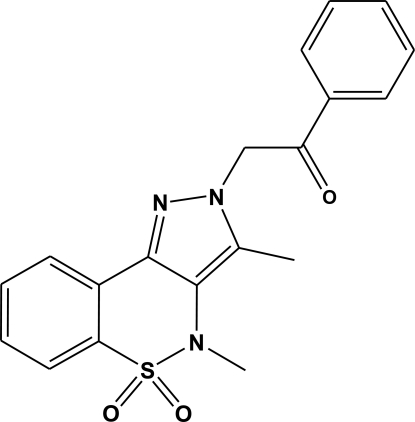
Experimental
Crystal data
C19H17N3O3S
M r = 367.42
Monoclinic,
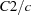
a = 24.380 (6) Å
b = 11.141 (4) Å
c = 14.996 (5) Å
β = 120.76 (2)°
V = 3500.1 (19) Å3
Z = 8
Mo Kα radiation
μ = 0.21 mm−1
T = 200 K
0.12 × 0.10 × 0.08 mm
Data collection
Nonius KappaCCD diffractometer
Absorption correction: multi-scan (SORTAV; Blessing, 1997 ▶) T min = 0.975, T max = 0.983
12615 measured reflections
3970 independent reflections
2847 reflections with I > 2σ(I)
R int = 0.073
Refinement
R[F 2 > 2σ(F 2)] = 0.069
wR(F 2) = 0.146
S = 1.15
3970 reflections
237 parameters
H-atom parameters constrained
Δρmax = 0.27 e Å−3
Δρmin = −0.44 e Å−3
Data collection: COLLECT (Hooft, 1998 ▶); cell refinement: DENZO (Otwinowski & Minor, 1997 ▶); data reduction: SCALEPACK (Otwinowski & Minor, 1997 ▶); program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 for Windows (Farrugia, 1997 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536812002188/pk2383sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536812002188/pk2383Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536812002188/pk2383Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C1—H1⋯O2i | 0.95 | 2.43 | 3.246 (5) | 144 |
| C9—H9B⋯O1ii | 0.98 | 2.46 | 3.413 (4) | 163 |
| C11—H11C⋯O1iii | 0.98 | 2.44 | 3.406 (4) | 168 |
Symmetry codes: (i) 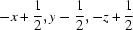 ; (ii)
; (ii) 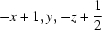 ; (iii)
; (iii) 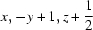 .
.
Acknowledgments
The authors are grateful to the Higher Education Commission, Pakistan, and the Institute of Chemistry, University of the Punjab, Lahore, Pakistan, for financial support.
supplementary crystallographic information
Comment
Both benzothiazines and pyrazoles are known as versatile biologically active heterocyclic nuclei. Pyrazoles are found to be cytotoxic agents (Ciciani et al., 2008), anti-tumor (Farag et al., 2008), anti-malarial (Cunico et al., 2006), etc. In continuation of our research interests in biologically active molecules (Ahmad et al., 2010), we have fused both of these heterocycles and herein report the synthesis and crystal structure of the title compound.
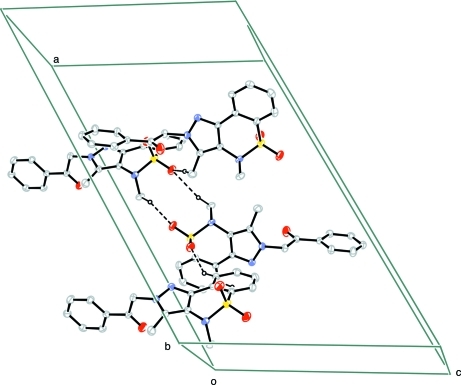
The bond distances and angles in the title molecule (Fig. 1) agree very well with the corresponding bond distances and angles reported in closely related compounds (Siddiqui et al., 2008). The heterocyclic thiazine ring adopts a half chair conformation with atoms S1 and N1 displaced by 0.530 (5) and 0.229 (6) Å, respectively, on opposite sides from the mean plane formed by the remaining ring atoms. The ethanone group O3/C12/C13/C14 is oriented at 3.8 (3) °, with the benzene ring (C14–C19) which lies almost perpendicular to the pyrazolyl ring (N2/N3/C7/C8/C10) with a dihedral between the two planes of 89.22 (11)°. The structure is devoid of classical hydrogen bonds. However, intermolecular hydrogen bonding interactions of C—H···O type are present (Table 1).
Experimental
Equimolar quantities of 3,4-dimethyl-2,4-dihydropyrazolo[4,3-c][1,2] benzothiazine 5,5-dioxide (1.0 g, 4.01 mmol) and corresponding phenacyl bromide (0.80 g, 4.01 mmol) were dissolved in acetonitrile (20 ml) followed by the addition of equimolar K2CO3 (0.55 g, 4.01 mmol). The mixture was subjected to reflux for 7 h. The completion of reaction was monitored with the help of TLC. The precipitates of the title compound were collected and washed with methanol. The crystals suitable for X-ray crystallographic analysis were grown from a solution of CHCl3:MeOH in 1:1 ratio.
Refinement
Though all the H atoms could be distinguished in the difference Fourier map, the H-atoms were included at geometrically idealized positions and refined in riding-model approximation with the following constraints: C—H = 0.95, 0.98 and 0.99 Å, for aryl, methyl and methylene H-atoms, respectively. The Uiso(H) were included at 1.5Ueq(C methyl) or 1.2Ueq(C non-methyl). The final difference map was essentially featureless.
Figures
Fig. 1.
The title molecule with displacement ellipsoids plotted at the 30% probability level.
Fig. 2.
A part of the unit cell showing intermolecular hydrogen bonding interactions as dashed lines. H-atoms not involved in hydrogen bonding have been excluded for clarity.
Crystal data
| C19H17N3O3S | F(000) = 1536 |
| Mr = 367.42 | Dx = 1.394 Mg m−3 |
| Monoclinic, C2/c | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -C 2yc | Cell parameters from 6235 reflections |
| a = 24.380 (6) Å | θ = 1.0–27.5° |
| b = 11.141 (4) Å | µ = 0.21 mm−1 |
| c = 14.996 (5) Å | T = 200 K |
| β = 120.76 (2)° | Block, colorless |
| V = 3500.1 (19) Å3 | 0.12 × 0.10 × 0.08 mm |
| Z = 8 |
Data collection
| Nonius KappaCCD diffractometer | 3970 independent reflections |
| Radiation source: fine-focus sealed tube | 2847 reflections with I > 2σ(I) |
| graphite | Rint = 0.073 |
| ω and φ scans | θmax = 27.5°, θmin = 2.1° |
| Absorption correction: multi-scan (SORTAV; Blessing, 1997) | h = −31→30 |
| Tmin = 0.975, Tmax = 0.983 | k = −14→14 |
| 12615 measured reflections | l = −18→19 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.069 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.146 | H-atom parameters constrained |
| S = 1.15 | w = 1/[σ2(Fo2) + (0.025P)2 + 10.0879P] where P = (Fo2 + 2Fc2)/3 |
| 3970 reflections | (Δ/σ)max < 0.001 |
| 237 parameters | Δρmax = 0.27 e Å−3 |
| 0 restraints | Δρmin = −0.44 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| S1 | 0.37478 (4) | 0.35736 (7) | 0.23368 (6) | 0.0377 (2) | |
| O1 | 0.41198 (11) | 0.3575 (2) | 0.18465 (19) | 0.0511 (6) | |
| O2 | 0.33464 (11) | 0.4586 (2) | 0.21844 (19) | 0.0470 (6) | |
| O3 | 0.42366 (12) | 0.1146 (2) | 0.65556 (19) | 0.0555 (7) | |
| N1 | 0.42423 (11) | 0.3433 (2) | 0.3603 (2) | 0.0348 (6) | |
| N2 | 0.31406 (11) | 0.2240 (2) | 0.43305 (19) | 0.0333 (6) | |
| N3 | 0.35687 (12) | 0.2864 (2) | 0.51876 (19) | 0.0337 (6) | |
| C1 | 0.26868 (14) | 0.0818 (3) | 0.2346 (2) | 0.0356 (7) | |
| H1 | 0.2551 | 0.0498 | 0.2788 | 0.043* | |
| C2 | 0.24943 (16) | 0.0283 (3) | 0.1398 (3) | 0.0445 (8) | |
| H2 | 0.2219 | −0.0394 | 0.1190 | 0.053* | |
| C3 | 0.26953 (17) | 0.0715 (3) | 0.0750 (3) | 0.0496 (9) | |
| H3 | 0.2563 | 0.0329 | 0.0106 | 0.059* | |
| C4 | 0.30886 (17) | 0.1711 (3) | 0.1036 (3) | 0.0447 (8) | |
| H4 | 0.3230 | 0.2010 | 0.0595 | 0.054* | |
| C5 | 0.32727 (14) | 0.2264 (3) | 0.1974 (2) | 0.0347 (7) | |
| C6 | 0.30809 (13) | 0.1828 (3) | 0.2652 (2) | 0.0311 (6) | |
| C7 | 0.33508 (13) | 0.2388 (3) | 0.3667 (2) | 0.0304 (6) | |
| C8 | 0.39001 (14) | 0.3110 (3) | 0.4110 (2) | 0.0320 (6) | |
| C9 | 0.48457 (15) | 0.2765 (3) | 0.3945 (3) | 0.0445 (8) | |
| H9A | 0.5120 | 0.2825 | 0.4701 | 0.067* | |
| H9B | 0.5067 | 0.3109 | 0.3614 | 0.067* | |
| H9C | 0.4748 | 0.1919 | 0.3747 | 0.067* | |
| C10 | 0.40399 (14) | 0.3389 (3) | 0.5091 (2) | 0.0354 (7) | |
| C11 | 0.45835 (17) | 0.4063 (3) | 0.5944 (3) | 0.0501 (9) | |
| H11A | 0.4820 | 0.4479 | 0.5668 | 0.075* | |
| H11B | 0.4869 | 0.3501 | 0.6487 | 0.075* | |
| H11C | 0.4420 | 0.4651 | 0.6236 | 0.075* | |
| C12 | 0.35231 (15) | 0.2791 (3) | 0.6109 (2) | 0.0365 (7) | |
| H12A | 0.3071 | 0.2675 | 0.5907 | 0.044* | |
| H12B | 0.3673 | 0.3555 | 0.6499 | 0.044* | |
| C13 | 0.39244 (14) | 0.1753 (3) | 0.6804 (2) | 0.0351 (7) | |
| C14 | 0.39025 (14) | 0.1515 (3) | 0.7764 (2) | 0.0345 (7) | |
| C15 | 0.35436 (16) | 0.2220 (3) | 0.8039 (2) | 0.0432 (8) | |
| H15 | 0.3320 | 0.2899 | 0.7632 | 0.052* | |
| C16 | 0.35119 (18) | 0.1929 (4) | 0.8912 (3) | 0.0551 (10) | |
| H16 | 0.3268 | 0.2414 | 0.9102 | 0.066* | |
| C17 | 0.38329 (16) | 0.0941 (3) | 0.9503 (3) | 0.0442 (8) | |
| H17 | 0.3802 | 0.0735 | 1.0091 | 0.053* | |
| C18 | 0.41977 (15) | 0.0256 (3) | 0.9241 (2) | 0.0389 (7) | |
| H18 | 0.4425 | −0.0417 | 0.9656 | 0.047* | |
| C19 | 0.42368 (15) | 0.0537 (3) | 0.8375 (2) | 0.0378 (7) | |
| H19 | 0.4492 | 0.0063 | 0.8200 | 0.045* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| S1 | 0.0417 (4) | 0.0377 (4) | 0.0452 (5) | 0.0021 (4) | 0.0305 (4) | 0.0067 (4) |
| O1 | 0.0548 (15) | 0.0607 (16) | 0.0576 (15) | 0.0012 (13) | 0.0430 (13) | 0.0104 (13) |
| O2 | 0.0550 (15) | 0.0386 (13) | 0.0570 (15) | 0.0097 (11) | 0.0356 (13) | 0.0128 (12) |
| O3 | 0.0629 (16) | 0.0654 (18) | 0.0503 (15) | 0.0271 (14) | 0.0377 (13) | 0.0125 (13) |
| N1 | 0.0318 (13) | 0.0371 (15) | 0.0427 (15) | −0.0028 (11) | 0.0243 (12) | 0.0012 (12) |
| N2 | 0.0305 (13) | 0.0380 (14) | 0.0324 (13) | 0.0011 (11) | 0.0169 (11) | 0.0020 (11) |
| N3 | 0.0353 (13) | 0.0373 (14) | 0.0313 (13) | 0.0017 (12) | 0.0190 (11) | 0.0019 (11) |
| C1 | 0.0288 (15) | 0.0397 (18) | 0.0354 (17) | 0.0004 (14) | 0.0144 (14) | 0.0027 (14) |
| C2 | 0.0388 (18) | 0.0427 (19) | 0.0447 (19) | −0.0034 (15) | 0.0160 (16) | −0.0040 (16) |
| C3 | 0.054 (2) | 0.053 (2) | 0.0355 (18) | 0.0026 (18) | 0.0183 (17) | −0.0076 (17) |
| C4 | 0.053 (2) | 0.050 (2) | 0.0370 (18) | 0.0111 (17) | 0.0275 (17) | 0.0050 (16) |
| C5 | 0.0332 (16) | 0.0378 (17) | 0.0358 (16) | 0.0070 (14) | 0.0196 (14) | 0.0080 (14) |
| C6 | 0.0265 (14) | 0.0351 (16) | 0.0311 (16) | 0.0044 (12) | 0.0143 (12) | 0.0051 (13) |
| C7 | 0.0276 (14) | 0.0348 (16) | 0.0315 (15) | 0.0022 (13) | 0.0171 (13) | 0.0034 (13) |
| C8 | 0.0322 (15) | 0.0333 (16) | 0.0350 (16) | −0.0010 (13) | 0.0204 (13) | 0.0001 (13) |
| C9 | 0.0341 (17) | 0.047 (2) | 0.059 (2) | 0.0022 (16) | 0.0292 (17) | 0.0043 (18) |
| C10 | 0.0341 (16) | 0.0352 (17) | 0.0391 (17) | 0.0011 (14) | 0.0203 (14) | 0.0011 (14) |
| C11 | 0.046 (2) | 0.054 (2) | 0.050 (2) | −0.0121 (18) | 0.0240 (18) | −0.0155 (18) |
| C12 | 0.0428 (17) | 0.0383 (17) | 0.0343 (16) | 0.0014 (15) | 0.0241 (15) | −0.0009 (14) |
| C13 | 0.0324 (15) | 0.0393 (17) | 0.0348 (16) | 0.0031 (14) | 0.0179 (14) | −0.0005 (14) |
| C14 | 0.0312 (15) | 0.0403 (17) | 0.0320 (16) | −0.0004 (14) | 0.0162 (13) | 0.0009 (14) |
| C15 | 0.0458 (19) | 0.050 (2) | 0.0403 (18) | 0.0174 (17) | 0.0265 (16) | 0.0124 (16) |
| C16 | 0.058 (2) | 0.070 (3) | 0.049 (2) | 0.025 (2) | 0.036 (2) | 0.015 (2) |
| C17 | 0.0404 (18) | 0.057 (2) | 0.0380 (18) | 0.0040 (17) | 0.0223 (16) | 0.0093 (17) |
| C18 | 0.0358 (16) | 0.0367 (17) | 0.0371 (17) | 0.0002 (14) | 0.0136 (14) | 0.0045 (14) |
| C19 | 0.0362 (17) | 0.0367 (18) | 0.0411 (18) | 0.0059 (14) | 0.0202 (15) | 0.0002 (14) |
Geometric parameters (Å, °)
| S1—O1 | 1.430 (2) | C8—C10 | 1.365 (4) |
| S1—O2 | 1.433 (2) | C9—H9A | 0.9800 |
| S1—N1 | 1.656 (3) | C9—H9B | 0.9800 |
| S1—C5 | 1.766 (3) | C9—H9C | 0.9800 |
| O3—C13 | 1.211 (4) | C10—C11 | 1.490 (4) |
| N1—C8 | 1.432 (3) | C11—H11A | 0.9800 |
| N1—C9 | 1.486 (4) | C11—H11B | 0.9800 |
| N2—C7 | 1.342 (3) | C11—H11C | 0.9800 |
| N2—N3 | 1.361 (3) | C12—C13 | 1.530 (4) |
| N3—C10 | 1.362 (4) | C12—H12A | 0.9900 |
| N3—C12 | 1.444 (4) | C12—H12B | 0.9900 |
| C1—C2 | 1.383 (4) | C13—C14 | 1.491 (4) |
| C1—C6 | 1.396 (4) | C14—C15 | 1.386 (4) |
| C1—H1 | 0.9500 | C14—C19 | 1.387 (4) |
| C2—C3 | 1.381 (5) | C15—C16 | 1.388 (4) |
| C2—H2 | 0.9500 | C15—H15 | 0.9500 |
| C3—C4 | 1.383 (5) | C16—C17 | 1.378 (5) |
| C3—H3 | 0.9500 | C16—H16 | 0.9500 |
| C4—C5 | 1.383 (4) | C17—C18 | 1.374 (4) |
| C4—H4 | 0.9500 | C17—H17 | 0.9500 |
| C5—C6 | 1.406 (4) | C18—C19 | 1.386 (4) |
| C6—C7 | 1.454 (4) | C18—H18 | 0.9500 |
| C7—C8 | 1.404 (4) | C19—H19 | 0.9500 |
| O1—S1—O2 | 118.56 (15) | N1—C9—H9C | 109.5 |
| O1—S1—N1 | 107.98 (14) | H9A—C9—H9C | 109.5 |
| O2—S1—N1 | 107.12 (14) | H9B—C9—H9C | 109.5 |
| O1—S1—C5 | 109.31 (15) | N3—C10—C8 | 104.9 (3) |
| O2—S1—C5 | 108.32 (14) | N3—C10—C11 | 123.6 (3) |
| N1—S1—C5 | 104.67 (14) | C8—C10—C11 | 131.4 (3) |
| C8—N1—C9 | 115.7 (3) | C10—C11—H11A | 109.5 |
| C8—N1—S1 | 110.62 (19) | C10—C11—H11B | 109.5 |
| C9—N1—S1 | 117.0 (2) | H11A—C11—H11B | 109.5 |
| C7—N2—N3 | 103.8 (2) | C10—C11—H11C | 109.5 |
| N2—N3—C10 | 113.6 (2) | H11A—C11—H11C | 109.5 |
| N2—N3—C12 | 118.5 (2) | H11B—C11—H11C | 109.5 |
| C10—N3—C12 | 127.5 (3) | N3—C12—C13 | 111.0 (2) |
| C2—C1—C6 | 120.0 (3) | N3—C12—H12A | 109.4 |
| C2—C1—H1 | 120.0 | C13—C12—H12A | 109.4 |
| C6—C1—H1 | 120.0 | N3—C12—H12B | 109.4 |
| C3—C2—C1 | 121.1 (3) | C13—C12—H12B | 109.4 |
| C3—C2—H2 | 119.4 | H12A—C12—H12B | 108.0 |
| C1—C2—H2 | 119.4 | O3—C13—C14 | 122.5 (3) |
| C2—C3—C4 | 120.0 (3) | O3—C13—C12 | 119.7 (3) |
| C2—C3—H3 | 120.0 | C14—C13—C12 | 117.8 (3) |
| C4—C3—H3 | 120.0 | C15—C14—C19 | 119.7 (3) |
| C3—C4—C5 | 119.1 (3) | C15—C14—C13 | 121.7 (3) |
| C3—C4—H4 | 120.4 | C19—C14—C13 | 118.6 (3) |
| C5—C4—H4 | 120.4 | C14—C15—C16 | 119.8 (3) |
| C4—C5—C6 | 121.7 (3) | C14—C15—H15 | 120.1 |
| C4—C5—S1 | 120.1 (2) | C16—C15—H15 | 120.1 |
| C6—C5—S1 | 118.1 (2) | C17—C16—C15 | 120.4 (3) |
| C1—C6—C5 | 117.9 (3) | C17—C16—H16 | 119.8 |
| C1—C6—C7 | 123.9 (3) | C15—C16—H16 | 119.8 |
| C5—C6—C7 | 118.0 (3) | C18—C17—C16 | 119.8 (3) |
| N2—C7—C8 | 110.7 (3) | C18—C17—H17 | 120.1 |
| N2—C7—C6 | 125.7 (3) | C16—C17—H17 | 120.1 |
| C8—C7—C6 | 123.5 (3) | C17—C18—C19 | 120.5 (3) |
| C10—C8—C7 | 107.0 (3) | C17—C18—H18 | 119.8 |
| C10—C8—N1 | 128.5 (3) | C19—C18—H18 | 119.8 |
| C7—C8—N1 | 124.5 (3) | C18—C19—C14 | 119.8 (3) |
| N1—C9—H9A | 109.5 | C18—C19—H19 | 120.1 |
| N1—C9—H9B | 109.5 | C14—C19—H19 | 120.1 |
| H9A—C9—H9B | 109.5 | ||
| O1—S1—N1—C8 | −164.8 (2) | C6—C7—C8—C10 | −174.9 (3) |
| O2—S1—N1—C8 | 66.5 (2) | N2—C7—C8—N1 | 179.7 (3) |
| C5—S1—N1—C8 | −48.4 (2) | C6—C7—C8—N1 | 3.3 (5) |
| O1—S1—N1—C9 | −29.4 (3) | C9—N1—C8—C10 | 74.8 (4) |
| O2—S1—N1—C9 | −158.1 (2) | S1—N1—C8—C10 | −149.2 (3) |
| C5—S1—N1—C9 | 87.0 (2) | C9—N1—C8—C7 | −103.0 (4) |
| C7—N2—N3—C10 | −0.4 (3) | S1—N1—C8—C7 | 33.0 (4) |
| C7—N2—N3—C12 | −173.6 (3) | N2—N3—C10—C8 | 1.3 (3) |
| C6—C1—C2—C3 | 1.3 (5) | C12—N3—C10—C8 | 173.8 (3) |
| C1—C2—C3—C4 | −0.9 (5) | N2—N3—C10—C11 | −176.5 (3) |
| C2—C3—C4—C5 | −0.3 (5) | C12—N3—C10—C11 | −4.0 (5) |
| C3—C4—C5—C6 | 1.3 (5) | C7—C8—C10—N3 | −1.6 (3) |
| C3—C4—C5—S1 | −177.4 (3) | N1—C8—C10—N3 | −179.7 (3) |
| O1—S1—C5—C4 | −27.1 (3) | C7—C8—C10—C11 | 176.0 (3) |
| O2—S1—C5—C4 | 103.5 (3) | N1—C8—C10—C11 | −2.2 (6) |
| N1—S1—C5—C4 | −142.5 (3) | N2—N3—C12—C13 | 90.0 (3) |
| O1—S1—C5—C6 | 154.2 (2) | C10—N3—C12—C13 | −82.2 (4) |
| O2—S1—C5—C6 | −75.3 (3) | N3—C12—C13—O3 | 1.9 (4) |
| N1—S1—C5—C6 | 38.8 (3) | N3—C12—C13—C14 | −176.9 (3) |
| C2—C1—C6—C5 | −0.3 (4) | O3—C13—C14—C15 | 179.8 (3) |
| C2—C1—C6—C7 | −174.6 (3) | C12—C13—C14—C15 | −1.4 (5) |
| C4—C5—C6—C1 | −0.9 (4) | O3—C13—C14—C19 | −2.1 (5) |
| S1—C5—C6—C1 | 177.8 (2) | C12—C13—C14—C19 | 176.6 (3) |
| C4—C5—C6—C7 | 173.6 (3) | C19—C14—C15—C16 | −1.2 (5) |
| S1—C5—C6—C7 | −7.7 (4) | C13—C14—C15—C16 | 176.8 (3) |
| N3—N2—C7—C8 | −0.7 (3) | C14—C15—C16—C17 | −0.3 (6) |
| N3—N2—C7—C6 | 175.7 (3) | C15—C16—C17—C18 | 1.4 (6) |
| C1—C6—C7—N2 | −18.5 (5) | C16—C17—C18—C19 | −1.1 (5) |
| C5—C6—C7—N2 | 167.3 (3) | C17—C18—C19—C14 | −0.4 (5) |
| C1—C6—C7—C8 | 157.4 (3) | C15—C14—C19—C18 | 1.5 (5) |
| C5—C6—C7—C8 | −16.8 (4) | C13—C14—C19—C18 | −176.6 (3) |
| N2—C7—C8—C10 | 1.5 (4) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C1—H1···O2i | 0.95 | 2.43 | 3.246 (5) | 144 |
| C9—H9B···O1ii | 0.98 | 2.46 | 3.413 (4) | 163. |
| C11—H11C···O1iii | 0.98 | 2.44 | 3.406 (4) | 168. |
Symmetry codes: (i) −x+1/2, y−1/2, −z+1/2; (ii) −x+1, y, −z+1/2; (iii) x, −y+1, z+1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: PK2383).
References
- Ahmad, M., Siddiqui, H. L., Zia-ur-Rehman, M. & Parvez, M. (2010). Eur. J. Med. Chem. 45, 698–704. [DOI] [PubMed]
- Blessing, R. H. (1997). J. Appl. Cryst. 30, 421–426.
- Ciciani, G., Coronnello, M., Guerrini, G., Selleri, S., Cantore, M., Failli, P., Mini, E. & Costanzo, A. (2008). Bioorg. Med. Chem. 16, 9409–9419. [DOI] [PubMed]
- Cunico, W., Cechinel, C. A., Bonacorso, H. G., Martins, M. A. P., Zanatta, N., Souza, M. V. N., Freitas, I. O., Soaresa, R. P. & Krettli, A. U. (2006). Bioorg. Med. Chem. 16, 649–653. [DOI] [PubMed]
- Farag, A. M., Mayhoub, A. S., Barakatb, S. E. & Bayomi, A. H. (2008). Bioorg. Med. Chem. Lett. 16, 881–889. [DOI] [PubMed]
- Farrugia, L. J. (1997). J. Appl. Cryst. 30, 565.
- Hooft, R. (1998). COLLECT Nonius BV, Delft, The Netherlands.
- Otwinowski, Z. & Minor, W. (1997). Methods in Enzymology, Vol. 276, Macromolecular Crystallography, Part A, edited by C. W. Carter Jr & R. M. Sweet, pp. 307–326. New York: Academic Press.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Siddiqui, W. A., Ahmad, S., Tariq, M. I., Siddiqui, H. L. & Parvez, M. (2008). Acta Cryst. C64, o4–o6. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536812002188/pk2383sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536812002188/pk2383Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536812002188/pk2383Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report