Abstract
People with schizophrenia (PSZ) exhibit signs of reduced working memory (WM) capacity. However, this may reflect an impairment in managing its content, e.g. preventing irrelevant information from taking up available storage space, rather than a true capacity reduction. We tested the ability to eliminate and update WM content in 38 PSZ and 30 healthy control subjects (HCS). Images of real-world objects were presented consecutively, and a tone cued the item most likely to be tested for memory. On half the trials, randomly intermixed, a second tone occurred. Participants were informed that the item cued by the second tone was now the most likely to be tested, and the item cued by the first tone now the least likely, providing incentive to eliminate the first cued item from WM. Both HCS and PSZ displayed a robust performance advantage for cued items. Unexpectedly, PSZ more efficiently removed the no-longer-essential item from WM than HCS. The magnitude of the WM clearance of this first cued item correlated with memory performance for the newly prioritized second cued item in PSZ, indicating that it was adaptive. However, WM clearance was not associated with WM capacity, ruling out the need to budget limited resources as an explanation for greater clearance in PSZ. A robust correlation between WM clearance and poverty of speech in PSZ instead suggests that the propensity to rapidly clear non-essential information and minimize the number of items in WM may be the reflection of a negative symptom trait. This finding may reflect a more general tendency of PSZ to focus processing more narrowly than HCS.
Keywords: schizophrenia, working memory, clearance, updating, capacity, alogia
1. Introduction
One of the most robust, measurable cognitive deficits in schizophrenia is a reduction in working memory (WM) storage capacity (Aleman et al., 1999; Barch, 2005; Gold et al. 1997, 2003; Goldman-Rakic, 1994; Lee and Park, 2005). However, performance limitations in tasks aimed at measuring WM capacity may not necessarily reflect the capacity of the store, per se, but rather the efficiency of its content management, i.e. the ability to flexibly shift items into and out of WM. Information encoded and maintained in WM does not reflect a passive storage of sensory information but depends on task demands (e.g. Droll et al., 2005; Makovski et al., 2008; Richard et al., 2008; Schmidt et al., 2002; Yotsumoto and Sekuler, 2006). Thus, WM content needs to be managed strategically so that information relevant to the current task is selectively encoded and maintained, and irrelevant stimuli are prevented from consuming WM resources.
Vogel et al. (2005) reported that the efficiency of such selection predicts individual differences in WM performance in healthy subjects: individuals who were unable to restrict WM to task-relevant information tended to have lower estimates of capacity, presumably because the presence of irrelevant items left fewer WM resources available for the maintenance of task-relevant items. We have applied this framework to ask whether the apparent capacity reduction in PSZ derives from deficits in their ability to limit WM to relevant items. In the most direct test of this hypothesis, PSZ displayed both intact selective encoding of relevant over irrelevant items and reduced capacity scores, indicating that reduced capacity was not secondary to impaired selection (Gold et al., 2006). However, we have also shown failures of selective encoding in PSZ when subjects were required to select non-salient over salient stimuli for storage in WM (Hahn et al., 2010). The inability to filter salient items was correlated with the degree of WM capacity reduction, suggesting that deficits in attentional selection for WM encoding can, in some instances, explain capacity reduction.
Strategic control of WM content goes beyond selective encoding and includes the reallocation of resources from items that are already stored in WM but are no longer relevant to newly relevant items. Findings by Landman et al. (2003) suggest that information in WM can be reprioritized based on changing task demands (but see also Matsukura et al., 2007; Matsukura and Hollingworth, in press). Similarly, Wolfe et al. (2007), using a very different type of paradigm, demonstrated that objects can be flexibly added and subtracted from a set of tracked objects. Furthermore, susceptibility to proactive interference was found to be a determinant of a low verbal WM span (Lustig et al., 2001; May et al., 1999), indicating that the clearance of items stored in WM is essential for its effective use. Thus, WM task performance appears to depend on the degree to which WM content can be flexibly cleared and updated based on changes in task demands. The ability to flexibly control WM content has been assessed in PSZ in the context of N-back paradigms, which require the continuous encoding and updating of consecutively presented items in WM to allow decisions of whether the current item is identical to the item presented N trials ago. PSZ have reliable deficits in this type of paradigm (e.g. Carter et al., 1998; Goldberg et al., 2003). However, N-back performance depends on many abilities beyond WM updating, such as the ability to sustain attention and to rapidly shift between encoding, maintenance, retrieval, and decision processes. Thus, it remains unclear whether the N-back deficit reflects a specific impairment in updating processes.
Using an approach developed by Maxcey-Richard and Hollingworth (under review), the current experiment tested directly whether PSZ display impairments in clearing and updating WM content, and whether this may be related to the reduced WM capacity reported in schizophrenia (see above).
2. Experimental Materials and Methods
2.1. Participants
Data from one PSZ were excluded because she had trouble staying awake. The remaining sample consisted of 38 individuals meeting Diagnostic and Statistical Manual of Mental Disorders-IV (American Psychiatric Association, 1994) criteria for schizophrenia (N=16 paranoid, 11 undifferentiated, 3 residual, 2 disorganized, 1 catatonic) or schizoaffective disorder (N=5), and 30 matched HCS. Diagnosis was established by combining information from a Structured Clinical Interview for DSM-IV (SCID; First et al., 2002) conducted by an experienced social worker working with a psychiatrist, with a review of all available diagnostic information at a consensus diagnosis meeting chaired by one of the coauthors (JMG). Demographic information is summarized in Table 1. Groups did not differ in age [t(66)=0.36, P=0.72], parental education [t(65)=0.45, P=0.66], sex (Chi-square P=0.70) or ethnicity (Chi-square P=0.58). However, PSZ had fewer years of education than HCS [t(66)=3.77, P<0.001].
Table 1.
Group Demographics (mean ± stdev)
| PSZ | HCS | |
|---|---|---|
| Age | 41.0 ± 10.8 (range 18–54) | 41.9 ± 9.8 (range 19–54) |
| Male : Female | 27 : 11 | 20 : 10 |
| AA : C : H: O a | 13 : 21 : 2: 2 | 9 : 18 : 2: 1 |
| Education (years) | 12.6 ± 2.5 | 14.8 ± 2.1 *** |
| Parental education b | 13.3 ± 2.8 c | 13.0 ± 2.6 |
| WASI | 98.1 ± 11.7 c | 113.4 ± 12.2 c *** |
| WRAT 4 standard score | 95.2 ± 12.7 c | 105.3 ± 15.8 c ** |
| WTAR standard score | 99.1 ± 14.8 c | 107.9 ± 14.7 c * |
| MATRICS total score | 32.4 ± 12.7 c | 52.8 ± 13.5 c *** |
| BPRSd total score | 35.7 ± 7.1 (range 23–49) | |
| SANSe total score | 29.4 ± 11.4 (range 7–54) | |
| LOFSf total score | 21.7 ± 5.1 (range 11–30) | |
| CDSg total score | 2.3 ± 3.2 (range 0–14) |
AA = African American; C = Caucasian; H = Hispanic; O = Other
average over mother’s and father’s years of education
data unavailable for 1 subject
P<0.05,
P<0.01,
P<0.001; significant difference between PSZ and HCS in independent samples t-test
Brief Psychiatric Rating Scale (Overall and Gorman, 1962)
Scale for the Assessment of Negative Symptoms (Andreasen, 1984)
Level Of Functioning Scale (Hawk et al., 1975)
Calgary Depression Scale (Addington et al., 1992)
The PSZ were clinically stable outpatients (see Table 1 for a description of clinical ratings). All were receiving antipsychotic medication: 5 were treated with first-generation antipsychotics, 32 with second-generation antipsychotics, and 1 with both. Twenty-two PSZ additionally received mood stabilizing medication, 12 anxiolytic and 6 antiparkinsonian medication. One patient received modafinil for sleep apnea, and 1 bromocriptine. Medication had not changed in the preceding four weeks. HCS were recruited from the community by cold-calling telephone numbers obtained from survey sampling companies and by word of mouth and had no Axis 1 or 2 diagnoses as established by a SCID conducted by a Master’s level clinical psychologist, had no self-reported family history of psychosis, and were not taking any psychotropic medication. Current substance abuse or dependence, mental retardation, neurological disorders or any medical disorder likely to impair cognitive function were exclusionary for all participants. All participants provided written informed consent. Before PSZ signed the consent form, the investigator formally evaluated basic understanding of study demands, risks, and what to do if experiencing distress or to end participation. This evaluation was done in the presence of a third-party witness. The study was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki).
2.2. Neuropsychological testing
Participants completed the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999), the Wide Range Achievement Test Reading (WRAT; Wilkinson and Robertson 2006), the Wechsler Test of Adult Reading (WTAR; Wechsler, 2001), and the MATRICS battery (Nuechterlein and Green, 2006). Neuropsychological testing was usually performed on a separate day to avoid fatigue. PSZ scored significantly lower than HCS on the WASI, WRAT, WTAR and MATRICS battery (see Table 1), and exhibited significant impairment in all MATRICS domains. Please note that all critical ANOVA interactions reported in the Results were still significant when any characterization measure that differed between groups (WASI, WRAT, WTAR, MATRICS total, years of education) was entered as a covariate.
2.3. Experimental paradigm
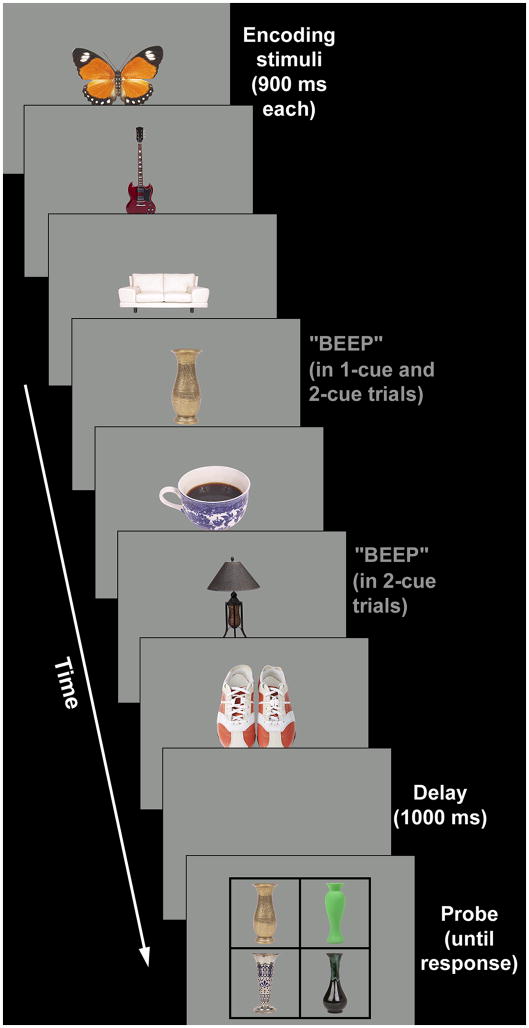
The task was conducted in a dimly illuminated room on a 17” CRT monitor with a 60 Hz refresh rate. Participants were seated approximately 70 cm from the monitor and responded with their dominant hand by mouse-click. The task stimuli consisted of images of real-world objects (1.4–4.3° wide and 2.3–4.3° high), presented against a grey background (Figure 1).
Figure 1.
An example of a single task trial. The object stimuli are shown enlarged relative to the size of the screen to be discernible in the figure. The size of the stimuli in the probe array was identical to that in the encoding array.
Each trial started with an encoding phase, during which seven objects were presented consecutively (900 ms each) at the center of the screen. Each object was drawn from a different basic-level category (e.g. sofa, vase, butterfly, shoes, etc.). Participants were asked to memorize the stimuli. After a 1000-ms delay, memory for one of the pictures was tested. Participants were told that any of the pictures could be tested, although the 1st and 2nd were never actually tested. The probe array consisted of four simultaneously presented pictures of the same object category (e.g. four sofas), but differing in color, shape and other details, laid out in 2 rows of 2 pictures. One of the four pictures was identical to the one presented during the encoding phase, and the task was to identify that picture by mouse-click. The probe array stayed on display until the participant responded. Because all four items in the probe array were from the same basic-level category (many were from the same subordinate-level category), verbal encoding could not easily be used to determine which item matched the original one (e.g. remembering the word “vase” would not have allowed a subject to choose the correct vase; see Figure 1). Previous experiments using naturalistic object stimuli and within-category memory tasks found no observable contribution of verbal encoding to memory performance (Hollingworth, 2003). Thus, although we cannot rule out the possibility that some verbal encoding occurred, it is unlikely that it was a central factor in performing the task.
To probe participants’ ability to manage the content of WM, a 150-ms auditory cue that came on simultaneously with the cued picture conveyed information about the likelihood with which certain items would be tested. In approximately half of the trials, only the 4th picture stimulus was cued (1-cue trials). In the other half, both the 4th and the 6th stimulus were cued (2-cue trials). Subjects were informed that in 1-cue trials, the cued picture was the most likely to be tested. After five 1-cue practice trials in which the 4th item was cued and tested, participants were informed that some trials had a second auditory cue, and that in 2-cue trials the picture cued by the first tone was now least likely to be tested and the picture cued by the second tone the most likely. Participants then performed 5 practice trials in which both the 4th and 6th objects were cued and the 6th object was tested. Table 2 lists the probabilities with which items 3 to 7 were tested in the final experimental task.
Table 2.
Percentage of trials in which a particular object was tested
| 1-cue trials | 2-cue trials | |
|---|---|---|
| 1st cued object (position 4) | 54% | 9% |
| 2nd cued object (position 6) | N/A | 54% |
| Uncued objects* | 11.5% | 12.3% |
except the 1st and 2nd objects, which were never tested
During the experimental section, both cue conditions were tested in a randomized, mixed fashion. Thus, when the position-4 cue came on, there was an approximately 50% chance that this item would be the only cued item, making it worthwhile to devote more resources to selectively retaining this item. In 2-cue trials, the second cue signaled the need to reallocate resources to the 6th object and encouraged the withdrawal of resources from the 4th object (i.e. to eliminate that object from WM). Thus, a flexible reallocation of WM capacity according to current task demands would be specifically reflected in the discarding of the position-4 object from WM. This pattern is easily discriminable from more general attentional impairment or encoding problems, which would manifest themselves in reduced performance across conditions. Participants practiced an additional nine 1-cue and nine 2-cue trials in which the different object positions were tested with approximately the same likelihood as in the experimental section (see Table 2). The final task was composed of 69 1-cue trials and 65 2-cue trials. The total task duration including practice was ~45 minutes.
To test whether the propensity to clear items from WM is related to WM capacity, we correlated each individual’s WM clearance effect (defined below) with a measure of WM capacity derived from a 60-trial change localization task, using the method of Gold et al. (2006, Experiment 5). Participants viewed an encoding array of four colored squares, arranged around a central cross, for 100 ms (see supplementary Figure S1). After a 900 ms delay during which only the central cross was on display, the four squares reappeared. The task was to mouse-click on the one square that had changed color. The larger a participant’s storage capacity, the more items are encoded in WM, and the greater is the probability that they will accurately select the changed item. Change localization performance is thus closely related to WM capacity.
2.4. Data analysis
The percentage of trials with a correct choice (response accuracy) was analyzed by several different mixed model ANOVAs as described in the Results section.
3. Results
3.1. One-Cue Condition
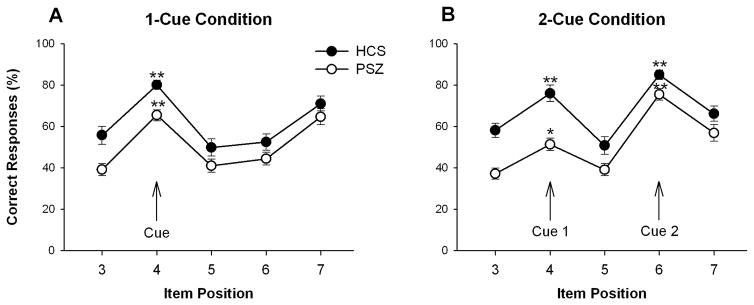
Both HCS and PSZ displayed a clear performance advantage for the position-4 picture relative to the immediately preceding and subsequent picture (Figure 2A), suggesting that both groups were able to use the cue to selectively store the cued item in WM. This was confirmed by a significant main effect of Tested Position (3 vs. 4 vs. 5) [F(2,132)=50.4, P<0.001] in a 2-factor ANOVA with Group (PSZ, HCS) as a between-subjects factor. The magnitude of this effect was nearly identical in PSZ and HCS, with no hint of a Group by Tested Position interaction [F(4,132)<1], despite lower overall performance in PSZ [Group: F(1,66)=19.1, P<0.001]. Both groups also displayed a pronounced recency effect as indicated by the relatively high accuracy for position-7 items.
Figure 2.
Average response accuracy (±SEM) of 38 people with schizophrenia (PSZ) and 30 healthy control subjects (HCS) for each tested object position on 1-cue trials (A) and 2-cue trials (B). Chance performance would be 25%, given that participants had to choose the correct picture from among four choices. The arrows mark the cued objects. * P<0.01, ** P<0.001, paired t-tests comparing accuracy for the cued object to accuracy averaged over the immediately preceding and subsequent object.
3.2. Two-Cue Condition
Both HCS and PSZ displayed a performance advantage for the cued items in positions 4 and 6 relative to their immediately preceding and subsequent items (Figure 2B). However, accuracy was lower for position-4 than position-6 objects, as confirmed by a significant main effect of Tested Position (4 vs. 6) [F(1,66)=37.0, P<0.001] in a 2-factor ANOVA with Group as a between-subjects factor. A significant difference between position 4 and 6 objects was seen in both HCS [t(29)=2.61, P<0.02] and PSZ [t(37)=6.035, P<0.001]. This was suggestive of WM clearance and updating, but it may also reflect an effect of item position per se, i.e. a recency effect. The cueing effect appeared to be of comparable magnitude between the two groups for the second cue (item position 6), but it appeared to be reduced in PSZ relative to HCS for the first cue (item position 4). This was confirmed by a significant Group × Tested Position interaction [F(1,66)=7.80, P<0.01]. Thus, PSZ more efficiently discarded items with a low probability of being tested.
3.3. Position-4 Item Across Cue Conditions
To confirm the above interpretation and disambiguate it from possible item position effects, we compared group differences for position-4 items between the 1-cue and 2-cue condition. Position 4 was the most likely to be tested in 1-cue trials, but became the least likely item to be tested in 2-cue trials. Consistent with a WM updating effect in 2-cue trials, performance for position-4 items was lower on 2-cue than on 1-cue trials, as confirmed by a main effect of Cue Condition [F(1,66)=14.8, P<0.001] in a 2-factor ANOVA limited to position-4 items, with Group as a between-subjects factor. However, this difference between the 1-cue and 2-cue condition was significant only in PSZ [t(37)=4.40, P<0.001] and not in HCS [t(29)=1.19, P>0.2], confirming that PSZ more efficiently cleared their WM of the no longer essential position 4 item. This was supported by an interaction of Group with Cue Condition [F(1,66)=4.47, P<0.04].
3.4. Correlations of WM Clearance
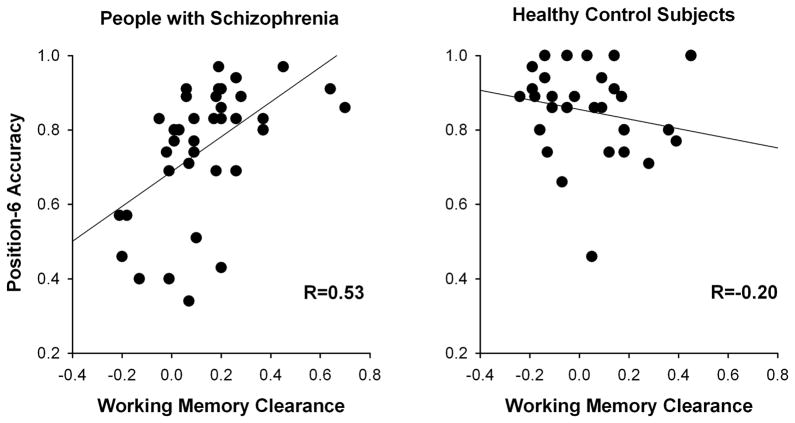
To test whether the clearance of the deprioritized position-4 item from WM aided accuracy for the newly prioritized position-6 item, we correlated each individual’s position-6 accuracy in 2-cue trials with the difference in position-4 accuracy between 1-cue and the 2-cue trials (reflecting the degree of clearance of the position-4 representation following the second cue, henceforward referred to as “WM clearance effect”). Figure 3 shows a significant positive correlation between the WM clearance effect and position-6 accuracy in PSZ (R=0.53, P<0.001) but not HCS (R=−0.20, P=0.29; Fisher's z-transformation test for difference in correlation: z=3.095, P<0.002). Thus, PSZ but not HCS appeared to benefit from clearing the position-4 representation from WM on 2-cue trials. The clearance effect did not correlate with overall performance accuracy, averaged across non-cued items (R=−0.13, P>0.4 in both PSZ and HCS).
Figure 3.
Relationship between working memory clearance of the deprioritized position-4 item (quantified as the difference in position-4 accuracy between 1-cue and 2-cue trials) and response accuracy for the newly prioritized position-6 item in 2-cue trials. Working memory clearance was associated with position-6 accuracy in people with schizophrenia but not in healthy control subjects.
The above finding may indicate that individuals with low WM capacity need to clear the position-4 representation to make resources available for the position-6 representation, whereas individuals with high WM capacity can store both items and do not need to clear the position-4 item. To test this hypothesis, we correlated each participant’s WM clearance effect with a measure of WM capacity derived from a change localization task with colored squares. The correlation was not significant in PSZ (R=0.19, P=0.27) or HCS (R=0.18, P=0.36), and the trends were in the opposite direction of what would have been expected if the above hypothesis was true. However, capacity correlated with overall response accuracy across all conditions in both PSZ (R=0.55, P<0.001) and HCS (R=0.51, P=0.006), indicating that the absence of association with the WM clearance effect did not reflect poor measurement reliability. These findings indicate that the larger WM clearance effect in PSZ was not born out of a greater necessity to budget WM capacity.
We further correlated the WM clearance effect with scores on the seven MATRICS domains and the four clinical assessment instruments (BPRS, SANS, LOFS, CDS, see Table 1). The only significant correlation was with the SANS total score (R=0.42, P<0.01; all other correlations P>0.2). The correlation with the SANS was fueled by the Alogia dimension (Alogia global score: R=0.45, P<0.004), and therein, by the Poverty of Speech item (R=0.48, P<0.002, Figure 4; all other items P>0.3). Note the direction of these correlations suggesting greater WM clearance among PSZ with more severe alogia.
Figure 4.
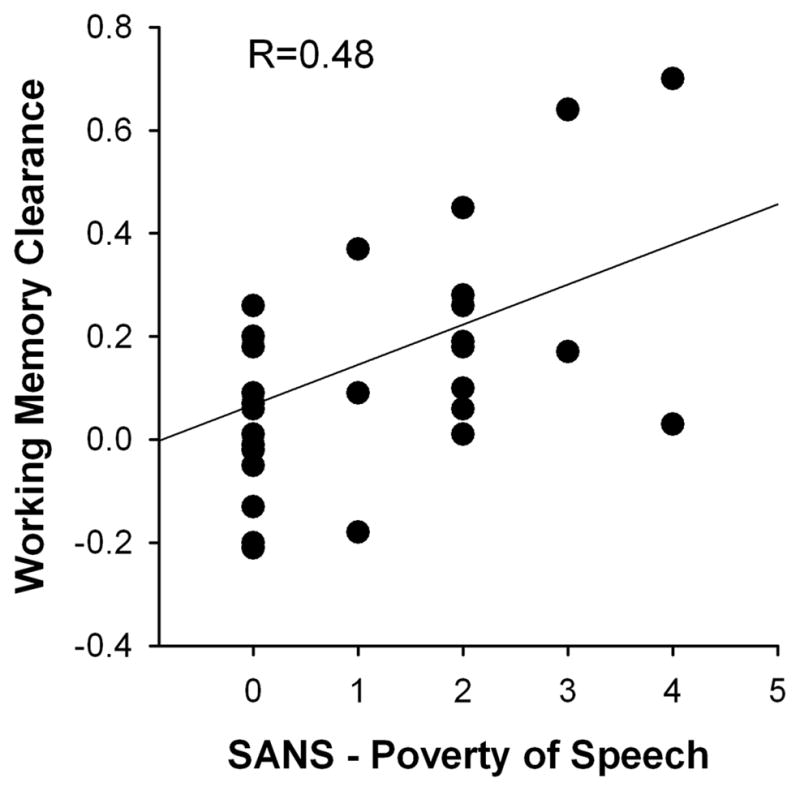
Relationship between the working memory clearance effect and the Poverty of Speech item of the Scale for the Assessment of Negative Symptoms (SANS) in 38 people with schizophrenia.
The WM clearance effect in PSZ did not correlate with haloperidol equivalents (P>0.6; Andreasen et al., 2010), and did not differ between PSZ who did and did not receive antidepressant [t(36)<1, independent samples t-test] or anxiolytic medication [t(36)=1.65, P>0.1].
4. Discussion
The present results were surprising in that, rather than exhibiting the hypothesized deficit in controlling, i.e. clearing and updating the contents of WM, PSZ displayed significantly greater WM clearance of deprioritized items whose likelihood of being tested was suddenly minimized. Moreover, the clearance of the deprioritized item from WM aided PSZ in encoding and maintaining the second cued, newly prioritized item, indicating that the greater WM clearance was adaptive in the context of the task. This clearly demonstrates that the basic mechanisms involved in clearing and updating WM content are largely intact in PSZ, and effectively rules out updating deficits as the basis of reduced WM capacity in schizophrenia.
Unlike in PSZ, WM clearance of the deprioritized item in HCS was not associated with better performance for the newly prioritized item. Thus, there appears to be a fundamental difference in the strategic usage of WM capacity between the two groups, with HCS displaying less WM clearance of deprioritized items, and obtaining less benefit from the clearance. A plausible explanation might have been that, due to their higher average WM capacity, HCS could afford the continued presence of the deprioritized item without compromising the storage of the newly prioritized stimulus. PSZ, in contrast, having less storage capacity, would be more dependent on the rapid clearance of the newly deprioritized item. While this seems like an attractive and logical possibility, we were unable to find supportive evidence for it. Specifically, we found no correlation between WM capacity derived from a secondary paradigm and the WM clearance effect in both groups. Thus, the more effective clearance of deprioritized items from WM in PSZ does not appear to constitute an acute response to task demands exceeding the available resources, but may reflect a more general trait.
It is intriguing that the WM clearance effect, despite its robustness and its association with item-6 accuracy in PSZ, was not associated with any of the cognitive domain measures obtained from neuropsychological tests. None of the MATRICS domain scores showed even a trend of an association (all Ps >0.2), a rare finding in light of commonly-observed generalized deficits in PSZ that drive cross-task correlations. Interestingly, there was a robust correlation between WM clearance in PSZ and the SANS, and specifically with poverty of speech, suggesting that the propensity to rapidly clear non-essential information from WM may be the reflection of a clinical trait in the negative symptom domain. This was, perhaps, a chance finding. However, the tendency to minimize the number of objects in WM at any moment in time does bear qualitative resemblance and potential explanatory power to the poverty of speech phenomenon. Such narrow focus could compromise the fluency of thought and expressive speech, resembling a kind of thought fragmentation in which there is a preference to consider small bits of information in isolation. Such narrow focus may also explain the previously reported association of object WM impairment with negative symptoms in partially remitted PSZ (Park et al., 2003), who resemble the current stable outpatient sample. Although only one item had to be encoded and maintained in this prior study, the presence of an intervening task in the delay period may have caused particular impairment in PSZ who tend to process information with a single-item focus, and these may have largely been negative-symptom patients. However, more research is needed to determine if this link of a specific cognitive processing style to a specific negative symptom is a reliable finding.
The tendency to focus narrowly on a subset of available information suggested by the present task may reflect a more general narrowing of information processing in PSZ that can be seen in other tasks. A recent study (Hahn et al., in press) reported that PSZ are impaired at spreading their attention broadly to encompass multiple locations (which, again, was not associated with WM capacity). Similarly, a recent event-related potential study of maintaining one of two items in WM found that neural activity associated with selecting the one over the other item was greater in PSZ than in HCS (Leonard et al., in preparation). Thus, in three completely different paradigms, PSZ focused attention more intensively on a single object or location than did HCS. The finding that this tendency to focus narrowly was not necessitated by WM capacity limitations suggests that it is a distinct cognitive trait in PSZ rather than an extreme expression of normal capacity variation.
The current findings constitute a rare example of a cognitive mechanism in which PSZ are not only unimpaired, but display seemingly superior task-adaptive behavior to HCS. At the same time, this finding may reflect an abnormality that crosses the boundary between circumscribed negative and cognitive symptoms in schizophrenia. The current paradigm appears to create a task condition in which performance profits from a clinical trait of at least some PSZ: the tendency to keep the current focus narrow and simple. This trait may be beneficial in certain laboratory paradigms, but may lead to impaired performance in many real-world situations that would profit from simultaneously considering multiple items. The findings emphasize the need for a nuanced and multidimensional view of WM deficits in schizophrenia.
Supplementary Material
An example of an encoding array or test array for the change localization task used to derive an independent measure of working memory capacity. On each trial, the encoding array was presented for 100 ms, followed by a 900-ms delay interval, and then a test array was presented. The test array was identical to the encoding array except that the color of one item was changed. In the test array, the mouse cursor was visible, and the task was to click on the item that had changed color. The test array remained visible until a response was made.
Acknowledgments
We thank Leeka Hubzin, Sharon M. August, Tatyana M. Matveeva, and Alexander N. Harvey for their help in the conduct of this study. We thank all volunteers who participated in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Andrew Hollingworth, Email: andrew-hollingworth@uiowa.edu.
Benjamin M. Robinson, Email: brobinso@mprc.umaryland.edu.
Samuel T. Kaiser, Email: skaiser@mprc.umaryland.edu.
Carly J. Leonard, Email: cjleonard@ucdavis.edu.
Valerie M. Beck, Email: valerie-beck@uiowa.edu.
Emily S. Kappenman, Email: eskappenman@ucdavis.edu.
Steven J. Luck, Email: sjluck@ucdavis.edu.
James M. Gold, Email: jgold@mprc.umaryland.edu.
References
- Addington D, Addington J, Maticka-Tyndale E, Joyce J. Reliability and validity of a depression rating scale for schizophrenics. Schizophr Res. 1992;6:201–208. doi: 10.1016/0920-9964(92)90003-n. [DOI] [PubMed] [Google Scholar]
- Aleman A, Hijman R, de Haan EH, Kahn RS. Memory impairment in schizophrenia: a meta-analysis. Am J Psychiatry. 1999;156:1358–1366. doi: 10.1176/ajp.156.9.1358. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders 4. American Psychiatric Press; Washington, DC: 1994. [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS) Iowa City, IA: University of Iowa; 1984. [Google Scholar]
- Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 2010;67:255–262. doi: 10.1016/j.biopsych.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM. The cognitive neuroscience of schizophrenia. Annu Rev Clin Psychol. 2005;1:321–353. doi: 10.1146/annurev.clinpsy.1.102803.143959. [DOI] [PubMed] [Google Scholar]
- Carter CS, Perlstein W, Ganguli R, Brar J, Mintun M, Cohen JD. Functional hypofrontality and working memory dysfunction in schizophrenia. Am J Psychiatry. 1998;155:1285–1287. doi: 10.1176/ajp.155.9.1285. [DOI] [PubMed] [Google Scholar]
- Droll JA, Hayhoe MM, Triesch J, Sullivan BT. Task demands control acquisition and storage of visual information. J Exp Psychol Hum Percept Perform. 2005;31:1416–1438. doi: 10.1037/0096-1523.31.6.1416. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Gold JM, Carpenter C, Randolph C, Goldberg TE, Weinberger DR. Auditory working memory and Wisconsin Card Sorting Test performance in schizophrenia. Arch Gen Psychiatry. 1997;54:159–165. doi: 10.1001/archpsyc.1997.01830140071013. [DOI] [PubMed] [Google Scholar]
- Gold JM, Wilk CM, McMahon RP, Buchanan RW, Luck SJ. Working memory for visual features and conjunctions in schizophrenia. J Abnorm Psychol. 2003;112:61–71. [PubMed] [Google Scholar]
- Gold JM, Fuller RL, Robinson BM, McMahon RP, Braun EL, Luck SJ. Intact attentional control of working memory encoding in schizophrenia. J Abnorm Psychol. 2006;115:658–673. doi: 10.1037/0021-843X.115.4.658. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Egan MF, Gscheidle T, Coppola R, Weickert T, Kolachana BS, Goldman D, Weinberger DR. Executive subprocesses in working memory: relationship to catechol-O-methyltransferase Val158Met genotype and schizophrenia. Arch Gen Psychiatry. 2003;60:889–896. doi: 10.1001/archpsyc.60.9.889. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Working memory dysfunction in schizophrenia. J Neuropsychiatry Clin Neurosci. 1994;6:348–357. doi: 10.1176/jnp.6.4.348. [DOI] [PubMed] [Google Scholar]
- Hahn B, Robinson BM, Kaiser ST, Harvey AN, Beck VM, Leonard CJ, Kappenman ES, Luck SJ, Gold JM. Failure of schizophrenia patients to overcome salient distractors during working memory encoding. Biol Psychiatry. 2010;68:603–609. doi: 10.1016/j.biopsych.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B, Robinson BM, Harvey AN, Kaiser ST, Leonard CJ, Luck SJ, Gold JM. Visuospatial attention in schizophrenia: Deficits in broad monitoring. J Abnorm Psychol. doi: 10.1037/a0023938. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawk AB, Carpenter WT, Jr, Strauss JS. Diagnostic criteria and five-year outcome in schizophrenia. A report from the International Pilot Study of schizophrenia. Arch Gen Psychiatry. 1975;32:343–347. doi: 10.1001/archpsyc.1975.01760210077005. [DOI] [PubMed] [Google Scholar]
- Hollingworth A. Failures of retrieval and comparison constrain change detection in natural scenes. J Exp Psychol Hum Percept Perform. 2003;29:388–403. doi: 10.1037/0096-1523.29.2.388. [DOI] [PubMed] [Google Scholar]
- Landman R, Spekreijse H, Lamme VA. Large capacity storage of integrated objects before change blindness. Vision Res. 2003;43:149–164. doi: 10.1016/s0042-6989(02)00402-9. [DOI] [PubMed] [Google Scholar]
- Lee J, Park S. Working memory impairments in schizophrenia: a meta-analysis. J Abnorm Psychol. 2005;114:599–611. doi: 10.1037/0021-843X.114.4.599. [DOI] [PubMed] [Google Scholar]
- Lustig C, May CP, Hasher L. Working memory span and the role of proactive interference. J Exp Psychol Gen. 2001;130:199–207. doi: 10.1037//0096-3445.130.2.199. [DOI] [PubMed] [Google Scholar]
- Makovski T, Sussman R, Jiang YV. Orienting attention in visual working memory reduces interference from memory probes. J Exp Psychol Learn Mem Cogn. 2008;34:369–380. doi: 10.1037/0278-7393.34.2.369. [DOI] [PubMed] [Google Scholar]
- Matsukura M, Hollingworth A. Does visual short-term memory have a high-capacity stage? Psychon Bull Rev. doi: 10.3758/s13423-011-0153-2. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukura M, Luck SJ, Vecera SP. Attention effects during visual short-term memory maintenance: protection or prioritization? Percept Psychophys. 2007;69:1422–1434. doi: 10.3758/bf03192957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May CP, Hasher L, Kane MJ. The role of interference in memory span. Mem Cognit. 1999;27:759–767. doi: 10.3758/bf03198529. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF. MATRICS Consensus Cognitive Battery, Manual. MATRICS Assessment Inc; Los Angeles, CA: 2006. [Google Scholar]
- Overall J, Gorman D. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- Park S, Puschel J, Sauter BH, Rentsch M, Hell D. Visual object working memory function and clinical symptoms in schizophrenia. Schizophr Res. 2003;59:261–268. doi: 10.1016/s0920-9964(02)00209-8. [DOI] [PubMed] [Google Scholar]
- Richard AM, Luck SJ, Hollingworth A. Establishing object correspondence across eye movements: Flexible use of spatiotemporal and surface feature information. Cognition. 2008;109:66–88. doi: 10.1016/j.cognition.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt BK, Vogel EK, Woodman GF, Luck SJ. Voluntary and automatic attentional control of visual working memory. Percept Psychophys. 2002;64:754–763. doi: 10.3758/bf03194742. [DOI] [PubMed] [Google Scholar]
- Vogel EK, McCollough AW, Machizawa MG. Neural measures reveal individual differences in controlling access to working memory. Nature. 2005;438:500–503. doi: 10.1038/nature04171. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) The Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- Wechsler D. Wechsler Test of Adult Reading (WTAR) The Psychological Corporation; San Antonio, TX: 2001. [Google Scholar]
- Wilkinson GS, Robertson GJ. Wide Range Achievement Test (WRAT) Vol. 4. Psychological Assessment Resources; Lutz, FL: 2006. [Google Scholar]
- Wolfe JM, Place SS, Horowitz TS. Multiple object juggling: Changing what is tracked during extended multiple object tracking. Psychon Bull Rev. 2007;14:344–349. doi: 10.3758/bf03194075. [DOI] [PubMed] [Google Scholar]
- Yotsumoto Y, Sekuler R. Out of mind, but not out of sight: intentional control of visual memory. Mem Cognit. 2006;34:776–786. doi: 10.3758/bf03193425. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
An example of an encoding array or test array for the change localization task used to derive an independent measure of working memory capacity. On each trial, the encoding array was presented for 100 ms, followed by a 900-ms delay interval, and then a test array was presented. The test array was identical to the encoding array except that the color of one item was changed. In the test array, the mouse cursor was visible, and the task was to click on the item that had changed color. The test array remained visible until a response was made.