Abstract
Streptococcus mutans, a dental caries pathogen, also causes endocarditis and is detected in atheroscelerotic plaque. We investigated the potential for an invasive strain of S. mutans, OMZ175, to accelerate plaque growth in apolipoprotein E deficient (ApoEnull) mice without and with balloon angioplasty (BA) injury, a model of restenosis. ApoEnull mice were divided into 4 groups (N = 10), 2 with and 2 without BA. One each of the BA and non-BA groups was infected with S. mutans (Sm). S. mutans DNA, plaque area, inflammatory cell invasion, and Toll-like receptor (TLR) expression were measured at 6-20 weeks post-infection. S. mutans genomic DNA was detected in the aorta, liver, spleen, and heart. Plaque growth was significantly increased in infected mice with BA (Sm+BA) vs. those in the non-infected groups (p < 0.03). Plaque size was increased after infection without BA (Sm), but did not reach significance. Aortic specimens from both S. mutans and Sm+BA groups displayed increased numbers of macrophages, and TLR4 expression was increased in BA mice. In conclusion, S. mutans infection accelerated plaque growth, macrophage invasion, and TLR4 expression after angioplasty. S. mutans may also be associated with atherosclerotic plaque growth in non-injured arteries.
Keywords: Streptococcus mutans, atherosclerosis, balloon angioplasty, macrophage invasion, Toll-like receptor 4, antibody response
Introduction
Atherosclerosis is a complex inflammatory disease afflicting medium- and large-sized arteries and is the leading cause of death in the United States (Lethbridge-Cejku et al., 2004; Rosamond et al., 2008). The fact that inflammation has a pivotal role in the development of atherosclerosis has intensified the search for chronic exposure to infectious organisms that have the potential to cause inflammation in vessels (Libby et al., 2002; Lucas et al., 2006). One type of atherosclerosis, restenosis, results from rapid plaque growth after percutaneous intervention with balloon angioplasty (BA) (Jones et al., 2009). This surgery causes local endothelial cell damage. One can postulate that local bacterial infections induce inflammatory cell invasion and promote arterial injury in both native and BA-treated vessels.
Numerous studies have demonstrated the presence of genomic DNA from several oral bacteria, including S. mutans, in human atherosclerotic lesions (Kozarov et al., 2006; Nakano et al., 2006, 2009). S. mutans is disseminated via bacteremia (Huang et al., 2008) and is also a causative agent of infective endocarditis (IE) (Nakano et al., 2007b). Significantly, S. mutans was the most predominant bacterial species detected in diseased heart valves and atheromatous plaques, with detection rates of 63% and 74%, respectively. We have also previously reported that S. mutans was detected with high prevalence in atheromatous plaque in 22.5% of younger patients (~27 yrs of age) and in 44.4% of older patients (~67 yrs of age) (Kozarov et al., 2006). In the Nakano et al. study, oral specimens from cardiovascular disease (CVD) patients were more complex, including multiple serotypes, and had far fewer serotype c isolates when compared with those from patients with no known CVD. These findings suggest that specific strains of S. mutans may play a role in the infection of cardiovascular tissues, and those individuals harboring these strains may be at higher risk for CVD (Nakano et al., 2007a,b).
Abranches and co-workers (2009, 2011) examined 30 strains of S. mutans and found that some serotype e and f strains were capable of invading human coronary artery endothelial cells (HCAEC). Of these, a serotype f strain, OMZ175, was the most invasive, whereas strains of serotype c were not invasive. They have also reported that a collagen binding protein, Cnm, is required for invasion (Abranches et al., 2011). However, the nature of in vivo interactions between S. mutans and cardiovascular cells and the role of invasive strains in the initiation and progression of atherosclerosis have not yet been investigated. The aim of this study was to examine the ability of S. mutans strain OMZ175 to infect and/or to accelerate atherogenesis in a pro-atherogenic mouse model. Effects of strain OMZ175 infection on plaque growth were examined with and without BA-induced vascular injury (Lucas et al., 1996; Petrov et al., 2005).
Materials & Methods
Bacterial Strain and Microbial Inocula
S. mutans strain OMZ175 was used in this study and was cultured and maintained for infection of mice as described previously (Abranches et al., 2009). The concentration of bacteria was determined quantitatively, and the organism was re-suspended in phosphate-buffered saline (pH 7.2) at 1 x 109 bacteria per mL.
Mice
Forty 10-week-old male ApoE-/- mice (Strain B6.129P2-ApoEtm1Unc/J) were obtained from The Jackson Laboratory (Bar Harbor, ME, USA) (Lucas et al., 1996; Li et al., 2002; Gibson et al., 2004). After 1 wk of acclimatization, they were fed a high-fat diet (HFD) (Atherogenic diet Teklad, Harlan, Indianapolis, IN, USA) for 8 wks (Fig. 1A). Mice were then randomized into 4 groups (Group I, S. mutans + BA; Group II, S. mutans alone; Group III, sham-infected with BA; and Group IV, sham-infected without BA). The control groups were shared with a complementary, but separate, study. All animal procedures were performed in accordance with policies of the Institutional Animal Care and Use Committee of the University of Florida.
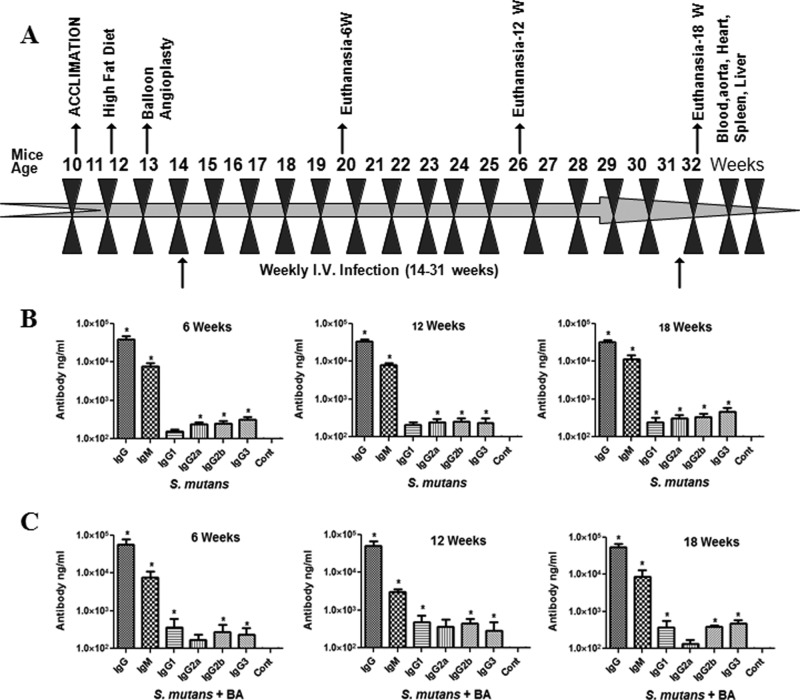
Figure 1.
Experimental design and antibody titers. 6, 12, and 18 weekly I.V. infections, euthanasia (6, 12, and 18 wks), and harvesting of tissues (A). Serum IgG, IgG isotypes, and IgM antibody levels in ApoE-/- mice intravenously infected with S. mutans without balloon angioplasty (BA) injury (B) or plus BA injury (C). Each bar represents the group mean (N = 3-5) antibody levels, and the vertical lines denote SD from the means. *Significantly more than sham-infected control mice (P < 0.01). ‘Cont’ indicates control mice.
Mouse Aortic Angioplasty Model
BA was performed on 2 groups of 13-week-old mice to induce endothelial cell injury in the aortas as previously described (Lucas et al., 1996). This vascular injury induces endothelial damage and inflammation, simulating restenosis in humans. All surgical procedures were performed with the mice under general anesthesia (ketamine/xylazine) and analgesia (Buprenorphine).
Streptococcus mutans Infection
Beginning at 14 wks of age, mice in Groups I and II were infected intravenously (via tail vein) once a week for either 6, 12, or 18 wks with strain OMZ175 (107 bacterial cells/mouse in 10 µL) (Fig. 1A). Control mice were sham-infected. One week after the last intravenous infection, 3-5 mice from each group were euthanized, and the aortas, hearts, spleens, and blood were collected for analysis.
Antibody Analysis
Serum from mice infected with S. mutans was used to determine immunoglobulin G (IgG), IgM, IgA, and IgG subclass antibody concentrations according to a standard ELISA protocol (Verma et al., 2010).
Detection of Genomic DNA from Aorta, Heart, and Spleen
DNA was isolated from aorta, heart, and spleen samples with the use of the EPICENTRE Master Pure DNA Purification Kit (EPICENTRE, Madison, WI, USA) with modifications, and PCR was performed (Appendix).
Histology
Hematoxylin-eosin-stained (H&E) sections of the aortic arch, thoracic aorta, and abdominal aorta were used for morphometric analysis. Plaque area was measured and the extent of atherosclerosis in the aortic sections of the mice was determined as previously described (Liu et al., 2000, 2004). The mean total cross-sectional intimal area or the mean intimal thickness normalized to medial thickness was calculated for each arterial section and used for statistical analyses. (For detailed methods, see the Appendix.)
Immunohistochemistry
Mouse aortic specimens were also assessed by immunohistochemical staining for the presence of macrophage and TLR4 expression and compared with smooth-muscle cells to assess inflammatory cell responses as previously described (Liu et al., 2000, 2004).
Statistical Analysis
Values are expressed as mean ± standard error (SE). An unpaired, two-tailed Student’s t test was used to compare groups of animals. Differences with p values less than 0.05 were considered statistically significant. Histological measurements were analyzed by analysis of variance (ANOVA) with the Statview program as described previously (Liu et al., 2000) with post hoc PLSD analysis. The antibody data are presented as means ± standard deviation (SD) (Prism 4, GraphPad software), with P values calculated by the Kruskal-Walis ANOVA with Dunn’s correction for multiple comparisons and the Mann-Whitney Student t test.
Results
Antibody Response to S. mutans
S. mutans infection (+/– BA) induced approximately a 10,000-fold increase in IgM antibody and significant IgG antibody responses at 6, 12, and 18 wks compared with sham-infected control mice, indicating S. mutans exposure (Figs. 1B, IC).
Detection of Genomic DNA from Aorta, Heart, and Spleen
Portions of the hearts, aortas, and spleens were examined for the presence of S. mutans genomic DNA by PCR. The PCR results (Table) demonstrated that amplicons for S. mutans were present in DNA isolated from the liver (9/10), heart (2/10), and thoracic aorta (6/10) from the Sm group. S. mutans was also detected in the liver (10/10), spleen (2/10), heart (2/10), and abdominal aorta (10/10) from Sm+BA mice.
Table.
Detection of S. mutans Genomic DNA in ApoEnull Mouse Tissue Infected with Invasive S. mutans OMZ175
| Specimen | Group I: S. mutans + Balloon angioplasty (N = 10) | Group II: S. mutans alone (N = 10) | ||||||
|---|---|---|---|---|---|---|---|---|
| Weeks | 6 (3) | 12 (3) | 18 (4) | Total | 6 (3) | 12 (3) | 18 (4) | Total |
| Liver | 3 | 3 | 4 | 10 | 2 | 3 | 4 | 9 |
| Spleen | 1 | 0 | 1 | 2 | 0 | 0 | 0 | 0 |
| Heart | 1 | 1 | 0 | 2 | 0 | 1 | 1 | 2 |
| Thoracic aorta | 0 | 0 | 0 | 0 | 3 | 3 | 0 | 6 |
| Abdominal aorta | 3 | 3 | 4 | 10 | 0 | 0 | 0 | 0 |
Numbers in brackets indicate number of ApoEnull mice at 6, 12, and 18 wks of intravenous infection.
Histomorphometric Analysis of Atherosclerotic Plaque
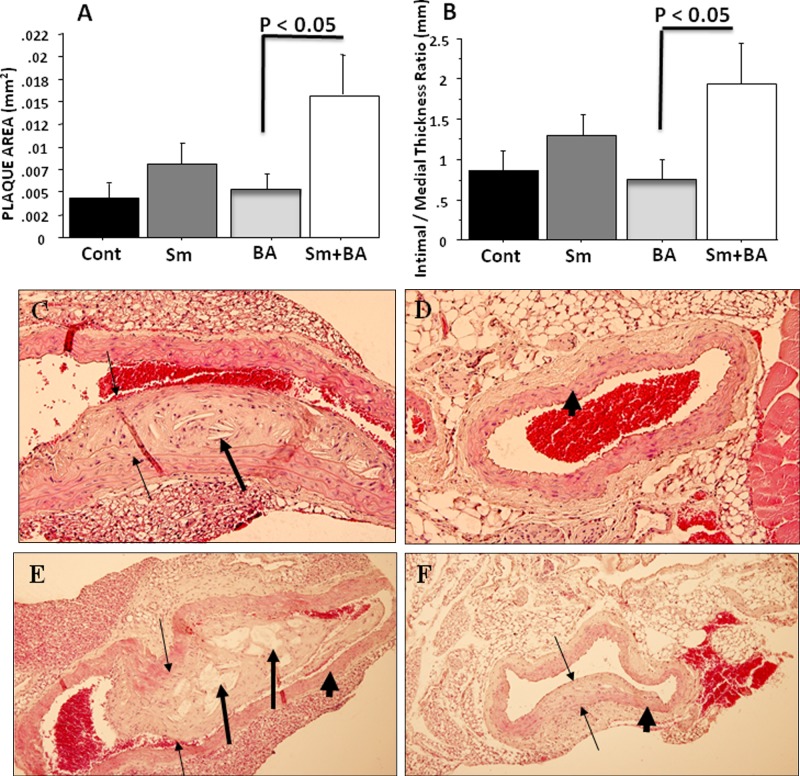
Combined analysis of all aortic specimens in mice with Sm+BA demonstrated a significant increase in plaque area (3×) (p < 0.05) when compared with BA with sham infection (Fig. 2A). The aortic plaques of the Sm group at 18 wks were larger than those of the corresponding control mice, but this difference, although demonstrating a clear trend, was not significant (Fig. 2A). With respect to the intimal/medial thickness, the plaques of the Sm mice were also larger than those of non-infected mice, but this again did not reach significance, whereas the Sm+BA mice had significantly increased (p < 0.05) intimal to medial thickness ratios when compared with control mice (Fig. 2B). Thus, S. mutans OMZ175 induced an increased plaque size, measured either as plaque area or intimal-to-medial-thickness ratios in BA-treated mice. In addition, there was a trend toward increased plaque size with S. mutans and no BA.
Figure 2.
Bar graphs of morphometric analysis of mean ascending, thoracic, and abdominal aortic plaque area at 18 wks follow-up (A) (N = 4). Total data comparison for plaque area was examined. Bar graphs of ascending, thoracic, and abdominal aorta intimal/medial thickness ratios (B) (N = 4). Total data comparison for intimal-medial-thickness ratios was examined. Sm indicates Streptococcus mutans; BA indicates balloon angioplasty (sham-infected control); and Cont indicates sham-infected control without BA. Histologic evaluation of H&E-stained plaque area in aortic cross-sections at 18 wks demonstrates increased plaque area and intimal/medial thickness ratios after BA in the presence of S. mutans infection (18 wks). (C) S. mutans (magnification 20×); (D) sham-infected control (20×); (E) S. mutans+BA (10×); (F) BA only (10×). Thin arrows indicate plaque margins, thick arrows indicate plaque with cholesterol crystals, and arrowheads indicate arterial wall.
The aortic sections were also assessed for vessel wall and lumen area as well as invading mononuclear cell count. As can be seen in Figs. 2C and 2E, the largest plaques were evident in S. mutans-infected groups. Histological sections of plaque growth in these infected groups demonstrated large plaques with embedded cholesterol crystals and invading inflammatory cells in all arterial layers, with associated loss of lumen area (Fig. 2E). In contrast, minimal plaques and no cholesterol crystals were visible in sections from the matched sham-infected control mice (Figs. 2D, 2F).
With respect to earlier times, there was a gradual increase in plaque size from 6 to 18 wks after BA with S. mutans infection, and with BA alone there was a gradual increase from the early time (6 wks) to late (18 wks) follow-ups. This increase over time was most marked for the thoracic aorta but was also seen in the abdominal aorta (data not shown).
Immunohistochemistry of Macrophage Infiltration
Recruited macrophages in atherosclerotic lesions are known to drive the pathophysiology of atherosclerosis. Immunohistochemistry of mouse abdominal aorta sections demonstrated a significant (p < 0.05) increase in invasion of macrophages in the adventitial area after 18 wks of both Sm+BA (Figs. 3A, 3B), Sm (Fig. 3C), and BA alone (Fig. 3D) (Lucas et al., 1996; Liu et al., 2004).
Figure 3.
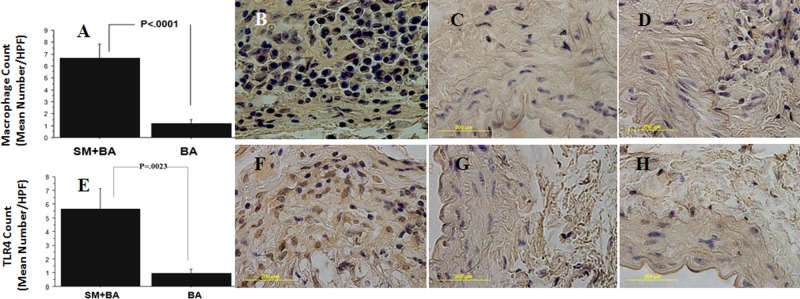
Bar graph of immunohistochemistry of ApoE-/- mice abdominal aorta sections showing significant (P < 0.001) increase in macrophage infiltration in the aortic adventitial area in S. mutans OMZ175-infected mice with BA compared with sham-infected control mice at 18 wks (A). Immunohistochemistry staining of significant macrophage infiltration in mice infected with S. mutans+BA (B), Sm without BA (C), and BA alone (D). Bar graph of TLR4 expression showing increased TLR4 in abdominal aortic adventitial area in S. mutans OMZ175-infected ApoE-null mice (18 wks) with BA (E). S. mutans infection of ApoE-null mice+BA in the abdominal aorta illustrates significant up-regulation of TLR4 (F), S. mutans (G), and control (sham-infected and no BA) mice (H).
Immunohistochemistry of TLR4
TLR4 is an initiator of cellular activation and inflammation, and its expression exerts an overall pro-atherogenic effect by promoting lipid and leukocyte accumulation within lesions. In our study, significant up-regulation of TLR4 expression (p < 0.05)occurred after 18 wks of S. mutans infection and BA vascular injury in the adventitial layers of abdominal aortic sections from the mice (Figs. 3E, 3F) when compared with S. mutans alone (Fig. 3G) and control mice (Fig. 3H).
Discussion
It is without dispute that S. mutans reaches cardiovascular tissues in humans. S. mutans is an etiologic agent of both bacteremia and infective endocarditis (IE) (Drangsholt, 1998; Li et al., 2000; Nakano et al., 2007b). The endothelium forms a continuous cellular sheet or organ interconnecting the heart, valves, and arterial linings. Thus, the direct interactions between oral bacteria and endothelial cells of the valves and arteries could have a significant effect on the progression of both valvular disease and atherosclerosis.
A pathogen’s ability to attach to and invade aortic endothelial cells in vitro and vascular tissues in vivo has previously been shown to be required for virulence in an animal model of atherosclerosis (Li et al., 2002; Gibson et al., 2004). The present study establishes an animal model for investigation of the pathogenesis of a chronic systemic infection and subsequent atherosclerosis by an invasive S. mutans strain. The results indicate that S. mutans OMZ175 produces significant pathology, including enhanced plaque and increased inflammatory responses when compared with control mice. This study establishes that infection with an invasive strain of S. mutans accelerates atherosclerotic plaque development and inflammatory cell invasion in this mouse model, with greatest increases when there is concomitant endothelial BA injury.
Our results may initially appear to be contradictory to those published recently by Zhang et al. (2010), who, also using the ApoEnull mouse model (but no BA), reported that S. mutans did not increase atherosclerotic plaque growth. The results demonstrated that atherosclerotic plaques in infected mice were virtually identical to those in the PBS control mice, whereas our results demonstrated significantly increased plaque size and area in mice with BA and demonstrated a trend toward increased plaque even without BA. Most significantly, Zhang et al. (2010) used S. mutans strain GS-5, a strain that we have previously reported cannot invade HCAECs. Thus, the results from Zhang et al. (2010) are consistent with and provide additional support for the hypothesis that only invasive S. mutans strains are associated with atherosclerotic pathology.
Interestingly, the pattern of detection of S. mutans DNA in tissues was different in mice subjected to BA compared with those exposed to S. mutans alone. In both cases, S. mutans genomic DNA was detected in the liver of almost all mice and in the hearts of 2 in each group. However, S. mutans genomic DNA was detected in the thoracic aortas in 6 out of 10 mice in the Sm group, but in none of the Sm+BA group. In contrast, S. mutans DNA was found in the abdominal aortas of all mice in the Sm+BA group, but in none of the abdominal aortas of the Sm group. The presence of genomic DNA in these tissues is suggestive, although not proof, of the presence of live bacteria. However, the presence of S. mutans genomic DNA in the abdominal aortas of Sm+BA mice corresponds to the induction of plaque and inflammation in these mice.
TLRs are a class of proteins that play a critical role in the activation of innate immunity by recognizing pathogenic invaders and are highly expressed in atherosclerotic tissues (Michelsen et al., 2004). Several in vivo studies have reported that another oral pathogen, P. gingivalis, was also found to accelerate atherosclerosis in ApoE-/- mice (Gibson et al., 2004), and infection correlated with increased expression of the innate immune response markers, TLR-2 and TLR-4 (Liu et al., 2008). Analysis of our data with S. mutans clearly demonstrated increased atherosclerotic plaque concomitant with the expression of TLR4 in aortic tissue.
Restenosis, one particular form of atherosclerosis after BA with stent implants in humans, results in recurrent blockage of arterial sites in 30% to 50% of patients (Counsell et al., 2004). This percutaneous intervention damages the endothelium and underlying smooth-muscle cells, exposing highly inflammatory and thrombotic connective tissue proteins such as collagen and glycosaminoglycans as well as lipid deposits (Liu et al., 2000). Analysis of the data indicates that BA significantly increases the interactions between S. mutans and the vasculature, suggesting that BA makes available additional receptors/tissues that allow for enhanced interactions between S. mutans OMZ175 and the luminal tissues of the aorta. Since analysis of our data suggested that S. mutans strain OMZ175 is more virulent with respect to atherosclerotic events in mice with BA than without BA, S. mutans may have a predilection for injured endothelium and may be a more significant pathogen with respect to some cardiovascular sites/diseases than others.
In conclusion, analysis of the data clearly documents the induction of chronic systemic inflammation with an invasive S. mutans strain and detection of S. mutans genomic DNA in infected mouse aorta samples. Most significantly, enhanced atherosclerotic plaque area, macrophage recruitment, and TLR4 expression were observed in mice after S. mutans infection. Analysis of these data, taken together, clearly indicates that S. mutans OMZ175 infection resulted in accelerated induction of atherosclerosis in these mice. Considering the epidemiological data in humans reported by Nakano et al. (2010), the present study adds considerable evidence for the involvement of specific S. mutans strains in human cardiovascular diseases.
Acknowledgments
The authors thank Mercedes Rivera and Joan Whitlock for their assistance.
Footnotes
This project was supported by U24 DE016509 from the NIDCR and by the University of Florida Opportunity Research and UF College of Dentistry seed funds.
The authors declare no potential conflict of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Abranches J, Zeng L, Belanger M, Rodrigues PH, Simpson-Haidaris PJ, Akin D, et al. (2009). Invasion of human coronary artery endothelial cells by Streptococcus mutans OMZ175. Oral Microbiol Immunol 24:141-145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abranches J, Miller JH, Martinez AR, Simpson-Haidaris PJ, Burne RA, Lemos JA. (2011). The collagen-binding protein Cnm is required for Streptococcus mutans adherence to and intracellular invasion of human coronary artery endothelial cells. Infect Immun 79:2277-2284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counsell A, Ghosh J, McCollum CC, Ashleigh R. (2004). Carotid stenting for restenosis after endarterectomy. Cardiovasc Intervent Radiol 34:488-492 [DOI] [PubMed] [Google Scholar]
- Drangsholt MT. (1998). A new causal model of dental diseases associated with endocarditis. Ann Periodontol 3:184-196 [DOI] [PubMed] [Google Scholar]
- Gibson FC, 3rd, Hong C, Chou HH, Yumoto H, Chen J, Lien E, et al. (2004). Innate immune recognition of invasive bacteria accelerates atherosclerosis in apolipoprotein E-deficient mice. Circulation 109:2801-2806 [DOI] [PubMed] [Google Scholar]
- Huang WC, Chen YY, Teng LJ, Lien HT, Chen JY, Chia JS. (2008). Chromosomal inversion between rrn operons among Streptococcus mutans serotype c oral and blood isolates. J Med Microbiol 57(Pt 2):198-206 [DOI] [PubMed] [Google Scholar]
- Jones WS, Washam JB, Meine TJ, Patel MR. (2009). Drug-eluting versus bare metal stenting in acute myocardial infarction. A clinical review. Minerva Cardioangiol 57:585-595 [PubMed] [Google Scholar]
- Kozarov E, Sweier D, Shelburne C, Progulske-Fox A, Lopatin D. (2006). Detection of bacterial DNA in atheromatous plaques by quantitative PCR. Microbes Infect 8:687-693 [DOI] [PubMed] [Google Scholar]
- Lethbridge-Cejku M, Schiller JS, Bernadel L. (2004). Summary health statistics for U.S. adults: National Health Interview Survey, 2002. Vital Health Stat 10 222: 1-151 [PubMed] [Google Scholar]
- Li L, Messas E, Batista EL, Jr, Levine RA, Amar S. (2002). Porphyromonas gingivalis infection accelerates the progression of atherosclerosis in a heterozygous apolipoprotein E-deficient murine model. Circulation 105:861-867 [DOI] [PubMed] [Google Scholar]
- Li X, Kolltveit KM, Tronstad L, Olsen I. (2000). Systemic diseases caused by oral infection. Clin Microbiol Rev 13:547-558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P, Ridker PM, Maseri A. (2002). Inflammation and atherosclerosis. Circulation 105:1135-1143 [DOI] [PubMed] [Google Scholar]
- Liu L, Lalani A, Dai E, Seet B, Macauley C, Singh R, et al. (2000). The viral anti-inflammatory chemokine-binding protein M-T7 reduces intimal hyperplasia after vascular injury. J Clin Invest 105:1613-1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Dai E, Miller L, Seet B, Lalani A, Macauley C, et al. (2004). Viral chemokine-binding proteins inhibit inflammatory responses and aortic allograft transplant vasculopathy in rat models. Transplantation 77:1652-1660 [DOI] [PubMed] [Google Scholar]
- Liu X, Ukai T, Yumoto H, Davey M, Goswami S, Gibson FC, 3rd, et al. (2008). Toll-like receptor 2 plays a critical role in the progression of atherosclerosis that is independent of dietary lipids. Atherosclerosis 196:146-154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas A, Liu L, Macen J, Nash P, Dai E, Stewart M, et al. (1996). Virus-encoded serine proteinase inhibitor SERP-1 inhibits atherosclerotic plaque development after balloon angioplasty. Circulation 94:2890-2900 [DOI] [PubMed] [Google Scholar]
- Lucas AR, Korol R, Pepine CJ. (2006). Inflammation in atherosclerosis: some thoughts about acute coronary syndromes. Circulation 113:e728-e732 [DOI] [PubMed] [Google Scholar]
- Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty TM, et al. (2004). Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci USA 101:10679-10684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano K, Inaba H, Nomura R, Nemoto H, Takeda M, Yoshioka H, et al. (2006). Detection of cariogenic Streptococcus mutans in extirpated heart valve and atheromatous plaque specimens. J Clin Microbiol 44:3313-3317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano K, Nemoto H, Nomura R, Homma H, Yoshioka H, Shudo Y, et al. (2007a). Serotype distribution of Streptococcus mutans a pathogen of dental caries in cardiovascular specimens from Japanese patients. J Med Microbiol 56(Pt 4):551-556 [DOI] [PubMed] [Google Scholar]
- Nakano K, Nomura R, Nemoto H, Mukai T, Yoshioka H, Shudo Y, et al. (2007b). Detection of novel serotype k Streptococcus mutans in infective endocarditis patients. J Med Microbiol 56(Pt 10):1413-1415 [DOI] [PubMed] [Google Scholar]
- Nakano K, Nemoto H, Nomura R, Inaba H, Yoshioka H, Taniguchi K, et al. (2009). Detection of oral bacteria in cardiovascular specimens. Oral Microbiol Immunol 24:64-68 [DOI] [PubMed] [Google Scholar]
- Nakano K, Nomura R, Matsumoto M, Ooshima T. (2010). Roles of oral bacteria in cardiovascular diseases—from molecular mechanisms to clinical cases: Cell-surface structures of novel serotype k Streptococcus mutans strains and their correlation to virulence. J Pharmacol Sci 113:120-125 [DOI] [PubMed] [Google Scholar]
- Petrov L, Laurila H, Hayry P, Vamvakopoulos JE. (2005). A mouse model of aortic angioplasty for genomic studies of neointimal hyperplasia. J Vasc Res 42:292-300 [DOI] [PubMed] [Google Scholar]
- Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, et al. (2008). Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 117:e25-e146 [DOI] [PubMed] [Google Scholar]
- Verma RK, Bhattacharyya I, Sevilla A, Lieberman I, Pola S, Nair M, et al. (2010). Virulence of major periodontal pathogens and lack of humoral immune protection in a rat model of periodontal disease. Oral Dis 16:686-695 [DOI] [PubMed] [Google Scholar]
- Zhang T, Kurita-Ochiai T, Hashizume T, Du Y, Oguchi S, Yamamoto M. (2010). Aggregatibacter actinomycetemcomitans accelerates atherosclerosis with an increase in atherogenic factors in spontaneously hyperlipidemic mice. FEMS Immunol Med Microbiol 59:143-151 [DOI] [PubMed] [Google Scholar]