Abstract
Mast cells occur in the brain and their number changes with reproductive status. While it has been suggested that brain mast cells contain the mammalian hypothalamic form of gonadotropin-releasing hormone (GnRH-I), it is not known whether mast cells synthesize GnRH-I de novo. In the present study, mast cells in the rat thalamus were immunoreactive to antisera generated against GnRH-I and the GnRH-I associated peptide (GAP); mast cell identity was confirmed by the presence of heparin, a molecule specific to mast cells, or serotonin. To test whether mast cells synthesize GnRH-I mRNA, in situ hybridization was performed using a GnRH-I cRNA probe, and the signal was identified as being within mast cells by the binding of avidin to heparin. GnRH-I mRNA was also found, using RT-PCR, in mast cells isolated from the peritoneal cavity. Given the function of GnRH-I in the regulation of reproduction, changes in the population of brain GnRH-I mast cells were investigated. While housing males with sexually receptive females for 2 h or 5 days resulted in a significant increase in the number of brain mast cells, the proportion of mast cells positive for GnRH-I was similar to that in males housed with a familiar male. These findings represent the first report showing that mast cells synthesize GnRH-I and that the mast cell increase seen in a reproductive context is the result of a parallel increase in GnRH-I positive and non-GnRH-I positive mast cells.
Keywords: neuromodulation, endocrine, degranulation, thalamus, median eminence
INTRODUCTION
Mast cells are bone marrow-derived multifunctional immune cells. In peripheral tissues mast cells have well-established roles in mediating inflammation and allergic responses and in providing immunity against parasites (Wasserman, 1989; Galli, 1993; Metcalfe et al., 1997). Mast cells also participate in defense against bacterial infection (Wedemeyer et al., 2000; Malaviya and Abraham, 2001).
Mast cells occur in the adult brain in both health and disease (Silver et al., 1996). In mammals, mast cells are found in the dura mater, meninges, and choroid plexus, and at perivascular locations and within the neural parenchyma in the thalamus (Dropp, 1972; Theoharides, 1990; Manning et al., 1994; Florenzano and Bentivoglio, 2000). In the murine model of multiple sclerosis (MS), experimental allergic encephalomyelitis (EAE), mast cells are found in proximity of MS plaques and play a role in the pathophysiology of this disease (Bebo et al., 1996; Secor et al., 2000).
Mature mast cells contain granules that store pre-formed effector molecules such as histamine, serotonin, serine proteases, exopeptidases, and neuropeptides (Stevens and Austen, 1989). When activated, mast cells release these granules in a process referred to as compound exocytosis or degranulation (Dvorak, 1991). Activation of mast cells also triggers de novo synthesis and secretion of leukotrienes, prostaglandins, cytokines, and chemotactic factors, which exert profound effects on the local microenvironment (Stevens and Austen, 1989). Mast cells accumulate diverse molecules and particles by endocytosis (e.g., antigen-aggregated IgE) (Furuichi et al., 1986), via high affinity uptake transporters (e.g., serotonin) (Chen et al., 2001) and by phagocytosis (e.g., bacteria and latex beads) (Czarnetzki, 1982).
Gonadotropin releasing hormone (GnRH-I) immunoreactive (IR) mast cells were found in the dove and mouse brain (Silverman et al., 1994b; Yang et al., 1999). This neurohormone regulates the hypothalamic-pituitarygonadal (HPG) axis and hence, reproduction. In the mammalian brain, GnRH-I neurons lie in a continuum in the medial septal, preoptic, and hypothalamic areas (Silverman et al., 1994a). GnRH-I is also expressed in peripheral organs such as the gonads and placenta (Khodr and Siler-Khodr, 1980; Oikawa et al., 1990; Peng et al., 1994), where it serves a variety of functions including regulation of steroid production in the gonads and tissue remodeling during implantation, among others.
The specific set of mediators produced by mast cells differs according to their activational stage, locale, and immune status of their microenvironment (Stevens and Austen, 1989; Galli, 1990; Friend et al., 1996). In addition, the number of mast cells in the brain changes in response to various behavioral stimuli. In the ringdove, courtship is associated with a dramatic increase in GnRH-I-IR mast cells in the medial habenula of the epithalamus (Zhuang et al., 1993). Moreover, an increase in mast cell number and in the proportion of activated mast cells occurs in the medial habenula following treatment with gonadal steroids (Wilhelm et al., 2000). In rats, cohabitation of a male with a sexually receptive female elicits an increase in the number of mast cells in dorsal thalamic nuclei (Asarian et al., 2002), as does mating followed by cohabitation of male mice with a female (Yang et al., 1999). An increase in thalamic mast cells was also found in postpartum female rats separated from their pups compared to age-matched virgin controls (Silverman et al., 2000), though this phenomenon may be due to nonreproductive parameters such as stress (Esposito et al., 2001).
Mast cell migration to tissues and degranulation therein is a mechanism of delivery of bioactive substances to specific sites in the body. The presence of GnRH-I in brain mast cells may point to a novel functional role of this neuropeptide. In the present study, we sought to determine if GnRH-I immunoreactivity in brain mast cells is due to its in situ synthesis. We then examined whether the number of brain mast cells and the proportion of GnRH-I-IR mast cells in the total population are influenced by a reproductive context.
MATERIALS AND METHODS
Experimental Protocol
Subjects were sexually naive adult male Long-Evans rats (Charles River Laboratories, Wilmington, MA) housed two/cage in a temperature- and humidity-controlled environment for at least 2 weeks before the start of experiments. The animals were maintained on a 12:12 h light/dark cycle (lights off 1900h) and were provided with food and water ad libitum. Males were paired with sexually receptive stimulus females for 2 h (n = 8; M+F, 2 h) or 5 days (n = 5; M+F, 5 d). Males that were housed with a familiar male (n = 8; M+M) served as controls.
Sexual receptivity was induced in ovariectomized females by s.c. injections of 10 μg estradiol 3-benzoate (E; Sigma, St. Louis, MO) at 12 pm on day 1 and 2 and 500 μg progesterone (P; Sigma) at 9 am on day 4 (Schumacher et al., 1991), and verified by testing manually for lordosis response 4 h after progesterone treatment. Paired animals were observed for 30 min, and only males whose female partner displayed lordosis, darting, and ear wiggling when approached by the males were used as subjects. Additional animals for immunocytochemical experiments as well as for in situ hybridization and RT-PCR analyses are indicated below.
Perfusion, Immunocytochemistry, and Histochemistry
Male rats were deeply anesthetized with a 1 mL i.p. injection of sodium pentobarbital (50 mg/mL; Henry Schein, Melville, NY) and were perfused transcardially with 100 mL of 0.9% saline followed by 300 mL of 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.3, all at room temperature. Brains were postfixed for 2 h and cryoprotected in 20% sucrose/phosphate buffer/0.1% sodium azide at 4°C.
Serial coronal sections (50 μm) were cut on a cryostat (Zeiss Microm HM 500 OM; Walldorf, Germany). All procedures described below were carried out at room temperature unless otherwise indicated.
Sections were viewed on a conventional/fluorescent microscope (Nikon Eclipse E800; Nikon, Inc., Melville, NJ), and images were captured using a SPOT cooled CCD camera (Diagnostic Instruments, Inc., Sterling Heights, MI). For scanning confocal fluorescence microscopy, images were captured using LSM 410 (Zeiss, Inc., Thornwood, NJ), with differential interference contrast (DIC) optics.
Mast Cell Markers
Histochemical detection of mast cells was accomplished by staining sections with acidic toluidine blue (TB) [0.2% Toluidine Blue O (Sigma), pH 2.0], which reacts with the sulfated proteoglycans in mast cell granules, or with Cy2-conjugated avidin (1:500, 1 h; Jackson Immunoresearch Labs, West Grove, PA), which binds to heparin, a mast cell specific glycosaminoglycan (Kjellen and Lindahl, 1991). In some experiments mast cell phenotype was confirmed by the presence of serotonin using an antibody raised in rabbit (1:5000; #20080 Lot 715499; Incstar, Still-water, CA).
Immunocytochemistry: GnRH-I in Mast Cells
Identification of GnRH-I-IR brain mast cells was made using a polyclonal antiserum to GnRH-I (guinea pig anti-GnRH-I, 1:2000, incubated overnight; #JUP3040; Advanced ChemTech Inc., Louisville, KY); antigen was detected with Cy3-conjugated donkey anti-guinea pig immunoglobulin (1:200, 1 h; Jackson Immunoresearch Labs). The antiserum to serotonin (vide supra) was added to the GnRH-I antibody and detected using Cy2-conjugated donkey anti-rabbit.
GnRH-I Prohormone in Mast Cells
Immunocytochemical localization of GnRH associated peptide (GAP), a segment of the GnRH-I prohormone (Kasten et al., 1996), was performed using a polyclonal antiserum [rabbit anti-rat GAP (amino acids 14 – 69); 1:2000, incubated overnight; IHC 7224; Peninsula Lab, Inc., Belmont, CA] and detected with Cy3-conjugated donkey anti-rabbit (1:200, 1 h; Jackson Immunoresearch Labs). The subjects were males paired with sexually receptive females for 5 days (n = 2).
Positive (and negative) controls for GnRH-I- and GAP-immunoreactivity consisted of sections of the medial pre-optic area (MPOA) and thalamus containing GnRH-I neuron cell bodies and mast cells, respectively, incubated in the presence (or absence) of the primary antibodies.
In Situ Hybridization
A digoxigenin- (DIG) labeled antisense cRNA probe comprising exons 1 through 4 of rat GnRH-I was generated using GnRH-I cDNA graciously provided by Dr. James Roberts and the MAXIscript™ in vitro transcription kit (Ambion, Inc., Austin, TX). In situ hybridization was performed as described previously (Hamada et al., 1999) on free-floating 30 μm serial sections collected through the extent of the hypothalamus and thalamus (bregma 1.6 mm to −6.0 mm; n = 3; M+F, 5 d) (Paxinos and Watson, 1986). The negative control consisted of omitting the anti-sense probe during the hybridization step; GnRH-I producing neurons in the MPOA served as a positive control. The sense probe was not used because the GnRH gene locus encodes a second gene (termed SH) that is transcribed from the opposite strand (Adelman et al., 1987), producing SH mRNA in the hypothalamus (Bond et al., 1989). Following hybridization and signal detection, the sections were incubated with avidin-Cy2. To distinguish the DIG signal from the mast cell nucleus, sections were counterstained for 10 min with 10−5 M 4,6 diamidino-2-phenylindole (DAPI; Sigma), a DNA-binding fluorescent dye.
RT-PCR
Peritoneal mast cells were chosen for RT-PCR as their phenotype resembles that of brain mast cells (Pang et al., 1996). Mast cells from the peritoneal cavity of male rats (n = 3) were isolated as previously described (Jippo et al., 2001). Briefly, male rats were deeply anesthetized and the peritoneal cavity was flushed with 30 mL of Tyrode’s buffer (23°C, pH = 7.4). Mast cells were separated by two successive centrifugations through a 22.5% (w/v) metrizamide gradient (density 1.12 g/mL, 400×g, 15 min; Sigma) in Tyrode’s buffer containing 0.1% gelatin. A >99% pure population of mast cells was obtained as confirmed by staining with TB. Total RNA from peritoneal mast cells was extracted using the RNAqueous™-4PCR kit (Ambion). The RNA (0.5 μg) was then reverse transcribed using oligo(dT) primers and reverse transcriptase (RETROscript™ Kit, Ambion). Rat GnRH-I, Carboxypeptidase A (CPA), a mast cell specific exopeptidase (Reynolds et al., 1989), which was used as a positive control, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA were PCR-amplified using the following primer pairs: 5′-TCC CTT TGG CTT TCA CAT CC-3′ and 5′-TGC CAT TTG ATC CTC CTC CT-3′ for GnRH-I, 5′-CAA TCA AAC TGC CAC CCA AC-3′ and 5′-ACG AAG CTC AAA GGC AAA TG-3′ for CPA, and 5′-TGC GAC TTC AAC AGC AAC TC -3′ and 5′-CAG CAA GGA CAC TGA GCA AG-3′ for GAPDH. Negative controls consisted of using RNA that was not reverse transcribed, which ensured that the samples were devoid of genomic DNA contamination. PCR was also performed in the absence of template DNA, which tested contamination in samples and reagents. To verify the sequence identity of the amplicons, the PCR products were sequenced directly using an ABI 3100 capillary sequencer (Applied Biosystems, Foster City, CA), and the sequences were aligned with the GnRH-I mRNA (GI: 6980967; Adelman et al., 1986) and CPA precursor mRNA (GI: 1698707; Lutzelschwab et al., 1997) sequences in the NCBI Entrez Nucleotides database using the pairwise BLAST program (Altschul et al., 1990).
Analysis of Mast Cell Numbers and Localization
Mast cells with visible nuclei were counted in TB-stained sections spanning the thalamus (bregma −0.05 mm to −5.9 mm) and mapped according to the atlas of Paxinos and Watson (1986), as previously reported (Asarian et al., 2002). Groups included M+M (n = 8), M+F 2 h (n = 8), and M+F 5 d (n = 5), as described above. Every second or third section was counted, and the number of cells was estimated using the Abercrombie correction factor (Abercrombie, 1946).
The proportion of GnRH-I-IR mast cells in the same experimental groups was determined in alternate sections through the midthalamus (bregma −2.66 to −3.64 mm) by calculating the number of double-labeled (GnRH-I- and serotonin-positive) compared to serotonin-positive mast cells. One-way ANOVA was used to determine significant differences in mast cell number among groups, followed by a t test to compare mast cell counts between individual groups.
RESULTS
Rat Brain Mast Cells Are IR for GnRH-I and GAP
Thalamic mast cells were IR for GnRH-I [Fig. 1(A)], and mast cell phenotype was confirmed by double-labeling with an antibody to serotonin [Fig. 1(B,C)]. All of the GnRH-I-IR mast cells contained serotonin, but the converse was not true (see below). Confocal microscopy revealed that GnRH-I and serotonin were sometimes, but not always, in the same mast cell granule [Fig. 1(C)]. When primary antisera were omitted no GnRH-I-IR neurons or mast cells were seen (data not shown).
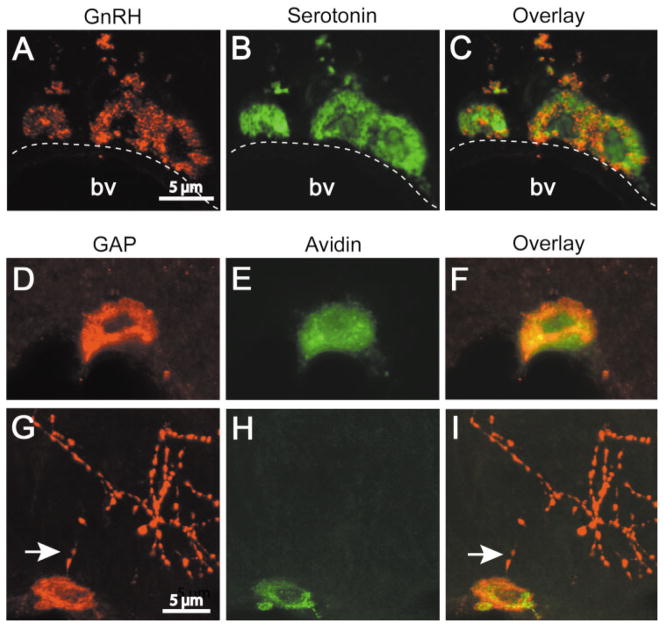
Figure 1.
GnRH-I and GAP immunoreactivity in mast cells in the rat thalamus and median eminence. (A–C) Confocal images (1 μm) of brain mast cells containing GnRH-I- (A) and serotonin- (B) IR. The overlay (C) shows that both labels occur in the same cell but not necessarily in the same mast cell granule. (D–I) Confocal images of GAP (D and G) and avidin (E and H) labeled mast cells in the thalamus and median eminence, respectively. Note that GAP and avidin staining occur in the same mast cell (F and I). GAP, but not avidin, is present in axons in the median eminence (G–I). Note the proximity of GAP positive axons (arrows) to mast cells. D–F and G–I are projections of 6 and 8 images (z axis, 1 μm), respectively. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
A subset of mast cells was IR for GAP, a fragment of the GnRH-I prohormone peptide [Fig. 1(D,G)]. GAP-IR was colocalized with the mast cell marker heparin as evidenced by the binding of avidin-Cy2 [Fig. 1(D–I)]. As with GnRH-I, GAP-IR was seen in some, but not all mast cell granules. GAP antiserum labeled GnRH-I neurons in the MPOA (not shown) and axons in the median eminence [Fig. 1(G)]. Avidin-Cy2 labeled mast cells but not GnRH-I neurons or their processes [Fig. 1(H,I)].
Mast Cells in the Brain Contain GnRH-I mRNA
In situ hybridization with a DIG-labeled GnRH-I cRNA probe revealed GnRH-I mRNA in neurons in MPOA [Fig. 2(A)] and in mast cells in the thalamus [Fig. 2(B,D,E,H)]. The mast cell phenotype was verified by the binding of avidin-Cy2 [Fig. 2(C,F)], and colocalization of DIG signal with avidin was shown using confocal fluorescence microscopy and DIC optics [Fig. 2(B–D)]. The DIG signal in the cell is located in the cytoplasm, above the DAPI-stained nucleus [Fig. 2(E–H)]. No background staining was detected in these sections. No signal was observed in sections in which probe was omitted (data not shown).
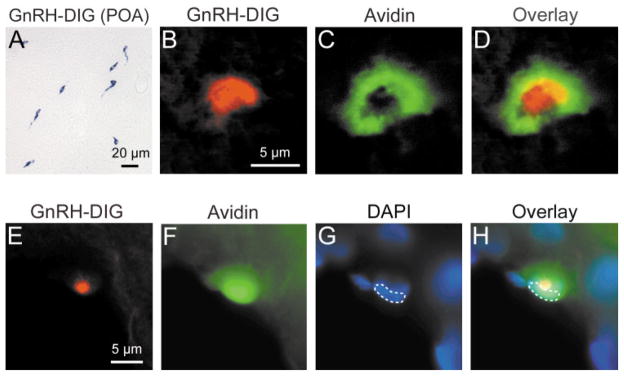
Figure 2.
GnRH-I mRNA detection in neurons and mast cells using in situ hybridization. (A) GnRH-I neurons in the MPOA visualized with DIG staining. (B) GnRH-I mRNA containing mast cell in the thalamus visualized with DIC optics to detect DIG staining (DIG label was inverted) in a 1 μm optical section. (C) Same mast cell, in the identical optical plane as in (B), visualized with fluorescence to detect avidin-Cy2. (D) Overlay of (B) and (C). (E–H) An individual thalamic mast cell visualized with single (E–G) and triple-label markers (H) using conventional fluorescence microscopy. (E–G) GnRH-I mRNA (DIG label, inverted), avidin-Cy2, and DAPI (outlined), respectively. (H) The DIG signal is found above the nucleus (outlined), but is within the cell cytoplasm. All 3 signals occur within the same cell. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Peritoneal Mast Cells Express GnRH-I mRNA
Amplification of GnRH-I, CPA, and GAPDH mRNA [Fig. 3(B,C)] was obtained by RT-PCR of purified peritoneal mast cells [Fig. 3(A)]. Lymphocytes were not seen in the peritoneal mast cell preparation. Alignment of the mRNA sequence obtained from the RT-PCR amplification of peritoneal mast cells using GnRH primers to the published rat GnRH-I sequence showed that it was identical to that of GnRH-I neurons (not shown). The sequence obtained using CPA specific primers was identical to the published sequence (not shown). Mast cells isolated from the peritoneal cavity were also IR for GnRH-I (data not shown).
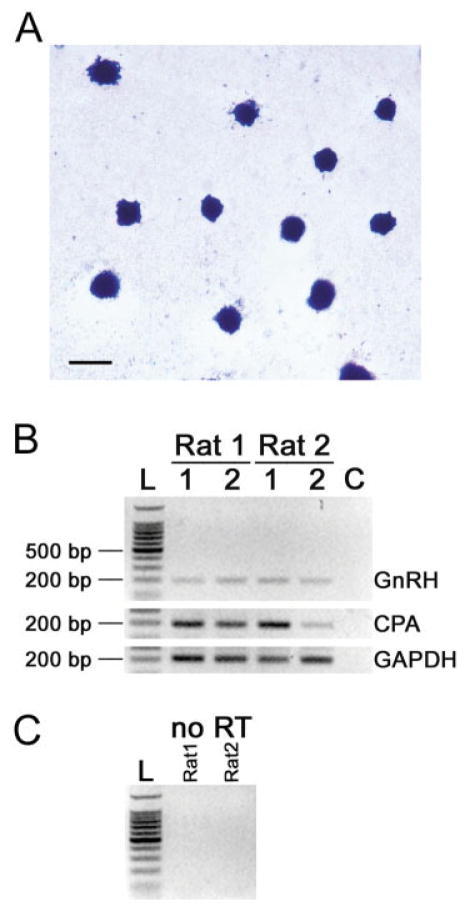
Figure 3.
Histochemical and RT-PCR analysis of peritoneal mast cells. (A) Mast cells isolated from the peritoneal cavity stained with acidic TB (scale bar = 10 μm). (B) RT-PCR of mRNA extracted from peritoneal mast cells. The figure shows PCR for GnRH-I, CPA, and GAPDH performed in duplicate of RNA extracted (labeled 1 and 2) from each of two rats. The size of the PCR products was 195-bp for GnRH-I, 191-bp for CPA and 200-bp for GAPDH. Left lane shows a 100 bp ladder (L). Amplification in the absence of template DNA (control) repeated with each of the three primer sets (lane C). (C) PCR of RNA from rat 1 and 2 that was not reverse transcribed (no RT) did not yield a product. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Behavioral State Influences Brain Mast Cells
The thalami of males paired with sexually receptive females for 2 h or 5 days had more TB-positive (TB+) mast cells compared to males housed with familiar males [p = 0.017; Fig. 4(A)], extending our previous observations (Asarian et al., 2002). This statistically significant increase in the mast cell population was confined to the midthalamic region (AP −2.66 to −3.64 mm) encompassing the centromedial (CM), centrolateral (CL), paracentral (PC), posterior (Po), ventrolateral (VL), ventral posterolateral (VPL), and ventral posteromedial (VPM) nuclei [Fig. 4(A)]. A similar increase was documented using serotonin immunocytochemistry [Fig. 4(B)].
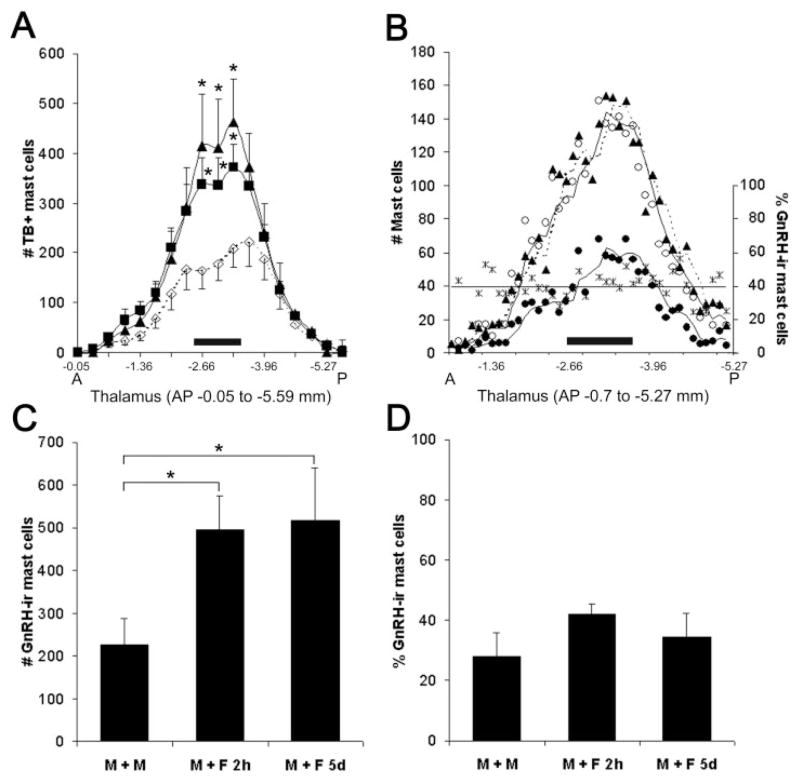
Figure 4.
Effect of cohabitation on thalamic mast cell number and phenotype. (A) Distribution of TB positive mast cells along the anterior- (A) posterior (P) extent of the thalamus in males exposed to sexually receptive females for 2 h (■), 5 days (▲), or cohabiting with familiar males (◇). The midthalamic region (−2.66 to −3.64 mm, represented by a solid horizontal bar) contained the most mast cells. Data points correspond to the average number of mast cells in bins spanning 300 μm. * Two hours and 5 days versus familiar males, p < 0.02. (B) For a representative animal (M+F 5 days), the distribution (left y axis; generated by a moving average trend line, Excel) of mast cells stained with various markers and the proportion (right y axis) of GnRH-I- IR mast cells along the A-P extent of the thalamus are shown. Each data point corresponds to the number of mast cells in each consecutive 50 μm section. For all three markers used, peak numbers of mast cells occur in the midthalamus (horizontal bar). Note that serotonin (○) and TB (▲) markers indicate similar number and distribution of mast cells. The number of mast cells double-labeled for GnRH-I and serotonin (●) shows the same general rostral to caudal distribution. The percent of double-labeled GnRH-I-and serotonin-IR mast cells (*) remains constant (represented by a best fit line). (C) The number of double-labeled (GnRH-I- and serotonin-) IR mast cells in the midthalamus is greater in male rats paired with females for 2 h (M+F 2 h) or 5 days (M+F 5 days) than in those housed with familiar males (M+M), * p < 0.05. (D) The proportion of GnRH-I- to serotonin-IR mast cells in the midthalamus is similar in all three experimental groups. For (A), (C), and (D), values represent the mean ±S.E.M.
GnRH-I-IR mast cells had a similar distribution to the entire mast cell population [Fig. 4(B)]. GnRH-I-IR mast cells also increased in the midthalamus when males cohabited with females [p = 0.036; Fig. 4(C)]. The number of GnRH-I-IR mast cells increased in proportion to the overall population [Fig. 4(D)].
Occasional TB+ and GnRH-I-IR mast cells were present in the median eminence in all three groups [Fig. 1(G–I)]. The change in the average number of TB+ mast cells in this area in M+M, M+F 2 h, and M+F 5 day approached significance (30.5 ± 9.0, 65.2 ± 19.4, and 12.4 ± 7.7, respectively; one-way ANOVA: p = 0.064).
DISCUSSION
It has previously been reported that brain mast cells are GnRH-I-IR in doves and mice (Zhuang et al., 1993; Yang et al., 1999); however, this work did not reveal whether mast cells synthesize GnRH-I or whether the peptide enters the mast cell granule by an endocytotic mechanism. The present study demonstrates that mast cells in the rat brain and in the peritoneal cavity synthesize GnRH-I. This finding is particularly interesting as the number of mast cells in the brain increases coincident with reproductive behavior (Silver et al., 1992; Zhuang et al., 1993; Silverman et al., 1994b) and treatment with gonadal steroids in doves (Wilhelm et al., 2000), mating and cohabitation in mice (Yang et al., 1999), and sexual and social stimuli from conspecifics in rats (Asarian et al., 2002). Consistent with previous findings, the increase in brain mast cells is rapid, is limited to specific nuclei in the dorsal thalamus, and is influenced by the stimulus condition presented by an opposite sex conspecific. A novel aspect revealed in the present work is that the proportion of GnRH-I-IR mast cells is constant irrespective of the behavioral state of the animal.
Brain Mast Cells Synthesize GnRH-I
The current experiments provide several lines of evidence that rat mast cells synthesize GnRH-I. First, brain mast cells are IR to antisera raised against the GnRH-I decapeptide. Second, and more importantly, these mast cells are also IR to antisera against GAP (a segment of the GnRH-I prohormone), suggesting that the protein is produced within the cell. It is unlikely that the immunoreactivity is due to GnRH-II, a second form of the neuropeptide encoded by a different gene, which is also found in rats and mice (Chen et al., 1998), as the GnRH-I GAP sequence (as demonstrated for tree shrew and human) is highly divergent from the GAP sequence of GnRH-II (Kasten et al., 1996; White et al., 1998). Third, the conclusion that mast cells synthesize GnRH-I is further supported by the in situ hybridization studies that employed a rat GnRH-I cRNA probe. Finally, RT-PCR of mRNA derived from peritoneal mast cells confirms that peripheral cells also synthesize GnRH-I. The GnRH-I mRNA sequence obtained from peritoneal mast cells is identical to that expressed by GnRH-I neurons. It is interesting to note that active forms of the GnRH-I peptide and mRNA are also synthesized by other hematopoietic cells, including lymphocytes and thymocytes (Emanuele et al., 1990; Azad et al., 1991; Maier et al., 1992; Chen et al., 1999). It is possible that the GnRH-I mRNA amplification of the peritoneal mast cell preparation obtained by RT-PCR was due to the presence of contamination by other hematopoietic cells. The immunocytochemistry showing peritoneal mast cells labeled for GnRH-I further supports our conclusion that peripheral mast cells produce GnRH-I and extends previous ultrastructural studies showing GnRH-I immunoreactivity in the Golgi apparatus as well as in secretory granules of brain mast cells (Silverman et al., 1994b, 2002).
GnRH-I Functions Outside of the HPG Axis
A growing body of evidence indicates that GnRH-I acts outside of the HPG axis, influencing neuronal excitability, behavioral responses, and neuroimmune interactions. For example, GnRH-I can change the electrical activity of neurons in vivo and in vitro. This was first shown in frog sympathetic ganglia, in which a GnRH-like peptide is released upon stimulation and mediates a slow synaptic potential (Jan and Jan, 1982). GnRH-I alters the firing pattern of neurons in the hippocampus (Palovcik and Phillips, 1986), where it also induces a long-duration depolarization associated with increased input resistance, a reduction in the slow after hyperpolarization (AHP), and a decrease in accommodation of CA1 neurons (Wong et al., 1990). Application of the peptide to slices of the preoptic area or hypothalamic ventromedial nucleus causes a long latency, excitatory action potential, as well as a modulation of responses to other neurotransmitters such as norepinephrine (Jennes et al., 1997). GnRH-I increases the excitability of olfactory receptor neurons in vitro by increasing the magnitude of a voltage-activated inward current (Eisthen et al., 2000). GnRH-I can act in paracrine/autocrine fashion and can regulate its own release and receptor expression as shown in immortalized GnRH neurons (GT1-7 cells) (Krsmanovic et al., 1993).
GnRH-I potentiates sexual behavior (Moss and McCann, 1973; Pfaff, 1973). When infused into the midbrain central gray, GnRH enhances sexual receptivity in female rats (Sakuma and Pfaff, 1980), even in hypophysectomized animals (Pfaff, 1973).
GnRH-I also acts as an immunomodulator (Marchetti et al., 1989; Jacobson et al., 1994). The action of GnRH-I as a mast cell activating molecule was discovered when GnRH-I and its analogues were used in the treatment of precocious puberty. Such treatment induced histamine secretion from mast cells, leading to cutaneous anaphylaxis (Sundaram et al., 1988; Rivier et al., 1986; Phillips et al., 1988). For this reason, novel GnRH analogues for the treatment of this disorder have been designed to circumvent these adverse clinical effects (Jiang et al., 2001). GnRH-I also stimulates lymphocyte proliferation (Marchetti et al., 1989; Morale et al., 1991) and interleukin-2 receptor expression (Batticane et al., 1991; Chen et al., 1999). Finally, GnRH-I and -II stimulate expression of the laminin receptor in normal T cells, followed by their adhesion to laminin and chemotaxis toward SDF-1α in vitro. Both peptides enhance T cell migration and entry into specific organs in vivo (Chen et al., 2002).
Activated (degranulating) mast cells are found in the brain under normal physiological conditions (Theoharides et al., 1995; Florenzano and Bentivoglio, 2000; Wilhelm et al., 2000). Release of mast cell granular contents into the brain parenchyma would make GnRH-I available in the thalamic nuclei where they are located (Florenzano and Bentivoglio, 2000; Asarian et al., 2002; present results) and in the median eminence (present study; Theoharides et al., 1995). At these sites, mast cell derived GnRH-I could act in a paracrine fashion as a neuromodulator as postulated earlier following the discoveries of GnRH-I axons and terminals in many brain regions (see Silverman et al., 1994a). It could also work in an autocrine manner modulating expression of cellular adhesion molecules and/or chemotaxis as in the case of T cells (Chen et al., 2002). It is therefore of interest to determine whether mast cells express on their surface a receptor for GnRH. Excluding the medial habenula of the epithalamus, GnRH-I receptor/binding sites have not been reported in the thalamus (Jennes and Conn, 1994). However, additional forms of GnRH receptors are being identified and one or more may be expressed in the thalamus (see review by Neill, 2002).
Brain Mast Cell Number
As in previous studies, the number of brain mast cells increases rapidly. The number of mast cells in the midthalamus of males following a 2 h exposure to a sexually receptive female is significantly greater than in males housed with familiar males. In doves, 2 h of courtship is sufficient to cause an increase in brain mast cells (Zhuang et al., 1993). Similarly, in rats, vital dye-labeled mast cells can enter the brain in as little as 1 h following an intravascular injection (Silverman et al., 2000). The increase in the mast cell population is apparently due to migration from the periphery, because no evidence of mast cell division was found using BrdU labeling (Silverman et al., 2002). The mature granule content and mediator expression indicate that the mast cells identified in this and in prior studies are differentiated beyond the precursor stage (Rodewald et al., 1996). Under normal conditions it is thought that mature mast cells do not circulate in the blood; hence, the source of this augmented population is likely to be via the pial sheath of the thalamic blood vessels (Lambracht-Hall et al., 1990; Manning et al., 1994).
Proportion of GnRH-I Mast Cells Does Not Change with State
The phenotype of mast cells is regulated by their local microenvironment. Changes in expression of mast cell stimulating factors, such as the kit ligand (stem cell factor), nerve growth factor, and interleukin-3, -4, -9, and -10, alter the content of stored mediators produced by mast cells (Galli, 2000). Therefore, the present study examined whether the proportion of thalamic mast cells containing GnRH-I varied with behavioral state. Although pairing of a male with a sexually receptive female increased the number of mast cells in the midthalamus, the proportion of cells that was GnRH-I-IR remained constant at 25% of the total across all states tested. This finding suggests that, at the time points tested, the expression of GnRH-I peptide in brain mast cells is not up-regulated and that the migration of mast cells from the periphery to the brain may not depend on their GnRH-I content or expression. These data also suggest that GnRH-I-IR mast cells are recruited into the same brain regions as other (e.g., serotonin containing) mast cells. However, there are two other possibilities: it is possible that only GnRH-I negative cells enter the CNS and once there activate GnRH-I synthesis. Alternatively, GnRH-I negative cells enter the CNS, but the resident thalamic mast cells start to express GnRH-I, thereby keeping the proportion constant.
In summary, the converging evidence derived from the variety of methods used in the present study demonstrates that mast cells in the rat brain transcribe the GnRH-I gene and that translation can be detected using antisera to GnRH-I and GAP. In addition, the number of mast cells increases in the brain in response to a behavioral stimulus, and the proportion of GnRH-I-IR mast cells is maintained. What role mast cell-derived GnRH-I might play in the thalamus, especially during times of mast cell number increase, is unknown. This work points to a novel mechanism of interaction between the cells of the immune and central nervous systems, in which non-neuronal effector cells such as mast cells are directed to specific areas of the brain and potentially affect both CNS and immune system function.
Acknowledgments
We thank Lori Asarian and Lance Kriegsfeld for critical reading of the manuscript, Tony Yuen for help with RT-PCR, and Honor Kirwan for technical assistance. The Optical Microscopy Facility at Columbia University was established by NIH Grants #S10 RR10506 and #S10 RR13701 and by the Lieber Foundation. It is supported by NIH Grant #P30 CA13696 to the Herbert Irving Comprehensive Cancer Center.
Contract grant sponsor: NIH; contract grant number: MH 54088 (A.J.S.).
Contract grant sponsor: NIH; contract grant number: MH 29380 (R.S.).
Contract grant sponsor: NIH; contract grant number: NS 43035 (M.K.).
Contract grant sponsor: Columbia University; Hormones: Biochemistry and Molecular Biology Fellowship; contract grant number: DK07328 (M.K.).
References
- Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:39–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- Adelman JP, Bond CT, Douglass J, Herbert E. Two mammalian genes transcribed from opposite strands of the same DNA locus. Science. 1987;235:1514–1517. doi: 10.1126/science.3547652. [DOI] [PubMed] [Google Scholar]
- Adelman JP, Mason AJ, Hayflick JS, Seeburg PH. Isolation of the gene and hypothalamic cDNA for the common precursor of gonadotropin-releasing hormone and prolactin release-inhibiting factor in human and rat. Proc Natl Acad Sci USA. 1986;83:179–183. doi: 10.1073/pnas.83.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Asarian L, Yousefzadeh E, Silverman AJ, Silver R. Stimuli from conspecifics influence brain mast cell population in male rats. Horm Behav. 2002;42:1–12. doi: 10.1006/hbeh.2002.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azad N, Emanuele NV, Halloran MM, Tentler J, Kelley MR. Presence of luteinizing hormone-releasing hormone (LHRH) mRNA in rat spleen lymphocytes. Endocrinology. 1991;128:1679–1681. doi: 10.1210/endo-128-3-1679. [DOI] [PubMed] [Google Scholar]
- Batticane N, Morale MC, Gallo F, Farinella Z, Marchetti B. Luteinizing hormone-releasing hormone signaling at the lymphocyte involves stimulation of interleukin-2 receptor expression. Endocrinology. 1991;129:277–286. doi: 10.1210/endo-129-1-277. [DOI] [PubMed] [Google Scholar]
- Bebo BF, Jr, Yong T, Orr EL, Linthicum DS. Hypothesis: a possible role for mast cells and their inflammatory mediators in the pathogenesis of autoimmune encephalomyelitis. J Neurosci Res. 1996;45:340–348. doi: 10.1002/(SICI)1097-4547(19960815)45:4<340::AID-JNR3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Bond CT, Hayflick JS, Seeburg PH, Adelman JP. The rat gonadotropin-releasing hormone: SH locus: structure and hypothalamic expression. Mol Endocrinol. 1989;3:1257–1262. doi: 10.1210/mend-3-8-1257. [DOI] [PubMed] [Google Scholar]
- Chen A, Ganor Y, Rahimipour S, Ben-Aroya N, Koch Y, Levite M. The neuropeptides GnRH-II and GnRH-I are produced by human T cells and trigger laminin receptor gene expression, adhesion, chemotaxis and homing to specific organs. Nat Med. 2002;8:1421–1426. doi: 10.1038/nm1202-801. [DOI] [PubMed] [Google Scholar]
- Chen A, Yahalom D, Ben-Aroya N, Kaganovsky E, Okon E, Koch Y. A second isoform of gonadotropin-releasing hormone is present in the brain of human and rodents. FEBS Lett. 1998;435:199–203. doi: 10.1016/s0014-5793(98)01064-3. [DOI] [PubMed] [Google Scholar]
- Chen HF, Jeung EB, Stephenson M, Leung PC. Human peripheral blood mononuclear cells express gonadotropin-releasing hormone (GnRH), GnRH receptor, and interleukin-2 receptor gamma-chain messenger ribonucleic acids that are regulated by GnRH in vitro. J Clin Endocrinol Metab. 1999;84:743–750. doi: 10.1210/jcem.84.2.5440. [DOI] [PubMed] [Google Scholar]
- Chen JJ, Li Z, Pan H, Murphy DL, Tamir H, Koepsell H, Gershon MD. Maintenance of serotonin in the intestinal mucosa and ganglia of mice that lack the high-affinity serotonin transporter: Abnormal intestinal motility and the expression of cation transporters. J Neurosci. 2001;21:6348–6361. doi: 10.1523/JNEUROSCI.21-16-06348.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czarnetzki BM. Release of mediators from purified rat mast cells during phagocytosis. Scand J Immunol. 1982;15:581–586. doi: 10.1111/j.1365-3083.1982.tb00687.x. [DOI] [PubMed] [Google Scholar]
- Dropp JJ. Mast cells in the central nervous system of several rodents. Anat Rec. 1972;174:227–237. doi: 10.1002/ar.1091740207. [DOI] [PubMed] [Google Scholar]
- Dvorak AM. Basophil and Mast Cell Degranulation and Recovery. In: Harris JR, editor. Blood Cell Biochemistry. Vol. 4. New York: Plenum Press; 1991. pp. 27–65. [Google Scholar]
- Eisthen HL, Delay RJ, Wirsig-Wiechmann CR, Dionne VE. Neuromodulatory effects of gonadotropin-releasing hormone on olfactory receptor neurons. J Neurosci. 2000;20:3947–3955. doi: 10.1523/JNEUROSCI.20-11-03947.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuele NV, Emanuele MA, Tentler J, Kirsteins L, Azad N, Lawrence AM. Rat spleen lymphocytes contain an immunoactive and bioactive luteinizing hormone-releasing hormone. Endocrinology. 1990;126:2482–2486. doi: 10.1210/endo-126-5-2482. [DOI] [PubMed] [Google Scholar]
- Esposito P, Gheorghe D, Kandere K, Pang X, Connolly R, Jacobson S, Theoharides TC. Acute stress increases permeability of the blood-brain-barrier through activation of brain mast cells. Brain Res. 2001;888:117–127. doi: 10.1016/s0006-8993(00)03026-2. [DOI] [PubMed] [Google Scholar]
- Florenzano F, Bentivoglio M. Degranulation, density and distribution of mast cells in the rat thalamus: A light and electron microscopic study in basal conditions and after intracerebroventricular administration of nerve growth factor. J Comp Neurol. 2000;424:651–669. [PubMed] [Google Scholar]
- Friend DS, Ghildyal N, Austen KF, Gurish MF, Matsumoto R, Stevens RL. Mast cells that reside at different locations in the jejunum of mice infected with Trichinella spiralis exhibit sequential changes in their granule ultra-structure and chymase phenotype. J Cell Biol. 1996;135:279–290. doi: 10.1083/jcb.135.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi K, Ra C, Isersky C, Rivera J. Comparative evaluation of the effect of pharmacological agents on endocytosis and coendocytosis of IgE by rat basophilic leukaemia cells. Immunology. 1986;58:105–110. [PMC free article] [PubMed] [Google Scholar]
- Galli SJ. New insights into “the riddle of the mast cells”: microenvironmental regulation of mast cell development and phenotypic heterogeneity. Lab Invest. 1990;62:5–33. [PubMed] [Google Scholar]
- Galli SJ. New concepts about the mast cell. N Engl J Med. 1993;328:257–265. doi: 10.1056/NEJM199301283280408. [DOI] [PubMed] [Google Scholar]
- Galli SJ. Mast cells and basophils. Curr Opin Hematol. 2000;7:32–39. doi: 10.1097/00062752-200001000-00007. [DOI] [PubMed] [Google Scholar]
- Hamada T, Ootomi M, Horikawa K, Niki T, Wakamatu H, Ishida N. The expression of the melatonin synthesis enzyme: arylalkylamine N-acetyltransferase in the supra-chiasmatic nucleus of rat brain. Biochem Biophys Res Commun. 1999;258:772–777. doi: 10.1006/bbrc.1999.0668. [DOI] [PubMed] [Google Scholar]
- Jacobson JD, Nisula BC, Steinberg AD. Modulation of the expression of murine lupus by gonadotropin-releasing hormone analogs. Endocrinology. 1994;134:2516–2523. doi: 10.1210/endo.134.6.8194477. [DOI] [PubMed] [Google Scholar]
- Jan LY, Jan YN. Peptidergic transmission in sympathetic ganglia of the frog. J Physiol. 1982;327:219–246. doi: 10.1113/jphysiol.1982.sp014228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennes L, Conn PM. Gonadotropin-releasing hormone and its receptors in rat brain. Front Neuroendocrinol. 1994;15:51–77. doi: 10.1006/frne.1994.1003. [DOI] [PubMed] [Google Scholar]
- Jennes L, Eyigor O, Janovick JA, Conn PM. Brain gonadotropin releasing hormone receptors: localization and regulation. Recent Prog Horm Res. 1997;52:475–491. [PubMed] [Google Scholar]
- Jiang G, Stalewski J, Galyean R, Dykert J, Schteingart C, Broqua P, Aebi A, Aubert ML, Semple G, Robson P, et al. GnRH antagonists: a new generation of long acting analogues incorporating p-ureido-phenylalanines at positions 5 and 6. J Med Chem. 2001;44:453–467. doi: 10.1021/jm0003900. [DOI] [PubMed] [Google Scholar]
- Jippo T, Lee YM, Ge Y, Kim DK, Okabe M, Kitamura Y. Tissue-dependent alteration of protease expression phenotype in murine peritoneal mast cells that were genetically labeled with green fluorescent protein. Am J Pathol. 2001;158:1695–1701. doi: 10.1016/S0002-9440(10)64125-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasten TL, White SA, Norton TT, Bond CT, Adelman JP, Fernald RD. Characterization of two new preproG-nRH mRNAs in the tree shrew: first direct evidence for mesencephalic GnRH gene expression in a placental mammal. Gen Comp Endocrinol. 1996;104:7–19. doi: 10.1006/gcen.1996.0135. [DOI] [PubMed] [Google Scholar]
- Khodr GS, Siler-Khodr TM. Placental luteinizing hormone-releasing factor and its synthesis. Science. 1980;207:315–317. doi: 10.1126/science.6985750. [DOI] [PubMed] [Google Scholar]
- Kjellen L, Lindahl U. Proteoglycans: Structure and interactions. Annu Rev Biochem. 1991;60:443–475. doi: 10.1146/annurev.bi.60.070191.002303. [DOI] [PubMed] [Google Scholar]
- Krsmanovic LZ, Stojilkovic SS, Mertz LM, Tomic M, Catt KJ. Expression of gonadotropin-releasing hormone receptors and autocrine regulation of neuropeptide release in immortalized hypothalamic neurons. Proc Natl Acad Sci USA. 1993;90:3908–3912. doi: 10.1073/pnas.90.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambracht-Hall M, Dimitriadou V, Theoharides TC. Migration of mast cells in the developing rat brain. Brain Res Dev Brain Res. 1990;56:151–159. doi: 10.1016/0165-3806(90)90077-c. [DOI] [PubMed] [Google Scholar]
- Lutzelschwab C, Pejler G, Aveskogh M, Hellman L. Secretory granule proteases in rat mast cells. Cloning of 10 different serine proteases and a carboxypeptidase A from various rat mast cell populations. J Exp Med. 1997;185:13–29. doi: 10.1084/jem.185.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier CC, Marchetti B, LeBoeuf RD, Blalock JE. Thymocytes express a mRNA that is identical to hypothalamic luteinizing hormone-releasing hormone mRNA. Cell Mol Neurobiol. 1992;12:447–454. doi: 10.1007/BF00711545. [DOI] [PubMed] [Google Scholar]
- Malaviya R, Abraham SN. Mast cell modulation of immune response to bacteria. Immunol Rev. 2001;179:16–24. doi: 10.1034/j.1600-065x.2001.790102.x. [DOI] [PubMed] [Google Scholar]
- Manning KA, Pienkowski TP, Uhlrich DJ. Histaminergic and non-histamine-immunoreactive mast cells within the cat lateral geniculate complex examined with light and electron microscopy. Neuroscience. 1994;63:191–206. doi: 10.1016/0306-4522(94)90016-7. [DOI] [PubMed] [Google Scholar]
- Marchetti B, Guarcello V, Morale MC, Bartoloni G, Raiti F, Palumbo G, Jr, Farinella Z, Cordaro S, Scapagnini U. Luteinizing hormone-releasing hormone (LHRH) agonist restoration of age-associated decline of thymus weight, thymic LHRH receptors, and thymocyte proliferative capacity. Endocrinology. 1989;125:1037–1045. doi: 10.1210/endo-125-2-1037. [DOI] [PubMed] [Google Scholar]
- Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77:1033–1079. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- Morale MC, Batticane N, Bartoloni G, Guarcello V, Farinella Z, Galasso MG, Marchetti B. Blockade of central and peripheral luteinizing hormone-releasing hormone (LHRH) receptors in neonatal rats with a potent LHRH-antagonist inhibits the morphofunctional development of the thymus and maturation of the cell-mediated and humoral immune responses. Endocrinology. 1991;128:1073–1085. doi: 10.1210/endo-128-2-1073. [DOI] [PubMed] [Google Scholar]
- Moss RL, McCann SM. Induction of mating behavior in rats by luteinizing hormone-releasing factor. Science. 1973;181:177–179. doi: 10.1126/science.181.4095.177. [DOI] [PubMed] [Google Scholar]
- Neill JD. GnRH and GnRH receptor genes in the human genome. Endocrinology. 2002;143:737–743. doi: 10.1210/endo.143.3.8705. [DOI] [PubMed] [Google Scholar]
- Oikawa M, Dargan C, Ny T, Hsueh AJW. Expression of gonadotropin-releasing hormone and prothymosin-messenger ribonucleic acid in the ovary. Endocrinology. 1990;127:2350–2356. doi: 10.1210/endo-127-5-2350. [DOI] [PubMed] [Google Scholar]
- Palovcik RA, Phillips MI. A biphasic excitatory response of hippocampal neurons to gonadotropin-releasing hormone. Neuroendocrinology. 1986;44:137–141. doi: 10.1159/000124636. [DOI] [PubMed] [Google Scholar]
- Pang X, Letourneau R, Rozniecki JJ, Wang L, Theoharides TC. Definitive characterization of rat hypothalamic mast cells. Neuroscience. 1996;73:889–902. doi: 10.1016/0306-4522(95)00606-0. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. In: The Rat Brain in Stereotaxic Coordinates. 2. Peng C, Fan NC, Ligier M, Vaananen J, Leung PCK, editors. San Diego, CA: Academic Press Inc; 1986. 1994. [Google Scholar]
- Expression and regulation of gonadotropin-releasing hormone (GnRH) and GnRH receptor messenger ribonucleic acids in human granulosaluteal cells. Endocrinology. 135:1740–1746. doi: 10.1210/endo.135.5.7956897. [DOI] [PubMed] [Google Scholar]
- Pfaff DW. Luteinizing hormone-releasing factor potentiates lordosis behavior in hypophysectomized ovariectomized female rats. Science. 1973;182:1148–1149. doi: 10.1126/science.182.4117.1148. [DOI] [PubMed] [Google Scholar]
- Phillips A, Hahn DW, McGuire JL, Ritchie D, Capetola RJ, Bowers C, Folkers K. Evaluation of the anaphylactoid activity of a new LHRH antagonist. Life Sci. 1988;43:883–888. doi: 10.1016/0024-3205(88)90263-9. [DOI] [PubMed] [Google Scholar]
- Reynolds DS, Stevens RL, Gurley DS, Lane WS, Austen KF, Serafin WE. Isolation and molecular cloning of mast cell carboxypeptidase A. A novel member of the carboxypeptidase gene family. J Biol Chem. 1989;264:20094–20099. [PubMed] [Google Scholar]
- Rivier JE, Porter J, Rivier CL, Perrin M, Corrigan A, Hook WA, Siraganian RP, Vale WW. New effective gonadotropin releasing hormone antagonists with minimal potency for histamine release in vitro. J Med Chem. 1986;29:1846–1851. doi: 10.1021/jm00160a008. [DOI] [PubMed] [Google Scholar]
- Rodewald HR, Dessing M, Dvorak AM, Galli SJ. Identification of a committed precursor for the mast cell lineage. Science. 1996;271:818–822. doi: 10.1126/science.271.5250.818. [DOI] [PubMed] [Google Scholar]
- Sakuma Y, Pfaff DW. LH-RH in the mesencephalic central grey can potentiate lordosis reflex of female rats. Nature. 1980;283:566–567. doi: 10.1038/283566a0. [DOI] [PubMed] [Google Scholar]
- Schumacher M, Coirini H, Pfaff DW, McEwen BS. Light-dark differences in behavioral sensitivity to oxytocin. Behav Neurosci. 1991;105:487–492. doi: 10.1037//0735-7044.105.3.487. [DOI] [PubMed] [Google Scholar]
- Secor VH, Secor WE, Gutekunst CA, Brown MA. Mast cells are essential for early onset and severe disease in a murine model of multiple sclerosis. J Exp Med. 2000;191:813–822. doi: 10.1084/jem.191.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver R, Ramos CL, Silverman AJ. Sexual behavior triggers the appearance of non-neuronal cells containing gonadotropin-releasing hormone-like immunoreactivity. J Neuroendocrinol. 1992;4:207–210. doi: 10.1111/j.1365-2826.1992.tb00160.x. [DOI] [PubMed] [Google Scholar]
- Silver R, Silverman AJ, Vitkovic L, Lederhendler II. Mast cells in the brain: Evidence and functional significance. Trends Neurosci. 1996;19:25–31. doi: 10.1016/0166-2236(96)81863-7. [DOI] [PubMed] [Google Scholar]
- Silverman AJ, Asarian L, Khalil M, Silver R. GnRH, brain mast cells and behavior. In: Parhar IS, editor. Gonadotropin-Releasing Hormone: Molecules and Receptors. Progress in Brain Research. Vol. 141. Amsterdam; Elsevier: 2002. pp. 317–327. [DOI] [PubMed] [Google Scholar]
- Silverman AJ, Livne I, Witkin JW. The Gonadotropin-Releasing Hormone (GnRH) Neuronal Systems: Immunocytochemistry and in situ hybridization. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. New York: Raven Press; 1994a. pp. 1683–1709. [Google Scholar]
- Silverman AJ, Millar RP, King JA, Zhuang X, Silver R. Mast cells with gonadotropin-releasing hormone-like immunoreactivity in the brain of doves. Proc Natl Acad Sci USA. 1994b;91:3695–3699. doi: 10.1073/pnas.91.9.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman AJ, Sutherland AK, Wilhelm M, Silver R. Mast cells migrate from blood to brain. J Neurosci. 2000;20:401–408. doi: 10.1523/JNEUROSCI.20-01-00401.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens RL, Austen KF. Recent advances in the cellular and molecular biology of mast cells. Immunol Today. 1989;10:381–386. doi: 10.1016/0167-5699(89)90272-7. [DOI] [PubMed] [Google Scholar]
- Sundaram K, Didolkar A, Thau R, Chaudhuri M, Schmidt F. Antagonists of luteinizing hormone releasing hormone bind to rat mast cells and induce histamine release. Agents Actions. 1988;25:307–313. doi: 10.1007/BF01965036. [DOI] [PubMed] [Google Scholar]
- Theoharides TC. Mast cells: the immune gate to the brain. Life Sci. 1990;46:607–617. doi: 10.1016/0024-3205(90)90129-f. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Spanos C, Pang X, Alferes L, Ligris K, Letourneau R, Rozniecki JJ, Webster E, Chrousos GP. Stress-induced intracranial mast cell degranulation: a corticotropin-releasing hormone-mediated effect. Endocrinology. 1995;136:5745–5750. doi: 10.1210/endo.136.12.7588332. [DOI] [PubMed] [Google Scholar]
- Wasserman SI. Mast cell-mediated inflammation in asthma. Ann Allergy. 1989;63:546–550. [PubMed] [Google Scholar]
- Wedemeyer J, Tsai M, Galli SJ. Roles of mast cells and basophils in innate and acquired immunity. Curr Opin Immunol. 2000;12:624–631. doi: 10.1016/s0952-7915(00)00154-0. [DOI] [PubMed] [Google Scholar]
- White RB, Eisen JA, Kasten TL, Fernald RD. Proc Natl Acad Sci USA. Vol. 95. 1998. Second gene for gonadotropin-releasing hormone in humans; pp. 305–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm M, King B, Silverman AJ, Silver R. Gonadal steroids regulate the number and activational state of mast cells in the medial habenula. Endocrinology. 2000;141:1178–1186. doi: 10.1210/endo.141.3.7352. [DOI] [PubMed] [Google Scholar]
- Wong M, Eaton MJ, Moss RL. Electrophysiological actions of luteinizing hormone-releasing hormone: intra-cellular studies in the rat hippocampal slice preparation. Synapse. 1990;5:65–70. doi: 10.1002/syn.890050106. [DOI] [PubMed] [Google Scholar]
- Yang M, Chien C, Lu K. Morphological, immunohistochemical and quantitative studies of murine brain mast cells after mating. Brain Res. 1999;846:30–39. doi: 10.1016/s0006-8993(99)01935-6. [DOI] [PubMed] [Google Scholar]
- Zhuang X, Silverman AJ, Silver R. Reproductive behavior, endocrine state, and the distribution of GnRH-like immunoreactive mast cells in dove brain. Horm Behav. 1993;27:283–295. doi: 10.1006/hbeh.1993.1021. [DOI] [PubMed] [Google Scholar]