Abstract
Objective
We investigated atheroprotective properties of apoE beyond its ability to lower plasma cholesterol. We hypothesized that apoE reduces atherosclerosis by decreasing lipid accumulation in circulating monocytes and the inflammatory state of monocytes and the vascular endothelium.
Methods and Results
We developed mice with spontaneous hyperlipidemia with and without plasma apoE: Hypomorphic apoE mice deficient in low-density lipoprotein receptor (Apoeh/hLdlr–/–) were compared to Apoe–/–Ldlr–/– mice. Despite 4-fold more plasma apoE than WT mice, Apoeh/hLdlr–/– mice displayed similar plasma cholesterol as Apoe–/–Ldlr–/– mice but developed 4-fold less atherosclerotic lesions by 5 months of age. The aortic arch of Apoeh/hLdlr–/– mice showed decreased endothelial expression of ICAM-1, PECAM-1, and JAM-A. In addition, Apoeh/hLdlr–/– mice had less circulating leukocytes and pro-inflammatory Ly6Chigh monocytes. These monocytes had decreased neutral lipid content and reduced surface expression of ICAM-1, VLA-4, and L-Selectin. Apoeh/hLdlr–/– mice displayed increased levels of apoA1-rich HDL that were potent in promoting cellular cholesterol efflux.
Conclusions
Our findings suggest that apoE reduces atherosclerosis in the setting of hyperlipidemia by increasing plasma apoA1-HDL that likely contribute to reduce intracellular lipid accumulation and thereby the activation of circulating leukocytes and the vascular endothelium.
Keywords: apolipoprotein E, atherosclerosis, monocytosis, HDL, apolipoprotein A1, intracellular lipid, flow cytometry, endothelial activation
As a ligand for the receptor-mediated clearance of remnant lipoproteins, apolipoprotein (apo) E is an important modulator of atherosclerosis 1. The best evidence is provided by the spontaneous hyperlipidemia and atherosclerosis in mice lacking apoE 2, 3. Beyond its participation in plasma cholesterol lowering, apoE is known to have anti-inflammatory properties 4-8. However, because of its ability to reduce plasma cholesterol, investigating mechanisms by which apoE regulates the progression of atherosclerosis in the setting of hyperlipidemia remains challenging. Several approaches that addressed this question succeeded by studying mice expressing low levels of plasma apoE (below the threshold required for plasma cholesterol lowering) derived from macrophages 9, 10 or the adrenal gland 8. However, unlike many of these murine models, human hyperlipidemia is accompanied by simultaneous accumulation of plasma apoE due to its high affinity for triglyceride-rich lipoproteins 11. Consequently, mechanisms by which high levels of plasma apoE could serve to reduce atherosclerosis in the setting of hyperlipidemia remain incompletely understood.
To address this issue, we developed mouse models of equal total plasma cholesterol with and without accumulation of plasma apoE. This was achieved by crossing Ldlr–/– mice with hypomorphic apoE mice (Apoeh/h) 12, and Apoe–/– mice to derive Apoeh/h Ldlr–/– and Apoe–/– Ldlr–/– mice. We studied these mice to investigate anti-inflammatory properties of apoE on circulating monocytes and the vascular endothelium and its overall effect on atherosclerosis progression.
Previous observations demonstrated that apoE reduced the expression of endothelial adhesion molecules responsible for the recruitment of circulating monocytes to athero-prone regions of the vasculature 7, 13, 14. In addition, recent evidence suggests that circulating monocytes, can be activated by intracellular lipid accumulation prior to their recruitment to athero-prone regions of the vasculature 15-17. Whether elevated levels of apoE in hyperlipidemic plasma impact on intracellular lipid levels and thereby the inflammatory state of circulating monocytes remains unknown. Thus, we hypothesized that apoE can reduce lipid accumulation in circulating monocytes and the inflammatory state of monocytes and the vascular endothelium and thereby decrease atherosclerosis progression independently of its ability to lower plasma cholesterol. Results of our study highlight novel properties of apoE on the inflammatory state of monocytes and the endothelium in hyperlipidemic mice.
Methods
Briefly, Ldlr–/– mice on a C57Bl/6J background (Jackson Laboratories, ME) were bred to hypomorphic apoE mice18 and to Apoe–/– mice on a C57Bl/6J background (Jackson Laboratories, ME) to create Apoeh/hLdlr–/– and Apoe–/–Ldlr–/– mice. Apoeh/hLdlr–/– and Apoe–/–Ldlr–/– mice were randomly intercrossed to establish lineages of littermate mice that contained approximately 85% of C57BL/6 and 15% of 129/SvJ genetic backgrounds. The San Francisco Veterans Administration Medical Center committee for animal care and welfare approved all procedures. All procedures including blood and tissue collection, plasma lipid and lipoprotein fractionation/isolation with fast protein liquid chromatography (FPLC) and sequential density ultracentrifugation, colorimetric assays used to measure plasma lipid levels, SDS-polyacrylamide gel electrophoresis (SDS-PAGE), Western blot, in vitro cholesterol efflux assay, histological and immunofluorescence quantification of atherosclerotic lesions and blood leukocyte analysis by flow cytometry were either performed as described previously12, 19, 20 or as described in the online data supplement: http://atvb.ahajournals.org.
Results
ApoE reduces atherosclerosis beyond lowering plasma cholesterol levels
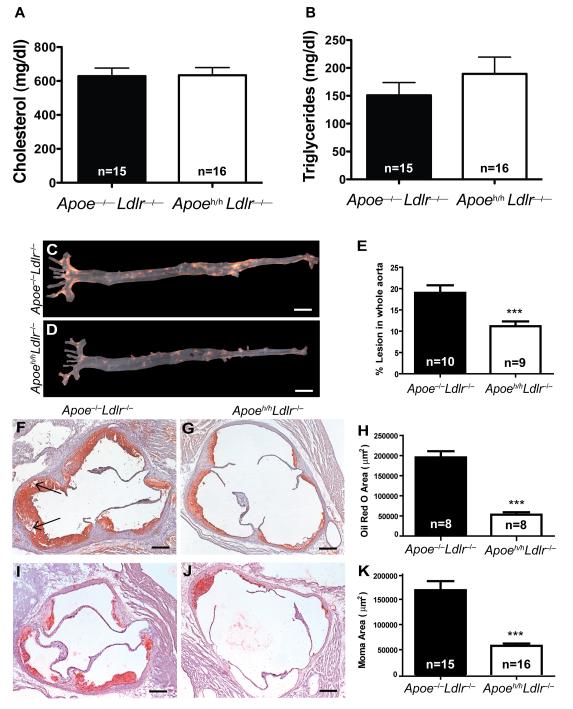
We previously described the Apoeh/h mouse in which a variant form of murine apoE 19 is expressed at 2-5% of normal levels 20. By breeding Apoeh/h and Apoe–/– mice to Ldlr–/– mice, we generated mice that develop spontaneous hyperlipidemia and atherosclerosis. Interestingly, Apoeh/hLdlr–/– mice do so despite accumulating 4-fold more plasma apoE than wild type (WT) mice (Figure I). When fed a chow diet containing 5.7% fat, Apoe–/–Ldlr–/– mice displayed slightly higher plasma cholesterol level (n=10; 584.9-1062mg/dl) than Apoeh/hLdlr–/– mice (n=24; 427.8-854.5mg/dl). However, feeding Apoe–/–Ldlr–/– and Apoeh/hLdlr–/– mice a chow diet of identical nutrient composition but containing 4.2% fat and 9% fat, respectively, brought their plasma cholesterol levels to a similar range. At 14-weeks of age (Figure IIA&B), both Apoeh/hLdlr–/– and Apoe–/–Ldlr–/– mice displayed similar levels of hypercholesterolemia and hypertryglyceridemia. By 20-weeks of age plasma cholesterol levels of Apoeh/hLdlr–/– mice reached 634.1±45.23mg/dl that closely matched those of Apoe–/–Ldlr–/– mice (628.9±47.48mg/dl; Figure 1A). Both groups of mice displayed similar plasma triglycerides levels (Apoeh/hLdlr–/– mice 189.4±30.08mg/dl, n=14; Apoe–/–Ldlr–/– mice 151.0±22.82mg/dl, n=16; (Figure 1B), body weight and blood glucose levels (results not shown).
Figure 1. Atherosclerosis burden.
Plasma cholesterol (A) and triglyceride (B) levels from fasted 20- week old Apoe–/–Ldlr–/– and Apoeh/hLdlr–/– mice. Representative Sudan IV staining of aorta (C,D&E, scale bar=5mm). Adjacent histological sections of aortic roots stained with oil red O (F,G&H, arrows=cholesterol crystals) and moma-2 (I,J&K, scale bar=200μm), mean ± sem, ***p<0.001.
The development of two mouse models with similar hypercholesterolemia and hypertriglyceridemia in presence and absence of elevated apoE levels enabled us to investigate how apoE can suppress atherosclerosis beyond lowering plasma cholesterol. Twenty-week old Apoeh/hLdlr–/– mice developed 1.7-fold less aortic sudanophilic positive lesions than Apoe–/–Ldlr–/– mice (Figure 1C-E). In addition, Apoeh/hLdlr–/– mice developed 4-fold less aortic root oil red O positive lesions than Apoe–/–Ldlr–/– mice (53,214±17,102μm2 versus 195,419±44,000μm2; Figure 1F-H). Beyond being more prominent, lesions of Apoe–/–Ldlr–/– mice also displayed evidence of necrotic cores containing crystallized cholesterol clefts (black arrows, Figure 1F). As shown in Figure 1I-K, aortic root lesions of Apoeh/hLdlr–/– mice also contained 3-fold less macrophage-positive lesion area than Apoe–/–Ldlr–/– mice (57,619±17,369μm2 versus 168,307±69,673 μm2). Taken together these results demonstrate that apoE reduces atherosclerosis progression independently of lowering plasma cholesterol.
ApoE reduces endothelial activation in the setting of hyperlipidemia
ApoE may contribute to reduce macrophage foam cell accumulation by limiting monocyte recruitment to the vascular wall. Thus, we investigated whether apoE reduces atherosclerosis progression in the setting of hyperlipidemia by reducing the expression of adhesion molecules on the endothelium. To test this hypothesis we measured the expression of Vascular Cell Adhesion Molecule-1 (VCAM-1), Intercellular Cell Adhesion Molecule-1 (ICAM-1), Platelet Endothelial Cell Adhesion-1 (PECAM-1) and Junctional Adhesion Molecule-A (JAM-A) on enface preparations of the proximal aorta of 14-week old Apoeh/h Ldlr–/– and Apoe–/– Ldlr–/– mice (see Figure VI for detailed procedures). We first observed heterogeneous expression of VCAM-1 and ICAM-1 on individual endothelial cells (EC) in both inner and outer curvatures of the aortic arch (Figure 2A&C). VCAM-1, ICAM-1 and JAM-A were expressed on the surface of ECs while PECAM-1, as expected, localized to endothelial cell-cell junctions (Figure 2A,C,E&G). Unexpectedly, we also observed PECAM-1 redistributing from the cell-cell junctions to the cell surface of EC in aortic arches of Apoe–/–Ldlr–/– mice (Figure 2E).
Figure 2. Endothelial inflammation.
Confocal images (8μm thick) of enface aortic arch (A,C,E,G) labeled with anti-VE-cadherin (green) and Hoechst (blue). Antibodies targeting VCAM-1 (A), ICAM-1 (C), PECAM-1 (E) or JAM-A (G) are shown in red (scale bar = 20μm). MFI per ECs (from 3 mice each; B,D,F&H). Mean ± sem, ****p<0.0001, one-way ANOVA. See Fig.IV for extra details on the procedure.
The quantification of the mean fluorescence intensity (MFI) for each inflammatory marker (Figure IV) revealed significantly lower expression levels of ICAM-1 (1.28-fold), PECAM-1 (4.5-fold) and JAM-A (11-fold) in Apoeh/hLdlr–/– mice than in Apoe–/–Ldlr–/– mice (Figure 2D,F&H). However, no significant difference in the expression level of VCAM-1 was observed between Apoe–/–Ldlr–/– and Apoeh/hLdlr–/– mice (Figure 2B). Taken together, our results suggest that plasma apoE contributes to the decrease expression of ICAM-1, PECAM-1 and JAM-A on vascular endothelium in the setting of hyperlipidemia.
ApoE reduces circulating leukocyte counts and monocyte activation
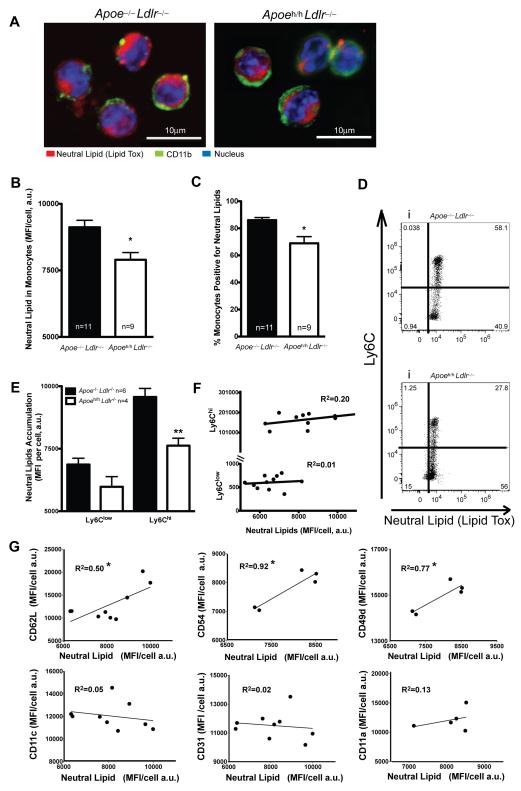
A lower count or activation of leukocytes and/or monocytes could also reduce monocyte recruitment to atheroma. To determine the effect of apoE on leukocytes, we analyzed with flow cytometry blood leukocyte count in our mouse models. Despite similar total plasma cholesterol, Apoeh/h Ldlr–/– mice displayed 30% less blood leukocytes than Apoe–/–Ldlr–/– mice (Figure 3A). This decrease in blood leukocytes arose from reduced numbers of monocytes, granulocytes and B cell, but not T cells in Apoeh/hLdlr–/– mice (Figure 3B). We also investigated potential differences in monocyte subtypes (defined by Ly6C expression; Figure 3C). Quantification of each monocyte subpopulation revealed 2-fold less Ly6Chigh monocytes in Apoeh/hLdlr–/– mice compared to Apoe–/–Ldlr–/– mice (Figure 3D).
Figure 3. Circulating leukocyte counts and monocyte activation.
Leukocyte counts in Apoeh/hLdlr–/– mice and Apoe–/–Ldlr–/– mice (A&B). Gating strategy (C) and monocyte Ly6C subtypes counts (two-way ANOVA and post-test, D). Monocyte expression level of CD62L, CD54, CD49d, CD11c, CD31 and CD11a, (E). Mean ± sem, *p<0.05.
We next measured the expression of cell surface markers of inflammation on circulating monocytes of both mouse models. CD54, CD49d and CD11a were present on the cell surface of most monocytes (~100%) in both groups of mice while CD62L, CD11c and CD31 were present on less than 30% of all monocytes. In addition, we observed a significant decrease in the level of expression of CD62L (32% less), CD54 (8% less) and CD49d (17% less) in Apoeh/hLdlr–/– monocytes (Figure 3E) compared to Apoe–/–Ldlr–/– mice. Taken together, these results demonstrate for the first time that plasma apoE accumulation contributes to reduce the number of circulating leukocytes, the expansion of Ly6Chigh monocytes, and the overall expression of key adhesion molecules on the surface of monocytes.
ApoE decreases lipid accumulation in circulating monocytes
In our mouse models neither a high fat diet nor a difference in plasma lipid levels could explain the reduced count in total leukocytes and Ly6Chigh monocytes and reduced surface expression of adhesion molecules on monocytes. Thus we hypothesized that plasma apoE reduces intracellular lipid accumulation. Confocal microscopy and flow cytometry analysis of isolated monocytes revealed less intracellular neutral lipid accumulation in Apoeh/hLdlr–/– monocytes than in Apoe–/– Ldlr–/– monocytes (Figure 4A&B). On average, a 16% decrease in neutral lipid accumulation per monocyte was observed in Apoeh/hLdlr–/– mice compared to Apoe–/–Ldlr–/– mice (Figure 4B). In addition, 17% fewer monocytes derived from Apoeh/hLdlr–/– mice accumulated detectable levels of neutral lipids (Figure 4C). A decrease in neutral lipid accumulation in inflammatory Ly6Chigh (20%) monocyte subtypes from Apoeh/hLdlr–/– mice was also observed (Figure 4E).
Figure 4. Lipid accumulation in monocytes.
Confocal images of monocytes isolated by FACS and labeled with LipidTox (red), FITC-anti-CD11b (green), and Hoechst (blue), scale bar =10μm; A). Neutral lipid accumulation in monocytes (B&C), Ly6Chigh and Ly6Clow subtypes (one-way ANOVA; D&E). Correlations between Lipid Tox and surface marker MFI (F&G). R2=R square; mean ± sem, *p<0.05, **p<0.01.
To establish a link between neutral lipid accumulation and an increase in inflammatory monocytes, we correlated the level of intracellular lipid to the surface expression of inflammatory markers. No significant correlations were observed between the expression level of Ly6C and intracellular neutral lipid accumulation (Figure 4F). In contrast, the surface expression of inflammatory markers such as CD62L, CD54 and CD49d positively correlated with neutral lipid accumulation in circulating monocytes. Increased neutral lipid levels correlated with a 50%, 92% and 77% increased expression of CD62L, CD54 and CD49d, respectively (Figure 4G). By comparison, the expression levels of CD11c, CD31 and CD11a, which showed no difference between Apoeh/hLdlr–/– mice and Apoe–/–Ldlr–/– mice monocytes, did not correlate with intracellular neutral lipid levels. Taken together these results demonstrate that plasma apoE decreases intracellular neutral lipid levels and thereby reduces the overall inflammatory phenotype of monocytes in Apoeh/hLdlr–/– mice.
ApoE modulates plasma lipoprotein composition
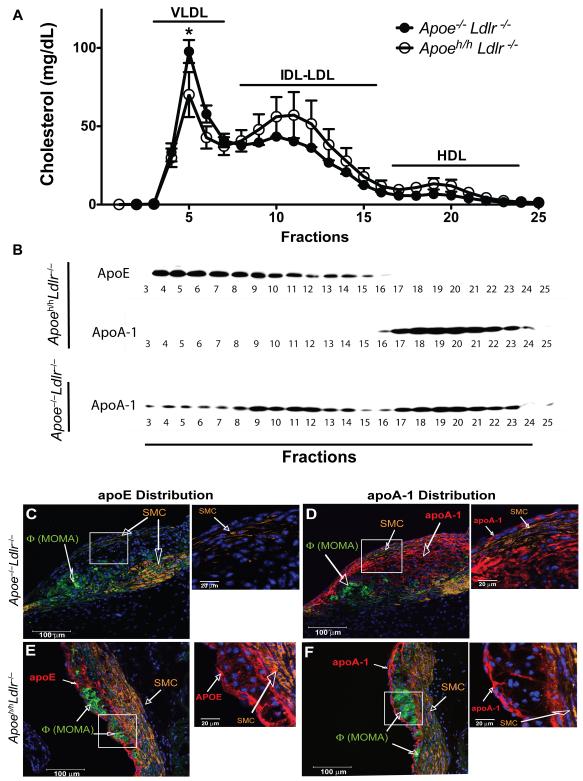
Next, we sought to investigate the potential effects of apoE on plasma lipoprotein composition. We first observed that apoE accumulation in plasma of Apoeh/hLdlr–/– mice modulated the lipoprotein cholesterol profile. The presence of apoE significantly reduced VLDL-cholesterol by 1.4-fold in Apoeh/hLdlr–/– mice (Figure 5A). In addition, Western blot analysis of plasma fractionated by FPLC (Figure 5B) revealed that in Apoeh/hLdlr–/– mice, apoE associates predominantly with VLDL, IDL and LDL. In contrast, in the absence of apoE, apoA-1 distributed amongst all lipoproteins classes (Figure 5B). To confirm these results, plasma lipoproteins were fractionated by sequential density ultracentrifugation and analyzed for their lipid and protein content. With this method, we found less VLDL-cholesterol (1.6-fold) but more LDL-(1.7-fold) and HDL-cholesterol (2-fold) content in Apoeh/hLdlr–/– mouse plasma than in Apoe–/–Ldlr–/– mouse plasma. Lastly, the presence of apoE increased significantly the protein content (1.6-fold) of HDL isolated from Apoeh/hLdlr–/– mice compared to that isolated from Apoe–/–Ldlr–/– mice (Table I).
Figure 5. Lipoproteins and atheroma composition.
Lipoprotein cholesterol distribution (A) and Western blot of fractionated plasma (n=3, B). Adjacent confocal images (2.6mm thick) of aortic root cross-sections labeled with anti-apoE (C&E) and anti-apoA1 (D&F) in red, anti-smooth muscle cell α- actin (orange), anti-moma-2 (green) and Hoechst (blue), insets=without moma-2.
These observations led us to question the potential local influence of apoE on the composition of atheroma. Results of our studies revealed the accumulation of apoE in aortic root lesions of Apoeh/hLdlr–/– mice (Figure 5C&E). ApoE immuno-reactivity was detected within ECs and macrophage cytoplasms but accumulated more prominently along the extracellular matrix surrounding macrophages and near the elastic lamina (Figure 5E, inset). ApoA1 also accumulated in lesions of both groups of mice but to a greater extent in lesions of Apoe–/–Ldlr–/– mice (Figure 5D&F). More specifically, apoA1 immunoreactivity localized mostly to ECs and extracellular matrix surrounding macrophages (Figure 5D&F, insets). Lastly, smooth muscle α-actin immunoreactivity was found only in the intimae of Apoe–/–Ldlr–/– mice, revealing the appearance of fibrous caps in the lesions, indicative of a more advanced lesional stage (Figure 5C&D, insets).
Enhanced cholesterol efflux capacity of apoA1-rich HDL
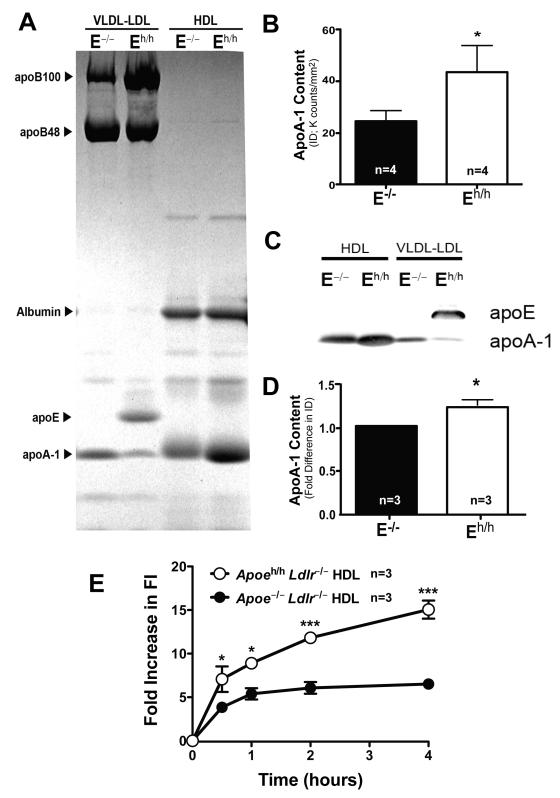
Finally, we compared the composition and cholesterol efflux capacity of plasma HDL isolated from Apoe–/–Ldlr–/– and Apoeh/hLdlr–/– mice by sequential density ultracentrifugation. Consistent with our findings with FPLC fractionated plasma (Figure 5B) we observed that the presence of apoE on the VLDL and LDL fractions of Apoeh/hLdlr–/– mice contributed to the redistribution of apoA1 to HDL (Figure 6A&C). We also observed a 1.5 fold enrichment of apoA-1 on HDL of Apoeh/hLdlr–/– mice compared to that of Apoe–/–Ldlr–/– mouse HDL (Figure 6B&D). Next, we performed cholesterol efflux experiments with the J7741 mouse macrophage cell line. When normalized for total protein content, HDL isolated from Apoeh/hLdlr–/– mouse plasma were 2.3-fold more potent at promoting cholesterol efflux than HDL isolated from Apoe–/–Ldlr–/– mouse plasma (Figure 6E). The low expression levels of apoE by macrophages of Apoeh/hLdlr–/– mice also contributed to enhance cholesterol efflux to HDL although to a lower extent. Less than a 5 fold increase in fluorescence intensity (FI) accumulated in media of freshly isolated peritoneal macrophages of Apoeh/hLdlr–/– mouse incubated with HDL from Apoe–/–Ldlr–/– mice (Fig.VIII) compared to 15 fold increase in FI accumulated in media of J7741 macrophages incubated with HDL from Apoeh/hLdlr–/– mice (Fig.6). Taken together, these results indicate that plasma apoE increases HDL-cholesterol and enriches HDL in apoA-1, which in turn enhances their potency to promote cellular cholesterol efflux. Thus, apoE-dependent enhancement of cholesterol efflux may at least partly explain the reduced accumulation of lipid within circulating monocytes and lesional macrophages in Apoeh/hLdlr–/– mice.
Figure 6. ApoA1-rich HDL.
Lipoproteins prepared by ultracentrifugation resolved by SDS-PAGE (coomassie blue: A; apoA-1 Integrated Density (ID) quantification: B) and blotted for apoE and apoA-1 (C&D). Fluorescence Intensity (FI) accumulation in media of J774.1 macrophages loaded with NBD-cholesterol and incubated with HDL (50μg protein/ml) from Apoeh/hLdlr–/– and Apoe–/–Ldlr–/– mice for 0.5, 1, 2 and 4 hours (E), mean ± sem, *p<0.05, ***p<0.001.
Discussion
We sought to uncover mechanisms by which apoE can suppress atherosclerosis beyond reducing plasma cholesterol. To this end, we developed a mouse model in which apoE accumulates in the setting of hyperlipidemia. With this model, we showed that plasma apoE contributes to decrease macrophage content in athero-prone regions of the vasculature by reducing lipid accumulation in circulating monocytes and the inflammatory state of both monocytes and the endothelium. Our findings suggest that apoE reduces lipid accumulation in circulating monocytes in part by raising levels of apoA1-rich HDL in plasma.
We generated two mouse models with equal total plasma cholesterol in the presence and absence of plasma apoE accumulation. Thus, these mouse models provided a new way to investigate atheroprotective properties of apoE beyond its ability to lower plasma cholesterol. Interestingly, despite having fourfold more plasma apoE than WT mice, Apoeh/hLdlr–/– mice remained hyperlipidemic when fed a chow diet, suggesting that alternative pathways of remnant lipoprotein clearance were defective in these mice (Figure I). Previous studies have shown that, in the absence of LDL receptor expression, hepatic production of apoE is required for the clearance of remnant lipoproteins by the LDL receptor-related protein (LRP) 21. Thus, the low hepatic expression of apoE in Apoeh/hLdlr–/– mice may explain the decrease in remnant lipoprotein clearance and plasma accumulation of apoE bound to lipoproteins. Nevertheless, as shown in Table 1 and Fig. 5, plasma apoE in Apoeh/hLdlr–/– mice contributed in some way to either increase VLDL clearance or its conversion to LDL cholesterol.
The elevated plasma apoE levels in the setting of hyperlipidemia reduced atherosclerosis in Apoeh/hLdlr–/– mice. One-way by which apoE could reduce macrophage foam cell accumulation within atherosclerotic lesions is by limiting monocyte recruitment. Accordingly, we demonstrated that the presence of apoE in hyperlipidemic mice decreased the expression of inflammatory molecules on endothelial cells (ECs) and monocytes that are known to enhance monocyte recruitment to athero-prone regions of the vasculature 22.
Evidence that apoE suppressed endothelial activation comes from reduced cell surface levels of ICAM-1, JAM-A and PECAM-1 on the endothelium of the proximal aorta of Apoeh/hLdlr–/– mice. Elevated endothelial expressions of such adhesion molecules are known to be pro-atherogenic 14, 23-25 and to mediate the recruitment of monocytes 22 to athero-prone regions of the vasculature including the inner curvature of the aortic arch 26. To our knowledge, this is the first report documenting a suppressive effect of apoE on the endothelial expression of JAM-A and PECAM-1. Moreover, in the presence of apoE, we found reduced expression of ICAM-1 but unchanged expression of VCAM-1 unlike results of previous studies 7, 13, 27.
In vitro studies have shown that apoE attenuates cytokine-induced expression of adhesion molecules including VCAM-1 and ICAM-1 by stimulating the endothelial production of nitric oxide 7, 27. More recently, Ma et al. showed a robust reduction of VCAM-1 and ICAM-1 gene expression in the whole aorta of hyperlipidemic mice expressing sub-physiological levels of plasma apoE 8. However, because of other cell types present in an intact aortic arch such as macrophages and smooth muscle cells, which also express VCAM-1 and ICAM-1 28, 29, it is difficult to attribute this decreased expression solely to the endothelium. In fact, it is possible that a decrease in macrophages and smooth muscle cells could have contributed to the overall decrease in VCAM-1 and ICAM-1 expression. Alternatively, in our model, the lack of attenuation of VCAM-1 expression in the endothelium of the aortic arch of Apoeh/hLdlr–/– mice may have been masked or diluted by the high degree of heterogeneity in the expression of VCAM-1 amongst ECs (Figure 2A). Accordingly, the analysis of VCAM-1 expression levels on individual ECs of the proximal aorta exposed two populations of ECs; one with higher and one with lower expression levels of VCAM-1. Additionally, this analysis revealed a lower expression level of VCAM-1 among the population of ECs expressing higher levels of VCAM-1 in Apoeh/hLdlr–/– compared to those of Apoe–/–Ldlr–/– mice (data not shown).
Hyperlipidemia and atherosclerosis have been associated with leukocytosis 30 and more recently with the increased recruitment of monocytes to athero-prone regions of the vasculature 17, 31, 32. Studies also demonstrated that an increase in cell survival and proliferation are responsible for hyperlipidemia-induced leukocytosis and monocytosis 16, 17, 33. A major finding of our study is that elevated plasma apoE levels contribute to reduce leukocyte counts in hyperlipidemic mice. Moreover, the presence of apoE was associated with a reduction in Ly-6Chigh inflammatory monocytes known to be recruited to atherosclerotic lesions 17, 32.
Enhanced expression of cell adhesion molecules on circulating monocytes contributes to atherosclerosis progression 22. Interestingly, we found that elevated apoE levels in hyperlipidemic plasma decreases the cell surface expression of some known inflammatory molecules 17, 34 but not that of others 16 on circulating monocytes. Studies of Swirski et al. have previously shown that Ly6Chigh monocytes consistently express CD62L but not CD11c 17. Accordingly, we observed that in addition to reducing levels of Ly6Chigh monocyte levels, apoE also reduced the cell surface expression of CD62L but not that of CD11c in circulating monocytes. We also found that apoE decreases the surface expression of CD49d, a key integrin involved in the arrest and adhesion of monocytes on inflamed endothelium of atherosclerotic lesions 22, 34. High cell surface expression of CD54, CD11a and CD31 on monocytes has also been associated with increased cell adhesion and atherosclerosis 22; however, in our hyperlipidemic mouse models, apoE solely decreased the expression of CD54. It is unclear why the presence of apoE reduces the expression of only a select set of adhesion molecules. Nevertheless, this observation constitutes a novel athero-protective property by which plasma apoE suppresses atherosclerosis.
A new concept has emerged demonstrating a direct association between cellular lipid loading and the activation of circulating monocytes 15-17, 35. Thus, we propose that elevated plasma apoE levels prevent lipid–induced activation of circulating monocytes in hyperlipidemic mice. Evidence for a role of apoE in this process comes from the lower neutral lipid content in circulating monocytes of Apoeh/hLdlr–/– mice. Additionally, we observed a striking correlation between the level of intracellular neutral lipid and the expression levels of adhesion molecules on circulating monocytes. Cellular lipid accumulation can results from at least three different dysfunctional mechanisms; i) the synthesis of lipids, ii) the uptake of lipoproteins, and iii) the mediation of cholesterol efflux by HDL. ApoE could modulate each one of these processes.
While examining plasma lipoproteins in both mouse models we observed an important apoE-mediated modulation of plasma lipoprotein composition. ApoA1, which distributed equally amongst all classes of lipoproteins in Apoe–/–Ldlr–/– mice, distributed almost exclusively to HDL in Apoeh/hLdlr–/– mice. As apoE bound preferentially to VLDL and LDL in Apoeh/hLdlr–/– mice, it likely displaced apoA1 from these particles, concentrating it onto HDL. Thus, the presence of apoE likely raised HDL cholesterol and apoA1 levels in the plasma of Apoeh/hLdlr–/– mice. Accordingly, HDL derived from Apoeh/hLdlr–/– mice displayed an enhanced ability to promote cellular cholesterol efflux. We also attempted to dissect the direct cellular contribution of apoE from that of its indirect influence on plasma lipoprotein. At first, we assessed the expression level of genes related to cellular lipid metabolism that could potentially be regulated by apoE in isolated macrophages and monocytes (Fig.VII). The effect of endogenous apoE on the expression level of relevant genes was minimal. We also performed cholesterol efflux experiments with peritoneal macrophages isolated from both Apoeh/hLdlr–/– and Apoe–/–Ldlr–/– mice. Although we cannot rule out the possibility that macrophage-derived apoE, even at low expression level, also contributed to enhance cholesterol efflux in Apoeh/hLdlr–/– mice (Fig.VIII), our results suggest that the apoA1-rich HDL had a greater impact on total cholesterol efflux. In light of previous reports documenting the importance of apoA1 in HDL-mediated suppression of leukocytosis and monocyte activation 33, 35, apoA1-rich HDL likely contributed to reduce leukocyte counts and the inflammatory state of monocytes observed in Apoeh/hLdlr–/– mice.
Because of the pleiotropic nature of plasma HDL, we cannot rule out the possibility that higher levels of plasma HDL also contributed to reduce atherosclerosis locally in the arterial wall of Apoeh/hLdlr–/– mice by suppressing endothelial activation and foam cell formation. Histological studies of atheromas by confocal microscopy revealed the presence of apoE in intracellular compartments and on the surface of macrophage foam cells. ApoA1 was detected in atheroma of both mouse models. However, as apoA1 resided primarily on HDL in Apoeh/hLdlr–/– mice, lesional apoA1 in atheromas of these mice likely contributed to the efflux of cholesterol from foam cells. In contrast, in Apoe–/–Ldlr–/– mice, lesional apoA1 resided primarily on pro-atherogenic apoB-containing lipoproteins. These particles were likely less effective at promoting cellular cholesterol efflux and thereby reverse cholesterol transport. Another interesting finding relates to reduced presence of fibrous caps and intimal smooth muscle cells in atheromas of Apoeh/hLdlr–/– mice (Figure 5). These results confirm and extend findings of earlier studies that reported a role for apoE in regulating the migration and proliferation of smooth muscle cells 36.
In conclusion, key results of our study highlight apoE’s capacity to reduce lipid-induced leukocyte counts and the inflammatory state of both monocytes and the vascular endothelium. We propose that these new roles of apoE derive in part from its ability to increase apoA1-rich HDL in plasma. Such HDL likely contributed to reduce lipid accumulation in monocytes and thereby their inflammatory state. Thus, these results provide new mechanistic insights to explain how apoE participates to reduce atherosclerosis beyond lowering plasma cholesterol levels.
Supplementary Material
Acknowledgements
We thank Drs. Karl Weisgraber and Michael Conte for critical review of the manuscript and Andrew C. Birkeland for technical assistance with our mouse colonies.
Sources of Funding This work was supported by Grants from the American Heart Association Western States affiliate (0565117Y), the National Institutes of Health HL089871, and a Merit Review grant from the Department of Veterans Affairs 5I01BX000532 to RLR, which were administered by the Northern California Institute for Research and Education, and with resources of the Veterans Affairs Medical Center, San Francisco, California. This work was also supported by the Foundation for Accelerated Vascular Research, and funds from the Department of Surgery at UCSF to RLR.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mahley RW, Rall SC. Apolipoprotein e: Far more than a lipid transport protein. Annu Rev Genomics Hum Genet. 2000;1:507–537. doi: 10.1146/annurev.genom.1.1.507. [DOI] [PubMed] [Google Scholar]
- 2.Plump AS, Smith JD, Hayek T, Aalto-Setälä K, Walsh A, Verstuyft JG, Rubin EM, Breslow JL. Severe hypercholesterolemia and atherosclerosis in apolipoprotein e-deficient mice created by homologous recombination in es cells. Cell. 1992;71:343–353. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- 3.Zhang SH, Reddick RL, Piedrahita JA, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein e. Science (New York, NY) 1992;258:468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- 4.Ali K, Middleton M, Puré E, Rader DJ. Apolipoprotein e suppresses the type i inflammatory response in vivo. Circ Res. 2005;97:922–927. doi: 10.1161/01.RES.0000187467.67684.43. [DOI] [PubMed] [Google Scholar]
- 5.Curtiss LK. Apoe in atherosclerosis : A protein with multiple hats. Arteriosclerosis, Thrombosis, and Vascular Biology. 2000;20:1852–1853. doi: 10.1161/01.atv.20.8.1852. [DOI] [PubMed] [Google Scholar]
- 6.Davignon J. Apolipoprotein e and atherosclerosis: Beyond lipid effect. Arteriosclerosis, Thrombosis, and Vascular Biology. 2005;25:267–269. doi: 10.1161/01.ATV.0000154570.50696.2c. [DOI] [PubMed] [Google Scholar]
- 7.Stannard AK, Riddell DR, Sacre SM, Tagalakis AD, Langer C, von Eckardstein A, Cullen P, Athanasopoulos T, Dickson G, Owen JS. Cell-derived apolipoprotein e (apoe) particles inhibit vascular cell adhesion molecule-1 (vcam-1) expression in human endothelial cells. J Biol Chem. 2001;276:46011–46016. doi: 10.1074/jbc.M104812200. [DOI] [PubMed] [Google Scholar]
- 8.Thorngate FE, Rudel LL, Walzem RL, Williams DL. Low levels of extrahepatic nonmacrophage apoe inhibit atherosclerosis without correcting hypercholesterolemia in apoe-deficient mice. Arteriosclerosis, Thrombosis, and Vascular Biology. 2000;20:1939–1945. doi: 10.1161/01.atv.20.8.1939. [DOI] [PubMed] [Google Scholar]
- 9.Fazio S, Babaev VR, Murray AB, Hasty AH, Carter KJ, Gleaves LA, Atkinson JB, Linton MF. Increased atherosclerosis in mice reconstituted with apolipoprotein e null macrophages. Proc Natl Acad Sci USA. 1997;94:4647–4652. doi: 10.1073/pnas.94.9.4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bellosta S, Mahley RW, Sanan DA, Murata J, Newland DL, Taylor JM, Pitas RE. Macrophage-specific expression of human apolipoprotein e reduces atherosclerosis in hypercholesterolemic apolipoprotein e-null mice. J Clin Invest. 1995;96:2170–2179. doi: 10.1172/JCI118271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahley RW. Apolipoprotein e: Cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- 12.Raffai RL, Weisgraber KH. Hypomorphic apolipoprotein e mice: A new model of conditional gene repair to examine apolipoprotein e-mediated metabolism. J Biol Chem. 2002;277:11064–11068. doi: 10.1074/jbc.M111222200. [DOI] [PubMed] [Google Scholar]
- 13.Ma Y, Malbon CC, Williams DL, Thorngate FE. Altered gene expression in early atherosclerosis is blocked by low level apolipoprotein e. PLoS ONE. 2008;3:e2503. doi: 10.1371/journal.pone.0002503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakashima Y, Raines EW, Plump AS, Breslow JL, Ross R. Upregulation of vcam-1 and icam-1 at atherosclerosis-prone sites on the endothelium in the apoe-deficient mouse. Arterioscler Thromb Vasc Biol. 1998;18:842–851. doi: 10.1161/01.atv.18.5.842. [DOI] [PubMed] [Google Scholar]
- 15.den Hartigh LJ, Connolly-Rohrbach JE, Fore S, Huser TR, Rutledge JC. Fatty acids from very low-density lipoprotein lipolysis products induce lipid droplet accumulation in human monocytes. J Immunol. 2010;184:3927–3936. doi: 10.4049/jimmunol.0903475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu H, Gower RM, Wang H, Perrard X-YD, Ma R, Bullard DC, Burns AR, Paul A, Smith CW, Simon SI, Ballantyne CM. Functional role of cd11c+ monocytes in atherogenesis associated with hypercholesterolemia. Circulation. 2009;119:2708–2717. doi: 10.1161/CIRCULATIONAHA.108.823740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raffaï RL, Hasty AH, Wang Y, Mettler SE, Sanan DA, Linton MF, Fazio S, Weisgraber KH. Hepatocyte-derived apoe is more effective than non-hepatocyte-derived apoe in remnant lipoprotein clearance. J Biol Chem. 2003;278:11670–11675. doi: 10.1074/jbc.M212873200. [DOI] [PubMed] [Google Scholar]
- 19.Raffai RL, Dong LM, Farese RV, Weisgraber KH. Introduction of human apolipoprotein e4 "domain interaction" into mouse apolipoprotein e. Proc Natl Acad Sci USA. 2001;98:11587–11591. doi: 10.1073/pnas.201279298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raffai RL, Loeb SM, Weisgraber KH. Apolipoprotein e promotes the regression of atherosclerosis independently of lowering plasma cholesterol levels. Arteriosclerosis, Thrombosis, and Vascular Biology. 2005;25:436–441. doi: 10.1161/01.ATV.0000152613.83243.12. [DOI] [PubMed] [Google Scholar]
- 21.Linton MF, Hasty AH, Babaev VR, Fazio S. Hepatic apo e expression is required for remnant lipoprotein clearance in the absence of the low density lipoprotein receptor. J Clin Invest. 1998;101:1726–1736. doi: 10.1172/JCI2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mestas J, Ley K. Monocyte-endothelial cell interactions in the development of atherosclerosis. Trends in Cardiovascular Medicine. 2008;18:228–232. doi: 10.1016/j.tcm.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goel R, Schrank BR, Arora S, Boylan B, Fleming B, Miura H, Newman PJ, Molthen RC, Newman DK. Site-specific effects of pecam-1 on atherosclerosis in ldl receptor-deficient mice. Arteriosclerosis, Thrombosis, and Vascular Biology. 2008;28:1996–2002. doi: 10.1161/ATVBAHA.108.172270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zernecke A, Liehn EA, Fraemohs L, von Hundelshausen P, Koenen RR, Corada M, Dejana E, Weber C. Importance of junctional adhesion molecule-a for neointimal lesion formation and infiltration in atherosclerosis-prone mice. Arteriosclerosis, Thrombosis, and Vascular Biology. 2006;26:e10–13. doi: 10.1161/01.ATV.0000197852.24529.4f. [DOI] [PubMed] [Google Scholar]
- 25.Hajra L, Evans AI, Chen M, Hyduk SJ, Collins T, Cybulsky MI. The nf-kappa b signal transduction pathway in aortic endothelial cells is primed for activation in regions predisposed to atherosclerotic lesion formation. Proc Natl Acad Sci USA. 2000;97:9052–9057. doi: 10.1073/pnas.97.16.9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiu J-J, Chien S. Effects of disturbed flow on vascular endothelium: Pathophysiological basis and clinical perspectives. Physiological Reviews. 2011;91:327–387. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mullick AE, Powers AF, Kota RS, Tetali SD, Eiserich JP, Rutledge JC. Apolipoprotein e3- and nitric oxide-dependent modulation of endothelial cell inflammatory responses. Arteriosclerosis, Thrombosis, and Vascular Biology. 2007;27:339–345. doi: 10.1161/01.ATV.0000253947.70438.99. [DOI] [PubMed] [Google Scholar]
- 28.Trogan E, Choudhury RP, Dansky HM, Rong JX, Breslow JL, Fisher EA. Laser capture microdissection analysis of gene expression in macrophages from atherosclerotic lesions of apolipoprotein e-deficient mice. Proc Natl Acad Sci USA. 2002;99:2234–2239. doi: 10.1073/pnas.042683999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Braun M, Pietsch P, Schrör K, Baumann G, Felix SB. Cellular adhesion molecules on vascular smooth muscle cells. Cardiovasc Res. 1999;41:395–401. doi: 10.1016/s0008-6363(98)00302-2. [DOI] [PubMed] [Google Scholar]
- 30.Coller BS. Leukocytosis and ischemic vascular disease morbidity and mortality: Is it time to intervene? Arteriosclerosis, Thrombosis, and Vascular Biology. 2005;25:658–670. doi: 10.1161/01.ATV.0000156877.94472.a5. [DOI] [PubMed] [Google Scholar]
- 31.Combadière C, Potteaux S, Rodero M, Simon T, Pezard A, Esposito B, Merval R, Proudfoot A, Tedgui A, Mallat Z. Combined inhibition of ccl2, cx3cr1, and ccr5 abrogates ly6c(hi) and ly6c(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation. 2008;117:1649–1657. doi: 10.1161/CIRCULATIONAHA.107.745091. [DOI] [PubMed] [Google Scholar]
- 32.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ ccr2, ccr5, and cx3cr1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yvan-Charvet L, Pagler T, Gautier EL, Avagyan S, Siry RL, Han S, Welch CL, Wang N, Randolph GJ, Snoeck HW, Tall AR. Atp-binding cassette transporters and hdl suppress hematopoietic stem cell proliferation. Science (New York, NY) 2010 doi: 10.1126/science.1189731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barringhaus KG, Phillips JW, Thatte JS, Sanders JM, Czarnik AC, Bennett DK, Ley KF, Sarembock IJ. Alpha4beta1 integrin (vla-4) blockade attenuates both early and late leukocyte recruitment and neointimal growth following carotid injury in apolipoprotein e (-/-) mice. Oncology. 2004;41:252–260. doi: 10.1159/000078646. [DOI] [PubMed] [Google Scholar]
- 35.Murphy AJ, Woollard KJ, Hoang A, Mukhamedova N, Stirzaker RA, Mccormick SPA, Remaley AT, Sviridov D, Chin-Dusting J. High-density lipoprotein reduces the human monocyte inflammatory response. Arteriosclerosis, Thrombosis, and Vascular Biology. 2008;28:2071–2077. doi: 10.1161/ATVBAHA.108.168690. [DOI] [PubMed] [Google Scholar]
- 36.Hui DY, Basford JE. Distinct signaling mechanisms for apoe inhibition of cell migration and proliferation. Neurobiol Aging. 2005;26:317–323. doi: 10.1016/j.neurobiolaging.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 37.Becker L, Gharib SA, Irwin AD, Wijsman E, Vaisar T, Oram JF, Heinecke JW. A macrophage sterol-responsive network linked to atherogenesis. Cell Metab. 2010;11:125–135. doi: 10.1016/j.cmet.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.