Abstract
Background
Positive peritoneal fluid cytology predicts poor outcome in patients with resected pancreatic cancer. Reverse transcription–polymerase chain reaction (RT-PCR) has been proposed as a more sensitive means of detection of peritoneal micrometastases than conventional cytology. The clinical significance of RT-PCR positivity in the absence of other evidence of peritoneal disease is unknown. The purpose of the current study was to determine the outcome RT-PCR positive/cytology-negative patients who underwent potentially curative resection.
Methods
Peritoneal washings were collected prospectively from 115 patients with pancreatic cancer undergoing diagnostic laparoscopy at a single institution. Specimens were analyzed by a cytopathologist and by RT-PCR for carcinoembryonic antigen (CEA).
Results
Of the 115 patients, 62 (54%) underwent R0 resection. Eleven of the 62 patients (18%) had peritoneal washings that were negative by conventional cytology but positive for CEA by RT-PCR. Those 11 patients experienced early peritoneal and overall disease recurrence versus those who were RT-PCR negative (P = 0.001, P = 0.003, respectively) independent of nodal status.
Conclusions
RT-PCR for CEA is a sensitive and specific method for the detection of clinically significant peritoneal micrometastases from pancreatic cancer and it might identify a subgroup of patients with otherwise negative findings at staging laparoscopy who might respond better to treatment other than primary surgical resection.
Pancreatic adenocarcinoma continues to have a dismal survival. Surgery is currently the only therapeutic modality that offers any potential for cure. Pancreatectomy is not without risk, with operative morbidity of 38–50% and mortality of 1–6%.1–3 Efforts should be made to identify those patients who may benefit from resection and to avoid unnecessary resection in patients who will not. Patients with subradiologic metastatic disease are destined for early recurrence after resection despite curative intent.
Computed tomography (CT), endoscopic ultrasound (EUS), and staging laparoscopy (SL) are the primary modalities used for preoperative assessment of patients with pancreatic cancer. Preoperative evaluation with CT has historically been associated with relatively low resectability rates at laparotomy. Recent studies from our institution and others report 80–90% resectability in patients with pancreatic cancer with high-resolution, multidetector CT for preoperative evaluation.4–7 CT scans and EUS allow for enhanced assessment of locally advanced disease and gross distant metastases, but despite technological advances still cannot provide accurate assessment of small metastatic deposits to the liver and peritoneum and micrometastatic disease in peritoneal fluid.
SL identifies radiographically occult metastatic disease in 12–21% of patients with pancreatic adenocarcinoma.7 Although the principle yield of SL is detection of subradiologic liver disease, a significant number of patients are found to have metastatic or micrometastatic disease to the peritoneum.8
In the absence of liver metastases, positive peritoneal cytology (PPC) is found in approximately 15% of patients with pancreatic cancer with radiographically resectable disease undergoing laparoscopy, and it predicts poor prognosis.9–12 Patients with PPC have the same poor prognosis after surgery as patients with grossly visible metastatic disease present at the time of resection.10 PPC is defined as stage IV disease in the 2002 American Joint Commission on Cancer staging system.13
PPC involves cytologic examination of peritoneal lavage fluid by a cytopathologist, with or without immunohistochemistry. This test is specific but lacks sensitivity and is highly dependent on both cytopathologist and cellularity of the sample. Many patients with pancreatic cancer with negative cytology develop early peritoneal recurrence after R0 resection of their primary tumors. This observation has lead to the investigation of more sensitive molecular techniques, such as microarray analysis and reverse transcription–polymerase chain reaction (RT-PCR), for detection of metastatic cancer cells in peritoneal fluid.14–17
We previously selected a panel of tumor markers associated with pancreatic cancer, including carcinoembryonic antigen (CEA), cytokeratin 7 (CK7), Kras2, and MUC1. CEA was the most sensitive and specific marker, whereas the others were highly nonspecific.18 We also found that > 10% of patients had positive findings by RT-PCR for CEA but had negative cytology and essentially no other evidence of metastatic disease.
The current study was designed to evaluate whether RT-PCR positive status is clinically significant in patients with pancreatic cancer with no other evidence of metastatic disease. In addition to standard SL with peritoneal fluid cytology, we performed RT-PCR for CEA mRNA on peritoneal lavage samples from patients with pancreatic adenocarcinoma. We then followed curatively resected patients to determine outcome.
MATERIALS AND METHODS
Patients
From March 2006 to January 2008, peritoneal washings were collected prospectively from patients who underwent laparoscopy for staging of pancreatic adenocarcinoma at our institution. Eligible patients were older than 18 years and had pancreatic cancer based on radiologic imaging studies and/or tissue diagnosis. Patients were identified preoperatively and provided informed consent, after Institutional Review Board approval. Patients were followed by interval physical examination, labs, and CT scan of the abdomen and pelvis. Time of recurrence was defined as the date of the first abnormal lab test or imaging study that indicated recurrent disease. In most cases, peritoneal recurrence was demonstrated as new peritoneal thickening or studding on CT scan.
Laparoscopic Evaluation
Patients underwent staging laparoscopy (SL) under general anesthesia. A 10-mm, 30-degree laparoscope was placed through the umbilical port. Additional trocars (5 mm) were placed in the right or left upper quadrants, or both. A systematic examination of the peritoneal cavity was performed. Normal saline was introduced into the right and left upper abdomen and pelvis and was aspirated after gentle agitation but before manipulation or biopsy of primary or metastatic tumor. In all cases, three samples from each site were collected and divided into two parts: half from each sample was sent for cytologic examination with conventional Papanicolaou staining, and half was transported on ice to the laboratory for RNA isolation. During the laparoscopic examination, any visible suspicious lesions were biopsied and sent for frozen and permanent section.
Cytologic Evaluation
Specimens for cytology were placed in Cytolyte fixative (Cytyc Corp; Marlborough, MA). After centrifugation for 10 min, the resulting cell pellet was fixed with PreservCyte (Cytyc Corp). Using the Thin Prep procedure, two slide preparations were made. The first was stained with a modified hematoxylin and eosin preparation, and the second using the Papanicolaou method.
RNA Isolation
Each of three peritoneal washing samples per patient was centrifuged at 800 rpm for 5 min at 4°C. The three pellets were combined and resuspended in a volume of 1 mL of supernatant. The combined sample was centrifuged again at 8000 ×g for 5 min at 4°C. The resulting pellet was processed according to the RNeasy Mini-Kit (Qiagen; Valencia CA) as described by the manufacturer. The pellet was resuspended by addition of Buffer RLT with beta-mercaptoethanol. Samples were homogenized by repeated passage through a blunt 20-gauge needle. Lysates were washed with 70% ethanol and transferred to the RNeasy mini-column, washed with buffer RW1, and eluted in a 30-μL volume of RNase-free water. The samples were stored at −80°C.
RT-PCR Controls
The gastric cancer cell line OCUM-2MD3 (Osaka City University Graduate School of Medicine; Osaka, Japan), known to strongly express CEA, was used as positive control for the RT-PCR assay. Cells were incubated in a humidified incubator at 37°C with 5% CO2 in RPMI media. Peritoneal washings were obtained from 26 patients undergoing laparoscopy for benign pathology to serve as negative controls.
RT-PCR
Reverse transcription was performed using the Taq-Man® Universal Reverse Transcriptase Master Mix (Applied Biosystems). RNA was quantified by spectrophotometry with the Nanodrop 1000 spectrophotometer (Thermo Scientific; Waltham, MA). RT-PCR was performed on each sample using random hexamer priming and TaqMan® Reverse Transcription Reagents (Applied Biosystems; Foster City, CA) on a ThermoHybrid thermocycler (Thermo Scientific). When available, 2 μg of RNA was used in each reaction. For those samples that did not contain 2 μg of RNA, the entire sample (approximately 28 μL volume) was used. For each reaction, sample RNA was amplified in a 100-μL reaction containing: 20 μL of deoxynucleotide triphosphate (2.5 mM each dNTP), 22 μL of MgCL2 (25 mM), 10 μL 10x-RT buffer, 5 μL random hexamers (50 μM), 2 μL RNase inhibitor (20 U/μL), 6.25 μL Multiscribe Reverse Transcriptase (50 U/μL), and a variable amount of free water to total 100 μL depending on the concentration of the RNA sample. The samples were transferred to the Thermo Hybrid thermocycler at 25°C for 10 min, 48°C for 30 min, and 95°C for 5 min. The cDNA samples were stored at −20°C.
TaqMan Assay-on-Demand Gene Expression Assay primers for carcinoembryonic antigen (CEA) mRNA and 18 s rRNA were purchased from Applied Biosystems. Real-time quantitative RT-PCR was performed using the ABI-PRISM 7900 HT Sequence Detection System (Applied Biosystems). DNA was amplified in a 20-μL reaction containing 1 μM of the appropriate primer, 2 μL cDNA, and TaqMan Gene Expression Master Mix. Each PCR reaction was subjected to 30 min at 48°C, 10 min at 95°C, followed by 40 cycles at 95°C for 15 s, and 60°C for 60 s. Each sample was assayed in triplicate with positive and negative PCR controls. The endogenous control gene, 18 s rRNA, was used to confirm the presence of mRNA in the peritoneal washing samples. A positive result was defined as amplification of CEA mRNA in less than 40 cycles of RT-PCR.
Statistical Analysis
RT-PCR status is reported as positive/negative and correlated with clinical/pathologic factors using a chi-square test. The probability of recurrence was estimated by the Kaplan-Meier method and compared across groups (RT-PCR, clinical or pathologic variables) using a log-rank test. Statistical significance is defined by P < 0.05.
RESULTS
Patient Characteristics
From March 2006 through February 2008, 125 patients underwent SL for biopsy-proven or presumed pancreatic adenocarcinoma and were entered into the study. Seven patients had intestinal type adenocarcinoma and three had cholangiocarcinoma on final pathology. These ten patients were excluded from the study. Among the remaining 115 patients, the median age was 68 years (range, 29–85). A total of 67 patients (58%) underwent curative resection in the form of pancreaticoduodenectomy (83%) or distal pancreatectomy (17%). Three of the patients underwent R1 resection, and two patients had positive peritoneal cytology in addition to positive RT-PCR. These five patients were excluded from recurrence and survival analyses.
Yield of Staging Laparoscopy
Of the 115 total patients, 32 (28%) were found to have metastatic disease at the time of SL by cytology, biopsy, or both. Within this group, a total of 13 patients (41%) had positive cytology. Three of the 13 had positive cytology only and ten had positive cytology and grossly visible peritoneal or liver metastases. Thus, among all patients undergoing SL, the yield of positive cytology by conventional Papanicolaou staining was 2.8%. The remaining 19 patients (59%) had grossly visible, biopsy-proven peritoneal or liver metastases but negative cytology.
Of the 26 patients who underwent laparoscopy for benign disease, all had negative cytology and 25 (96%) were RT-PCR negative. One patient (4%) who underwent prophylactic hysterectomy and bilateral oophorectomy had a false-positive result.
RT-PCR Results
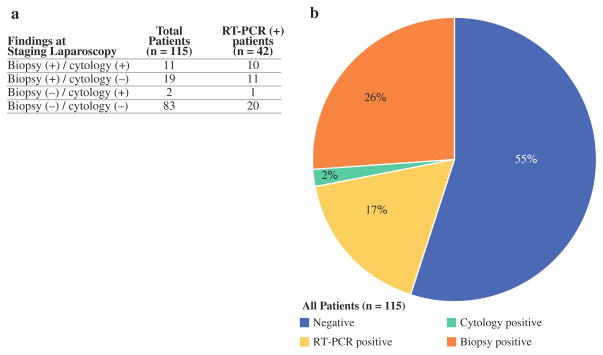
In total, 42 patients (37%) were found to have micro-metastatic disease by RT-PCR at the time of SL. Of these patients, 22 (52%) also had positive findings by cytology or biopsy of gross disease. RT-PCR therefore identified an additional 20 patients (17%) over standard SL. A total of 63 patients (55%) had no evidence of metastatic disease by biopsy, cytology, or RT-PCR (Figs. 1, 2).
FIG. 1.
a Comparison of results of biopsy and peritoneal fluid cytology performed in staging laparoscopy with RT-PCR results in 115 patients with pancreatic adenocarcinoma. b 28% of patients were found to have unresectable disease at staging laparoscopy. An additional 17% of patients had CEA mRNA in peritoneal fluid detected by RT-PCR as their only evidence of metastatic disease
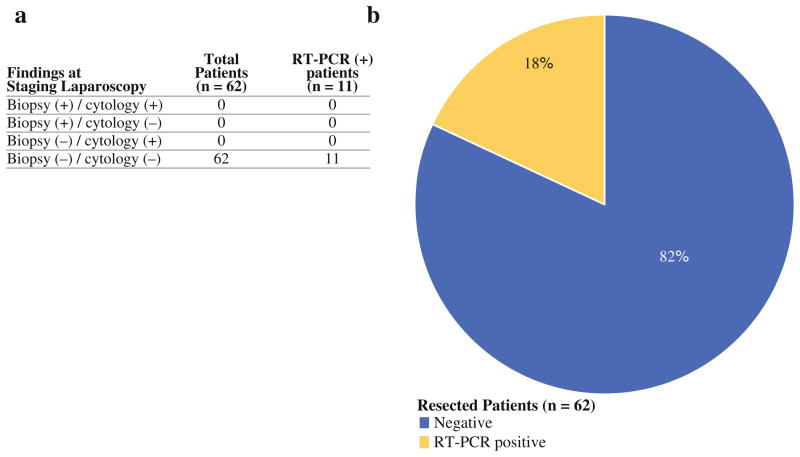
FIG. 2.
a Of 62 patients who underwent R0 resection, no patients had positive findings at staging laparoscopy but 11 patients had peritoneal fluid positive for CEA by RT-PCR. b 18% of patients who underwent R0 resection had evidence of micrometastatic disease to the peritoneum detected by RT-PCR for CEA mRNA
Of the 29 patients with grossly visible, biopsy-proven metastases, 9 (31%) had gross metastases to the peritoneum/carcinomatosis. An additional four patients had positive cytology with no gross peritoneal metastases. Of the 13 patients with peritoneal disease by standard staging laparoscopy, 11 (85%) were RT-PCR positive. An additional 31 patients were RT-PCR positive with no other evidence of metastatic disease to the peritoneum (Table 1).
TABLE 1.
Diagnosis of peritoneal metastasis by standard staging laparoscopy and RT-PCR
| Findings at staging laparoscopy | RT-PCR status
|
||
|---|---|---|---|
| (+) n = 42 | (−) n = 73 | ||
| Peritoneal biopsy | (+) | 9 | 0 |
| Cytology | (+) | 2 | 2 |
| Peritoneal biopsy and cytology | (−) | 31 | 71 |
Nineteen patients (66%) had gross metastatic disease confined to the liver at the time of SL. Within this group, 1 patient (5%) was cytology positive and 12 (63%) were RT-PCR positive. One patient had biopsy-proven metastatic disease to grossly enlarged hepatic artery lymph nodes and was both cytology and RT-PCR negative.
Correlation Between Clinical Factors and RT-PCR
Table 2 summarizes the correlation between positive CEA-mRNA by RT-PCR in peritoneal washings and various clinical features including T stage, presence of peritoneal and hepatic metastases, cytology, and stage. Molecular positivity by RT-PCR correlated significantly with clinical T stage, the presence of peritoneal and hepatic metastases, and cytology status.
TABLE 2.
Relationship between RT-PCR status and various clinical indicators of advanced disease at staging laparoscopy in 112 patients with pancreatic adenocarcinoma
| RT-PCR status
| |||
|---|---|---|---|
| Clinical features | Positive (n = 42) | Negative (n = 73) | P |
| Tumor size | |||
| Tx | 6 | 3 | 0.02 |
| T1–T2 | 1 | 14 | |
| T3–T4 | 35 | 56 | |
| Gross peritoneal metastasis | |||
| Negative | 33 | 73 | < 0.001 |
| Positive | 9 | 0 | |
| Cytology | |||
| Negative | 31 | 71 | < 0.001 |
| Positive | 11 | 2 | |
| Hepatic metastasis | |||
| Negative | 30 | 66 | 0.02 |
| Positive | 12 | 7 | |
| Clinical stage | |||
| I–IIA | 10 | 25 | 0.295 |
| IIB–IV | 32 | 48 | |
Recurrence and Survival
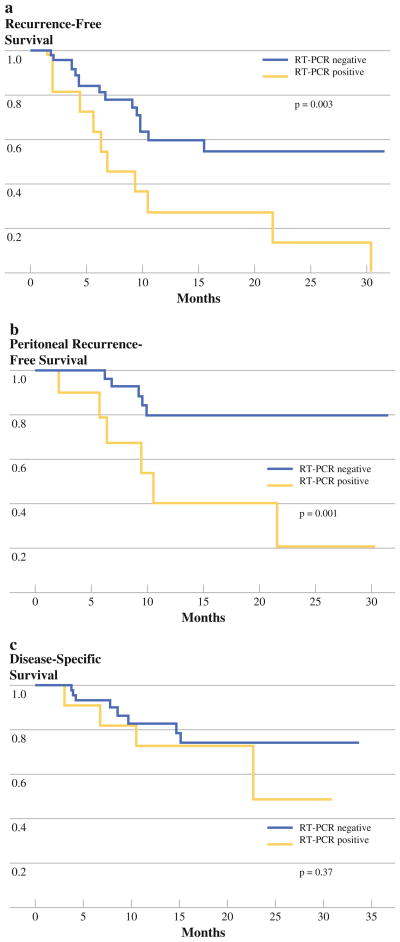
Disease recurrence was analyzed in the 62 patients who underwent R0 resection. Within this group, 11 patients had washings positive for CEA-mRNA by RT-PCR as their only evidence of metastatic disease. These 11 patients experienced a significantly higher rate of recurrence (at any site) postoperatively than the 51 RT-PCR negative patients (P = 0.003) in a median follow-up period of 10.3 (range 1.2–33.8) months (Fig. 3a). The majority of first site recurrences in the 11 patients with positive RT-PCR were peritoneal (Fig. 3b). Survival also was analyzed in the 62 patients who underwent R0 resection. A trend toward decreased survival was observed in the RT-PCR positive patients but did not reach statistical significance (P = 0.37; Fig. 3c). A univariate analysis was performed to evaluate various clinicopathologic factors known to be associated with prognosis in the 62 patients that underwent curative resection (Table 3). RT-PCR positive status and degree of differentiation of the primary tumor significantly correlated with early disease recurrence (P = 0.01, P = 0.01; respectively).
FIG. 3.
a and b Kaplan-Meier curves demonstrate significantly increased incidence of early overall and peritoneal recurrence in RT-PCR positive patients (P = 0.003, P = 0.001; respectively). c Kaplan-Meier curve demonstrates a trend toward decreased disease-free survival in RT-PCR positive patients
TABLE 3.
Univariate analysis of factors associated with time to recurrence in 62 patients undergoing R0 resection for pancreatic adenocarcinoma
| Parameter | Median time to recurrence (mo) | 95% CI (lower bound) | P |
|---|---|---|---|
| Tumor depth | |||
| T1–T2 | NR | NR | 0.15 |
| T3 | 10.5 | 9.4 | |
| Differentiation | |||
| Well to moderate | 21.7 | 10.5 | 0.01 |
| Poor | 5.8 | 4.1 | |
| Lymph node metastasis | |||
| Negative | 30.4 | 9.4 | 0.19 |
| Positive | 10.5 | 6.9 | |
| Vascular invasion | |||
| Negative | 30.4 | 9.4 | 0.11 |
| Positive | 9.9 | 6.7 | |
| Perineural invasion | |||
| Negative | NR | NR | 0.09 |
| Positive | 10.5 | 6.7 | |
| Pathologic stage | |||
| I–IIA | 30.4 | 9.4 | 0.38 |
| IIB–IV | 10.5 | 6.9 | |
| RT-PCR | |||
| Negative | NR | 9.9 | 0.01 |
| Positive | 6.9 | 5.8 | |
DISCUSSION
SL provides important staging information in the pre-operative assessment of pancreatic cancer patients by identifying patients with liver and peritoneal metastatic disease that cannot be detected by standard high-quality radiologic assessment.4,9,19,20 Patients who are found to have subradiologic metastatic disease are very unlikely to benefit from pancreatic resection. Conventional cytology is currently accepted for assessment of peritoneal micrometastatic disease. However, cytology lacks sensitivity, because many patients with negative cytology develop early peritoneal recurrence.
We have previously demonstrated that real-time quantitative RT-PCR can be used as a potentially more sensitive method of detection of cancer cells in peritoneal washings of patients who undergo SL for staging of pancreatic cancer. We optimized this assay using a panel of probes and found that CEA to be the most sensitive and specific marker.18 We did not measure CEA levels in the samples because it has been shown that measured peritoneal fluid CEA levels are not prognostic of peritoneal recurrence and the accuracy of measured CEA is inferior to that of RT-PCR-detected CEA.21 The current study reports the clinical significance of CEA mRNA in peritoneal fluid of patients with pancreatic cancer who undergo curative resection with no other evidence of metastatic disease.
In our entire cohort of 115 patients, RT-PCR for CEA increased the yield of detection of peritoneal micrometastases by 17% compared with standard SL. The majority of patients found to have gross metastatic disease and/or positive cytology at laparoscopy also were RT-PCR positive. The few who were not might have had tumors that did not express CEA. Additionally, many of these patients had liver metastases that were biopsied at laparoscopy representing a hematogenous route of metastasis rather than transperitoneal. It has been shown that in patients with gastric cancer, CEA levels in peritoneal fluid are independent of those in sera.22 We, therefore, did not correlate RT-PCR positivity with serum CEA levels in this study. These observations imply that RT-PCR should not replace gross examination of the peritoneal cavity and conventional cytology as components of SL but should be added to maximize detection of metastatic disease. In the entire cohort, RT-PCR positive status correlated with other indicators of advanced disease, including advanced clinical T stage, positive cytology, and the presence of peritoneal and hepatic metastases.
In patients who underwent curative resection with no other evidence of metastatic disease, detection of CEA-mRNA by RT-PCR predicted early peritoneal recurrence. In addition, RT-PCR positive status demonstrated a trend toward decreased overall survival. Katsuragi and colleagues similarly demonstrated poor prognostic impact of RT-PCR-based detection of peritoneal disease in patients with gastric cancer, but this is the first study to demonstrate this finding in patients with pancreatic cancer.14 Poor differentiation was the only other variable associated with early recurrence in our study. We do not know whether the early recurrence is being independently predicted by the RT-PCR status, or whether it is driven by the degree of differentiation. We have too few patients for a multivariate analysis at this time.
We have demonstrated that the addition of RT-PCR to SL in the preoperative assessment of patients with pancreatic cancer identifies a subgroup with clinically relevant micrometastatic peritoneal disease not detected by conventional cytology that is at high risk for early recurrence and possibly early death after R0 resection. If these observations can be expanded and validated, RT-PCR of peritoneal washings may be another useful technique to help select pancreas cancer patients at risk for early recurrence, for whom treatment other than upfront surgical resection may be appropriate.
Acknowledgments
The authors thank all participating attending surgeons at Memorial Sloan-Kettering Cancer Center (Dr. William Jarnagin, Dr. Vivian Strong, Dr. Michael D’Angelica, and Dr. Ronald Dematteo), as well as the operating room nurses and staff for their coordinated and diligent effort in specimen collection and processing, which made this study possible.
References
- 1.House MG, Fong Y, Arnaoutakis DJ, et al. Preoperative predictors for complications after pancreaticoduodenectomy: impact of BMI and body fat distribution. J Gastrointest Surg. 2008;12(2):270–8. doi: 10.1007/s11605-007-0421-7. [DOI] [PubMed] [Google Scholar]
- 2.Williams TK, Rosato EL, Kennedy EP, et al. Impact of obesity on perioperative morbidity and mortality after pancreaticoduodenectomy. J Am Coll Surg. 2009;208(2):210–7. doi: 10.1016/j.jamcollsurg.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 3.Bentrem DJ, Brennan MF. Outcomes in oncologic surgery: does volume make a difference? World J Surg. 2005;29(10):1210–6. doi: 10.1007/s00268-005-7991-x. [DOI] [PubMed] [Google Scholar]
- 4.Mayo SC, Austin DF, Sheppard BC, et al. Evolving preoperative evaluation of patients with pancreatic cancer: does laparoscopy have a role in the current era? J Am Coll Surg. 2009;208(1):87–95. doi: 10.1016/j.jamcollsurg.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 5.Soriano A, Castells A, Ayuso C, et al. Preoperative staging and tumor resectability assessment of pancreatic cancer: prospective study comparing endoscopic ultrasonography, helical computed tomography, magnetic resonance imaging, and angiography. Am J Gastroenterol. 2004;99(3):492–501. doi: 10.1111/j.1572-0241.2004.04087.x. [DOI] [PubMed] [Google Scholar]
- 6.Howard TJ, Chin AC, Streib EW, et al. Value of helical computed tomography, angiography, and endoscopic ultrasound in determining resectability of periampullary carcinoma. Am J Surg. 1997;174(3):237–41. doi: 10.1016/s0002-9610(97)00132-3. [DOI] [PubMed] [Google Scholar]
- 7.White R, Winston C, Gonen M, et al. Current utility of staging laparoscopy for pancreatic and peripancreatic neoplasms. J Am Coll Surg. 2008;206(3):445–50. doi: 10.1016/j.jamcollsurg.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 8.Tani M, Kawai M, Miyazawa M, et al. Liver metastasis as an initial recurrence has no impact on the survival of patients with resectable pancreatic adenocarcinoma. Langenbecks Arch Surg. 2009;394(2):249–53. doi: 10.1007/s00423-008-0296-4. [DOI] [PubMed] [Google Scholar]
- 9.Jimenez RE, Warshaw AL, Fernandez-Del Castillo C. Laparoscopy and peritoneal cytology in the staging of pancreatic cancer. J Hepatobiliary Pancreat Surg. 2000;7(1):15–20. doi: 10.1007/s005340050148. [DOI] [PubMed] [Google Scholar]
- 10.Ferrone CR, Haas B, Tang L, et al. The influence of positive peritoneal cytology on survival in patients with pancreatic adenocarcinoma. J Gastrointest Surg. 2006;10(10):1347–53. doi: 10.1016/j.gassur.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 11.Yachida S, Fukushima N, Sakamoto M, et al. Implications of peritoneal washing cytology in patients with potentially resectable pancreatic cancer. Br J Surg. 2002;89(5):573–8. doi: 10.1046/j.1365-2168.2002.02061.x. [DOI] [PubMed] [Google Scholar]
- 12.Merchant NB, Conlon KC, Saigo P, et al. Positive peritoneal cytology predicts unresectability of pancreatic adenocarcinoma. J Am Coll Surg. 1999;188(4):421–6. doi: 10.1016/s1072-7515(98)00327-5. [DOI] [PubMed] [Google Scholar]
- 13.Greene FL. AJCC cancer staging manual. 6 [Google Scholar]
- 14.Katsuragi K, Yashiro M, Sawada T, et al. Prognostic impact of PCR-based identification of isolated tumour cells in the peritoneal lavage fluid of gastric cancer patients who underwent a curative R0 resection. Br J Cancer. 2007;97(4):550–6. doi: 10.1038/sj.bjc.6603909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dalal KM, Woo Y, Kelly K, et al. Detection of micrometastases in peritoneal washings of gastric cancer patients by the reverse transcriptase polymerase chain reaction. Gastric Cancer. 2008;11(4):206–13. doi: 10.1007/s10120-008-0483-6. [DOI] [PubMed] [Google Scholar]
- 16.Mori K, Aoyagi K, Ueda T, et al. Highly specific marker genes for detecting minimal gastric cancer cells in cytology negative peritoneal washings. Biochem Biophys Res Commun. 2004;313(4):931–7. doi: 10.1016/j.bbrc.2003.12.025. [DOI] [PubMed] [Google Scholar]
- 17.Reis-Filho JS, de Lander Schmitt FC. Fluorescence in situ hybridization, comparative genomic hybridization, and other molecular biology techniques in the analysis of effusions. Diagn Cytopathol. 2005;33(5):294–9. doi: 10.1002/dc.20278. [DOI] [PubMed] [Google Scholar]
- 18.Dalal KM, Woo Y, Galanis C, et al. Detection of micrometastases in peritoneal washings of pancreatic cancer patients by the reverse transcriptase polymerase chain reaction. J Gastrointest Surg. 2007;11(12):1598–606. doi: 10.1007/s11605-007-0283-z. [DOI] [PubMed] [Google Scholar]
- 19.Shah D, Fisher WE, Hodges SE, et al. Preoperative prediction of complete resection in pancreatic cancer. J Surg Res. 2008;147(2):216–20. doi: 10.1016/j.jss.2008.02.061. [DOI] [PubMed] [Google Scholar]
- 20.Ahmed SI, Bochkarev V, Oleynikov D, Sasson AR. Patients with pancreatic adenocarcinoma benefit from staging laparoscopy. J Laparoendosc Adv Surg Tech A. 2006;16(5):458–63. doi: 10.1089/lap.2006.16.458. [DOI] [PubMed] [Google Scholar]
- 21.Yonemura Y, Endou Y, Fujimura T, et al. Diagnostic value of preoperative RT-PCR-based screening method to detect carcinoembryonic antigen-expressing free cancer cells in the peritoneal cavity from patients with gastric cancer. ANZ J Surg. 2001;71(9):521–8. doi: 10.1046/j.1440-1622.2001.02187.x. [DOI] [PubMed] [Google Scholar]
- 22.Nishiyama M, Takashima I, Tanaka T, et al. Carcinoembryonic antigen levels in the peritoneal cavity: useful guide to peritoneal recurrence and prognosis for gastric cancer. World J Surg. 1995;19(1):133–7. doi: 10.1007/BF00316997. [DOI] [PubMed] [Google Scholar]