Abstract
The title compound, C37H31ClN4O4, crystallizes with two molecules (A and B) in the asymmetric unit. The pyrrole rings in both molecules are connected via cis fusion, whereas one ring has a twisted conformation and the other assumes a half-chair conformation. In the crystal, the A molecules form inversion dimers via a pair of C—H⋯Cl interactions, while the B molecules form chains propagating in [1 0], via C—H⋯O interactions. In the crystal, there are also a number of C—H⋯π interactions present.
0], via C—H⋯O interactions. In the crystal, there are also a number of C—H⋯π interactions present.
Related literature
For the bioactivity of pyrazole derivatives, see: Sullivan et al. (2006 ▶); Patel et al. (2010 ▶); Siu et al. (2008 ▶). For conformation studies, see: Nardelli (1983 ▶).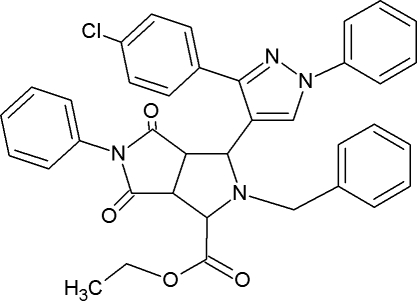
Experimental
Crystal data
C37H31ClN4O4
M r = 631.11
Triclinic,
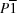
a = 12.8293 (8) Å
b = 13.3467 (8) Å
c = 22.0754 (11) Å
α = 83.897 (4)°
β = 81.585 (5)°
γ = 62.213 (6)°
V = 3304.9 (3) Å3
Z = 4
Mo Kα radiation
μ = 0.16 mm−1
T = 293 K
0.2 × 0.2 × 0.2 mm
Data collection
Oxford Diffraction Xcalibur Eos diffractometer
Absorption correction: multi-scan (CrysAlis PRO; Oxford Diffraction, 2009 ▶) T min = 0.978, T max = 0.984
29939 measured reflections
15365 independent reflections
7600 reflections with I > 2σ(I)
R int = 0.027
Refinement
R[F 2 > 2σ(F 2)] = 0.067
wR(F 2) = 0.225
S = 1.00
15365 reflections
831 parameters
259 restraints
H-atom parameters constrained
Δρmax = 0.88 e Å−3
Δρmin = −0.57 e Å−3
Data collection: CrysAlis PRO (Oxford Diffraction, 2009 ▶); cell refinement: CrysAlis PRO; data reduction: CrysAlis PRO; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: PLATON (Spek, 2009 ▶); software used to prepare material for publication: PLATON and publCIF (Westrip, 2010 ▶).
Supplementary Material
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536812002450/su2345sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536812002450/su2345Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536812002450/su2345Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
Cg1, Cg2 and Cg3 are the centroids of the C10–C15, C27′–C32′ and C33′–C38′ rings, respectively.
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C7—H7⋯O44′ | 0.98 | 2.45 | 3.311 (3) | 146 |
| C42′—H42A⋯Cl1′i | 0.96 | 2.76 | 3.707 (10) | 170 |
| C14′—H14′⋯Cg1 | 0.93 | 2.79 | 3.653 (4) | 154 |
| C17′—H17′⋯Cg2ii | 0.93 | 2.95 | 3.738 (4) | 143 |
| C29—H29⋯Cg1iii | 0.93 | 2.87 | 3.633 (3) | 140 |
| C42′—H42B⋯Cg3iv | 0.96 | 2.80 | 3.866 (9) | 153 |
Symmetry codes: (i) 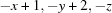 ; (ii)
; (ii) 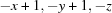 ; (iii)
; (iii) 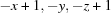 ; (iv)
; (iv) 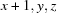 .
.
Acknowledgments
PK and SA thank the UGC, India, for financial support.
supplementary crystallographic information
Comment
Pyrazoles exhibit a variety of pharmacological properties for e.g antibacterial and anti-inflammatory activities (Sullivan et al., 2006; Patel et al., 2010). One of the pyrazole derivatives shows nucleosidase inhibitory activity against Staphylococcus aureus (Siu et al., 2008). In view of their importance, the crystal structure determination of the title compound was carried out and the results are presented herein.
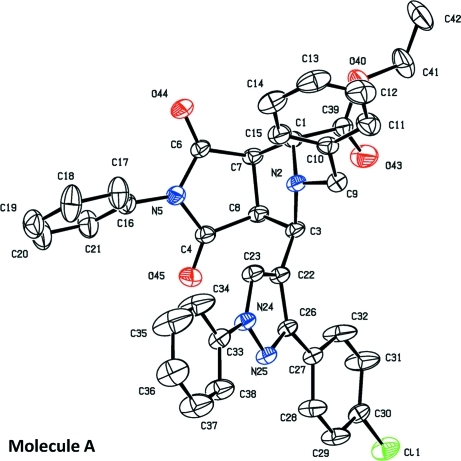
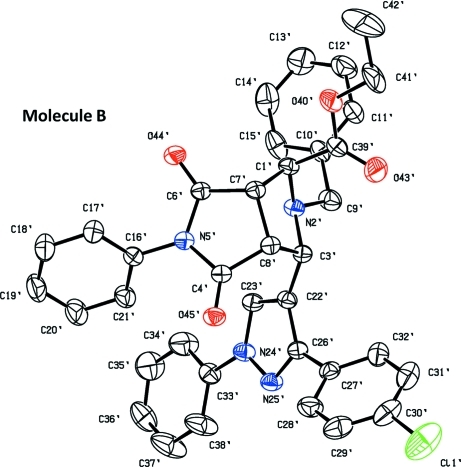
The molecular structure of the two independent molecules (A and B) of the title compound are shown in Figs. 1 and 2, respectively. In each molecule the pyrazole rings, (N24,N25,C26,C22,C23) in A and (N24',N25',C26',C22',C23') in B, are planar. In molecule A it is almost coplanar with the chlorophenyl ring attached to C26 as well as the phenyl ring attached at N24, with dihedral angles of 3.91 (16) and 4.88 (17) °, respectively. However, in molecule B the pyrazole ring is inclined to the chlorophenyl ring by 48.71 (17) °, and by 5.42 (18) ° to the phenyl ring at N24'.
The torsion angle H8—C8—C7—H7 in molecule A is 12.23 °, and H8'-C8'-C7'-H7' in molecule B is -14.81 °, which defines the ring fusion in the pyrrolo-pyrrole moieties as cis.
The pyrrole rings [(N5,C6—C8,C4) in A, and (N5',C6'-C8',C4') in B] assume half-chair (or envelope) conformations, with atoms C8 and C8' at the flap in molecules A and B, respectively, whereas the other pyrrole rings [(N2,C1,C7,C8,C3) in A and (N2',C1',C7',C8',C3') in B] have twisted conformations: defined by the asymmetry parameters (Nardelli, 1983), DS (N2) = 0.086 (2) Å and D2 (C8) = 0.010 (1) Å in molecule A, and DS (C3') = 0.018 (3) and D2 (C8') = 0.073 (2) in molecule B.
The partial double bond character of bonds C6—N5 [1.386 (3) Å] and C6'-N5' [1.390 (4) Å], and N5—C4 [1.395 (3) Å] and N5'-C4' [1.393 (3) Å], shows a high degree of electron delocalization.
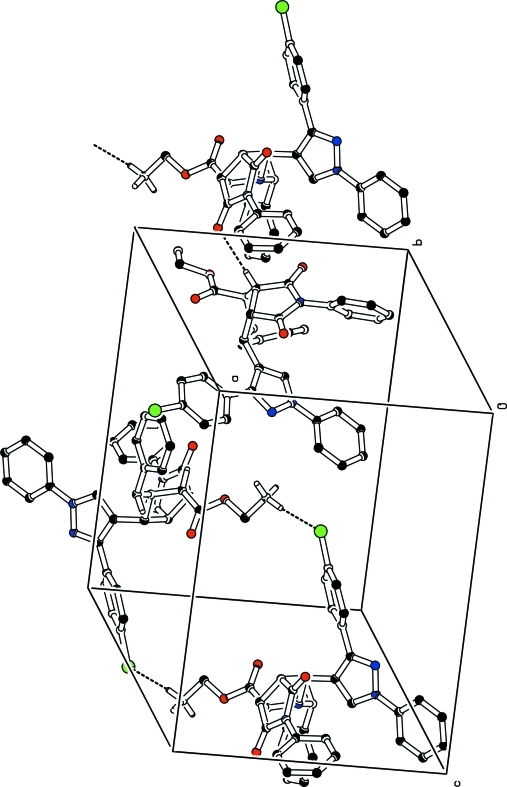
In the crystal, the A molecules form inversion dimers via a pair of C—H···Cl interactions, while the B molecules form chains, propagating in [1 - 1 0], via C—H···O interactions (Fig. 3 and Table 1). There are also a number of C—H···π interactions present (Table 1).
Experimental
A mixture of pyrazole aldehyde (0.3 g), benzylethylglycinate (0.177 g) and maleimide (0.158 g) was refluxed in toluene(15 ml) until completion of the reaction as evidenced by TLC analysis. The solvent was evaporated under reduced pressure. The crude product was purified by column chromatography on silica gel [Merck, 100–200 mesh, ethylacetate–petroleum ether (10:90)] to afford pure product. Crystals, suitable for X-ray analysis, where obtained by slow evaporation of a solution in ethylacetate.
Refinement
The NH H-atom was located in a difference electron-density map and was freely refined. The C-bound H-atoms were included in calculated positions and treated as riding atoms: C—H = 0.95, 0.98, 0.99 and 1.00 Å for CH(aromatic), CH3, CH2 and CH(methine) H-atoms, respectively, with Uiso(H) = k × Ueq(parent C-atom), where k = 1.5 for CH3 H-atoms and k = 1.2 for all other H-atoms. A potential solvent accessible void of 135.8 Å3 was detected but no residual electon density could be located in the final difference Fourier map.
Figures
Fig. 1.
The molecular structure of one of the two independent molecules (A) of the title compound, showing 30% probability displacement ellipsoids and the atom numbering scheme [H atoms have been omitted for clarity].
Fig. 2.
The molecular structure of the other independent molecules (B) of the title compound, showing 30% probability displacement ellipsoids and the atom numbering scheme [H atoms have been omitted for clarity].
Fig. 3.
A partial view of the crystal packing of the title compound. H atoms not involved in the C—H···O and C—H···.Cl interactions (dashed lines) have been omitted for clarity.
Crystal data
| C37H31ClN4O4 | V = 3304.9 (3) Å3 |
| Mr = 631.11 | Z = 4 |
| Triclinic, P1 | F(000) = 1320 |
| Hall symbol: -P 1 | Dx = 1.268 Mg m−3 |
| a = 12.8293 (8) Å | Mo Kα radiation, λ = 0.71073 Å |
| b = 13.3467 (8) Å | θ = 3.0–29.3° |
| c = 22.0754 (11) Å | µ = 0.16 mm−1 |
| α = 83.897 (4)° | T = 293 K |
| β = 81.585 (5)° | Triclinic, colourless |
| γ = 62.213 (6)° | 0.2 × 0.2 × 0.2 mm |
Data collection
| Oxford Diffraction Xcalibur Eos diffractometer | 15365 independent reflections |
| Radiation source: fine-focus sealed tube | 7600 reflections with I > 2σ(I) |
| graphite | Rint = 0.027 |
| Detector resolution: 15.9821 pixels mm-1 | θmax = 29.3°, θmin = 2.8° |
| ω scans | h = −17→17 |
| Absorption correction: multi-scan (CrysAlis PRO; Oxford Diffraction, 2009) | k = −17→18 |
| Tmin = 0.978, Tmax = 0.984 | l = −29→27 |
| 29939 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.067 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.225 | H-atom parameters constrained |
| S = 1.00 | w = 1/[σ2(Fo2) + (0.1261P)2] where P = (Fo2 + 2Fc2)/3 |
| 15365 reflections | (Δ/σ)max = 0.004 |
| 831 parameters | Δρmax = 0.88 e Å−3 |
| 259 restraints | Δρmin = −0.57 e Å−3 |
Special details
| Geometry. Bond distances, angles etc. have been calculated using the rounded fractional coordinates. All su's are estimated from the variances of the (full) variance-covariance matrix. The cell e.s.d.'s are taken into account in the estimation of distances, angles and torsion angles |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Cl1 | 0.99273 (7) | −0.39783 (6) | 0.32740 (4) | 0.0719 (3) | |
| O40 | 0.52582 (19) | 0.46815 (17) | 0.31405 (10) | 0.0661 (7) | |
| O43 | 0.6376 (2) | 0.28330 (19) | 0.30436 (14) | 0.0945 (11) | |
| O44 | 0.20936 (17) | 0.42683 (15) | 0.25533 (10) | 0.0611 (6) | |
| O45 | 0.39488 (19) | 0.04084 (15) | 0.27550 (11) | 0.0650 (7) | |
| N2 | 0.41464 (18) | 0.28538 (16) | 0.37365 (9) | 0.0402 (5) | |
| N5 | 0.27486 (19) | 0.23366 (17) | 0.26915 (10) | 0.0444 (5) | |
| N24 | 0.33633 (19) | 0.03102 (17) | 0.45976 (10) | 0.0440 (6) | |
| N25 | 0.44097 (18) | −0.06358 (17) | 0.44880 (9) | 0.0416 (6) | |
| C1 | 0.4250 (2) | 0.3578 (2) | 0.32078 (12) | 0.0417 (6) | |
| C3 | 0.4882 (2) | 0.16583 (19) | 0.35570 (11) | 0.0399 (6) | |
| C4 | 0.3811 (2) | 0.1361 (2) | 0.27651 (12) | 0.0430 (6) | |
| C6 | 0.2887 (2) | 0.3313 (2) | 0.26336 (11) | 0.0409 (6) | |
| C7 | 0.4163 (2) | 0.29884 (19) | 0.26863 (11) | 0.0399 (5) | |
| C8 | 0.4720 (2) | 0.1727 (2) | 0.28676 (11) | 0.0397 (5) | |
| C9 | 0.4357 (3) | 0.3100 (2) | 0.43141 (12) | 0.0499 (7) | |
| C10 | 0.3352 (3) | 0.4161 (2) | 0.45940 (12) | 0.0469 (7) | |
| C11 | 0.3575 (3) | 0.4766 (3) | 0.49836 (15) | 0.0679 (10) | |
| C12 | 0.2657 (4) | 0.5719 (3) | 0.52623 (17) | 0.0879 (11) | |
| C13 | 0.1525 (4) | 0.6075 (3) | 0.51712 (15) | 0.0859 (11) | |
| C14 | 0.1278 (3) | 0.5487 (3) | 0.47838 (15) | 0.0740 (10) | |
| C15 | 0.2188 (3) | 0.4535 (2) | 0.44965 (14) | 0.0579 (9) | |
| C16 | 0.1640 (2) | 0.2316 (2) | 0.27036 (13) | 0.0479 (7) | |
| C17 | 0.0737 (3) | 0.2876 (3) | 0.31495 (15) | 0.0740 (13) | |
| C18 | −0.0322 (3) | 0.2809 (4) | 0.31833 (17) | 0.0928 (16) | |
| C19 | −0.0471 (3) | 0.2206 (3) | 0.27818 (18) | 0.0815 (15) | |
| C20 | 0.0444 (3) | 0.1650 (3) | 0.2319 (2) | 0.0911 (16) | |
| C21 | 0.1498 (3) | 0.1710 (3) | 0.22826 (17) | 0.0713 (11) | |
| C22 | 0.4488 (2) | 0.0895 (2) | 0.39715 (11) | 0.0409 (6) | |
| C23 | 0.3406 (2) | 0.1223 (2) | 0.42914 (12) | 0.0450 (7) | |
| C26 | 0.5105 (2) | −0.0294 (2) | 0.41068 (10) | 0.0379 (6) | |
| C27 | 0.6304 (2) | −0.1164 (2) | 0.38973 (11) | 0.0385 (6) | |
| C28 | 0.6655 (3) | −0.2281 (2) | 0.40817 (14) | 0.0610 (9) | |
| C29 | 0.7764 (3) | −0.3134 (2) | 0.39048 (15) | 0.0639 (9) | |
| C30 | 0.8549 (2) | −0.2896 (2) | 0.35297 (12) | 0.0472 (7) | |
| C31 | 0.8267 (3) | −0.1788 (3) | 0.33514 (18) | 0.0875 (12) | |
| C32 | 0.7165 (3) | −0.0943 (3) | 0.35405 (18) | 0.0828 (12) | |
| C33 | 0.2419 (2) | 0.0237 (2) | 0.50002 (12) | 0.0482 (7) | |
| C34 | 0.1347 (3) | 0.1166 (3) | 0.5099 (2) | 0.1044 (13) | |
| C35 | 0.0450 (3) | 0.1096 (4) | 0.5502 (2) | 0.1265 (16) | |
| C36 | 0.0626 (3) | 0.0098 (3) | 0.58066 (19) | 0.0963 (13) | |
| C37 | 0.1694 (3) | −0.0818 (3) | 0.57148 (19) | 0.0973 (14) | |
| C38 | 0.2599 (3) | −0.0766 (3) | 0.53084 (16) | 0.0732 (10) | |
| C39 | 0.5429 (3) | 0.3636 (2) | 0.31210 (14) | 0.0520 (8) | |
| C41 | 0.6313 (4) | 0.4863 (4) | 0.3002 (2) | 0.0993 (16) | |
| C42 | 0.5998 (4) | 0.6009 (4) | 0.3140 (2) | 0.115 (2) | |
| Cl1' | 0.18611 (12) | 0.99456 (13) | −0.21002 (6) | 0.1397 (6) | |
| O40' | 0.5436 (2) | 0.76797 (18) | 0.17379 (12) | 0.0793 (10) | |
| O43' | 0.3934 (3) | 0.8848 (2) | 0.12233 (14) | 0.0982 (11) | |
| O44' | 0.52736 (19) | 0.44261 (17) | 0.17319 (9) | 0.0612 (8) | |
| O45' | 0.34709 (17) | 0.54348 (15) | −0.00136 (8) | 0.0515 (6) | |
| N2' | 0.28545 (19) | 0.73543 (18) | 0.15662 (9) | 0.0438 (6) | |
| N5' | 0.42346 (18) | 0.47404 (17) | 0.09055 (9) | 0.0408 (6) | |
| N24' | 0.0349 (2) | 0.66935 (19) | 0.08555 (10) | 0.0496 (7) | |
| N25' | 0.0304 (2) | 0.72321 (19) | 0.02929 (10) | 0.0512 (8) | |
| C1' | 0.4098 (2) | 0.7020 (2) | 0.16074 (12) | 0.0438 (6) | |
| C3' | 0.2686 (2) | 0.7506 (2) | 0.09111 (12) | 0.0427 (6) | |
| C4' | 0.3813 (2) | 0.5558 (2) | 0.04336 (11) | 0.0401 (7) | |
| C6' | 0.4795 (2) | 0.5021 (2) | 0.13029 (11) | 0.0410 (7) | |
| C7' | 0.4713 (2) | 0.6166 (2) | 0.10997 (11) | 0.0413 (6) | |
| C8' | 0.3883 (2) | 0.6590 (2) | 0.05994 (11) | 0.0410 (6) | |
| C9' | 0.1991 (3) | 0.8294 (3) | 0.19409 (13) | 0.0584 (8) | |
| C10' | 0.2239 (3) | 0.8099 (2) | 0.26041 (12) | 0.0492 (8) | |
| C11' | 0.2617 (3) | 0.8750 (3) | 0.28592 (14) | 0.0611 (10) | |
| C12' | 0.2873 (4) | 0.8558 (3) | 0.34542 (16) | 0.0811 (13) | |
| C13' | 0.2759 (4) | 0.7703 (3) | 0.38064 (16) | 0.0872 (15) | |
| C14' | 0.2399 (4) | 0.7033 (3) | 0.35620 (16) | 0.0884 (13) | |
| C15' | 0.2135 (3) | 0.7228 (3) | 0.29611 (15) | 0.0712 (13) | |
| C16' | 0.4186 (2) | 0.3680 (2) | 0.09344 (11) | 0.0430 (7) | |
| C17' | 0.5183 (3) | 0.2670 (2) | 0.10030 (13) | 0.0536 (8) | |
| C18' | 0.5120 (3) | 0.1660 (3) | 0.10246 (17) | 0.0737 (9) | |
| C19' | 0.4051 (4) | 0.1673 (3) | 0.09795 (18) | 0.0830 (13) | |
| C20' | 0.3065 (3) | 0.2682 (3) | 0.09114 (19) | 0.0809 (13) | |
| C21' | 0.3113 (3) | 0.3694 (3) | 0.08934 (15) | 0.0600 (9) | |
| C22' | 0.1635 (2) | 0.7362 (2) | 0.08089 (12) | 0.0430 (7) | |
| C23' | 0.1146 (2) | 0.6758 (2) | 0.11719 (12) | 0.0460 (8) | |
| C26' | 0.1077 (2) | 0.7639 (2) | 0.02693 (12) | 0.0458 (8) | |
| C27' | 0.1305 (2) | 0.8226 (2) | −0.03069 (13) | 0.0488 (7) | |
| C28' | 0.1491 (3) | 0.7735 (3) | −0.08581 (14) | 0.0713 (12) | |
| C29' | 0.1704 (4) | 0.8235 (3) | −0.14082 (16) | 0.0844 (13) | |
| C30' | 0.1673 (3) | 0.9274 (3) | −0.14000 (17) | 0.0805 (10) | |
| C31' | 0.1513 (3) | 0.9783 (3) | −0.08606 (18) | 0.0755 (9) | |
| C32' | 0.1346 (3) | 0.9237 (2) | −0.03113 (16) | 0.0605 (9) | |
| C33' | −0.0345 (2) | 0.6111 (2) | 0.10326 (13) | 0.0518 (9) | |
| C34' | −0.0336 (4) | 0.5605 (4) | 0.16032 (17) | 0.0858 (15) | |
| C35' | −0.1015 (4) | 0.5030 (4) | 0.1764 (2) | 0.0999 (16) | |
| C36' | −0.1702 (4) | 0.4989 (3) | 0.13663 (18) | 0.0868 (16) | |
| C37' | −0.1668 (4) | 0.5460 (4) | 0.0797 (2) | 0.113 (2) | |
| C38' | −0.0995 (4) | 0.6011 (4) | 0.06219 (18) | 0.0948 (18) | |
| C39' | 0.4460 (3) | 0.7962 (3) | 0.14954 (14) | 0.0559 (9) | |
| C41' | 0.5952 (4) | 0.8477 (3) | 0.1627 (3) | 0.126 (2) | |
| C42' | 0.6759 (7) | 0.8153 (7) | 0.2110 (4) | 0.230 (5) | |
| H1 | 0.35830 | 0.43410 | 0.32390 | 0.0500* | |
| H3 | 0.57150 | 0.14300 | 0.35970 | 0.0480* | |
| H7 | 0.45600 | 0.31410 | 0.23000 | 0.0480* | |
| H8 | 0.54770 | 0.13030 | 0.26200 | 0.0480* | |
| H11 | 0.43500 | 0.45340 | 0.50610 | 0.0820* | |
| H12 | 0.28330 | 0.61170 | 0.55180 | 0.1060* | |
| H13 | 0.09190 | 0.67050 | 0.53650 | 0.1030* | |
| H14 | 0.04980 | 0.57260 | 0.47130 | 0.0890* | |
| H15 | 0.20060 | 0.41490 | 0.42360 | 0.0690* | |
| H17 | 0.08310 | 0.32980 | 0.34270 | 0.0890* | |
| H18 | −0.09320 | 0.31840 | 0.34870 | 0.1110* | |
| H19 | −0.11780 | 0.21600 | 0.28110 | 0.0980* | |
| H20 | 0.03420 | 0.12410 | 0.20370 | 0.1090* | |
| H21 | 0.21060 | 0.13440 | 0.19760 | 0.0860* | |
| H23 | 0.27900 | 0.19590 | 0.42990 | 0.0540* | |
| H28 | 0.61210 | −0.24690 | 0.43360 | 0.0730* | |
| H29 | 0.79690 | −0.38780 | 0.40450 | 0.0770* | |
| H31 | 0.88180 | −0.16120 | 0.31050 | 0.1050* | |
| H32 | 0.69880 | −0.01950 | 0.34250 | 0.0990* | |
| H34 | 0.12190 | 0.18520 | 0.48930 | 0.1250* | |
| H35 | −0.02790 | 0.17350 | 0.55660 | 0.1520* | |
| H36 | 0.00190 | 0.00460 | 0.60740 | 0.1150* | |
| H37 | 0.18250 | −0.14970 | 0.59290 | 0.1170* | |
| H38 | 0.33250 | −0.14080 | 0.52450 | 0.0880* | |
| H41A | 0.69310 | 0.43300 | 0.32460 | 0.1190* | |
| H41B | 0.66120 | 0.47400 | 0.25720 | 0.1190* | |
| H42D | 0.66840 | 0.61350 | 0.30520 | 0.1730* | |
| H42E | 0.57080 | 0.61230 | 0.35670 | 0.1730* | |
| H42F | 0.53920 | 0.65320 | 0.28950 | 0.1730* | |
| H91 | 0.50760 | 0.31860 | 0.42540 | 0.0600* | |
| H92 | 0.44870 | 0.24600 | 0.46010 | 0.0600* | |
| H1' | 0.43180 | 0.66330 | 0.20060 | 0.0530* | |
| H9'1 | 0.12020 | 0.83850 | 0.19190 | 0.0700* | |
| H9'2 | 0.20120 | 0.89890 | 0.17780 | 0.0700* | |
| H3' | 0.25720 | 0.82640 | 0.07600 | 0.0510* | |
| H7' | 0.54920 | 0.61100 | 0.09430 | 0.0500* | |
| H8' | 0.41910 | 0.69170 | 0.02440 | 0.0490* | |
| H11' | 0.27020 | 0.93330 | 0.26230 | 0.0730* | |
| H12' | 0.31260 | 0.90110 | 0.36190 | 0.0970* | |
| H13' | 0.29250 | 0.75770 | 0.42120 | 0.1040* | |
| H14' | 0.23310 | 0.64440 | 0.38000 | 0.1060* | |
| H15' | 0.18880 | 0.67710 | 0.27970 | 0.0850* | |
| H17' | 0.59010 | 0.26640 | 0.10350 | 0.0640* | |
| H18' | 0.57950 | 0.09740 | 0.10690 | 0.0880* | |
| H19' | 0.40040 | 0.09960 | 0.09950 | 0.1000* | |
| H20' | 0.23490 | 0.26860 | 0.08760 | 0.0970* | |
| H21' | 0.24340 | 0.43780 | 0.08540 | 0.0720* | |
| H23' | 0.13260 | 0.64490 | 0.15630 | 0.0550* | |
| H28' | 0.14720 | 0.70470 | −0.08580 | 0.0860* | |
| H29' | 0.18640 | 0.78780 | −0.17740 | 0.1010* | |
| H31' | 0.15170 | 1.04790 | −0.08640 | 0.0910* | |
| H32' | 0.12610 | 0.95600 | 0.00580 | 0.0730* | |
| H34' | 0.01190 | 0.56430 | 0.18830 | 0.1030* | |
| H35' | −0.09960 | 0.46710 | 0.21500 | 0.1200* | |
| H36' | −0.21870 | 0.46430 | 0.14830 | 0.1040* | |
| H37' | −0.21140 | 0.54090 | 0.05160 | 0.1350* | |
| H38' | −0.09770 | 0.63190 | 0.02240 | 0.1140* | |
| H41C | 0.53380 | 0.92540 | 0.16650 | 0.1510* | |
| H41D | 0.63860 | 0.83960 | 0.12220 | 0.1510* | |
| H42A | 0.70100 | 0.87250 | 0.21220 | 0.3450* | |
| H42B | 0.74390 | 0.74400 | 0.20190 | 0.3450* | |
| H42C | 0.63500 | 0.80850 | 0.25000 | 0.3450* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Cl1 | 0.0458 (5) | 0.0517 (4) | 0.0859 (6) | 0.0009 (3) | 0.0072 (4) | −0.0054 (4) |
| O40 | 0.0564 (13) | 0.0607 (9) | 0.0939 (16) | −0.0375 (10) | −0.0080 (11) | −0.0036 (11) |
| O43 | 0.0357 (10) | 0.0613 (11) | 0.171 (3) | −0.0162 (9) | −0.0045 (14) | 0.0191 (15) |
| O44 | 0.0438 (10) | 0.0392 (8) | 0.0867 (14) | −0.0064 (8) | −0.0167 (10) | 0.0044 (10) |
| O45 | 0.0625 (14) | 0.0385 (8) | 0.0938 (15) | −0.0203 (9) | −0.0183 (12) | −0.0030 (11) |
| N2 | 0.0364 (11) | 0.0327 (8) | 0.0446 (8) | −0.0112 (8) | −0.0053 (8) | 0.0045 (6) |
| N5 | 0.0361 (8) | 0.0405 (7) | 0.0519 (12) | −0.0138 (7) | −0.0048 (9) | −0.0011 (10) |
| N24 | 0.0363 (9) | 0.0383 (9) | 0.0452 (12) | −0.0097 (7) | −0.0001 (8) | 0.0059 (8) |
| N25 | 0.0366 (9) | 0.0355 (8) | 0.0427 (12) | −0.0105 (7) | −0.0010 (8) | 0.0053 (8) |
| C1 | 0.0355 (10) | 0.0324 (9) | 0.0530 (10) | −0.0138 (9) | −0.0041 (10) | 0.0060 (8) |
| C3 | 0.0293 (12) | 0.0318 (8) | 0.0488 (10) | −0.0075 (9) | −0.0038 (8) | 0.0061 (6) |
| C4 | 0.0388 (10) | 0.0360 (8) | 0.0485 (13) | −0.0141 (8) | −0.0014 (11) | 0.0013 (12) |
| C6 | 0.0342 (10) | 0.0369 (8) | 0.0431 (13) | −0.0104 (7) | −0.0030 (10) | 0.0029 (11) |
| C7 | 0.0341 (9) | 0.0348 (9) | 0.0431 (9) | −0.0127 (8) | 0.0014 (10) | 0.0066 (8) |
| C8 | 0.0303 (9) | 0.0327 (8) | 0.0457 (8) | −0.0085 (7) | 0.0029 (10) | 0.0020 (8) |
| C9 | 0.0500 (13) | 0.0464 (13) | 0.0524 (11) | −0.0207 (10) | −0.0108 (12) | 0.0019 (9) |
| C10 | 0.0576 (11) | 0.0405 (12) | 0.0418 (13) | −0.0231 (10) | −0.0053 (11) | 0.0044 (8) |
| C11 | 0.0867 (18) | 0.0617 (17) | 0.0631 (19) | −0.0383 (14) | −0.0120 (15) | −0.0079 (12) |
| C12 | 0.127 (2) | 0.0618 (19) | 0.069 (2) | −0.0348 (18) | −0.012 (2) | −0.0181 (14) |
| C13 | 0.1104 (19) | 0.0545 (19) | 0.0526 (19) | −0.0069 (18) | 0.0003 (18) | −0.0021 (13) |
| C14 | 0.0654 (15) | 0.0603 (18) | 0.066 (2) | −0.0073 (12) | 0.0003 (15) | 0.0053 (13) |
| C15 | 0.0548 (12) | 0.0467 (15) | 0.0644 (18) | −0.0176 (11) | −0.0078 (13) | 0.0029 (11) |
| C16 | 0.0397 (9) | 0.0518 (15) | 0.0510 (14) | −0.0206 (10) | −0.0068 (9) | 0.0030 (10) |
| C17 | 0.0485 (15) | 0.114 (3) | 0.0647 (19) | −0.0408 (18) | 0.0053 (12) | −0.0236 (16) |
| C18 | 0.0514 (17) | 0.164 (4) | 0.071 (2) | −0.056 (2) | 0.0044 (14) | −0.0201 (19) |
| C19 | 0.0572 (18) | 0.106 (3) | 0.097 (3) | −0.051 (2) | −0.0158 (14) | 0.0085 (17) |
| C20 | 0.059 (2) | 0.095 (3) | 0.130 (3) | −0.036 (2) | −0.0201 (16) | −0.032 (2) |
| C21 | 0.0515 (16) | 0.078 (2) | 0.088 (2) | −0.0281 (17) | −0.0078 (14) | −0.0256 (16) |
| C22 | 0.0357 (11) | 0.0341 (8) | 0.0440 (12) | −0.0103 (8) | −0.0026 (9) | 0.0047 (9) |
| C23 | 0.0376 (11) | 0.0357 (9) | 0.0490 (15) | −0.0092 (8) | 0.0010 (10) | 0.0052 (10) |
| C26 | 0.0377 (9) | 0.0353 (8) | 0.0317 (12) | −0.0108 (7) | −0.0029 (8) | 0.0053 (9) |
| C27 | 0.0378 (10) | 0.0342 (8) | 0.0345 (12) | −0.0103 (7) | −0.0022 (9) | 0.0020 (9) |
| C28 | 0.0506 (14) | 0.0372 (9) | 0.073 (2) | −0.0106 (9) | 0.0170 (13) | 0.0092 (13) |
| C29 | 0.0538 (14) | 0.0348 (11) | 0.077 (2) | −0.0060 (8) | 0.0112 (13) | 0.0088 (13) |
| C30 | 0.0372 (11) | 0.0403 (9) | 0.0478 (15) | −0.0053 (8) | −0.0025 (9) | 0.0003 (11) |
| C31 | 0.0523 (16) | 0.0482 (10) | 0.117 (3) | −0.0026 (10) | 0.0322 (17) | 0.0227 (18) |
| C32 | 0.0513 (16) | 0.0400 (11) | 0.116 (3) | −0.0030 (9) | 0.0279 (16) | 0.0257 (17) |
| C33 | 0.0376 (11) | 0.0519 (12) | 0.0438 (15) | −0.0144 (9) | 0.0010 (9) | 0.0068 (11) |
| C34 | 0.0508 (17) | 0.0717 (18) | 0.129 (3) | 0.0025 (12) | 0.0331 (19) | 0.039 (2) |
| C35 | 0.053 (2) | 0.102 (2) | 0.150 (4) | 0.0010 (17) | 0.041 (2) | 0.048 (3) |
| C36 | 0.0572 (18) | 0.102 (2) | 0.104 (3) | −0.0301 (15) | 0.0238 (19) | 0.024 (2) |
| C37 | 0.067 (2) | 0.084 (2) | 0.114 (3) | −0.0288 (14) | 0.022 (2) | 0.034 (2) |
| C38 | 0.0505 (16) | 0.0581 (14) | 0.090 (2) | −0.0177 (12) | 0.0116 (14) | 0.0205 (15) |
| C39 | 0.0395 (11) | 0.0479 (10) | 0.0669 (18) | −0.0207 (8) | −0.0076 (12) | 0.0098 (13) |
| C41 | 0.079 (2) | 0.108 (2) | 0.146 (4) | −0.071 (2) | −0.020 (2) | −0.001 (3) |
| C42 | 0.137 (4) | 0.121 (3) | 0.145 (4) | −0.104 (3) | −0.034 (3) | 0.004 (3) |
| Cl1' | 0.1165 (10) | 0.1634 (12) | 0.1110 (9) | −0.0587 (9) | −0.0035 (7) | 0.0712 (9) |
| O40' | 0.0692 (15) | 0.0680 (14) | 0.125 (2) | −0.0462 (12) | −0.0362 (13) | 0.0044 (13) |
| O43' | 0.106 (2) | 0.0794 (15) | 0.141 (2) | −0.0654 (16) | −0.0595 (17) | 0.0412 (15) |
| O44' | 0.0796 (16) | 0.0643 (12) | 0.0524 (11) | −0.0411 (12) | −0.0272 (10) | 0.0127 (9) |
| O45' | 0.0554 (12) | 0.0591 (11) | 0.0388 (9) | −0.0232 (10) | −0.0085 (8) | −0.0077 (8) |
| N2' | 0.0392 (9) | 0.0510 (12) | 0.0437 (8) | −0.0217 (9) | −0.0044 (7) | −0.0079 (8) |
| N5' | 0.0392 (12) | 0.0475 (8) | 0.0379 (10) | −0.0222 (9) | −0.0025 (8) | −0.0015 (8) |
| N24' | 0.0433 (13) | 0.0610 (14) | 0.0512 (11) | −0.0288 (11) | −0.0075 (9) | −0.0022 (9) |
| N25' | 0.0463 (14) | 0.0624 (14) | 0.0525 (11) | −0.0300 (11) | −0.0132 (9) | 0.0017 (10) |
| C1' | 0.0426 (10) | 0.0542 (11) | 0.0429 (11) | −0.0283 (9) | −0.0082 (9) | −0.0014 (9) |
| C3' | 0.0396 (10) | 0.0444 (12) | 0.0464 (9) | −0.0200 (8) | −0.0096 (8) | −0.0005 (9) |
| C4' | 0.0365 (14) | 0.0501 (10) | 0.0338 (11) | −0.0207 (11) | −0.0004 (8) | −0.0030 (7) |
| C6' | 0.0381 (15) | 0.0497 (10) | 0.0384 (12) | −0.0235 (11) | −0.0038 (9) | 0.0007 (8) |
| C7' | 0.0340 (10) | 0.0508 (10) | 0.0439 (12) | −0.0241 (8) | −0.0034 (7) | 0.0004 (7) |
| C8' | 0.0394 (10) | 0.0501 (10) | 0.0369 (9) | −0.0247 (9) | −0.0027 (7) | 0.0021 (7) |
| C9' | 0.0509 (14) | 0.0591 (16) | 0.0616 (10) | −0.0195 (12) | −0.0037 (11) | −0.0192 (9) |
| C10' | 0.0483 (17) | 0.0491 (15) | 0.0522 (9) | −0.0247 (13) | 0.0071 (11) | −0.0166 (9) |
| C11' | 0.085 (2) | 0.0578 (18) | 0.0555 (13) | −0.0450 (17) | −0.0045 (15) | −0.0081 (12) |
| C12' | 0.111 (3) | 0.088 (2) | 0.0618 (16) | −0.056 (2) | −0.0160 (19) | −0.0147 (15) |
| C13' | 0.111 (3) | 0.094 (3) | 0.0454 (16) | −0.040 (2) | 0.0015 (17) | −0.0057 (14) |
| C14' | 0.123 (3) | 0.072 (2) | 0.0636 (15) | −0.049 (2) | 0.0254 (18) | −0.0033 (15) |
| C15' | 0.093 (3) | 0.0675 (19) | 0.0682 (14) | −0.055 (2) | 0.0236 (16) | −0.0202 (13) |
| C16' | 0.0453 (12) | 0.0479 (9) | 0.0381 (13) | −0.0239 (9) | −0.0005 (11) | −0.0049 (10) |
| C17' | 0.0466 (12) | 0.0501 (9) | 0.0608 (17) | −0.0215 (9) | −0.0002 (13) | −0.0003 (14) |
| C18' | 0.0722 (17) | 0.0481 (10) | 0.096 (2) | −0.0252 (13) | −0.006 (2) | −0.0002 (17) |
| C19' | 0.093 (2) | 0.0553 (12) | 0.116 (3) | −0.0464 (14) | −0.015 (2) | −0.001 (2) |
| C20' | 0.0733 (18) | 0.0692 (15) | 0.120 (3) | −0.0486 (13) | −0.017 (2) | 0.002 (2) |
| C21' | 0.0487 (13) | 0.0548 (12) | 0.083 (2) | −0.0282 (11) | −0.0091 (16) | −0.0048 (16) |
| C22' | 0.0373 (11) | 0.0462 (14) | 0.0465 (11) | −0.0184 (10) | −0.0051 (9) | −0.0087 (10) |
| C23' | 0.0392 (15) | 0.0540 (16) | 0.0466 (11) | −0.0220 (12) | −0.0059 (10) | −0.0050 (11) |
| C26' | 0.0381 (15) | 0.0487 (14) | 0.0520 (10) | −0.0196 (12) | −0.0106 (9) | −0.0011 (10) |
| C27' | 0.0376 (15) | 0.0534 (14) | 0.0558 (9) | −0.0202 (13) | −0.0144 (11) | 0.0057 (9) |
| C28' | 0.092 (3) | 0.088 (2) | 0.0516 (10) | −0.054 (2) | −0.0213 (17) | 0.0060 (11) |
| C29' | 0.091 (3) | 0.108 (2) | 0.0539 (11) | −0.046 (2) | −0.0200 (18) | 0.0158 (13) |
| C30' | 0.051 (2) | 0.095 (2) | 0.0797 (11) | −0.027 (2) | −0.0111 (18) | 0.0342 (13) |
| C31' | 0.0502 (19) | 0.0660 (18) | 0.1050 (11) | −0.0282 (17) | −0.0058 (19) | 0.0224 (13) |
| C32' | 0.0459 (17) | 0.0531 (15) | 0.0804 (12) | −0.0226 (14) | −0.0057 (15) | 0.0036 (11) |
| C33' | 0.0412 (16) | 0.0592 (17) | 0.0589 (13) | −0.0271 (13) | 0.0006 (11) | −0.0073 (12) |
| C34' | 0.096 (3) | 0.123 (3) | 0.0724 (17) | −0.081 (2) | −0.0190 (19) | 0.0219 (19) |
| C35' | 0.112 (3) | 0.122 (3) | 0.099 (2) | −0.087 (3) | −0.016 (2) | 0.029 (2) |
| C36' | 0.098 (3) | 0.098 (3) | 0.096 (2) | −0.076 (3) | 0.0174 (19) | −0.020 (2) |
| C37' | 0.142 (4) | 0.181 (5) | 0.090 (2) | −0.137 (4) | −0.010 (2) | −0.007 (3) |
| C38' | 0.116 (3) | 0.156 (4) | 0.0716 (19) | −0.112 (3) | −0.020 (2) | 0.010 (2) |
| C39' | 0.0579 (17) | 0.0580 (12) | 0.0654 (19) | −0.0362 (14) | −0.0133 (13) | −0.0021 (11) |
| C41' | 0.108 (3) | 0.079 (3) | 0.236 (6) | −0.070 (3) | −0.060 (4) | −0.004 (3) |
| C42' | 0.239 (8) | 0.261 (8) | 0.319 (10) | −0.196 (8) | −0.152 (7) | 0.025 (7) |
Geometric parameters (Å, °)
| Cl1—C30 | 1.741 (3) | C21—H21 | 0.9300 |
| Cl1'—C30' | 1.746 (4) | C23—H23 | 0.9300 |
| O40—C39 | 1.312 (3) | C28—H28 | 0.9300 |
| O40—C41 | 1.467 (6) | C29—H29 | 0.9300 |
| O43—C39 | 1.191 (4) | C31—H31 | 0.9300 |
| O44—C6 | 1.220 (3) | C32—H32 | 0.9300 |
| O45—C4 | 1.202 (3) | C34—H34 | 0.9300 |
| O40'—C41' | 1.478 (6) | C35—H35 | 0.9300 |
| O40'—C39' | 1.308 (5) | C36—H36 | 0.9300 |
| O43'—C39' | 1.199 (4) | C37—H37 | 0.9300 |
| O44'—C6' | 1.208 (3) | C38—H38 | 0.9300 |
| O45'—C4' | 1.193 (3) | C41—H41A | 0.9700 |
| N2—C1 | 1.466 (3) | C41—H41B | 0.9700 |
| N2—C3 | 1.488 (3) | C42—H42E | 0.9600 |
| N2—C9 | 1.442 (4) | C42—H42F | 0.9600 |
| N5—C16 | 1.432 (4) | C42—H42D | 0.9600 |
| N5—C6 | 1.386 (3) | C1'—C7' | 1.534 (3) |
| N5—C4 | 1.395 (3) | C1'—C39' | 1.517 (5) |
| N24—N25 | 1.358 (3) | C3'—C8' | 1.564 (4) |
| N24—C23 | 1.349 (3) | C3'—C22' | 1.499 (4) |
| N24—C33 | 1.430 (4) | C4'—C8' | 1.507 (4) |
| N25—C26 | 1.338 (3) | C6'—C7' | 1.505 (3) |
| N2'—C1' | 1.457 (4) | C7'—C8' | 1.524 (4) |
| N2'—C3' | 1.472 (3) | C9'—C10' | 1.513 (4) |
| N2'—C9' | 1.463 (4) | C10'—C11' | 1.373 (5) |
| N5'—C16' | 1.440 (3) | C10'—C15' | 1.382 (5) |
| N5'—C4' | 1.393 (3) | C11'—C12' | 1.369 (5) |
| N5'—C6' | 1.390 (4) | C12'—C13' | 1.365 (6) |
| N24'—C23' | 1.358 (4) | C13'—C14' | 1.366 (7) |
| N24'—C33' | 1.424 (4) | C14'—C15' | 1.385 (5) |
| N24'—N25' | 1.362 (3) | C16'—C17' | 1.372 (4) |
| N25'—C26' | 1.328 (4) | C16'—C21' | 1.384 (5) |
| C1—C7 | 1.506 (4) | C17'—C18' | 1.383 (5) |
| C1—C39 | 1.534 (5) | C18'—C19' | 1.382 (7) |
| C3—C8 | 1.553 (3) | C19'—C20' | 1.364 (6) |
| C3—C22 | 1.509 (4) | C20'—C21' | 1.377 (5) |
| C4—C8 | 1.511 (4) | C22'—C23' | 1.368 (4) |
| C6—C7 | 1.505 (4) | C22'—C26' | 1.406 (4) |
| C7—C8 | 1.524 (3) | C26'—C27' | 1.487 (4) |
| C9—C10 | 1.517 (4) | C27'—C28' | 1.381 (4) |
| C10—C15 | 1.379 (6) | C27'—C32' | 1.374 (4) |
| C10—C11 | 1.382 (5) | C28'—C29' | 1.377 (5) |
| C11—C12 | 1.393 (5) | C29'—C30' | 1.371 (5) |
| C12—C13 | 1.341 (7) | C30'—C31' | 1.376 (5) |
| C13—C14 | 1.379 (6) | C31'—C32' | 1.389 (5) |
| C14—C15 | 1.397 (5) | C33'—C34' | 1.363 (5) |
| C16—C17 | 1.373 (5) | C33'—C38' | 1.373 (6) |
| C16—C21 | 1.378 (5) | C34'—C35' | 1.396 (8) |
| C17—C18 | 1.393 (6) | C35'—C36' | 1.354 (7) |
| C18—C19 | 1.347 (6) | C36'—C37' | 1.348 (6) |
| C19—C20 | 1.404 (6) | C37'—C38' | 1.367 (8) |
| C20—C21 | 1.382 (6) | C41'—C42' | 1.484 (11) |
| C22—C26 | 1.425 (3) | C1'—H1' | 0.9800 |
| C22—C23 | 1.353 (4) | C3'—H3' | 0.9800 |
| C26—C27 | 1.473 (4) | C7'—H7' | 0.9800 |
| C27—C32 | 1.388 (5) | C8'—H8' | 0.9800 |
| C27—C28 | 1.375 (3) | C9'—H9'1 | 0.9700 |
| C28—C29 | 1.376 (4) | C9'—H9'2 | 0.9700 |
| C29—C30 | 1.341 (5) | C11'—H11' | 0.9300 |
| C30—C31 | 1.375 (4) | C12'—H12' | 0.9300 |
| C31—C32 | 1.373 (5) | C13'—H13' | 0.9300 |
| C33—C38 | 1.368 (4) | C14'—H14' | 0.9300 |
| C33—C34 | 1.362 (5) | C15'—H15' | 0.9300 |
| C34—C35 | 1.381 (6) | C17'—H17' | 0.9300 |
| C35—C36 | 1.361 (6) | C18'—H18' | 0.9300 |
| C36—C37 | 1.350 (5) | C19'—H19' | 0.9300 |
| C37—C38 | 1.384 (6) | C20'—H20' | 0.9300 |
| C41—C42 | 1.443 (7) | C21'—H21' | 0.9300 |
| C1—H1 | 0.9800 | C23'—H23' | 0.9300 |
| C3—H3 | 0.9800 | C28'—H28' | 0.9300 |
| C7—H7 | 0.9800 | C29'—H29' | 0.9300 |
| C8—H8 | 0.9800 | C31'—H31' | 0.9300 |
| C9—H91 | 0.9700 | C32'—H32' | 0.9300 |
| C9—H92 | 0.9700 | C34'—H34' | 0.9300 |
| C11—H11 | 0.9300 | C35'—H35' | 0.9300 |
| C12—H12 | 0.9300 | C36'—H36' | 0.9300 |
| C13—H13 | 0.9300 | C37'—H37' | 0.9300 |
| C14—H14 | 0.9300 | C38'—H38' | 0.9300 |
| C15—H15 | 0.9300 | C41'—H41C | 0.9700 |
| C17—H17 | 0.9300 | C41'—H41D | 0.9700 |
| C18—H18 | 0.9300 | C42'—H42A | 0.9600 |
| C19—H19 | 0.9300 | C42'—H42B | 0.9600 |
| C20—H20 | 0.9300 | C42'—H42C | 0.9600 |
| C39—O40—C41 | 116.5 (3) | O40—C41—H41A | 110.00 |
| C39'—O40'—C41' | 117.1 (3) | O40—C41—H41B | 110.00 |
| C1—N2—C9 | 115.9 (2) | C42—C41—H41A | 110.00 |
| C3—N2—C9 | 114.8 (2) | C42—C41—H41B | 110.00 |
| C1—N2—C3 | 107.05 (18) | H41A—C41—H41B | 108.00 |
| C4—N5—C6 | 112.4 (2) | C41—C42—H42F | 110.00 |
| C4—N5—C16 | 122.9 (2) | H42E—C42—H42F | 109.00 |
| C6—N5—C16 | 124.6 (2) | H42D—C42—H42E | 109.00 |
| N25—N24—C23 | 110.7 (2) | H42D—C42—H42F | 110.00 |
| N25—N24—C33 | 119.8 (2) | C41—C42—H42D | 110.00 |
| C23—N24—C33 | 129.5 (2) | C41—C42—H42E | 109.00 |
| N24—N25—C26 | 105.6 (2) | N2'—C1'—C7' | 101.5 (2) |
| C1'—N2'—C9' | 116.1 (2) | N2'—C1'—C39' | 116.0 (2) |
| C3'—N2'—C9' | 114.7 (2) | C7'—C1'—C39' | 110.1 (2) |
| C1'—N2'—C3' | 107.4 (2) | N2'—C3'—C8' | 103.9 (2) |
| C6'—N5'—C16' | 124.6 (2) | N2'—C3'—C22' | 110.9 (2) |
| C4'—N5'—C6' | 112.5 (2) | C8'—C3'—C22' | 113.1 (2) |
| C4'—N5'—C16' | 122.7 (2) | O45'—C4'—N5' | 125.0 (2) |
| C23'—N24'—C33' | 128.2 (2) | O45'—C4'—C8' | 127.9 (2) |
| N25'—N24'—C23' | 111.4 (2) | N5'—C4'—C8' | 107.1 (2) |
| N25'—N24'—C33' | 120.3 (2) | O44'—C6'—N5' | 124.8 (2) |
| N24'—N25'—C26' | 104.7 (2) | O44'—C6'—C7' | 126.8 (3) |
| N2—C1—C39 | 113.5 (2) | N5'—C6'—C7' | 108.4 (2) |
| C7—C1—C39 | 110.6 (2) | C1'—C7'—C6' | 112.0 (2) |
| N2—C1—C7 | 101.6 (2) | C1'—C7'—C8' | 105.9 (2) |
| N2—C3—C8 | 102.79 (18) | C6'—C7'—C8' | 104.3 (2) |
| N2—C3—C22 | 109.7 (2) | C3'—C8'—C4' | 113.5 (2) |
| C8—C3—C22 | 116.5 (2) | C3'—C8'—C7' | 104.4 (2) |
| O45—C4—N5 | 124.9 (3) | C4'—C8'—C7' | 104.93 (19) |
| O45—C4—C8 | 127.3 (3) | N2'—C9'—C10' | 111.9 (3) |
| N5—C4—C8 | 107.8 (2) | C9'—C10'—C11' | 121.1 (3) |
| O44—C6—N5 | 124.7 (3) | C9'—C10'—C15' | 120.6 (3) |
| O44—C6—C7 | 126.7 (2) | C11'—C10'—C15' | 118.3 (3) |
| N5—C6—C7 | 108.6 (2) | C10'—C11'—C12' | 121.3 (3) |
| C6—C7—C8 | 104.8 (2) | C11'—C12'—C13' | 120.1 (4) |
| C1—C7—C8 | 106.6 (2) | C12'—C13'—C14' | 119.8 (4) |
| C1—C7—C6 | 110.3 (2) | C13'—C14'—C15' | 120.2 (4) |
| C3—C8—C4 | 112.1 (2) | C10'—C15'—C14' | 120.2 (4) |
| C3—C8—C7 | 105.01 (19) | N5'—C16'—C17' | 120.8 (3) |
| C4—C8—C7 | 104.6 (2) | N5'—C16'—C21' | 118.9 (2) |
| N2—C9—C10 | 113.8 (3) | C17'—C16'—C21' | 120.3 (3) |
| C9—C10—C15 | 122.4 (3) | C16'—C17'—C18' | 119.9 (4) |
| C11—C10—C15 | 117.3 (3) | C17'—C18'—C19' | 119.8 (3) |
| C9—C10—C11 | 120.3 (3) | C18'—C19'—C20' | 119.8 (4) |
| C10—C11—C12 | 121.0 (4) | C19'—C20'—C21' | 121.0 (4) |
| C11—C12—C13 | 121.5 (4) | C16'—C21'—C20' | 119.2 (3) |
| C12—C13—C14 | 118.6 (4) | C3'—C22'—C23' | 127.5 (2) |
| C13—C14—C15 | 120.7 (4) | C3'—C22'—C26' | 127.0 (2) |
| C10—C15—C14 | 120.9 (3) | C23'—C22'—C26' | 104.6 (2) |
| N5—C16—C17 | 119.3 (3) | N24'—C23'—C22' | 107.5 (2) |
| N5—C16—C21 | 120.1 (3) | N25'—C26'—C22' | 111.9 (2) |
| C17—C16—C21 | 120.6 (3) | N25'—C26'—C27' | 118.9 (2) |
| C16—C17—C18 | 119.6 (3) | C22'—C26'—C27' | 129.1 (3) |
| C17—C18—C19 | 120.7 (4) | C26'—C27'—C28' | 118.9 (2) |
| C18—C19—C20 | 119.7 (4) | C26'—C27'—C32' | 122.4 (3) |
| C19—C20—C21 | 119.9 (4) | C28'—C27'—C32' | 118.7 (3) |
| C16—C21—C20 | 119.5 (3) | C27'—C28'—C29' | 121.9 (3) |
| C3—C22—C26 | 130.1 (2) | C28'—C29'—C30' | 118.0 (3) |
| C23—C22—C26 | 104.4 (2) | Cl1'—C30'—C29' | 118.1 (3) |
| C3—C22—C23 | 125.5 (2) | Cl1'—C30'—C31' | 120.0 (3) |
| N24—C23—C22 | 109.0 (2) | C29'—C30'—C31' | 121.9 (3) |
| N25—C26—C27 | 117.4 (2) | C30'—C31'—C32' | 118.7 (3) |
| C22—C26—C27 | 132.2 (2) | C27'—C32'—C31' | 120.7 (3) |
| N25—C26—C22 | 110.3 (2) | N24'—C33'—C34' | 120.6 (3) |
| C28—C27—C32 | 115.4 (3) | N24'—C33'—C38' | 120.4 (3) |
| C26—C27—C28 | 119.8 (3) | C34'—C33'—C38' | 119.0 (4) |
| C26—C27—C32 | 124.7 (2) | C33'—C34'—C35' | 119.5 (4) |
| C27—C28—C29 | 122.7 (3) | C34'—C35'—C36' | 120.9 (4) |
| C28—C29—C30 | 120.2 (2) | C35'—C36'—C37' | 118.7 (5) |
| Cl1—C30—C31 | 119.8 (3) | C36'—C37'—C38' | 121.7 (5) |
| C29—C30—C31 | 119.7 (3) | C33'—C38'—C37' | 120.0 (4) |
| Cl1—C30—C29 | 120.45 (19) | O40'—C39'—O43' | 123.9 (4) |
| C30—C31—C32 | 119.4 (4) | O40'—C39'—C1' | 110.7 (3) |
| C27—C32—C31 | 122.4 (3) | O43'—C39'—C1' | 125.4 (4) |
| N24—C33—C34 | 120.8 (3) | O40'—C41'—C42' | 104.3 (5) |
| N24—C33—C38 | 120.0 (3) | N2'—C1'—H1' | 110.00 |
| C34—C33—C38 | 119.2 (3) | C7'—C1'—H1' | 110.00 |
| C33—C34—C35 | 120.5 (4) | C39'—C1'—H1' | 110.00 |
| C34—C35—C36 | 120.4 (4) | N2'—C3'—H3' | 110.00 |
| C35—C36—C37 | 119.0 (4) | C8'—C3'—H3' | 110.00 |
| C36—C37—C38 | 121.3 (4) | C22'—C3'—H3' | 110.00 |
| C33—C38—C37 | 119.6 (3) | C1'—C7'—H7' | 111.00 |
| O40—C39—O43 | 124.6 (4) | C6'—C7'—H7' | 111.00 |
| O40—C39—C1 | 111.3 (3) | C8'—C7'—H7' | 111.00 |
| O43—C39—C1 | 124.1 (3) | C3'—C8'—H8' | 111.00 |
| O40—C41—C42 | 108.9 (4) | C4'—C8'—H8' | 111.00 |
| C7—C1—H1 | 110.00 | C7'—C8'—H8' | 111.00 |
| C39—C1—H1 | 110.00 | N2'—C9'—H9'1 | 109.00 |
| N2—C1—H1 | 110.00 | N2'—C9'—H9'2 | 109.00 |
| N2—C3—H3 | 109.00 | C10'—C9'—H9'1 | 109.00 |
| C8—C3—H3 | 109.00 | C10'—C9'—H9'2 | 109.00 |
| C22—C3—H3 | 109.00 | H9'1—C9'—H9'2 | 108.00 |
| C6—C7—H7 | 112.00 | C10'—C11'—H11' | 119.00 |
| C8—C7—H7 | 112.00 | C12'—C11'—H11' | 119.00 |
| C1—C7—H7 | 112.00 | C11'—C12'—H12' | 120.00 |
| C4—C8—H8 | 112.00 | C13'—C12'—H12' | 120.00 |
| C7—C8—H8 | 112.00 | C12'—C13'—H13' | 120.00 |
| C3—C8—H8 | 112.00 | C14'—C13'—H13' | 120.00 |
| H91—C9—H92 | 108.00 | C13'—C14'—H14' | 120.00 |
| C10—C9—H92 | 109.00 | C15'—C14'—H14' | 120.00 |
| C10—C9—H91 | 109.00 | C10'—C15'—H15' | 120.00 |
| N2—C9—H91 | 109.00 | C14'—C15'—H15' | 120.00 |
| N2—C9—H92 | 109.00 | C16'—C17'—H17' | 120.00 |
| C12—C11—H11 | 119.00 | C18'—C17'—H17' | 120.00 |
| C10—C11—H11 | 120.00 | C17'—C18'—H18' | 120.00 |
| C13—C12—H12 | 119.00 | C19'—C18'—H18' | 120.00 |
| C11—C12—H12 | 119.00 | C18'—C19'—H19' | 120.00 |
| C12—C13—H13 | 121.00 | C20'—C19'—H19' | 120.00 |
| C14—C13—H13 | 121.00 | C19'—C20'—H20' | 119.00 |
| C15—C14—H14 | 120.00 | C21'—C20'—H20' | 120.00 |
| C13—C14—H14 | 120.00 | C16'—C21'—H21' | 120.00 |
| C10—C15—H15 | 120.00 | C20'—C21'—H21' | 120.00 |
| C14—C15—H15 | 120.00 | N24'—C23'—H23' | 126.00 |
| C16—C17—H17 | 120.00 | C22'—C23'—H23' | 126.00 |
| C18—C17—H17 | 120.00 | C27'—C28'—H28' | 119.00 |
| C17—C18—H18 | 120.00 | C29'—C28'—H28' | 119.00 |
| C19—C18—H18 | 120.00 | C28'—C29'—H29' | 121.00 |
| C20—C19—H19 | 120.00 | C30'—C29'—H29' | 121.00 |
| C18—C19—H19 | 120.00 | C30'—C31'—H31' | 121.00 |
| C21—C20—H20 | 120.00 | C32'—C31'—H31' | 121.00 |
| C19—C20—H20 | 120.00 | C27'—C32'—H32' | 120.00 |
| C16—C21—H21 | 120.00 | C31'—C32'—H32' | 120.00 |
| C20—C21—H21 | 120.00 | C33'—C34'—H34' | 120.00 |
| C22—C23—H23 | 126.00 | C35'—C34'—H34' | 120.00 |
| N24—C23—H23 | 125.00 | C34'—C35'—H35' | 120.00 |
| C29—C28—H28 | 119.00 | C36'—C35'—H35' | 120.00 |
| C27—C28—H28 | 119.00 | C35'—C36'—H36' | 121.00 |
| C28—C29—H29 | 120.00 | C37'—C36'—H36' | 121.00 |
| C30—C29—H29 | 120.00 | C36'—C37'—H37' | 119.00 |
| C30—C31—H31 | 120.00 | C38'—C37'—H37' | 119.00 |
| C32—C31—H31 | 120.00 | C33'—C38'—H38' | 120.00 |
| C31—C32—H32 | 119.00 | C37'—C38'—H38' | 120.00 |
| C27—C32—H32 | 119.00 | O40'—C41'—H41C | 111.00 |
| C33—C34—H34 | 120.00 | O40'—C41'—H41D | 111.00 |
| C35—C34—H34 | 120.00 | C42'—C41'—H41C | 111.00 |
| C36—C35—H35 | 120.00 | C42'—C41'—H41D | 111.00 |
| C34—C35—H35 | 120.00 | H41C—C41'—H41D | 109.00 |
| C35—C36—H36 | 121.00 | C41'—C42'—H42A | 109.00 |
| C37—C36—H36 | 120.00 | C41'—C42'—H42B | 109.00 |
| C36—C37—H37 | 119.00 | C41'—C42'—H42C | 109.00 |
| C38—C37—H37 | 119.00 | H42A—C42'—H42B | 110.00 |
| C37—C38—H38 | 120.00 | H42A—C42'—H42C | 109.00 |
| C33—C38—H38 | 120.00 | H42B—C42'—H42C | 110.00 |
| C41—O40—C39—O43 | 5.6 (5) | C21—C16—C17—C18 | 1.4 (5) |
| C41—O40—C39—C1 | −174.1 (3) | N5—C16—C21—C20 | 176.7 (3) |
| C39—O40—C41—C42 | −170.8 (3) | C17—C16—C21—C20 | −1.3 (5) |
| C39'—O40'—C41'—C42' | 161.3 (4) | C16—C17—C18—C19 | −0.3 (6) |
| C41'—O40'—C39'—O43' | −4.5 (5) | C17—C18—C19—C20 | −0.8 (6) |
| C41'—O40'—C39'—C1' | 175.5 (3) | C18—C19—C20—C21 | 0.9 (6) |
| C3—N2—C1—C39 | −76.4 (3) | C19—C20—C21—C16 | 0.1 (5) |
| C1—N2—C3—C8 | −35.2 (3) | C3—C22—C26—N25 | −178.3 (2) |
| C9—N2—C1—C7 | 171.9 (2) | C23—C22—C26—C27 | 178.4 (3) |
| C9—N2—C1—C39 | 53.2 (3) | C3—C22—C26—C27 | 0.2 (5) |
| C3—N2—C1—C7 | 42.3 (3) | C23—C22—C26—N25 | 0.0 (3) |
| C1—N2—C9—C10 | 74.3 (3) | C26—C22—C23—N24 | 0.0 (3) |
| C3—N2—C9—C10 | −160.0 (2) | C3—C22—C23—N24 | 178.4 (2) |
| C9—N2—C3—C8 | −165.4 (2) | N25—C26—C27—C28 | 1.7 (4) |
| C9—N2—C3—C22 | 70.1 (3) | N25—C26—C27—C32 | −174.9 (3) |
| C1—N2—C3—C22 | −159.7 (2) | C22—C26—C27—C28 | −176.6 (3) |
| C6—N5—C4—O45 | −171.1 (3) | C22—C26—C27—C32 | 6.8 (5) |
| C6—N5—C4—C8 | 9.8 (3) | C32—C27—C28—C29 | −2.8 (5) |
| C4—N5—C6—C7 | −1.4 (3) | C26—C27—C32—C31 | −179.3 (3) |
| C16—N5—C6—O44 | −4.1 (4) | C26—C27—C28—C29 | −179.7 (3) |
| C16—N5—C6—C7 | 175.9 (2) | C28—C27—C32—C31 | 4.0 (5) |
| C4—N5—C16—C17 | 118.1 (3) | C27—C28—C29—C30 | −1.1 (5) |
| C4—N5—C16—C21 | −60.0 (4) | C28—C29—C30—C31 | 3.8 (5) |
| C6—N5—C16—C17 | −59.0 (4) | C28—C29—C30—Cl1 | −176.9 (3) |
| C6—N5—C16—C21 | 123.0 (3) | C29—C30—C31—C32 | −2.6 (5) |
| C16—N5—C4—C8 | −167.6 (2) | Cl1—C30—C31—C32 | 178.1 (3) |
| C4—N5—C6—O44 | 178.6 (2) | C30—C31—C32—C27 | −1.4 (6) |
| C16—N5—C4—O45 | 11.5 (4) | N24—C33—C34—C35 | −178.1 (3) |
| N25—N24—C23—C22 | 0.0 (3) | C38—C33—C34—C35 | −0.4 (6) |
| N25—N24—C33—C34 | −177.5 (3) | N24—C33—C38—C37 | 177.6 (3) |
| N25—N24—C33—C38 | 4.8 (4) | C34—C33—C38—C37 | −0.2 (5) |
| C33—N24—C23—C22 | 179.1 (3) | C33—C34—C35—C36 | 0.1 (7) |
| C23—N24—N25—C26 | −0.1 (3) | C34—C35—C36—C37 | 0.8 (7) |
| C33—N24—N25—C26 | −179.2 (2) | C35—C36—C37—C38 | −1.4 (6) |
| C23—N24—C33—C38 | −174.2 (3) | C36—C37—C38—C33 | 1.2 (6) |
| C23—N24—C33—C34 | 3.5 (5) | N2'—C1'—C7'—C6' | −79.5 (3) |
| N24—N25—C26—C22 | 0.1 (3) | N2'—C1'—C7'—C8' | 33.6 (2) |
| N24—N25—C26—C27 | −178.7 (2) | C39'—C1'—C7'—C6' | 157.1 (3) |
| C3'—N2'—C1'—C39' | 77.0 (3) | C39'—C1'—C7'—C8' | −89.8 (3) |
| C9'—N2'—C1'—C7' | −172.1 (2) | N2'—C1'—C39'—O40' | 158.6 (2) |
| C9'—N2'—C1'—C39' | −52.9 (3) | N2'—C1'—C39'—O43' | −21.4 (4) |
| C1'—N2'—C3'—C8' | 34.1 (2) | C7'—C1'—C39'—O40' | −87.0 (3) |
| C1'—N2'—C9'—C10' | −51.9 (4) | C7'—C1'—C39'—O43' | 93.0 (4) |
| C3'—N2'—C9'—C10' | −178.2 (3) | N2'—C3'—C8'—C4' | 102.3 (2) |
| C9'—N2'—C3'—C8' | 164.7 (3) | N2'—C3'—C8'—C7' | −11.3 (3) |
| C9'—N2'—C3'—C22' | −73.4 (3) | C22'—C3'—C8'—C4' | −18.1 (3) |
| C1'—N2'—C3'—C22' | 155.9 (2) | C22'—C3'—C8'—C7' | −131.7 (2) |
| C3'—N2'—C1'—C7' | −42.3 (2) | N2'—C3'—C22'—C23' | −24.9 (3) |
| C6'—N5'—C4'—O45' | 167.6 (3) | N2'—C3'—C22'—C26' | 167.8 (2) |
| C6'—N5'—C4'—C8' | −12.1 (3) | C8'—C3'—C22'—C23' | 91.4 (3) |
| C16'—N5'—C6'—C7' | 176.6 (2) | C8'—C3'—C22'—C26' | −75.9 (3) |
| C4'—N5'—C16'—C17' | 127.5 (3) | O45'—C4'—C8'—C3' | 83.4 (3) |
| C4'—N5'—C16'—C21' | −52.7 (4) | O45'—C4'—C8'—C7' | −163.2 (3) |
| C6'—N5'—C16'—C17' | −46.2 (4) | N5'—C4'—C8'—C3' | −96.8 (3) |
| C6'—N5'—C16'—C21' | 133.6 (3) | N5'—C4'—C8'—C7' | 16.6 (3) |
| C16'—N5'—C4'—C8' | 173.5 (2) | O44'—C6'—C7'—C1' | −58.5 (4) |
| C4'—N5'—C6'—O44' | −176.9 (3) | O44'—C6'—C7'—C8' | −172.6 (3) |
| C4'—N5'—C6'—C7' | 2.3 (3) | N5'—C6'—C7'—C1' | 122.3 (2) |
| C16'—N5'—C6'—O44' | −2.6 (4) | N5'—C6'—C7'—C8' | 8.2 (3) |
| C16'—N5'—C4'—O45' | −6.8 (4) | C1'—C7'—C8'—C3' | −13.5 (3) |
| N25'—N24'—C33'—C34' | −177.6 (3) | C1'—C7'—C8'—C4' | −133.1 (2) |
| N25'—N24'—C33'—C38' | 5.1 (4) | C6'—C7'—C8'—C3' | 104.9 (2) |
| C23'—N24'—N25'—C26' | −0.6 (3) | C6'—C7'—C8'—C4' | −14.8 (3) |
| C33'—N24'—N25'—C26' | −177.6 (2) | N2'—C9'—C10'—C11' | 111.5 (4) |
| N25'—N24'—C23'—C22' | 0.4 (3) | N2'—C9'—C10'—C15' | −65.9 (5) |
| C33'—N24'—C23'—C22' | 177.1 (2) | C9'—C10'—C11'—C12' | −178.2 (4) |
| C23'—N24'—C33'—C34' | 5.9 (5) | C15'—C10'—C11'—C12' | −0.8 (6) |
| C23'—N24'—C33'—C38' | −171.4 (3) | C9'—C10'—C15'—C14' | 178.0 (4) |
| N24'—N25'—C26'—C27' | 176.4 (2) | C11'—C10'—C15'—C14' | 0.5 (6) |
| N24'—N25'—C26'—C22' | 0.6 (3) | C10'—C11'—C12'—C13' | 0.3 (7) |
| N2—C1—C7—C8 | −32.2 (3) | C11'—C12'—C13'—C14' | 0.6 (7) |
| C39—C1—C7—C6 | −158.2 (2) | C12'—C13'—C14'—C15' | −0.8 (7) |
| C39—C1—C7—C8 | 88.5 (2) | C13'—C14'—C15'—C10' | 0.3 (6) |
| N2—C1—C7—C6 | 81.0 (2) | N5'—C16'—C17'—C18' | −179.5 (3) |
| N2—C1—C39—O40 | −121.1 (2) | C21'—C16'—C17'—C18' | 0.7 (4) |
| N2—C1—C39—O43 | 59.2 (4) | N5'—C16'—C21'—C20' | 179.0 (3) |
| C7—C1—C39—O40 | 125.5 (2) | C17'—C16'—C21'—C20' | −1.2 (5) |
| C7—C1—C39—O43 | −54.3 (4) | C16'—C17'—C18'—C19' | −0.3 (5) |
| N2—C3—C8—C7 | 13.6 (3) | C17'—C18'—C19'—C20' | 0.3 (6) |
| C22—C3—C8—C4 | 20.6 (3) | C18'—C19'—C20'—C21' | −0.7 (6) |
| N2—C3—C8—C4 | −99.3 (2) | C19'—C20'—C21'—C16' | 1.2 (6) |
| C8—C3—C22—C23 | −93.0 (3) | C3'—C22'—C23'—N24' | −169.6 (2) |
| C8—C3—C22—C26 | 85.0 (3) | C26'—C22'—C23'—N24' | 0.0 (3) |
| N2—C3—C22—C26 | −158.9 (3) | C3'—C22'—C26'—N25' | 169.3 (2) |
| C22—C3—C8—C7 | 133.6 (2) | C3'—C22'—C26'—C27' | −6.0 (4) |
| N2—C3—C22—C23 | 23.2 (4) | C23'—C22'—C26'—N25' | −0.4 (3) |
| O45—C4—C8—C3 | −79.6 (3) | C23'—C22'—C26'—C27' | −175.6 (2) |
| O45—C4—C8—C7 | 167.2 (3) | N25'—C26'—C27'—C28' | −46.2 (4) |
| N5—C4—C8—C3 | 99.4 (2) | N25'—C26'—C27'—C32' | 134.3 (3) |
| N5—C4—C8—C7 | −13.8 (3) | C22'—C26'—C27'—C28' | 128.7 (3) |
| N5—C6—C7—C8 | −7.4 (3) | C22'—C26'—C27'—C32' | −50.8 (4) |
| O44—C6—C7—C1 | 58.3 (3) | C26'—C27'—C28'—C29' | 179.9 (4) |
| O44—C6—C7—C8 | 172.6 (2) | C32'—C27'—C28'—C29' | −0.6 (6) |
| N5—C6—C7—C1 | −121.8 (2) | C26'—C27'—C32'—C31' | −177.3 (3) |
| C1—C7—C8—C3 | 11.4 (3) | C28'—C27'—C32'—C31' | 3.1 (5) |
| C1—C7—C8—C4 | 129.6 (2) | C27'—C28'—C29'—C30' | −3.0 (7) |
| C6—C7—C8—C3 | −105.6 (2) | C28'—C29'—C30'—Cl1' | −176.5 (4) |
| C6—C7—C8—C4 | 12.6 (2) | C28'—C29'—C30'—C31' | 4.2 (7) |
| N2—C9—C10—C15 | 29.6 (4) | Cl1'—C30'—C31'—C32' | 178.9 (3) |
| N2—C9—C10—C11 | −153.3 (3) | C29'—C30'—C31'—C32' | −1.8 (6) |
| C15—C10—C11—C12 | −0.4 (5) | C30'—C31'—C32'—C27' | −2.0 (6) |
| C9—C10—C11—C12 | −177.7 (3) | N24'—C33'—C34'—C35' | −179.4 (4) |
| C9—C10—C15—C14 | 177.1 (3) | C38'—C33'—C34'—C35' | −2.1 (6) |
| C11—C10—C15—C14 | −0.1 (4) | N24'—C33'—C38'—C37' | −179.4 (4) |
| C10—C11—C12—C13 | 1.0 (6) | C34'—C33'—C38'—C37' | 3.3 (6) |
| C11—C12—C13—C14 | −1.1 (6) | C33'—C34'—C35'—C36' | −1.5 (7) |
| C12—C13—C14—C15 | 0.5 (5) | C34'—C35'—C36'—C37' | 3.7 (7) |
| C13—C14—C15—C10 | 0.1 (5) | C35'—C36'—C37'—C38' | −2.5 (7) |
| N5—C16—C17—C18 | −176.6 (3) | C36'—C37'—C38'—C33' | −1.0 (7) |
Hydrogen-bond geometry (Å, °)
| Cg1, Cg2 and Cg3 are the centroids of the C10–C15, C27'–C32' and C33'–C38' rings, respectively. |
| D—H···A | D—H | H···A | D···A | D—H···A |
| C7—H7···O44' | 0.98 | 2.45 | 3.311 (3) | 146 |
| C42'—H42A···Cl1'i | 0.96 | 2.76 | 3.707 (10) | 170 |
| C14'—H14'···Cg1 | 0.93 | 2.79 | 3.653 (4) | 154 |
| C17'—H17'···Cg2ii | 0.93 | 2.95 | 3.738 (4) | 143 |
| C29—H29···Cg1iii | 0.93 | 2.87 | 3.633 (3) | 140 |
| C42'—H42B···Cg3iv | 0.96 | 2.80 | 3.866 (9) | 153 |
Symmetry codes: (i) −x+1, −y+2, −z; (ii) −x+1, −y+1, −z; (iii) −x+1, −y, −z+1; (iv) x+1, y, z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: SU2345).
References
- Nardelli, M. (1983). Acta Cryst. C39, 1141–1142.
- Oxford Diffraction (2009). CrysAlis PRO Oxford Diffraction Ltd, Yarnton, England.
- Patel, C. K., Rami, C. S., Panigrahi, B. & Patel, C. N. (2010). J. Chem. Pharm. Res. 2, 73–78.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Siu, K. K. W., Lee, J. E., Smith, G. D., Horvatin-Mrakovcic, C. & Howell, P. L. (2008). Acta Cryst. F64, 343–350. [DOI] [PMC free article] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Sullivan, T. J., Truglio, J. J., Boyne, M. E., Novichenok, P., Zhang, X., Stratton, C. F., Li, H., Kaur, T., Amin, A., Johnson, F., Slayden, R. A., Kisker, C. & Tonge, P. J. (2006). Chem. Biol. 1, 43–53. [DOI] [PubMed]
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536812002450/su2345sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536812002450/su2345Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536812002450/su2345Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report