Abstract
Objective
The purpose of this study was to evaluate the primary resistance rates of recent clinical Helicobacter pylori isolates to the most commonly used antibiotics in Iran.
Materials and Methods
210 patients presenting with gastric maladies between January and July of 2009 were enrolled in this study. Endoscopy was performed, and biopsy specimens were collected from each patient for subsequent bacterial culture of H. pylori. Single colony isolates from each patient were then used for antimicrobial susceptibility testing. The disk diffusion method was used to determine susceptibility patterns.
Results
197 of the patients were H. pylori positive (93.8%). The rates of resistance to tetracycline, amoxicillin, ciprofloxacin, metronidazole, clarithromycin, and furizoladone were 37.1%, 23.9%, 34.5%, 65.5%, 45.2%, and 61.4%, respectively. A significant association between amoxicillin resistance and disease state (P <0.05) was identified. Furthermore, some double, triple, quadruple, and quintuple combinations of antibiotic resistance were found to be associated with disease state.
Conclusions
This study evaluated the prevalence of H. pylori resistance to the most commonly prescribed antibiotics used in Iran and showed that resistance rates were generally higher than previously reported. This data adds to the growing body of evidence that suggests there is increasing antibiotic resistance among H. pylori isolates, which likely is responsible for the decreasing efficacy of anti-H. pylori therapy at the local and global level. Hence, there is a need for continued monitoring of resistance patterns, especially at the local level, and for incorporation of that information into treatment regimens for H. pylori infections.
Keywords: Helicobacter, antibiotic resistance, Iran, multidrug resistance, amoxicillin, metronidazole
Introduction
Helicobacter pylori is a Gram negative, microaerophilic, spiral shaped bacterium that infects the gastric mucosa of over 50% of the population worldwide; infection rates can be as high as 90% in developing countries (Dunn, et al., 1997). Infection with H. pylori usually occurs during childhood, and an infected individual typically remains infected throughout their life unless a specific anti-H. pylori therapy is administered (Blaser, 1990). While infections with H. pylori are mainly asymptomatic, diseases in symptomatic individuals range from milder forms (gastritis) to more severe states (peptic ulcer disease) to the most severe states (gastric cancer in the form of gastric adenocarcinoma or mucosa-associated lymphoid tissue lymphoma) (Blaser, 1990). Indeed, numerous findings support the fact that H. pylori eradication from the stomach results in significant regression of these associated gastroduodenal disorders (Leodolter, et al., 2001).
Currently, triple therapy, consisting of a proton pump inhibitor with clarithromycin and either metronidazole or amoxicillin administered for one to two weeks, is recommended for use in treating H. pylori (Malfertheiner, et al., 2007). However, treatment of H. pylori is complicated by the increasing rates of antibiotic resistance being reported worldwide (Megraud, 2004) and is likely responsible, at least in part, to the overall decreasing treatment efficacy (O’Connor, et al., 2010). Compounding this problem is the high variability of antibiotic resistance rates from one region of the world to the next; stark differences are often observed between developed and developing countries. For example, over a ten year period in China, the prevalence of amoxicillin resistance decreased from 2.1% in 2000 to 0.3% in 2009, while clarithromycin and metronidazole resistance both increased from 12.8 to 23.8% and 12.8 to 56.6%, respectively, over the same ten year period (Gao, et al., 2010). Similarly to what was seen in China, in Japan resistance rates to clarithromycin increased to approximately 30% from 1996 to 2004, and the rate has remained fairly constant to the present day (Horiki, et al., 2009). In the Unites States, clarithromycin resistance rates are low, ranging from 10–15%, while metronidazole resistance rates range from 20–40% and resistance to amoxicillin is rare (Osato, et al., 2001). A recent report in Spain showed that the rate of clarithromycin resistance was 35.6% in patient isolates of H. pylori (Agudo, et al., 2010). In contrast, the rate of resistance to metronidazole in Saudi Arabia in 2008 was 69.5%, which is higher than the rate generally reported for developed countries (Vakil and Megraud, 2007), while clarithromycin and amoxicillin resistance rates were similar at 21% and 0%, respectively (Marie, 2008). Given the broad diversity in antibiotic resistance patterns for H. pylori around the globe, it is highly unlikely that one “best option” treatment regimen could be successful worldwide. This evidence has prompted some researchers to suggest that physicians should use the treatments that work best within their local geographic area (Graham and Fischbach, 2010).
In keeping with this notion and because it would be impractical and costly to test the antibiotic susceptibility patterns for each individual seeking anti-H. pylori treatment, it is important for clinicians to know the local resistance rates of H. pylori. In this way, the likelihood of treatment eradication success can be improved through the use of antibiotics that are known to be most effective in the region. In order to approach H. pylori treatment in this manner, the local resistance rates must be known. In many countries, like those discussed above, H. pylori susceptibility surveillance is commonly performed and reported, while in other countries, like Iran, information regarding antibiotic resistance rates is relatively scarce (Saberi-Firoozi, 2006). This is despite the fact that in Iran most of the patients that are admitted for gastric disorders are H. pylori positive (Mohammadi, et al., 2005, Saberi-Firoozi, 2006). Given the chronic nature of infection with this bacterium, the problems with eradication of the organism as a result of antibiotic resistance (Siavoshi, et al., 2006), and the relatively small amount of information concerning resistance rates in the country (Saberi-Firoozi, 2006), we sought to define the most effective antibiotic regimen for H. pylori eradication in Iran by determining the antibiotic susceptibility patterns of H. pylori to the six antibiotics most commonly utilized by Iranian clinicians.
Materials and Methods
Patients
A total of 210 patients suffering from gastritis, gastric ulcer, duodenal ulcer, or gastric cancer, who were admitted to Tooba Medical Center and Imam Khomeini hospital (Sari, Iran) for routine endoscopy between January and July of 2009, were evaluated in this study. A detailed questionnaire was completed by all patients, and informed consent was obtained from patients prior to endoscopy. Demographic data which included gender, age, socioeconomic status, consumption of bottled mineral water, and whether the individual was breast fed as a child, were collected for all participating patients that were H. pylori positive. Patients who reported the use of antibiotics within three months prior to entering the study were excluded from the analysis. All 210 antral biopsies were sent for bacterial culture; 197 were H. pylori positive and thus eligible for further study. Among the 197 H. pylori positive samples, 76 were from gastritis patients, 54 were from duodenal ulcer patients, 32 were from gastric ulcer patients, and 35 were from gastric cancer patients. Endoscopic findings and pathology data were used as criteria for the clinical diagnosis of these conditions (Block, 2004, Dixon, et al., 1996). This study was approved by the ethical committee of Tarbiat Modares University, Iran.
Bacterial Culture
Antral biopsy specimens were transported on ice in sterile thioglycolate broth (Merck, Germany) and then quickly shipped to the diagnostic laboratory in a cold flask within three hours post endoscopy. The resulting tissue was homogenized to ensure distribution of the bacteria within each biopsy specimen, and 200μL of the homogenate was then spread on Columbia agar plates (Merck, Germany) supplemented with 5% defibrinated sheep blood (Jihad daneshgahi, Tehran, Iran), 8% fetal calf serum (Gibco, USA), and one CAMP Selectatab [Skirrow] (Mast Group Ltd., UK) per 250mL agar media. Bacterial plates were incubated at 37°C under microaerophilic conditions in a CO2 incubator (Binder, USA) with high humidity for up to ten days. Bacteriological identification was completed based on classic positive biochemical tests for catalase, oxidase, and urease, as well as Gram staining and visual examination for typical colony morphology. To prevent any complications due to mixed susceptibility patterns from different isolates from a single patient, a single colony from each patient was used for antibiotic susceptibility testing. All resulting strains were stored at −20°C until needed for further investigation.
Antibiotic Susceptibility Testing
The disk diffusion method was utilized in the determination of the resistance status of each H. pylori isolate (Megraud and Lehours, 2007, Mohammadi, et al., 2005). Each H. pylori strain was grown on solid media, and suspensions were made in normal saline to an equivalent of 2 McFarland units. 50μL of each suspension was then spread on Muller-Hinton agar (Merck, Germany) plates supplemented with 5% defibrinated sheep blood (Bahar-Azma, Tehran, Iran) and 8% fetal calf serum (Gibco, USA). Discs impregnated with 5μg metronidazole, 2μg clarithromycin, 2μg amoxicillin, 1μg furazolidone, 10μg tetracycline, and 1μg ciprofloxacin (Mast Group Ltd., UK) were then placed on the surface of the agar. The plates were allowed to dry for ten minutes, and plates were incubated for three to six days under microaerophilic conditions as detailed above for isolation of the bacteria from biopsy specimens (Megraud and Lehours, 2007). Following incubation, the diameters of the zones of inhibition around each disc were measured, and the breakpoints for resistance were considered as previously described (Boyanova, et al., 2000, Elviss, et al., 2005, Wolle, et al., 2002). The well characterized H. pylori strain, ATCC43504, was utilized as a control to ensure the validity of the susceptibility tests in our study.
Statistical Analysis
The Pearson’s Chi Square test was performed on this data set to determine if there was any association between disease state and antibiotic resistance (either as single antibiotics or combinations of antibiotics). Additionally, analysis of association between antibiotic resistance and the demographic factors included in the questionnaire as detailed above was also performed using Pearson’s Chi Square test. Log Linear modeling and cluster analysis was also performed on these data to determine if higher order associations existed. The statistical analysis of these data was conducted using SPSS software version 18 (SPSS Inc., Chicago, IL).
Results
Among the 210 patients, suffering from gastritis, gastric ulcers, duodenal ulcers, or gastric cancer, admitted to the study from January to July 2009, H. pylori was successfully isolated from 197 individuals; therefore, the prevalence of H. pylori infection was 93.8% within symptomatic patients in this region of Iran. The age of patients who were H. pylori positive ranged from 18 to 74 years old with a mean age of 40.7 years. The distribution of men and women within the H. pylori infected population was similar with 95 women (48.2%) and 102 men (51.8%), which indicates that both genders were equally represented within this study (Table 1). The distribution of men and women among patients who were of low socioeconomic status, consumed bottled mineral water, and were breast fed as infants was also similar (Table 2).
Table 1.
Epidemiological Breakdown of Antibiotic Resistance Patterns
| AMXa | CIP | CLR | FUR | MTZ | TET | Total | |
|---|---|---|---|---|---|---|---|
| No. isolates | 47 (23.9%) | 68 (34.5%) | 89 (45.2%) | 121 (61.4%) | 129 (65.5%) | 73 (37.1%) | 197 |
| Age Range | 20–71 | 18–71 | 18–74 | 18–71 | 18–74 | 18–68 | 18–74 |
| Mean Age | 40.9 | 39.4 | 39.6 | 40 | 38.9 | 37.7 | 40.7 |
| Males | 23 | 39 | 51 | 64 | 67 | 42 | 102 |
| Age Range | 20–68 | 18–71 | 18–74 | 18–71 | 18–74 | 18–68 | 18–74 |
| Mean Age | 39.2 | 40.9 | 40.7 | 39.6 | 38.6 | 37.9 | 40.4 |
| Females | 24 | 29 | 38 | 57 | 62 | 31 | 95 |
| Age Range | 21–71 | 19–67 | 18–71 | 18–71 | 18–67 | 18–64 | 18–71 |
| Mean Age | 42.5 | 37.4 | 38.2 | 40.5 | 39.3 | 37.3 | 41 |
AMX - amoxicillin, CIP - ciprofloxacin, CLR - clarithromycin, FUR - furizoladone, MTZ - metronidazole, and TET - tetracycline
The number of isolates is indicated and the corresponding percentage of the total each represents is given in parentheses.
Table 2.
Epidemiological Breakdown of Demographics
| Low Socioeconomic Status | Mineral Water Consumption | Breast Fed as Infant | |
|---|---|---|---|
| Total | 124 (62.9%)a | 59 (29.9%) | 63 (32.0%) |
| Males | 63 | 31 | 39 |
| Females | 61 | 28 | 24 |
The number of isolates in each group is indicated and the corresponding percentage of the total each represents is given in parentheses.
Histopathological analysis and diagnosis of each biopsy specimen revealed that of the 197 H. pylori infected individuals, 76 suffered from gastritis (38.6%), 32 suffered from gastric ulcers (16.2%), 54 suffered from duodenal ulcers (27.4%), and 35 suffered from gastric cancer (17.8%). No statistically significant associations were found between the particular disease state and gender. Likewise, disease state was not associated with any of the other demographic information obtained from the patients: low socioeconomic status, bottled mineral water consumption, or having been breast fed as an infant.
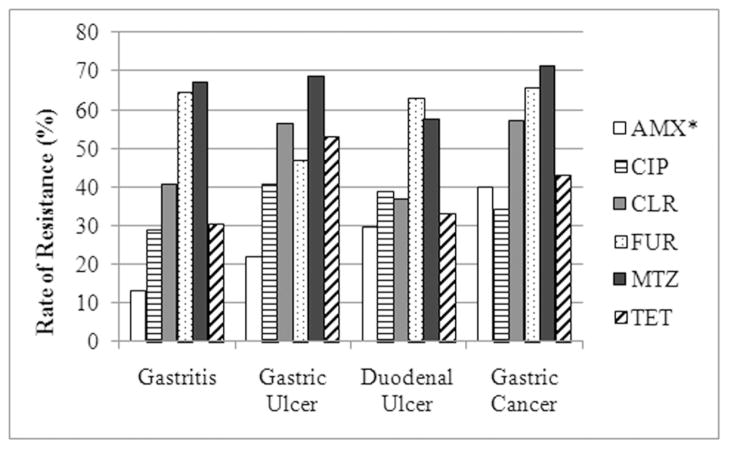
Single colony isolates were successfully obtained from each biopsy specimen and were subsequently used to determine the antibiotic resistance status of the bacteria from each patient. The rates of resistance to amoxicillin, ciprofloxacin, clarithromycin, furazolidone, metronidazole, and tetracycline were determined to be 23.9%, 34.5%, 45.2%, 61.4%, 65.5%, and 37.1%, respectively. No statistically significant associations were found between any of the individual antibiotics and gender, bottled mineral water consumption, or low socioeconomic status. However, furizoladone and tetracycline resistance were found to be associated with whether the patients had been breast fed or not during infancy (P < 0.05). The trends suggest that patients who were not breast fed were more likely to carry tetracycline sensitive isolates, while patients who were breast fed were more likely to carry furizoladone resistant isolates. Resistance to none of the other antibiotics was found to be associated with this demographic trait. While the rates of resistance to each antibiotic varied across disease date (Figure 1), only amoxicillin resistance showed a statistical association with disease state (P < 0.05).
FIG. 1.
The Rate of Antimicrobial Resistance across Disease State. The relative rate of resistance across the indicated H. pylori associated disease states is indicated as a percent of the total number of isolates from within that disease state. The antibiotics used in this study are abbreviated as follows: amoxicillin – AMX, ciprofloxacin – CIP, clarithromycin – CLR, furizoladone – FUR, metronidazole – MTZ, and tetracycline – TET. An asterisk indicates that a statistical association was found between the antibiotic and disease state.
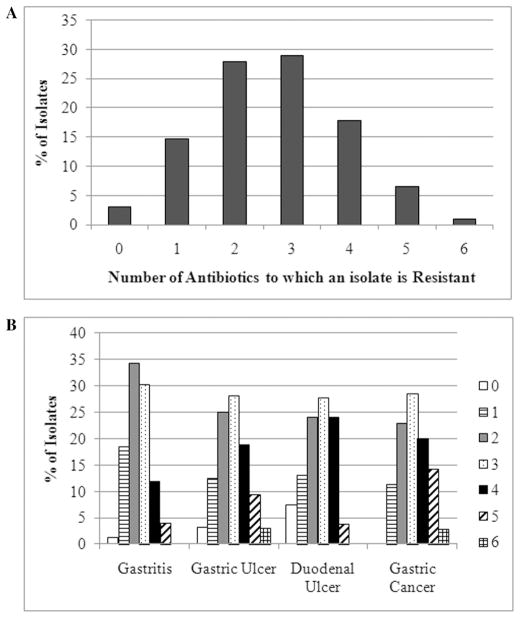
Of the 197 H. pylori positive biopsy isolates, six isolates were sensitive to all six of the tested antibiotics (3%), 29 were resistant to only one antibiotic (14.7%), 55 were resistant to two antibiotics (27.9%), 57 were resistant to three antibiotics (28.9%), 35 were resistant to four antibiotics (17.8%), 13 were resistant to five antibiotics (6.6%), and 2 were resistant to all six antibiotics (1.0%) (Figure 2A). While the distribution of the total number of antibiotics to which the strains were resistant varied across disease state (Figure 2B), there was no statistical association between these two factors. However, because of the high number of multidrug resistant H. pylori isolates, we wondered if there were any combinations of antibiotics that were associated with the identified disease states; therefore, each possible combination of double, triple, quadruple, quintuple, and sextuple resistance patterns were analyzed. Of these, a number of combinations were found to show a statistically significant association (P < 0.05) with disease state (Table 3), but no specific disease state could be linked to the resistance combinations. While the distribution of disease states among the strains that were resistant to two or three antibiotics did not vary much, the majority of isolates resistant to four or five antibiotics showed a distinct trend towards being isolated from patients suffering from gastric ulcers or gastric cancer. In addition, because of the commonalities among some of the significant resistance combinations, Log Linear modeling was performed to determine if any higher order associations existed between the combinations of antibiotic resistance and disease state. No higher order interactions were identified. Similarly, cluster analysis was performed on these data, and no statistically significant clusters were determined.
FIG. 2.
Total Number of Multidrug Resistant Strains. Panel A: the total numbers of strains that were resistant to none or from one to six of the examined antibiotics are given as a percent of the total number of isolates. Panel B: the number of strains that were resistant to none or from one to six of the antibiotics as a percent within a given disease state.
Table 3.
Combinations of Antibiotic Resistance that were Statistically Associated with Disease State*
| Combination | Total no. isolates | Gastritis | Gastric Ulcer | Duodenal Ulcer | Gastric Cancer |
|---|---|---|---|---|---|
| Total | 197 | 76 | 32 | 54 | 35 |
|
| |||||
| Double Resistance | |||||
|
| |||||
| AMX, CLR | 20 | 4 (5.3)a | 4 (12.5) | 3 (5.6) | 9 (25.7) |
| AMX, FUR | 31 | 6 (7.9) | 3 (9.4) | 12 (22.2) | 10 (28.6) |
| TET, CIP | 23 | 4 (5.3) | 8 (25) | 8 (14.8) | 3 (8.6) |
| TET, CLR | 37 | 10 (13.2) | 11 (34.4) | 7 (13.0) | 9 (25.7) |
| MTZ, CLR | 58 | 19 (25.0) | 14 (43.8) | 11 (20.4) | 14 (40.0) |
| Triple Resistance | |||||
|
| |||||
| AMX, CLR, FUR | 13 | 1 (1.3) | 3 (9.4) | 2 (3.7) | 7 (20.0) |
| AMX, CLR, TET | 10 | 1 (1.3) | 4 (12.5) | 1 (1.9) | 4 (11.4) |
| CLR, FUR, TET | 24 | 7 (9.2) | 6 (18.8) | 3 (5.6) | 8 (22.9) |
| CLR, MTZ, TET | 28 | 5 (6.6) | 9 (28.1) | 6 (11.1) | 8 (22.9) |
| Quadruple Resistance | |||||
|
| |||||
| AMX, CIP, CLR, TET | 5 | 1 (1.3) | 3 (9.4) | 0 (0) | 1 (2.9) |
| AMX, CLR, FUR, MTZ | 7 | 0 (0) | 2 (6.3) | 1 (1.9) | 4 (11.4) |
| AMX, CLR, FUR, TET | 7 | 0 (0) | 3 (9.4) | 0 (0) | 4 (11.4) |
| AMX, FUR, MTZ, TET | 13 | 3 (3.9) | 2 (6.3) | 2 (3.7) | 6 (17.1) |
| CLR, FUR, MTZ, TET | 18 | 3 (3.9) | 5 (15.6) | 3 (5.6) | 7 (20.0) |
| Quintuple Resistance | |||||
|
| |||||
| AMX, CLR, FUR, MTZ, TET | 5 | 0 (0) | 2 (6.3) | 0 (0) | 3 (8.6) |
P < 0.05
AMX - amoxicillin, CIP - ciprofloxacin, CLR - clarithromycin, FUR - furizoladone, MTZ - metronidazole, and TET - tetracycline
The numbers in parentheses represent the percentage of isolates with the given antibiotic resistance pattern out of the total number of isolates from the given disease state.
Discussion
With over 50% of the world’s population infected, H. pylori is a human pathogen of significance in the medical world. Given the sheer number of infected individuals and the potential for severe disease outcome in the form of gastric cancer, it is critical that effective treatments remain available. However, the efficacy of treatment regimes is on the decline in most areas of the world due to increasing antibiotic resistance among H. pylori isolates. This fact, coupled with the wide geographical diversity among resistance patterns (Boyanova and Mitov, 2010), highlights the importance of understanding local antibiotic resistance patterns when clinicians prescribe anti-H. pylori therapy.
The overall prevalence of H. pylori infection in Iran varies geographically but remains high; rates range from 42% to 89% (Malekzadeh, et al., 2009). In the present study, 93.8% of the gastric biopsy specimens yielded H. pylori positive cultures, suggesting that the prevalence of infection is extremely high in the northern region of Iran. Because of this high rate of infection, we sought to gain a better understanding of the antibiotic resistance patterns of H. pylori to the six most commonly used antibiotics in this area: amoxicillin, ciprofloxacin, clarithromycin, furizoladone, metronidazole, and tetracycline.
Amoxicillin is recommended for anti-H. pylori triple therapy in areas where metronidazole resistance is high. Worldwide, resistance to amoxicillin is rare (Boyanova and Mitov, 2010), yet in our study we found that 23.9% of the H. pylori isolates were resistant to this antibiotic. This rate of resistance is intermediate as compared to other studies conducted in the Mazandaran region of northern Iran, which reported resistance rates of 6.8% (Talebi Bezmin Abadi, et al., 2010) and 35% (Talebi Bezmin Adabi, 2009). Recent reports from southern Iran indicate similar rates of amoxicillin resistance (about 20%) (Farshad, et al., 2010, Kohanteb, et al., 2007) as compared to central Iran where amoxicillin resistance is lower (7.3%) (Siavoshi, et al., 2010). Interestingly, in our study amoxicillin resistance tended to show a higher prevalence among gastric cancer isolates as opposed to gastritis isolates (Table 1). Overall, it appears that while resistance to amoxicillin varies within Iran, the rates are still relatively low, suggesting that this drug may continue to be useful.
Ciprofloxacin belongs to the fluoroquinolone group of antibiotics that are generally used as part of rescue therapy for treating H. pylori infections when first and second line therapies have failed (Jones, 2008). Resistance to fluoroquinolones is generally very low (<10%) worldwide (Boyanova and Mitov, 2010). In our population, 34.5% of the bacterial isolates were resistant to ciprofloxacin, which is much higher than typically found. This rate is slightly higher than the 25% rate of ciprofloxacin resistance recently reported for this same region in northern Iran (Talebi Bezmin Adabi, 2009), but is considerably higher than the 4.7% resistance rate recently reported in southern Iran (Kohanteb, et al., 2007). To the best of our knowledge, no studies have yet examined the rate of ciprofloxacin resistance in central Iran. The discrepancy between ciprofloxacin resistance rates between the northern and southern portions of Iran further stresses the importance of monitoring local resistance rates, since anti-H. pylori therapy using ciprofloxacin in southern Iran is very likely to be successful but less likely to be so in northern Iran.
Clarithromycin is utilized in the recommended first line triple therapies against H. pylori. In this study, 45.2% of all H. pylori isolates were resistant to clarithromycin, which is an increase over the rates previously reported for this area (25–30%) (Talebi Bezmin Abadi, et al., 2010, Talebi Bezmin Adabi, 2009), and is drastically higher than the recently reported rates of resistance in southern and central Iran, which are <10% (Kohanteb, et al., 2007, Siavoshi, et al., 2010). In fact, this rate of resistance is at the upper end of what is described worldwide (4.0% to 49.2%) (Boyanova and Mitov, 2010). This increase in clarithromycin resistance in northern Iran is troubling given how important this antibiotic is in the treatment of H. pylori infection. However, given that cross-resistance is possible within the macrolide antibiotic group (Hulten, et al., 1997) and that erythromycin is commonly prescribed in Iran, especially in children, it may not be surprising that clarithromycin resistance is increasing among H. pylori isolates in this country. At 45.2% clarithromycin resistance, use of this antibiotic should be carefully considered as it is not likely to be very effective at least within the northern areas of Iran.
The rate of furizoladone resistance among H. pylori isolates in this study was determined to be 61.4%, which is higher than the 24% resistance rate previously reported for this region in Iran (Talebi Bezmin Adabi, 2009). It is also drastically higher than the rates reported in 2010 for central and southern Iran, 4.5% and 9.4%, respectively (Kohanteb, et al., 2007, Siavoshi, et al., 2010). This high level of furizoladone resistance presents a striking treatment problem for clinicians in Iran where furazolidone was shown to be a viable alternative to metronidazole (Malekzadeh, et al., 2000). Additionally, furizoladone has been shown to be a suitable replacement for clarithromycin in anti-H. pylori therapies (Fakheri, et al., 2001, Riahizadeh, et al., 2010). Furthermore, it has the added benefit of being inexpensive and readily available in developing countries (Segura, et al., 1997). The increased rate of furizoladone resistance seen in this study suggests that the usefulness of this antibiotic on its own or as an alternative to metronidazole is decreasing.
Metronidazole is a commonly used antibiotic in the treatment of H. pylori infection and is part of the classic triple therapy regimen. 65.5% of the H. pylori isolates examined in this study showed metronidazole resistance, which is similar to two other reports of approximately 70% (Talebi Bezmin Abadi, et al., 2010, Talebi Bezmin Adabi, 2009). Compared to recent reports from other regions in Iran, our rate of metronidazole resistance is similar to what is seen in the south (72.6%) (Kohanteb, et al., 2007), but is higher than what is seen in the central portion of the country (55.6%) (Siavoshi, et al., 2010). On the whole, metronidazole resistance rates vary greatly around the world, and developing countries tend to have higher rates of resistance (Boyanova and Mitov, 2010). While the rate of metronidazole resistance has remained constant within the northern parts of Iran, the present rate is high enough to suggest that this antibiotic may no longer be useful for treatment of H. pylori.
The rate of tetracycline resistance in this study was determined to be 37.1%, a striking jump in resistance as compared to the 9% reported previously within this same region in Iran (Talebi Bezmin Abadi, et al., 2010). In 2010 the rate of tetracycline resistance in southern Iran was reported as 4.7% (Kohanteb, et al., 2007). However, in central Iran the rate was 38.1% (Siavoshi, et al., 2010). Based on these combined data, it is difficult to determine whether the rate of tetracycline resistance is rising in Iran or whether there are just large differences among the regions. Tetracycline resistance among H. pylori isolates is largely non-existent around the world; when present, it is generally less than 5%. Therefore, it is surprising that rates over 35% were seen in Iran. The discrepancy in the rates of tetracycline resistance warrant continued surveillance of H. pylori isolates from all over Iran to determine the continued usefulness of this antibiotic.
While there is limited data on the patterns of antibiotic resistance in H. pylori isolates from infected individuals in Iran, there is even less data available on the rates of multidrug resistant isolates. Of the reports containing this type of information, there are only a handful of isolates that have been reported from southern Iran (Farshad, et al., 2010, Kohanteb, et al., 2007) and from northern Iran (Talebi Bezmin Abadi, et al., 2010). In the present study, we found that 14.7% of the isolates were resistant to only 1 antibiotic and 3% were resistant to no antibiotics. This implies that the vast majority (82.3%) were multidrug resistant. The alarmingly high number of multidrug resistant strains will likely complicate the development and use of an optimal anti-H. pylori treatment regimen. Moreover, several of the antibiotic combinations in the multidrug resistant strains were found to have a statistical association with disease state (Table 3). It should however be noted that since all possible combinations of single to sextuple resistance patterns were analyzed and some of those combinations had very small numbers of isolates, some of the statistically significant combinations could be the result of Type 1 error. This being said, distinct trends were present. Compared to double and triple resistant strains that were evenly spread across disease state, the quadruple and quintuple resistant isolates tended to come from gastric ulcer and gastric cancer patients. Furthermore, quadruple and quintuple resistance patterns tended to be less prevalent among gastritis and duodenal ulcer patients, which was not the case among the double and triple resistant isolates. The exception to this appears to be amoxicillin, clarithromycin and tetracycline resistant isolates, where the highest number of isolates were from gastric ulcer and gastric cancer patients. Interestingly, if you add furizoladone resistance to this combination, there were no longer any isolates from duodenal ulcer or gastritis patients. This trend also held true for the metronidazole, amoxicillin, clarithromycin, tetracycline, and furizoladone resistance combination. Taken together these data suggest that while no specific disease state could be determined to vary significantly among the multidrug resistant isolates, there was a trend towards increased drug resistance among gastric ulcer and gastric cancer patients.
The sheer number of strains that were resistant to multiple antibiotics also suggests that future treatment of H. pylori may very well become more complicated if the rates of resistance to any given antibiotic continue to rise as well as if the number of multidrug resistant strains increases. Indeed, if new treatment regimens and/or new antibiotics are not developed, even though it would be time consuming and costly, it may become necessary to perform susceptibility testing on H. pylori isolated from infected patients prior to the start of treatment.
The most commonly prescribed anti-H. pylori therapy in Iran consists of a proton pump inhibitor or ranitidine and bismuth citrate combined with clarithromycin and either amoxicillin or metronidazole. Based on our data, it is clear that metronidazole and furizoladone are not the best antibiotics to use in the treatment of H. pylori infections in northern Iran; we observed rates of resistance of over 60% among the isolates. A better therapeutic option would be to use amoxicillin, ciprofloxacin, and/or tetracycline in either a triple therapy or quadruple therapy regimen because the rates of resistance for these antibiotics were each less than 38%. Clarithromycin resistance was present in 45.2% of the isolates studied, so this antibiotic may still be useful in treating H. pylori in the northern region of Iran. It may in fact become necessary to switch to a sequential therapy strategy if the standard simultaneous therapies prove ineffective; there is a growing body of evidence to suggest that sequential therapy may be superior to the current regimens (Gisbert, et al., 2010). Additionally, the use of bismuth in addition to a proton pump inhibitor should be continued as the evidence for the effectiveness of this agent continues to grow (Graham and Fischbach, 2010, O’Connor, et al., 2010). The data presented here highlight the continued need for monitoring of H. pylori resistance patterns in the northern region of Iran, so as to provide the best possible eradication options for those individuals suffering from H. pylori associated diseases.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. C. Olsen for critical review of the statistical analysis of this data. We thank the research branch of Tarbiat Modares University and especially Dr. Fathollahi for their kind support of this project through an Elite Student Grant (2009). Research in the laboratory of D. Scott Merrell is made possible by grant AI065529 from the NIAID. The contents of this article are the sole responsibility of the authors and do not necessarily represent the official views of the Department of Defense.
References
- Agudo S, Perez-Perez G, Alarcon T, Lopez-Brea M. High prevalence of clarithromycin-resistant helicobacter pylori strains and risk factors associated with resistance in madrid, spain. J Clin Microbiol. 2010;48:3703–3707. doi: 10.1128/JCM.00144-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser MJ. Epidemiology and pathophysiology of campylobacter pylori infections. Rev Infect Dis. 1990;12(Suppl 1):S99–106. doi: 10.1093/clinids/12.supplement_1.s99. [DOI] [PubMed] [Google Scholar]
- Block B, Schachschal G, Schmidt H. Endoscopy of the upper gi tract: A training manual. Thieme; New Yord: 2004. [Google Scholar]
- Boyanova L, Mitov I. Geographic map and evolution of primary helicobacter pylori resistance to antibacterial agents. Expert Rev Anti Infect Ther. 2010;8:59–70. doi: 10.1586/eri.09.113. [DOI] [PubMed] [Google Scholar]
- Boyanova L, Stancheva I, Spassova Z, Katzarov N, Mitov I, Koumanova R. Primary and combined resistance to four antimicrobial agents in helicobacter pylori in sofia, bulgaria. J Med Microbiol. 2000;49:415–418. doi: 10.1099/0022-1317-49-5-415. [DOI] [PubMed] [Google Scholar]
- Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated sydney system. International workshop on the histopathology of gastritis, houston 1994. Am J Surg Pathol. 1996;20:1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- Dunn BE, Cohen H, Blaser MJ. Helicobacter pylori. Clin Microbiol Rev. 1997;10:720–741. doi: 10.1128/cmr.10.4.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elviss NC, Owen RJ, Breathnach A, Palmer C, Shetty N. Helicobacter pylori antibiotic-resistance patterns and risk factors in adult dyspeptic patients from ethnically diverse populations in central and south london during 2000. J Med Microbiol. 2005;54:567–574. doi: 10.1099/jmm.0.45896-0. [DOI] [PubMed] [Google Scholar]
- Fakheri H, Malekzadeh R, Merat S, Khatibian M, Fazel A, Alizadeh BZ, Massarrat S. Clarithromycin vs. Furazolidone in quadruple therapy regimens for the treatment of helicobacter pylori in a population with a high metronidazole resistance rate. Aliment Pharmacol Ther. 2001;15:411–416. doi: 10.1046/j.1365-2036.2001.00931.x. [DOI] [PubMed] [Google Scholar]
- Farshad S, Alborzi A, Japoni A, Ranjbar R, Hosseini Asl K, Badiee P, Amin Shahidi M, Hosseini M. Antimicrobial susceptibility of helicobacter pylori strains isolated from patients in shiraz, southern iran. World J Gastroenterol. 2010;16:5746–5751. doi: 10.3748/wjg.v16.i45.5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Cheng H, Hu F, Li J, Wang L, Yang G, Xu L, Zheng X. The evolution of helicobacter pylori antibiotics resistance over 10 years in beijing, china. Helicobacter. 2010;15:460–466. doi: 10.1111/j.1523-5378.2010.00788.x. [DOI] [PubMed] [Google Scholar]
- Gisbert JP, Calvet X, O’Connor A, Megraud F, O’Morain CA. Sequential therapy for helicobacter pylori eradication: A critical review. J Clin Gastroenterol. 2010;44:313–325. doi: 10.1097/MCG.0b013e3181c8a1a3. [DOI] [PubMed] [Google Scholar]
- Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010;59:1143–1153. doi: 10.1136/gut.2009.192757. [DOI] [PubMed] [Google Scholar]
- Horiki N, Omata F, Uemura M, Suzuki S, Ishii N, Iizuka Y, Fukuda K, Fujita Y, Katsurahara M, Ito T, Cesar GE, Imoto I, Takei Y. Annual change of primary resistance to clarithromycin among helicobacter pylori isolates from 1996 through 2008 in japan. Helicobacter. 2009;14:86–90. doi: 10.1111/j.1523-5378.2009.00714.x. [DOI] [PubMed] [Google Scholar]
- Hulten K, Gibreel A, Skold O, Engstrand L. Macrolide resistance in helicobacter pylori: Mechanism and stability in strains from clarithromycin-treated patients. Antimicrob Agents Chemother. 1997;41:2550–2553. doi: 10.1128/aac.41.11.2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KR, Cha J, Merrell DS. Who’s winning the war? Molecular mechanisms of antibiotic resistance in helicobacter pylori. Current Drug Therapy. 2008;3:190–203. doi: 10.2174/157488508785747899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohanteb J, Bazargani A, Saberi-Firoozi M, Mobasser A. Antimicrobial susceptibility testing of helicobacter pylori to selected agents by agar dilution method in shiraz-iran. Indian J Med Microbiol. 2007;25:374–377. doi: 10.4103/0255-0857.37342. [DOI] [PubMed] [Google Scholar]
- Leodolter A, Kulig M, Brasch H, Meyer-Sabellek W, Willich SN, Malfertheiner P. A meta-analysis comparing eradication, healing and relapse rates in patients with helicobacter pylori-associated gastric or duodenal ulcer. Aliment Pharmacol Ther. 2001;15:1949–1958. doi: 10.1046/j.1365-2036.2001.01109.x. [DOI] [PubMed] [Google Scholar]
- Malekzadeh R, Ansari R, Vahedi H, Siavoshi F, Alizadeh BZ, Eshraghian MR, Vakili A, Saghari M, Massarrat S. Furazolidone versus metronidazole in quadruple therapy for eradication of helicobacter pylori in duodenal ulcer disease. Aliment Pharmacol Ther. 2000;14:299–303. doi: 10.1046/j.1365-2036.2000.00709.x. [DOI] [PubMed] [Google Scholar]
- Malekzadeh R, Derakhshan MH, Malekzadeh Z. Gastric cancer in iran: Epidemiology and risk factors. Arch Iran Med. 2009;12:576–583. [PubMed] [Google Scholar]
- Malfertheiner P, Megraud F, O’Morain C, Bazzoli F, El-Omar E, Graham D, Hunt R, Rokkas T, Vakil N, Kuipers EJ. Current concepts in the management of helicobacter pylori infection: The maastricht iii consensus report. Gut. 2007;56:772–781. doi: 10.1136/gut.2006.101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie MAM. Patterns of helicobacter pylori resistance to metronidazole, clarithromycin and amoxicillin in saudi arabia. Journal of Bacteriology and Virology. 2008;38:173–178. [Google Scholar]
- Megraud F. H pylori antibiotic resistance: Prevalence, importance, and advances in testing. Gut. 2004;53:1374–1384. doi: 10.1136/gut.2003.022111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megraud F, Lehours P. Helicobacter pylori detection and antimicrobial susceptibility testing. Clin Microbiol Rev. 2007;20:280–322. doi: 10.1128/CMR.00033-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi M, Doroud D, Mohajerani N, Massarrat S. Helicobacter pylori antibiotic resistance in iran. World J Gastroenterol. 2005;11:6009–6013. doi: 10.3748/wjg.v11.i38.6009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor A, Gisbert JP, McNamara D, O’Morain C. Treatment of helicobacter pylori infection 2010. Helicobacter. 2010;15(Suppl 1):46–52. doi: 10.1111/j.1523-5378.2010.00774.x. [DOI] [PubMed] [Google Scholar]
- Osato MS, Reddy R, Reddy SG, Penland RL, Malaty HM, Graham DY. Pattern of primary resistance of helicobacter pylori to metronidazole or clarithromycin in the united states. Arch Intern Med. 2001;161:1217–1220. doi: 10.1001/archinte.161.9.1217. [DOI] [PubMed] [Google Scholar]
- Riahizadeh S, Malekzadeh R, Agah S, Zendehdel N, Sotoudehmanesh R, Ebrahimi-Dariani N, Pourshams A, Vahedi H, Mikaeli J, Khatibian M, Massarrat S. Sequential metronidazole-furazolidone or clarithromycin-furazolidone compared to clarithromycin-based quadruple regimens for the eradication of helicobacter pylori in peptic ulcer disease: A double-blind randomized controlled trial. Helicobacter. 2010;15:497–504. doi: 10.1111/j.1523-5378.2010.00798.x. [DOI] [PubMed] [Google Scholar]
- Saberi-Firoozi M, Nejabat M. Experiences with helicobacter pylori treatment in iran. Iran J Med Sci. 2006;31:181–185. [Google Scholar]
- Segura AM, Gutierrez O, Otero W, Angel A, Genta RM, Graham DY. Furazolidone, amoxycillin, bismuth triple therapy for helicobacter pylori infection. Aliment Pharmacol Ther. 1997;11:529–532. doi: 10.1046/j.1365-2036.1997.00172.x. [DOI] [PubMed] [Google Scholar]
- Siavoshi F, Safari F, Doratotaj D, Khatami GR, Fallahi GH, Mirnaseri MM. Antimicrobial resistance of helicobacter pylori isolates from iranian adults and children. Arch Iran Med. 2006;9:308–314. [PubMed] [Google Scholar]
- Siavoshi F, Saniee P, Latifi-Navid S, Massarrat S, Sheykholeslami A. Increase in resistance rates of H. pylori isolates to metronidazole and tetracycline--comparison of three 3-year studies. Arch Iran Med. 2010;13:177–187. [PubMed] [Google Scholar]
- Talebi Bezmin Abadi A, Mobarez AM, Taghvaei T, Wolfram L. Antibiotic resistance of helicobacter pylori in mazandaran, north of iran. Helicobacter. 2010;15:505–509. doi: 10.1111/j.1523-5378.2010.00795.x. [DOI] [PubMed] [Google Scholar]
- Talebi Bezmin Adabi A, Mohabati Mobarez A, Ajami AAGH, Ali Reza R, Taghvaei T. Evaluation on antibiotic resistance of helicobacter pylori isolated from patients admitted to tooba medical center, sari. Journal of Mazandaran University of Medical Sciences. 2009;19:26–32. [Google Scholar]
- Vakil N, Megraud F. Eradication therapy for helicobacter pylori. Gastroenterology. 2007;133:985–1001. doi: 10.1053/j.gastro.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Wolle K, Leodolter A, Malfertheiner P, Konig W. Antibiotic susceptibility of helicobacter pylori in germany: Stable primary resistance from 1995 to 2000. J Med Microbiol. 2002;51:705–709. doi: 10.1099/0022-1317-51-8-705. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.