Abstract
Bisphosphonates are therapeutic agents in the treatment of post-menopausal osteoporosis. Although they have been associated with delayed healing in injured tissues, inappropriate femoral fractures, and osteonecrosis of the jaw (ONJ), the pathophysiological mechanisms involved are not clear. Our hypothesis is that alendronate, a member of the N-containing bisphosphonates, indirectly inhibits osteoblast function through the coupling of osteoclasts to osteoblasts by ephrinB-EphB interaction. We found that alendronate increased gene and protein expression of ephrinB1 and EphB1, as well as B3, in femurs of adult mice injected with alendronate (10 µg/100 g/wk) for 8 weeks. Alendronate suppressed the expression of bone sialoprotein (BSP) and osteonectin in both femurs and bone marrow osteoblastic cells of mice. After elimination of pre-osteoclasts from bone marrow cells, alendronate did not affect osteoblast differentiation, indicating the need for pre-osteoclasts for alendronate’s effects. Alendronate stimulated EphB1 and EphB3 protein expression in osteoblasts, whereas it enhanced ephrinB1 protein in pre-osteoclasts. In addition, a reverse signal by ephrinB1 inhibited osteoblast differentiation and suppressed BSP gene expression. Thus, alendronate, through its direct effects on the pre-osteoclast, appears to regulate expression of ephrinB1, which regulates and acts through the EphB1, B3 receptors on the osteoblast to suppress osteoblast differentiation.
Keywords: bone biology, cell biology, gene expression, osteoblast(s), osteoclast(s), osteonecrosis
Introduction
Bisphosphonates are potent inhibitors of bone resorption and are used in the treatment of bone diseases (Park et al., 2009). Alendronate acts on osteoclast function and survival by binding and blocking the enzyme farnesyl diphosphate synthase in the HMG-CoA reductase pathway (Fleisch, 2002; van Beek et al., 2003). Although they are associated with inappropriate femoral fractures, the pathophysiological mechanisms are not known.
Cell-surface molecules, such as receptors and their associated ligands, play a role in the maintenance of bone homeostasis (Edwards and Mundy, 2008). EphrinB2 and EphB4 mediate osteoclast-osteoblast interactions by simultaneous signal transduction in both cells. Eph receptors are members of the receptor tyrosine kinase family, and their cell-surface ligands, ephrins, are involved in a variety of cell communications (Noren et al., 2009). EphrinB1 and ephrinB2 are strongly expressed during osteoclast differentiation. EphrinB2 expressed on osteoclasts stimulates ephrin-Eph bidirectional signaling in the osteoblast and induces osteoblast differentiation as a forward signal. In contrast, a reverse signal from EphB4 on the osteoblast inhibits osteoclast formation (Zhao et al., 2006). Interestingly, the interaction of ephrinA2-EphA2 inhibits osteoblast differentiation at the initiation phase of bone remodeling, whereas ephrinA2-EphA2 stimulate osteoclast formation (Irie et al., 2009).
Here, we have identified that alendronate inhibited normal physiological bone remodeling and turnover through the coupling of ephrinB1-EphBs.
Materials & Methods
Mice
Two-month-old C57Bl/6 mice were obtained from Taconic (Hudson, NY, USA). Mice were assigned to two different groups with 15 mice per group. Each received either saline or alendronate (10 µg/100g/wk) subcutaneously for 8 wks and then was sacrificed by CO2 narcosis. The dose of alendronate used in humans is approximately 1 mg/kg/wk orally (Huang et al., 2005). Our dosage in mice approximated the human dose (Samadfam et al., 2007).
Cell Culture
Bone marrow osteoblastic cells were flushed from the tibiae and femurs of mice, plated in growth medium (αMEM supplemented with 10% FBS, 100 international units/mL penicillin, 100 µg/mL streptomycin, and 2 mM glutamine), and incubated at 37°C in 5% CO2. Elimination of myeloid cells was performed with a Dynabeads Biotin Binder (Invitrogen, Carlsbad, CA, USA) with CD11b antibody (eBioscience, San Diego, CA, USA), according to the manufacturer’s instructions. For immunofluorescent staining, cells were fixed in 4% paraformaldehyde in PBS. Primary antibodies for anti-F4/80 conjugated to Alexa Fluor 488 and anti-CD45 conjugated to PE (eBioscience, San Diego, CA, USA) were used at a dilution of 1:100. Mouse primary osteoblasts were derived from mouse calvariae by digestion in 0.1 mg/mL collagenase P, 25 mg/mL, or 50 mg/mL trypsin. The cells were re-plated in αMEM supplemented with 10% FBS, 50 µg/mL ascorbic acid, and 5 mM β-glycerol phosphate. To stimulate forward signaling, ephrin B-Fc or Fc fragments (R&D, Minneapolis, MN, USA) were clustered with anti-Fc antibody at 2 µg/mL on the plates prior to the addition of cells unless otherwise indicated.
Von Kossa Staining
Cells were fixed with 100% ethanol, and then stained with silver nitrate solution for 60 min at 37°C and exposed to bright light. Images were analyzed with Image analysis software (SPOT Advanced; Diagnostic Instruments, Inc., Sterling Heights, MI, USA).
Real-time RT-PCR
Total RNA from the spongiosa of femurs or bone marrow osteoblasts was isolated with TRIzol reagent. Total RNA was reverse-transcribed to cDNA with the Superscript kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. All were amplified by the addition of cDNA to the PCR mixture containing each primer and the SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA). The sequences used are listed in the Appendix Table. The PCR reactions and program were described in a previous publication (Shimizu et al., 2010).
Western Blot
Distal femurs were homogenized with lysis buffer at 4ºC. Proteins were separated by SDS-PAGE and transferred to polyvinylidene difluoride membranes. Proteins were detected by SuperSignal West Dura Extended Duration Substrate (Thermo Scientific, Rockford, IL, USA) according to the manufacturer’s instructions.
Immunohistochemistry
Femurs were fixed in 4% formaldehyde and decalcified in 10% EDTA. Frozen sections (10 µm) were cut longitudinally. Immunohistochemical staining was performed by the ABC staining system (Santa Cruz Biotechnology, Santa Cruz, CA, USA) according to the manufacturer’s instructions. The sections were incubated with anti-ephrinB1 (AF473, R&D, Minneapolis, MN, USA), anti-EphB1 (M-19), and anti-EphB3 (7E5) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) antibodies or normal IgG as negative control at 4°C. The sections were incubated with biotin-labeled anti-IgG. Staining was completed with 5-minute incubation with 3,3′-diaminobenzidine (DAB). TRAP staining was performed with a leukocyte acid phosphatase kit from Sigma (St. Louis, MO, USA), according to the manufacturer’s instructions. The sections were incubated at 37°C in staining solution for 1 hr. Cells positive for TRAP and having more than 3 nuclei were considered as TRAP-positive pre-osteoclasts microscopically.
Statistical Analysis
All results are expressed as means ± SE of triplicate measurements, with all experiments being repeated at least 3 times. Statistical analyses were carried out by Student’s t test.
Results
Alendronate Inhibited Osteoblast-specific Gene Expression in Mice
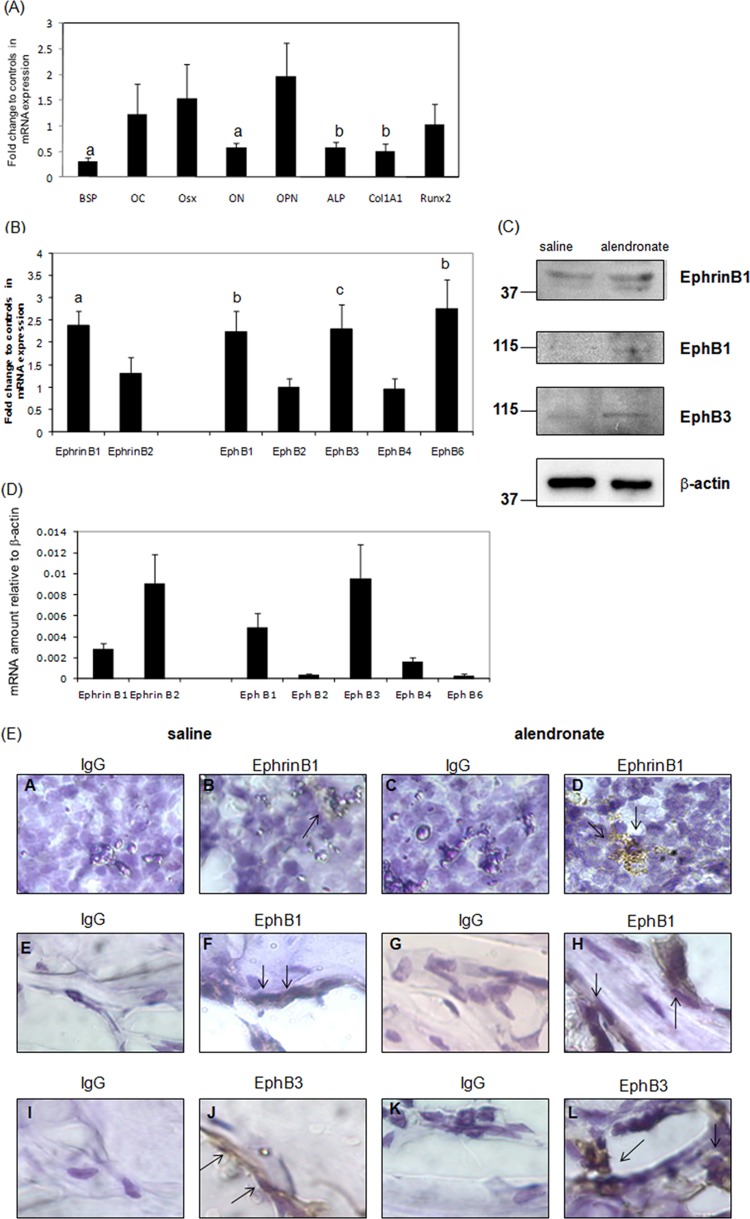
The numbers and sizes of TRAP-positive cells and osteoclast marker genes in femurs of alendronate-injected mice were decreased compared with those in saline-injected mice, whereas hematoxylin-eosin staining did not differ (Appendix Fig. 1). We found that BSP, osteonectin (ON), alkaline phosphatase (ALP), and type 1 collagen alpha 1 (Col1A1) gene expression were significantly decreased in femurs of alendronate-injected mice compared with saline-injected controls (Fig. 1A).
Figure 1.
Alendronate inhibits osteoblast gene expression in vivo and alters ephrin/Eph gene and protein expression. Two-month-old C57Bl/6 mice received either saline or alendronate (10 µg/100 g/wk) subcutaneously for 8 wks. Total RNA was extracted from primary spongiosae of saline- or alendronate-injected mice. RNAs were measured by real-time RT-PCR. The relative levels of mRNAs were normalized to β-actin and then expressed as fold stimulation over controls. Error bars represent ± SEM of 6 animals. (A) Osteoblast genes. a, p < 0.001; b, p < 0.03; c, p < 0.04 compared with saline-injected animals. (B) Ephrin and Eph genes. a, p < 0.002; b, p < 0.01; and c, p < 0.03 compared with saline-injected animals. (C) EphrinB1, EphB1, and B3 protein expression. (D) Relative levels of ephrins and Ephs in femurs of saline-injected animals compared with β-actin. BSP, bone sialoprotein; OC, osteocalcin; Osx, osterix; ON, osteonectin; OPN, osteopontin; ALP, Alkaline phosphatase. (E) Femurs were isolated from saline (A, B, E, F, I, and J) or alendronate (C, D, G, H, K, and L)-injected mice. Sections were incubated with anti-IgG (A, C, E, G, I, and K), anti-ephrinB1 (B and D), anti-EphB1 (F and H), or anti-EphB3 (J and L). Staining was completed with 3,3′-diaminobenzidine (DAB). Sections were counter-stained with hematoxylin. Black arrowheads show ephrinB1, EphB1, or EphB3 protein expression. Magnification, x 600.
Ligands and Receptors in Bone Were Affected by Alendronate
We found that alendronate changed expression of these genes, with enhanced ephrinB1, EphB1, B3, and B6 gene expression, but not ephrinB2 (Fig. 1B). Moreover, alendronate stimulated ephrinB1, EphB1, B3 protein expression compared with saline-injected controls (Fig. 1C). We also showed the relative gene expression of ephrins and Ephs under basal conditions in femurs. EphrinB1, B2 or EphB1, B3, B4 genes were more abundant than EphB2 and B6 genes, indicating that these genes may be important for regulating bone metabolism (Fig. 1D). In addition, alendronate stimulated ephrinB1 protein level in monocytes or pre-osteoclasts of bone marrow, whereas it enhanced EphB1 and EphB3 protein in osteoblasts of trabecular bone (Fig. 1E).
Alendronate Affected Osteoblast Differentiation and Mineralization
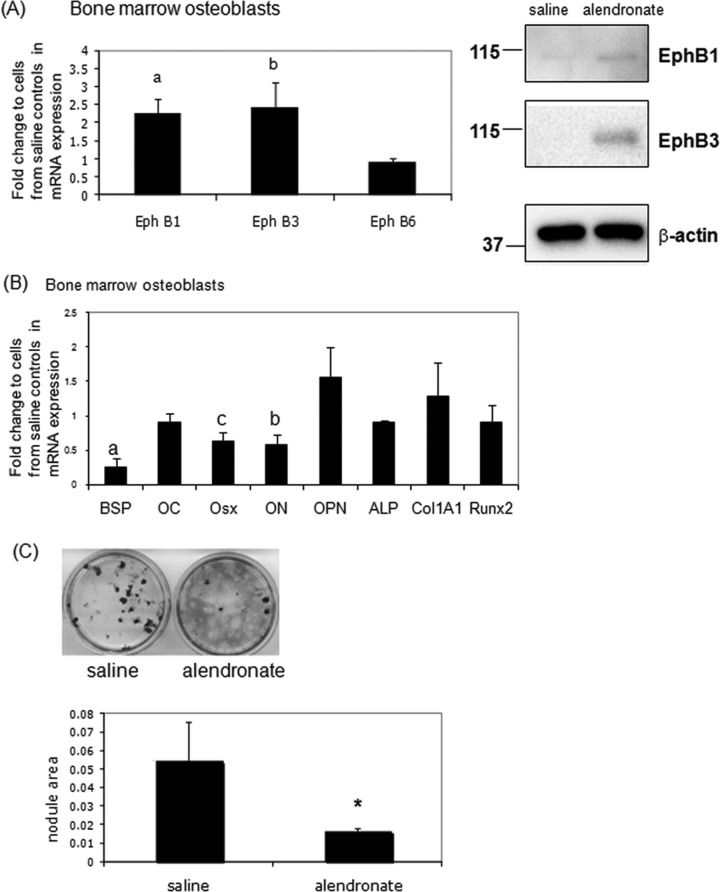
We found that EphB1 and B3 gene and protein levels were enhanced in bone marrow osteoblastic cells from alendronate-injected mice, whereas EphB6 was unchanged (Fig. 2A). Next, we examined bone marker genes from the same cells. The gene expression of BSP, ON, and osterix (Osx) was decreased in bone marrow osteoblasts from alendronate-injected mice (Fig. 2B). Bone nodules were decreased in cells from alendronate-injected mice compared with saline-injected mice (Fig. 2C). We hypothesize that alendronate affects osteoblast development indirectly through crosstalk from the osteoclast to the osteoblast.
Figure 2.
Osteoblast differentiation from bone marrow cells of alendronate-injected animals is impaired. Bone marrow osteoblastic cells from tibia or femur of saline- or alendronate-injected mice were cultured with 50 µg/mL ascorbic acid and 5 mM β-glycerophosphate for 21 days, and then total RNA was extracted. RNAs were measured by real-time RT-PCR. The relative levels of mRNAs were normalized to β-actin and then expressed as fold stimulation over control. Error bars represent ± SEM of 8 animals. (A) Eph gene and protein expression compared with cells from saline-injected mice. a, p < 0.01 and b, p < 0.05 compared with controls. (B) Osteoblast gene markers. a, p < 0.001; b, p < 0.01; and c, p < 0.05 compared with cells from saline-injected mice. (C) (upper) Bone marrow osteoblastic cells formed bone nodules as shown by von Kossa staining. (lower) Quantitation of nodule areas, *p < 0.04 vs. cells from saline-injected mice, by SPOT Advanced software and microscopy.
Alendronate Affects Osteoblast Differentiation through Pre-osteoclasts
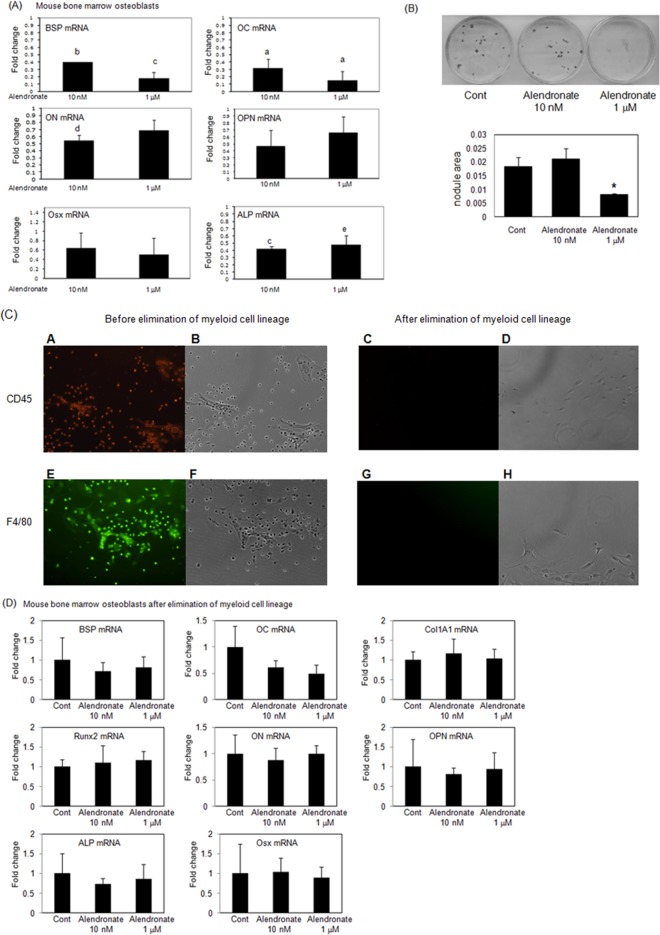
To investigate how alendronate directly or indirectly affects osteoblast differentiation in vivo, we investigated the effect of alendronate (10 nM or 1 µM) using mouse bone marrow cells. Alendronate at 10 nM inhibited osteoblast marker genes such as BSP, OC, ON, and ALP genes (Fig. 3A). Alendronate at 1 µM also suppressed BSP, OC, and ALP gene expression, but not ON, OPN, or Osx genes. Moreover, alendronate at 10 nM slightly inhibited the formation of bone nodules, whereas a high concentration (1 µM) completely inhibited nodule formation (Fig. 3B). Alendronate did not affect early osteoblast differentiation (Appendix Fig. 2). We hypothesize that inhibition of osteoblast differentiation and mineralization was associated with effects through osteoclast precursors. To test this hypothesis, we cultured the bone marrow cells so that pre-osteoclasts would be eliminated and then examined the effect of alendronate. We confirmed deletion of myeloid cells using immunofluorescent staining with F4/80 (macrophage marker) and CD45 (pan-hematopoietic marker) (Fig. 3C). We found that alendronate did not change gene expression after the elimination of myeloid cells (Fig. 3D). These results support the hypothesis that alendronate acts on pre-osteoclasts which interact with osteoblast precursors, thus inhibiting osteoblast differentiation and mineralization.
Figure 3.
Alendronate affects osteoblast differentiation through pre-osteoclasts in vitro. (A) Mouse bone marrow osteoblastic cells were cultured with or without alendronate (10 nM or 1 µM) for 21 days. RNAs were measured by real-time RT-PCR. The relative levels of mRNAs were normalized to β-actin and then expressed as fold stimulation over control. Error bars represent ± SEM of three independent experiments. a, p < 0.001; b, p < 0.002; c, p < 0.004; d, p < 0.005; and e, p < 0.02 compared with control. (B) (upper) Mouse bone marrow osteoblasts formed bone nodules as shown by von Kossa staining. (lower) Quantitation of nodule areas, *p < 0.05 vs. cells from saline-injected mice, with SPOT Advanced software and microscopy. (C) After elimination of myeloid lineage cells, bone marrow osteoblastic cells were cultured with or without alendronate (10 nM or 1 µM) for 21 days. Primary antibodies against F4/80 or CD45 were used at a dilution of 1:100. Primary antibodies were conjugated to either green-fluorescent Alexa Fluor 488 dye (E and G) for F4/80 or PE-fluorescent dye (A and C) for CD45. B, D, F, and H represent the phase-contrast micrographs of A, C, E, and G, respectively. Magnification x100. (D) RNAs were measured by real-time RT-PCR. The relative levels of mRNAs were normalized to β-actin and then expressed as fold stimulation over control. Error bars represent ± SEM of 3 independent experiments.
EphrinB1 Affects Osteoblast Differentiation in vitro
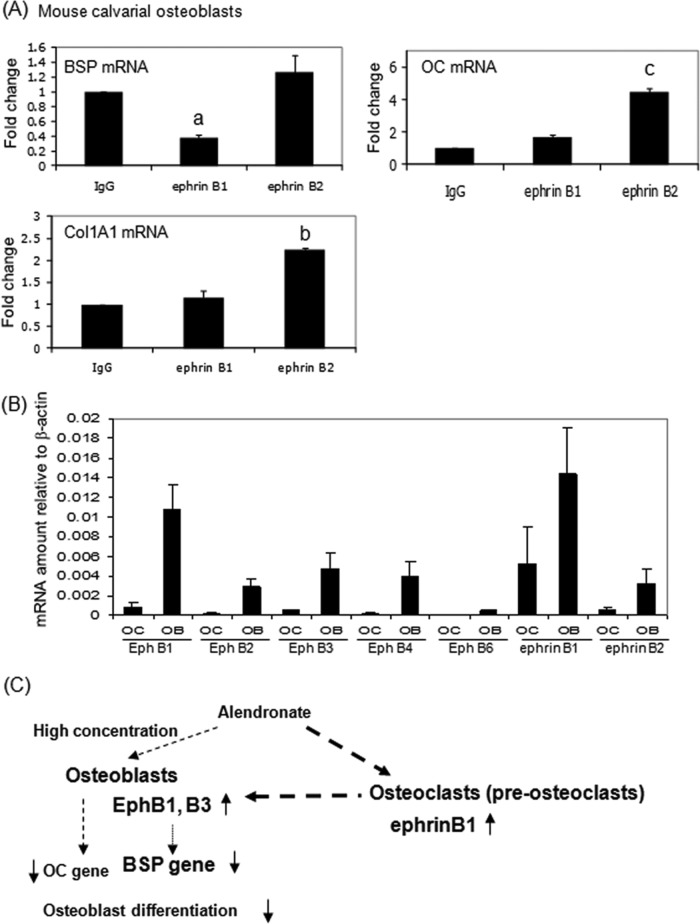
EphrinB1 strongly inhibited BSP gene expression, but ephrinB2 did not (Fig. 4A). In contrast, ephrinB2 significantly stimulated OC, ALP, and Col1A1 gene expression. We also examined the gene expression of ephrins and Ephs in osteoblasts and osteoclasts relative to β-actin. EphrinB1 was more highly expressed than ephrinB2 in differentiated osteoclasts (Fig. 4B). These results suggest that ephrinB1 expressed by osteoclasts inhibits osteoblast differentiation, especially BSP gene expression.
Figure 4.
Ephrin B1 inhibits bone sialoprotein (BSP) gene expression in vitro. (A) To stimulate forward or reverse signaling, ephrin B1-Fc, ephrin B2-Fc, or IgG-Fc fragments were clustered with anti-Fc antibody at 2 µg/mL for 1 hr, and then primary mouse osteoblasts were cultured with 50 µg/mL ascorbic acid and 10 mM β-glycerophosphate for 17 days on the clustered proteins. RNAs were measured by real-time RT-PCR. The relative levels of mRNAs were normalized to β-actin and then expressed as fold stimulation over control. Error bars represent ± SEM of 3 experiments. a, p < 0.001; b, p<0.003; and c, p < 0.02 compared with IgG. (B) Eph and ephrin expression in osteoblasts and osteoclasts. The relative levels of mRNAs were normalized to β-actin. OB, primary mouse osteoblasts; OC, mouse osteoclasts derived from mouse bone marrow. (C) Schematic representation of osteoclast-osteoblast interactions and the effect of alendronate. Alendronate stimulated ephrinB1 gene expression in osteoclasts, which interacts with EphB1 or B3 receptors on osteoblasts to inhibit osteoblast function, especially BSP.
Discussion
Alendronate-associated femoral fractures arise from sites of cortical stress reactions (Schneider, 2009). Rats injected with alendronate had delayed new bone formation compared with the control group (Hikita et al., 2009). We postulated that the inhibition of osteoclast functions by bisphosphonate results in an inhibition of normal physiological bone remodeling and turnover and may induce inappropriate femoral fractures and ONJ.
In particular, osteoblast-osteoclast interactions are very important for the regulation of bone remodeling. EphrinB2 produced by osteoclasts acts on EphB4 in osteoblasts to promote osteoblast differentiation (Zhao et al., 2006). Moreover, the blockage of ephrinB2/EphB4 interaction reduces mineralization of osteoblasts in vitro (Allan et al., 2008). Irie et al. (2009) have recently reported that ephrinA2- EphA2 interaction promotes the initiation phase of bone remodeling by enhancing osteoclast differentiation and suppressing osteoblast differentiation. Thus, a communicating interaction between osteoclasts and osteoblasts regulates differentiation in both cells through their exchange of ephrin/Eph signals.
In this study, we obtained data establishing that mRNA for ephrinB1 was increased in femurs of mice after alendronate injections. We also found that EphB1 and EphB3 gene expression was stimulated in femurs or differentiating mouse bone marrow osteoblastic cells from the alendronate-injected mice. At the same time, several osteoblast differentiation markers were decreased in the same samples. Therefore, the effect of alendronate on ephrinB1/EphB1 or B3 interaction may cause the inhibition of osteoblast differentiation and mineralization. Analysis of our data revealed that alendronate indirectly inhibited osteoblast differentiation in vitro through pre-osteoclasts. These results indicate that the expression of ephrinB1 in pre-osteoclasts inhibited osteoblast differentiation and mineralization, especially the BSP gene. BSP is a member of the SIBLING family and is essentially expressed in the initial stage of trabecular bone and other mineralized tissues (Chen et al., 1992; Fisher et al., 2001). The protein regulates the nucleation of hydroxyapatite at the mineralization front of bone (Hunter and Goldberg, 1993). Why should ephrinB1 in pre-osteoclasts particularly affect the BSP gene? Activation of the EphB receptor via ephrinB1 may affect bone remodeling and turnover at an early stage, as does ephrinA2-EphA2 interaction. BSP is also an Arg-Gly-Asp (RGD)-containing peptide, and it is involved in osteoclastogenesis and increases osteoclast differentiation and activity through αvβ3 integrin (Valverde et al., 2005; Malaval et al., 2008). We found that alendronate inhibited β3 integrin gene expression in vivo but not αv integrin (data not shown). Eph receptors regulate integrin activity; activation of EphA2, B2, B3, and B4 resulted in a decrease in integrin activation and cellular adhesion (Zou et al., 1999; Miao et al., 2005). Thus, activation of Eph receptors in osteoblasts may regulate integrin activity and consequently regulate BSP-gene expression (Zhao et al., 2006).
We found that alendronate at a high concentration suppressed OC, BSP, ON, and ALP genes in bone marrow osteoblastic cells, which are a mixed culture. After depletion of pre-osteoclasts, alendronate had no effect on gene expression, indicating that this is an indirect effect. Iwata et al. (2006) have shown that bisphosphonates suppress bone formation. Intracortical remodeling of the alveolar portion of the mandible was significantly suppressed by zoledronate treatment (Kubek et al., 2010). Zoledronate suppressed bone formation in the jaws without causing osteocyte death (Huja et al., 2009). Since zoledronate has the same inhibitory actions on osteoclasts as alendronate, it may also affect ephrinB-EphB interaction. To study whether alendronate directly affects osteoblasts, we treated rat calvarial primary osteoblasts in vitro with alendronate for 21 days. At the higher concentration, alendronate significantly inhibited OC gene expression while not affecting other genes (Appendix Fig. 3A). High concentrations of aminobisphosphonates, such as ≥ 1 µM, have a cytotoxic inhibitory effect on osteoblast growth and function (Orriss et al., 2009). Moreover, high doses of zoledronic acid decreased osteoblast viability and function (Pozzi et al., 2009). However, we found that total RNA amounts were not significantly different with 10 nM or 1 µM alendronate (Appendix Fig. 3B), indicating that it is not toxic for these cells at the lower doses. Therefore, our results suggest that alendronate indirectly affects osteoblast differentiation and mineralization.
A lack of ephrinB1 results in perinatal lethality associated with a range of phenotypes, including defects in neural-crest-cell-derived tissues, incomplete body wall closure, and abnormal skeletal patterning. Neural-crest-cell-derived tissues are the source of the frontal bone osteoprogenitor population, and ephrinB1 and ephrinB2 control migration of these cells (Davy et al., 2004, 2006). Targeted deletion of the ephrinB1 gene in type 1 alpha 2 collagen-producing cells in vivo led to the conclusion that ephrinB1 stimulates osteoblast differentiation through EphB2 in bone marrow stromal cells (Xing et al., 2010). Although ephrinB1 is expressed in both osteoclasts and osteoblasts, in this study we show that ephrinB1 functions in osteoclasts through Eph receptors in osteoblasts. We show that ephrinB1-Fc suppressed osteoblast differentiation, whereas ephrinB2-Fc stimulated osteoblast marker genes. Analysis of our data leads us to propose that activation of EphB receptors by ephrinB1 in osteoclasts or pre-osteoclasts suppresses osteoblast differentiation, and that this is increased by alendronate.
In summary, our findings provide evidence that alendronate suppresses osteoblast differentiation indirectly. Alendronate regulates ephrinB1 gene expression in osteoclasts, which interacts with EphB1 or B3 receptors on osteoblasts to inhibit osteoblast function. Alendronate may affect osteoclast precursors, which then act on osteoblast precursors in bone marrow cells through ephrinB1-EphB interactions. We conclude that alendronate affects physiological bone metabolism through the crosstalk of the osteoclast and osteoblast.
Acknowledgments
We greatly appreciate Dr. Louis M. Lin and Dr. George Jong at the New York University College of Dentistry for their comments on the manuscript.
Footnotes
This work was supported in part by NIH grant DK47420 (to N.C.P.).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Allan EH, Hausler KD, Wei T, Gooi JH, Quinn JM, Crimeen-Irwin B, et al. (2008). EphrinB2 regulation by PTH and PTHrP revealed by molecular profiling in differentiating osteoblasts. J Bone Miner Res 23:1170-1181 [DOI] [PubMed] [Google Scholar]
- Chen J, Shapiro HS, Sodek J. (1992). Development expression of bone sialoprotein mRNA in rat mineralized connective tissues. J Bone Miner Res 7:987-997 [DOI] [PubMed] [Google Scholar]
- Davy A, Aubin J, Soriano P. (2004). Ephrin-B1 forward and reverse signaling are required during mouse development. Genes Dev 18:572-583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davy A, Bush JO, Soriano P. (2006). Inhibition of gap junction communication at ectopic Eph/ephrin boundaries underlies craniofrontonasal syndrome. PLoS Biol 4:e315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards CM, Mundy GR. (2008). Eph receptors and ephrin signaling pathways: a role in bone homeostasis. Int J Med Sci 5:263-272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher LW, Torchia DA, Fohr B, Young MF, Fedarko NS. (2001). Flexible structures of SIBLING proteins, bone sialoprotein, and osteopontin. Biochem Biophys Res Commun 280:460-465 [DOI] [PubMed] [Google Scholar]
- Fleisch H. (2002). Development of bisphosphonates. Breast Cancer Res 4:30-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikita H, Miyazawa K, Tabuchi M, Kimura M, Goto S. (2009). Bisphosphonate administration prior to tooth extraction delays initial healing of the extraction socket in rats. J Bone Miner Metab 27:663-672 [DOI] [PubMed] [Google Scholar]
- Huang RC, Khan SN, Sandhu HS, Metzl JA, Cammisa FP, Jr, Zheng F, et al. (2005). Alendronate inhibits spine fusion in a rat model. Spine (Phila Pa 1976) 30:2516-2522 [DOI] [PubMed] [Google Scholar]
- Huja SS, Fernandez SA, Phillips C, Li Y. (2009). Zoledronic acid decreases bone formation without causing osteocyte death in mice. Arch Oral Biol 54:851-856 [DOI] [PubMed] [Google Scholar]
- Hunter GK, Goldberg HA. (1993). Nucleation of hydroxyapatite by bone sialoprotein. Proc Natl Acad Sci USA 90:8562-8565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie N, Takada Y, Watanabe Y, Matsuzaki Y, Naruse C, Asano M, et al. (2009). Bidirectional signaling through ephrinA2-EphA2 enhances osteoclastogenesis and suppresses osteoblastogenesis. J Biol Chem 284:14637-14644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata K, Li J, Follet H, Phipps RJ, Burr DB. (2006). Bisphosphonates suppress periosteal osteoblast activity independently of resorption in rat femur and tibia. Bone 39:1053-1058 [DOI] [PubMed] [Google Scholar]
- Kubek DJ, Burr DB, Allen MR. (2010). Ovariectomy stimulates and bisphosphonates inhibit intracortical remodeling in the mouse mandible. Orthod Craniofac Res 13:214-222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaval L, Wade-Gueye NM, Boudiffa M, Fei J, Zirngibl R, Chen F, et al. (2008). Bone sialoprotein plays a functional role in bone formation and osteoclastogenesis. J Exp Med 205:1145-1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao H, Strebhardt K, Pasquale EB, Shen TL, Guan JL, Wang B. (2005). Inhibition of integrin-mediated cell adhesion but not directional cell migration requires catalytic activity of EphB3 receptor tyrosine kinase. Role of Rho family small GTPases. J Biol Chem 280:923-932 [DOI] [PubMed] [Google Scholar]
- Noren NK, Yang NY, Silldorff M, Mutyala R, Pasquale EB. (2009). Ephrin-independent regulation of cell substrate adhesion by the EphB4 receptor. Biochem J 422:433-442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orriss IR, Key ML, Colston KW, Arnett TR. (2009). Inhibition of osteoblast function in vitro by aminobisphosphonates. J Cell Biochem 106:109-118 [DOI] [PubMed] [Google Scholar]
- Park IH, Ro J, Nam BH, Kwon Y, Lee KS. (2009). Potential antitumor effects of nitrogen-containing bisphosphonate in hormone receptor negative breast cancer patients with bone metastases. BMC Cancer 9:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzi S, Vallet S, Mukherjee S, Cirstea D, Vaghela N, Santo L, et al. (2009). High-dose zoledronic acid impacts bone remodeling with effects on osteoblastic lineage and bone mechanical properties. Clin Cancer Res 15:5829-5839 [DOI] [PubMed] [Google Scholar]
- Samadfam R, Xia Q, Goltzman D. (2007). Co-treatment of PTH with osteoprotegerin or alendronate increases its anabolic effect on the skeleton of oophorectomized mice. J Bone Miner Res 22:55-63 [DOI] [PubMed] [Google Scholar]
- Schneider JP. (2009). Bisphosphonates and low-impact femoral fractures: current evidence on alendronate-fracture risk. Geriatrics 64:18-23 [PubMed] [Google Scholar]
- Shimizu E, Selvamurugan N, Westendorf JJ, Olson EN, Partridge NC. (2010). HDAC4 represses matrix metalloproteinase-13 transcription in osteoblastic cells, and parathyroid hormone controls this repression. J Biol Chem 285:9616-9626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde P, Tu Q, Chen J. (2005). BSP and RANKL induce osteoclastogenesis and bone resorption synergistically. J Bone Miner Res 20:1669-1679 [DOI] [PubMed] [Google Scholar]
- van Beek ER, Cohen LH, Leroy IM, Ebetino FH, Lowik CW, Papapoulos SE. (2003). Differentiating the mechanisms of antiresorptive action of nitrogen containing bisphosphonates. Bone 33:805-811 [DOI] [PubMed] [Google Scholar]
- Xing W, Kim J, Wergedal J, Chen ST, Mohan S. (2010). Ephrin B1 regulates bone marrow stromal cell differentiation and bone formation by influencing TAZ transactivation via complex formation with NHERF1. Mol Cell Biol 30:711-721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Irie N, Takada Y, Shimoda K, Miyamoto T, Nishiwaki T, et al. (2006). Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab 4:111-121 [DOI] [PubMed] [Google Scholar]
- Zou JX, Wang B, Kalo MS, Zisch AH, Pasquale EB, Ruoslahti E. (1999). An Eph receptor regulates integrin activity through R-Ras. Proc Natl Acad Sci USA 96:13813-13818 [DOI] [PMC free article] [PubMed] [Google Scholar]