Abstract
Purpose:
The purpose of the study was to explore optimal combinations of currently actively developed drugs for dually targeting the Ras → Raf → MAPK kinase (MEK) → MAPK/ERK (MAPK) and the phosphatidylinositol 3-kinase/Akt pathways as effective treatments for thyroid cancer.
Experimental Design:
We tested the combinations of the Akt inhibitors MK2206 or perifosine with the BRAFV600E inhibitor PLX4032 or the MEK1/2 inhibitor AZD6244 in thyroid cancer cells harboring both the BRAFV600E and PIK3CA mutations.
Results:
We found that MK2206 could potently, when used alone, and synergistically, when combined with either PLX4032 or AZD6244, inhibit thyroid cancer cell growth with all the combination index values lower than 1. Perifosine could potently inhibit thyroid cancer cell growth when used alone, but a strong antagonism occurred between this drug and PLX4032 or AZD6244 in the inhibition of thyroid cancer cell growth with all combination index values higher than 1. Combinations of MK2206 with PLX4032 or AZD6244 dramatically enhanced G1 cell cycle arrest induced by each drug alone. However, G2 cell cycle arrest uniquely induced by perifosine alone and G1 cell cycle arrest induced by PLX4032 or AZD6244 were both reversed by combination treatments, providing a mechanism for their antagonism. All these drugs could correspondingly inhibit the MAPK and phosphatidylinositol 3-kinase/Akt signalings, confirming their expected target effects.
Conclusions:
We demonstrated, unexpectedly, opposite outcomes of MK2206 and perifosine in their combinational treatments with BRAFV600E/MEK inhibitors in thyroid cancer cells. The data may help appropriate selection of these prominent drugs for clinical trials of combination therapies for thyroid cancer.
The Ras → Raf → MAPK kinase (MEK) → MAPK/ERK (MAPK) pathway, driven by the BRAFV600E mutation and other genetic alterations, plays a fundamental role in thyroid tumorigenesis (1, 2). The phosphatidylinositol 3-kinase (PI3K)/Akt pathway, driven by various genetic alterations, such as PIK3CA mutations, similarly plays an important role in this process (3, 4). Concurrence of genetic alterations in the MAPK and PI3K/Akt pathways is common in aggressive thyroid cancers (5–8). In fact, about 80% of cases of anaplastic thyroid cancer, the most aggressive and lethal thyroid cancer, harbored genetic mutations that could potentially dually activate the MAPK and PI3K/Akt pathways (8). This provides a strong molecular basis for a well-proposed therapeutic strategy of simultaneously targeting the two pathways using combination drugs for thyroid cancer (1, 9, 10). The need for such a drug combination strategy is also supported by the results from several recent single-agent clinical trials on thyroid cancer in which only partial response was achieved and was generally seen in less than 50% of cases (11–14).
Several prominent inhibitors of the MAPK and PI3K/Akt pathway have been individually tested in clinical trials on various human cancers and in preclinical studies on thyroid cancer cells. For example, the BRAFV600E-selective inhibitor PLX4032 showed great promises in treating metastatic melanoma in recent clinical trials (15, 16). Preclinical studies also demonstrated potent BRAFV600E-selective inhibition of thyroid cancer cell growth by this drug (17, 18). AZD6244 is a potent MEK1/2 inhibitor that has well-proven patient tolerance in clinical trials although its effect as a single drug seemed to be limited in several cancers (19). Akt inhibitors MK2206 and perifosine showed promising preclinical antitumor activities (20–23) and are currently under active clinical development (24, 25). The two Akt inhibitors act through different mechanisms. MK2206 is an allosteric Akt inhibitor with high Akt selectivity. Perifosine is an alkylphospholipid that targets the pleckstrin homology domain of Akt and blocks its membrane translocation, hence preventing Akt phosphorylation and activation (26). Both MK2206 and perifosine showed potent inhibitory effects on the proliferation of thyroid cancer cells when used alone, particularly in cells harboring genetic alterations that activate the PI3K/Akt pathway (21, 23). These encouraging preclinical results temptingly suggest that combination of these Akt inhibitors with BRAFV600E/MEK inhibitors would provide a more effective treatment for thyroid cancer. However, given the different mechanisms involved in the inhibition of the PI3K/Akt pathway by MK2206 and perifosine, the outcomes of their combination with the MAPK pathway inhibitors in thyroid cancer seem to be uncertain.
In the present study, we used thyroid cancer cell lines to examine the feasibility of combining the Akt inhibitors MK2206 or perifosine with the BRAFV600E inhibitor PLX4032 or the MEK inhibitor AZD6244 to dually target the MAPK and PI3K/Akt pathways as a therapeutic strategy for thyroid cancer.
Materials and Methods
Cell lines and reagents
The anaplastic thyroid cancer cell line OCUT1 was provided by Dr. Naoyoshi Onoda (Osaka City University Graduate School of Medicine, Osaka, Japan) and the papillary thyroid cancer cell line K1 was provided by Dr. David Wynford-Thomas (University of Wales College of Medicine, Cardiff, UK). The OCUT1 cell line harbored a homozygous PIK3CAH1047R mutation and the K1 cell line harbored a homozygous PIK3CAE542K mutation. Both cell lines harbored a heterozygous BRAFV600E mutation. Cells were cultured in RPMI 1640 supplemented with 10% fetal bovine serum in 5% CO2 at 37 C. MK2206 was purchased from ChemieTek (Indianapolis, IN), perifosine and AZD4244 were from Selleck Chemicals (Houston, TX), and PLX4032 was from Plexxikon Inc. (Berkeley, CA). MK2206, AZD6244, and PLX4032 were dissolved in dimethylsulfoxide and perifosine in PBS, all in 10 mm stock.
Western blotting
Cells were washed with PBS and lysed in radioimmunoprecipitation assay buffer supplemented with 1% phenylmethylsulfonyl fluoride, 1% protease inhibitor cocktail, and 1% sodium orthovanadate (Santa Cruz Biotechnology, Santa Cruz, CA). Cell lysate proteins were quantified, denatured, and resolved on 10% SDS-PAGE and transferred onto polyvinylidene difluoride membranes (Amersham Pharmacia Biotech, Piscataway, NJ). Membranes were blocked with 5% nonfat milk in PBS with 0.1% Tween 20 and blotted with primary antibodies: antiphospho-ERK (sc-7383), antiphospho-Akt1/2/3(Ser 473) (sc-7985-R), anti-p21 (sc-397), anticyclin D1 (sc-718), and antiactin (sc-1616-R) (all from Santa Cruz Biotechnology); antiphospho-p70S6K (9205), antiphospho-4E-binding protein 1 (4EBP1) (2855), and anti-p27 Kip1 (3688) (all from Cell Signaling Technology, Beverly, MA). Membranes were washed with PBS with 0.1% Tween 20 and incubated with horseradish peroxidase-conjugated antirabbit (sc-2204) or antimouse (sc-2005) secondary antibodies (Santa Cruz Biotechnology). Signals were visualized using the enhanced chemiluminescence detection system (Amersham Pharmacia Biotech). Protein band intensities were quantified using the Image J software (Wayne Rasband, The National Institute of Mental Health, Bethesda, MD).
Cell proliferation assay and drug combination analysis
Cell proliferation was performed using 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay. Briefly, cells were plated on 96-well plates in triplicates 1 d before treating drugs were added at indicated concentrations, individually or in combinations. The medium and drug were replenished every 24 h. After 5 d of treatments, 10 μl of 5 mg/ml MTT (Invitrogen, Carlsbad, CA) was added to the cell culture and incubated for 4 h at 37 C, followed by the addition of 100 μl of 10% sodium dodecyl sulfate for overnight incubation. Absorbance was measured at the test wavelength of 570 nm and the reference wavelength of 670 nm. CalcuSyn software (Biosoft, Great Shelford, Cambridge, UK) was used to calculate the combination index (CI) and isobologram to quantitatively determine the effect of drug interactions, according to the median-effect method of Chou and Talalay (27) and as described (28). CI values less than 1, 1, and greater than 1 represent synergism, additivity, and antagonism, respectively. The isobologram is formed by plotting the concentrations of each drug required for 50% inhibition (ED50) on the x- and y-axis, respectively, and connecting them to draw a line segment, which is ED50 isobologram. Combination data points that fall on, below, and above the line segment represent additivity, synergism, and antagonism, respectively.
Cell cycle analysis
DNA content analysis was performed by propidium iodide (PI) staining and flow cytometry measurement. Cells were cultured in 10-cm dish 1 d before treating drugs were added at indicated concentrations, individually or in combination. Twenty-four hours later, cells were harvested and gently washed with cold PBS containing 2% fetal bovine serum and fixed in 70% cold ethanol. Cells were then pelleted, washed, and stained with PI/ribonuclease staining buffer (BD Biosciences, San Jose, CA) for 15 min at room temperature. Fluorescences were measured by flow cytometry and analyzed by the Cellquest software (BD Biosciences).
Cell apoptosis
Cell apoptosis was analyzed using the annexin V-fluorescein isothiocyanate (FITC) apoptosis detection kit (BD Biosciences) following the instructions of the manufacturer. In brief, cells were plated in six-well plates and treated with various drugs for the indicated times. Both floating and attached cells were harvested and washed with cold PBS, followed by incubation with FITC annexin V and PI in 1× binding buffer for 15 min at room temperature and subsequent flow cytometry.
Results
MK2206 synergistically inhibited the proliferation of thyroid cancer cells when combined with PLX4032 or AZD6244, whereas perifosine antagonized their effects
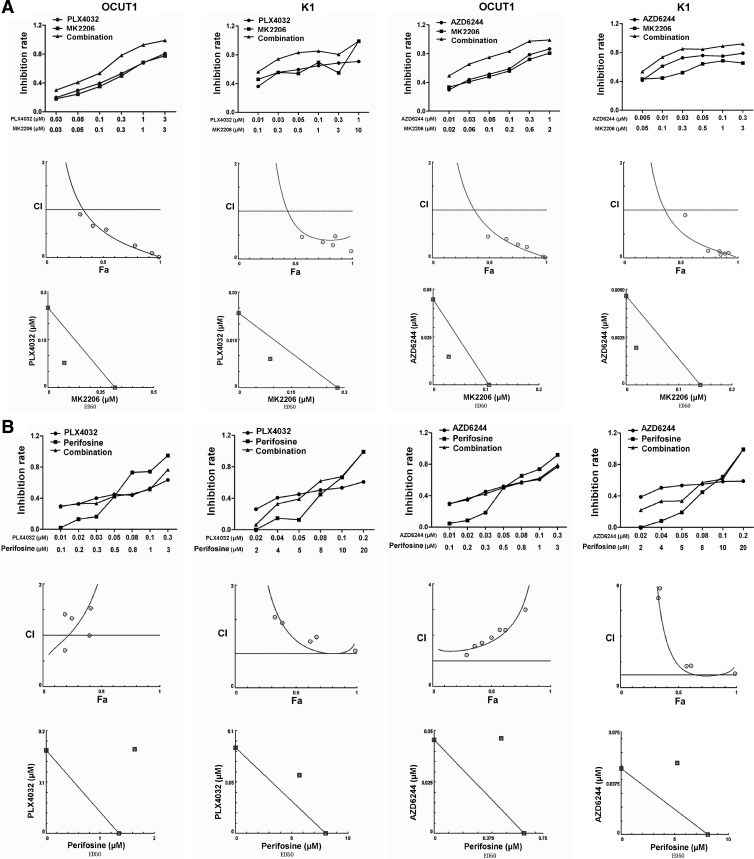
As shown in Fig. 1A, combination of MK2206 with either PLX4032 or AZD6244 significantly potentiated the inhibitory effect of any single drug on cell proliferation of OCUT1 and K1 cells, which both harbored BRAFV600E and PIK3CA mutations (Fig. 1A, upper panel). This is consistent with a recent report on the synergism between MK2206 and AZD6244 in the inhibition of lung cancer cells (29) as well as the synergism of Akt knockdown and AZD6244 in the inhibition of melanoma cells (30). Virtually all the CI values with the combinations of MK2206 and PLX4032 or MK2206 and AZD6244 in the two cells were much lower than 1, with averages at ED50 of 0.63 and 0.79 in the two cells, respectively, for the former combination, and 0.45 and 0.61 in the two cells for the latter combination (Fig. 1A, middle panel and Table 1). In the isobologram analysis, all the data points of different combinations were located below the isobologram line connecting the ED50 points of the individual drugs on the x- and y-axis (Fig. 1A, lower panel). These results were consistent with a strong synergism of MK2206 with PLX4032 or AZD6244 in inhibiting OCUT1 and K1 cells (Fig. 1A, lower panel). We also tested MK2206 with AZD6244 in SW1736, FTC133, WRO, and KAT18 cells. SW1736 cells harbor only BRAF mutation, FTC133 cells harbor only PTEN alterations, and WRO and KAT18 cells harbor no known genetic alterations in the MAPK and PI3K/Akt pathways. No or only weak synergism of these inhibitors was observed in these cells (data not shown).
Fig. 1.
MK2206 synergistically inhibited the proliferation of thyroid cancer cells when combined with PLX4032 or AZD6244, whereas perifosine antagonized their effects. A, OCUT1 and K1 cells were treated with MK2206, PLX4032, and AZD6244, individually or in the indicated combinations, with fixed molecular ratios at indicated concentrations for 5 d, followed by an MTT assay. Upper panel, Cell proliferation inhibition rates [defined by OD value (control − treatment)/control] of MK2206 (represented by square symbols), PLX4032, or AZD6244 (circles) and the indicated combinations (triangles). Middle panel, Combination index plot for the effects of the combined use of MK2206 with PLX4032 (the left two panels) or AZD6244 (the right two panels). Fa, Fractional effect. Lower panel, ED50 isobologram for the combination of MK2206 with PLX4032 or AZD6244. B, OCUT1 and K1 cells were treated with perifosine, PLX4032, or AZD6244 as similarly described in A. Upper panel, Cell proliferation inhibition rates of perifosine (represented by square symbols), PLX4032, or AZD6244 (circles) and the indicated combinations (triangles). Middle panel, Combination index plot for the effects of combination of perifosine with PLX4032 (the left two panels) or AZD6244 (the right two panels). Fa, Fractional effect. Lower panel, ED50 isobologram for the combination of perifosine with PLX4032 or AZD6244.
Table 1.
Combination index values of MK2206 with PLX4032 or AZD6244 as well as perifosine with PLX4032 or AZD6244 in thyroid cancer cells
| Drug combination | Cell line | Combination index |
||
|---|---|---|---|---|
| ED50 | ED75 | ED90 | ||
| MK2206/PLX4032 | OCUT1 | 0.63 | 0.24 | 0.09 |
| K1 | 0.79 | 0.39 | 0.39 | |
| MK2206/AZD6244 | OCUT1 | 0.45 | 0.22 | 0.11 |
| K1 | 0.61 | 0.15 | 0.07 | |
| Perifosine/PLX4032 | OCUT1 | 2.01 | 5.12 | 18.27 |
| K1 | 1.45 | 1.16 | 1.34 | |
| Perifosine/AZD6244 | OCUT1 | 2.05 | 3.87 | 9.25 |
| K1 | 2.99 | 1.22 | 1.41 | |
When perifosine was combined either with PLX4032 or AZD6244 in the two cells, cell inhibition rates were actually lower than those achieved with individual drugs (Fig. 1B, upper panel). The CI values of combinations of perifosine with PLX4032 or AZD6244 were virtually all higher than 1, with averages at ED50 of 2.01 and 1.45 in the two cells, respectively, for the former combination and 2.05 and 2.99 for the latter combination (Fig. 1B, middle panel, and Table 1). The combination data points in the isobologram were all located above the isobologram line at ED50 in both cells (Fig. 1B, lower panel). These results were consistent with a strong antagonism between perifosine and BRAFV600E/MEK inhibitors in inhibiting the thyroid cancer cells.
Effects of the Akt inhibitors and BRAFV600E/MEK inhibitors, individually or in combinations, on the MAPK and PI3K/Akt signalings in thyroid cancer cells
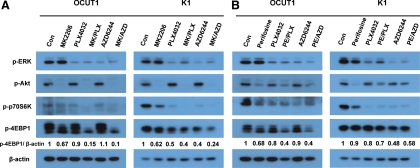
As shown in Fig. 2A, in both OCUT1 and K1 cells, treatment with MK2206 at 1 μm for 24 h caused and maintained complete inhibition of phosphorylated (p)-Akt. Treatment with PLX4032 at 0.5 μm or AZD6244 at 0.2 μm for 24 h caused and maintained dramatic inhibition of p-ERK. Combination of MK2206 with PLX4032 or AZD6244 effectively inhibited both p-Akt and p-ERK and, interestingly, enhanced the inhibitory effect of each single drug on p-p70S6K and p-4EBP1, two downstream effectors in the PI3K/Akt pathways (Fig. 2A), suggesting a stronger inhibition of the PI3K/Akt signaling by combination use of MK2206 with PLX4032 or AZD6244. Perifosine at 3 μm in OCUT1 cells and 10 μm in K1 cells nearly completely inhibited p-Akt (Fig. 2B). When combined with PLX4032 or AZD6244, the effect of perifosine remained in OCUT1 cells and seemed to be slightly diminished in K1 cells (Fig. 2B), although the inhibition of p-p70S6K by the combination treatments remained in both cells. The inhibition of p-ERK by PLX4032 or AZD6244 remained in the presence of perifosine in both cells (Fig. 2B). The inhibitory effects of Akt inhibitors on 4EBP1 were more profound in OCUT1 cells than in K1 cells, although the effects of these inhibitors on Akt phosphorylation were significant in both cells. Although 4EBP1 is thought to be coupled to Akt signaling in many cells, this coupling does not seem to be strong in K1 cells. Also, the p-ERK level with PLX4032 or AZD6244 alone was somehow slightly lower than that with their combination with MK2206 or perifosine, although, compared with control vehicle treatments, it was dramatically reduced under all these conditions. The mechanism for this phenomenon is unclear.
Fig. 2.
Effects of the Akt inhibitors and BRAFV600E/MEK inhibitors, used individually or in combinations, on the MAPK and PI3K/Akt signalings in thyroid cancer cells. A, OCUT1 and K1 cells were treated with 1 μm MK2206, 0.5 μm PLX4032, or 0.2 μm AZD6244, individually or in the indicated combinations (MK/PLX and MK/AZD, respectively) for 24 h, followed by cell lysis in radioimmunoprecipitation assay buffer. Cell lysates were subjected to Western blotting with antibodies against p-ERK, p-Akt, p-p70S6K, p-4EBP1, or β-actin. β-Actin was used for quality control. Con, Control. B, OCUT1 and K1 cells were treated with perifosine (3 μm for OCUT1 cells and 10 μm for K1 cells), 0.5 μm PLX4032, and 0.2 μm AZD6244, individually or in the indicated combinations (PE/PLX and PE/AZD, respectively) for 24 h, followed by Western blotting as described in A. Relative quantification of p-4EBP1, as shown underneath the band, was determined by the ratio of the density of p-4EBP1 of different treatments to control vehicle treatments, which had already been divided by the density of their respective β-actin bands for protein correction.
Effects of the Akt inhibitors and BRAFV600E/MEK inhibitors, individually or in combinations, on cell cycle of thyroid cancer cells
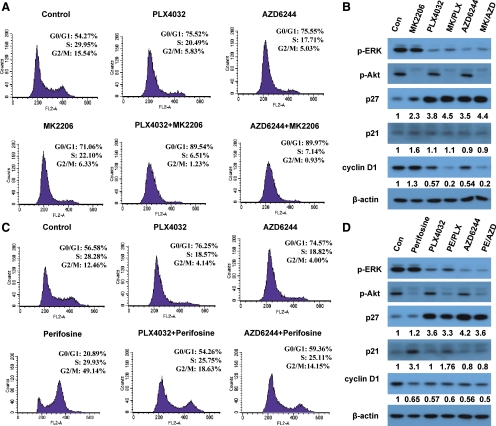
We next examined the effects of the various inhibitors on cell cycles. As shown in Fig. 3A, using OCUT1 cells as a representative, PLX4032, AZD6244, and MK2206 could each individually increase cell percentages in G0/G1phase, from 54.27% in the control cells to 75.52, 75.55, and 71.06% in the treated cells, respectively. Correspondingly, the S phase and G2/M phase cell percentages were decreased, from 29.95 and 15.54% in the control cells to 20.49 and 5.83%, 17.71 and 5.03%, and 22.1 and 6.33% in the treated cells, respectively. Combination of MK2206 with PLX4032 or AZD6244 drove further the G0/G1, S, and G2/M phases to 89.54, 6.51, and 1.23%, or to 89.97, 7.14, and 0.93%, respectively. Therefore, the combination use of MK2206 with BRAFV600E/MEK inhibitors virtually completely arrested cell cycle in the G0/G1 phase. Analysis on cell cycle regulators showed that the level of p27Kip1, which is required for G1 arrest (31), was significantly increased by treatment of cells with all the three drugs administered individually, although MK2206 showed a relatively modest effect (Fig. 3B). Combination of MK2206 with PLX4032 or AZD6244 caused a more pronounced expression of p27 than each drug alone. In comparison, the other Cip/Kip family member p21Waf1 showed comparable protein levels among different drug treatments (Fig. 3B). With the G0/G1 cell cycle arrest, the G1/S phase transition regulator cyclin D1 was correspondingly decreased in most of these treatment conditions (Fig. 3B). It is notable that although MK2206 itself had no inhibitory effect on cyclin D1 and PLX4032 or AZD6244 could only partially inhibit cyclin D1, the combination of MK2206 with either of the latter two nearly completely inhibited cyclin D1 expression. These results demonstrated that the inhibitory effects of the combined use of MK2206 with PLX4032 or AZD6244 on cell growth were mediated mainly by G1 cell cycle arrest with corresponding changes in the expression of cell cycle regulators p27Kip1 and cyclin D1.
Fig. 3.
Effects of MK2206, perifosine, PLX4032, or AZD6244 and their indicated combinations on cell cycle of thyroid cancer cells. A, OCUT1 cells were treated with 1 μm MK2206, 0.5 μm PLX4032, or 0.2 μm AZD6244, individually or in the indicated combinations, for 24 h. Cells were then collected, stained with PI, and subjected to flow cytometry. B, Effects of MK2206, PLX4032, and AZD6244 on the expression of cell cycle regulators. OCUT1 cells were treated with 1 μm MK2206, 0.5 μm PLX4032, and 0.2 μm AZD6244, individually or in the indicated combinations, for 24 h. Cell proteins were subjected to Western blotting, using antibodies against p-ERK, p-Akt, p27, p21, cyclin D1, or β-actin. β-Actin was used for quality control. Con, Control. C, OCUT1 cells were treated with 3 μm perifosine, 0.5 μm PLX4032, and 0.2 μm AZD6244, individually or in the indicated combinations, for 24 h and subjected to cell cycle analyses as in A. D, OCUT1 cells were treated with 3 μm perifosine, 0.5 μm PLX4032, and 0.2 μm AZD6244, individually or in the indicated combinations, for 24 h, followed by Western blotting analyses as in B. Relative quantification of p27, p21, and cyclin D1 shown below the corresponding bands was determined by the ratio of the density of the indicated proteins of different treatments to vehicle control treatments, which had already been divided by the density of their respective β-actin bands.
Perifosine alone uniquely caused a dramatic increase in G2/M phase of OCUT1 cells, from 12.46% in the control cells to 49.14% in the treated cells (Fig. 3C), accompanied by a decrease in the G0/G1phase, from 56.58 to 20.89%, suggesting G2/M arrest. G2 phase arrest by perifosine was also seen in other cancer cells (32, 33). Interestingly, when combined with PLX4032 or AZD6244, perifosine caused a cell cycle pattern very similar to that of control cells that were not treated with any drug, with a smaller cell percentage in G0/G1 phase than that induced by PLX4032 or AZD6244 alone and a smaller cell percentage in G2/M phase than that induced by perifosine alone (Fig. 3C). Given the better cell growth with combined use of perifosine with the BRAFV600E/MEK inhibitors than each drug alone, i.e. antagonism between the former and the latter (Fig. 1), the results in Fig. 3C suggested that the combination use of perifosine with the BRAFV600E/MEK inhibitors reversed the cell cycle arrest induced by each drug alone. To further confirm this, we examined the expression level of cell cycle regulators. Expression of p27Kip1 was markedly increased in cells treated with PLX4032 or AZD6244 alone (Fig. 3, B and D). However, in contrast to the enhanced effects seen with the combination use of MK2206 with BRAFV600E/MEK inhibitors on p27Kip1 expression (Fig. 3B), perifosine reduced the expression of p27Kip1 induced by PLX4032 or AZD6244 (Fig. 3D). Because p27Kip1 is required for G1 arrest (31), these results suggested that the G1 arrest of cells induced by PLX4032 or AZD6244 could be diminished by perifosine, thus reversing the inhibition of cell growth. The G2/M phase arrest by perifosine was reported to be p21 dependent in some tumor cells (32, 33). Indeed, we observed a marked elevation in the expression of p21 in OCUT1 cells treated with perifosine alone (Fig. 3D), in association with cell cycle arrest in the G2/M phase (Fig. 3C). Interestingly, this perifosine-induced expression of p21 was significantly reduced when used in combination with PLX4032 or AZD6244 (Fig. 3D). This may explain the reversal of perifosine-induced G2/M cell cycle arrest by PLX4032 or AZD6244 (Fig. 3C). Unlike MK2206, which enhanced the inhibition of cyclin D1 expression by BRAFV600E/MEK inhibitors, the combination of perifosine with BRAFV600E/MEK inhibitors did not show further effect on cyclin D1 expression compared with each individual drug (Fig. 3D). These inhibitors and their combinations showed, overall, similar effects on cell cycles of K1 cells as seen in OCUT1 cells (data not shown).
Effects of the Akt inhibitors and BRAFV600E/MEK inhibitors, individually or in combinations, on cell apoptosis of thyroid cancer cells
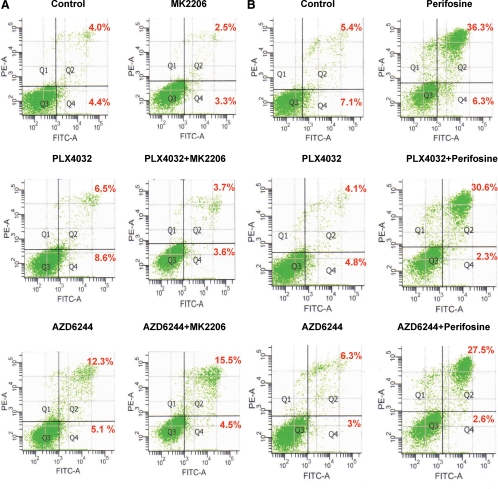
MK2206 or PLX4032 or their combination did not induce significant apoptosis of OCUT1 cells (Fig. 4A). AZD6244 induced only a modest cell apoptosis, which was slightly enhanced by MK2206. Therefore, the inhibition of thyroid cancer cell growth by these inhibitors, either alone or in their combinations, was mainly through cell cycle arrest but not cell apoptosis. In contrast, perifosine could induce apoptosis of cancer cells, including thyroid cancer cells (21, 32). We similarly observed marked apoptosis of OCUT1 cells induced by perifosine (Fig. 4B). Interestingly, this apoptosis tended to be diminished by combined treatment with PLX4032 or AZD6244. This was seen in both early cell apoptosis (from 6.3% with perifosine to 2.3 and 2.6% with combination treatments with the two drugs, respectively) and late cell apoptosis (from 36.3% with perifosine to 30.6 and 27.5% with combined treatments with the two drugs, respectively) (Fig. 4B). This result is different from the synergistic effects of perifosine with another MEK inhibitor PD184352 on leukemia cell apoptosis (34), suggesting that different cancer cells may respond differently. The compromise of perifosine-induced cell apoptosis by PLX4032 or AZD6244 might be another reason for the antagonism between perifosine and the BRAFV600E/MEK inhibitors in the inhibition of thyroid cancer cell growth (Fig. 1B). Overall, K1 cells showed similar apoptotic responses to the treatments with these inhibitors (data not shown).
Fig. 4.
Effects of the Akt inhibitors and BRAFV600E/MEK inhibitors, used individually or in combinations, on cell apoptosis of thyroid cancer cells. A, OCUT1 cells were treated with 1 μm MK2206, 0.5 μm PLX4032, and 0.2 μm AZD6244, individually or in the indicated combinations, for 48 h. Cells were collected and stained with FITC annexin V and PI, followed by flow cytometry. The percentages of early apoptotic (bottom right quarter) and late apoptotic (top right) cells are presented. B, OCUT1 cells were treated with 3 μm perifosine, 0.5 μm PLX4032, and 0.2 μm AZD6244, individually or in the indicated combinations, for 24 h, followed by apoptosis analysis as in A.
Discussion
It has become a highly advocated therapeutic strategy to simultaneously target the MAPK and PI3K/Akt pathways using drug combinations for treatments of thyroid cancer (1, 9, 10). This strategy would likely improve the low therapeutic efficiency achieved with single-agent treatments in clinical trials on cancer (35), including thyroid cancer (11–14). The present study tested this potential therapeutic strategy for thyroid cancer. Several prominent drug inhibitors of the MAPK and PI3K/Akt pathways are being actively developed for anticancer use. In particular, the Akt inhibitors MK2206 and perifosine, the BRAFV600E inhibitor PLX4032, and the MEK1/2 inhibitor AZD6244 are among the main drugs in this category that have dominated recent clinical and preclinical studies as single agents (15–26). Although there has been limited preclinical testing and no clinical studies on their combinations, it is expected that combination use of these drugs will be a main theme in the upcoming rounds of clinical trials on human cancers. The goal of the present study was to identify appropriate combinations of drugs to dually target the MAPK and PI3K/Akt pathways in thyroid cancer cells.
We demonstrated that MK2206, an allosteric Akt-specific inhibitor, could alone potently inhibit thyroid cancer cell growth as recently shown (23) and profoundly synergize with PLX4032 or AZD6244 in inhibiting thyroid cancer cells harboring activating mutations in both the PI3K/Akt and MAPK pathways. This synergism was absent or weak in thyroid cancer cells that harbored single or no mutations in the two pathways. This genetic preferentiality is similar to the genetic-potentiated synergism between the MEK inhibitor RDEA119 and the mammalian target of rapamycin inhibitor temsirolimus in the inhibition of thyroid cancer cells (28). This may be expected, given the genetic dependence of these inhibitors when used individually in thyroid cancer cells (17, 18, 23, 36).
A previous study from our group also demonstrated synergistic inhibitory effects of the Akt inhibitor IV and the MEK inhibitor U0126 on the growth of melanoma cells harboring genetic alterations in both the MAPK and PI3K/Akt pathways (37). A synergistic inhibitory effect of dual small interfering RNA knockdown of BRAF and Akt1/2 was also seen in these cells. Gopal et al. (30) reported a synergism between Akt knockdown and AZD6244 in the inhibition of melanoma cells. In a recent study by Meng et al. (29), MK2206 and AZD6244 were found to not only synergistically inhibit cell growth but also promote cell apoptosis of lung cancer cells. Akt inhibitors currently under clinical development may have significant toxicity at their effective doses (38), which may potentially limit their clinical application. The synergistic effects of MK2206 and BRAFV600E/MEK inhibitors support a therapeutic strategy for thyroid cancer in which a lower dose of individual drugs in combination could achieve effective therapy with reduced drug toxicities.
We expected similar synergism between perifosine and the BRAFV600E/MEK inhibitors in inhibiting thyroid cancer cells. However, we found the contrary to be true; although perifosine alone could potently and efficaciously inhibit growth and promote apoptosis of thyroid cancer cells, an antagonism between perifosine and the BRAFV600E/MEK inhibitors was observed instead. G1 and G2/M cell cycle arrests individually induced by these drugs were reversed by their combination with corresponding changes in the expression of related cell cycle regulators. It is interesting that this happened, even though under these conditions the signalings of the MAPK and PI3K/Akt pathways remained suppressed. We observed a similar antagonistic effect of perifosine with PLX4032 in the thyroid cancer cell line SW1736 (data not shown), which did not harbor mutations in the PI3K/Akt pathway but harbored BRAFV600E mutation and exhibited a resistance to perifosine in Akt inhibition (21). These results suggest that the antagonistic effects of perifosine observed in the present study likely do not depend on Akt. Perifosine is a signal transduction modulator that also has non-Akt targets, such as c-Jun N-terminal kinase and mammalian target of rapamycin signaling components (39, 40). It would be interesting to investigate in the future whether these targets are involved in the antagonistic effects of perifosine.
In summary, we demonstrate that the combination of MK2206 with PLX4032 or AZD6244 to dually target the MAPK and PI3K/Akt pathways is an effective strategy for synergistic inhibition of thyroid cancer cells that harbor mutations in both pathways. In contrary, perifosine may not be an appropriate agent for combination therapies with BRAFV600E/MEK inhibitors for thyroid cancer due to their antagonism. For its strong PI3K/Akt genetic-dependent inhibition of thyroid cancer cells (21), the use of perifosine as a single drug therapy may also prove to be effective.
Acknowledgments
We thank Plexxikon Inc. (Berkeley, CA) for providing us with PLX4032. We also thank Drs. Onoda N. and Wynford-Thomas for kindly providing us the accessibility to the cell lines used in this study.
This work was supported by National Institutes of Health Specialized Program of Research Excellence (SPORE) Grant CA-96784 and National Institutes of Health Grant R01CA134225 (to M.X.).
Disclosure Summary: The authors declare no conflict of interest.
Footnotes
- CI
- Combination index
- 4EBP1
- 4E-binding protein 1
- FITC
- fluorescein isothiocyanate
- MEK
- MAPK kinase
- MTT
- 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide
- p
- phosphorylated
- PI
- propidium iodide
- PI3K
- phosphatidylinositol 3-kinase.
References
- 1. Xing M. 2007. BRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endocr Rev 28:742–762 [DOI] [PubMed] [Google Scholar]
- 2. Tang KT, Lee CH. 2010. BRAF mutation in papillary thyroid carcinoma: pathogenic role and clinical implications. J Chin Med Assoc 73:113–128 [DOI] [PubMed] [Google Scholar]
- 3. Paes JE, Ringel MD. 2008. Dysregulation of the phosphatidylinositol 3-kinase pathway in thyroid neoplasia. Endocrinol Metab Clin North Am 37:375–387, viii-ix [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xing M. 2010. Genetic alterations in the phosphatidylinositol-3 kinase/Akt pathway in thyroid cancer. Thyroid 20:697–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hou P, Liu D, Shan Y, Hu S, Studeman K, Condouris S, Wang Y, Trink A, El-Naggar AK, Tallini G, Vasko V, Xing M. 2007. Genetic alterations and their relationship in the phosphatidylinositol 3-kinase/Akt pathway in thyroid cancer. Clin Cancer Res 13:1161–1170 [DOI] [PubMed] [Google Scholar]
- 6. Costa AM, Herrero A, Fresno MF, Heymann J, Alvarez JA, Cameselle-Teijeiro J, Garcia-Rostan G. 2008. BRAF mutation associated with other genetic events identifies a subset of aggressive papillary thyroid carcinoma. Clin Endocrinol (Oxf) 68:618–634 [DOI] [PubMed] [Google Scholar]
- 7. Santarpia L, El-Naggar AK, Cote GJ, Myers JN, Sherman SI. 2008. Phosphatidylinositol 3-kinase/akt and ras/raf-mitogen-activated protein kinase pathway mutations in anaplastic thyroid cancer. J Clin Endocrinol Metab 93:278–284 [DOI] [PubMed] [Google Scholar]
- 8. Liu Z, Hou P, Ji M, Guan H, Studeman K, Jensen K, Vasko V, El-Naggar AK, Xing M. 2008. Highly prevalent genetic alterations in receptor tyrosine kinases and phosphatidylinositol 3-kinase/akt and mitogen-activated protein kinase pathways in anaplastic and follicular thyroid cancers. J Clin Endocrinol Metab 93:3106–3116 [DOI] [PubMed] [Google Scholar]
- 9. Xing M. 2008. Recent advances in molecular biology of thyroid cancer and their clinical implications. Otolaryngol Clin North Am 41:1135–1146, ix [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xing M. 2009. Genetic-targeted therapy of thyroid cancer: a real promise. Thyroid 19:805–809 [DOI] [PubMed] [Google Scholar]
- 11. Sherman SI, Wirth LJ, Droz JP, Hofmann M, Bastholt L, Martins RG, Licitra L, Eschenberg MJ, Sun YN, Juan T, Stepan DE, Schlumberger MJ. 2008. Motesanib diphosphate in progressive differentiated thyroid cancer. N Engl J Med 359:31–42 [DOI] [PubMed] [Google Scholar]
- 12. Cohen EE, Rosen LS, Vokes EE, Kies MS, Forastiere AA, Worden FP, Kane MA, Sherman E, Kim S, Bycott P, Tortorici M, Shalinsky DR, Liau KF, Cohen RB. 2008. Axitinib is an active treatment for all histologic subtypes of advanced thyroid cancer: results from a phase II study. J Clin Oncol 26:4708–4713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kloos RT, Ringel MD, Knopp MV, Hall NC, King M, Stevens R, Liang J, Wakely PE, Jr, Vasko VV, Saji M, Rittenberry J, Wei L, Arbogast D, Collamore M, Wright JJ, Grever M, Shah MH. 2009. Phase II trial of sorafenib in metastatic thyroid cancer. J Clin Oncol 27:1675–1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bible KC, Suman VJ, Molina JR, Smallridge RC, Maples WJ, Menefee ME, Rubin J, Sideras K, Morris JC, 3rd, McIver B, Burton JK, Webster KP, Bieber C, Traynor AM, Flynn PJ, Goh BC, Tang H, Ivy SP, Erlichman C. 2010. Efficacy of pazopanib in progressive, radioiodine-refractory, metastatic differentiated thyroid cancers: results of a phase 2 consortium study. Lancet Oncol 11:962–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, O'Dwyer PJ, Lee RJ, Grippo JF, Nolop K, Chapman PB. 2010. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med 363:809–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bollag G, Hirth P, Tsai J, Zhang J, Ibrahim PN, Cho H, Spevak W, Zhang C, Zhang Y, Habets G, Burton EA, Wong B, Tsang G, West BL, Powell B, Shellooe R, Marimuthu A, Nguyen H, Zhang KY, Artis DR, Schlessinger J, Su F, Higgins B, Iyer R, D'Andrea K, Koehler A, Stumm M, Lin PS, Lee RJ, Grippo J, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, Chapman PB, Flaherty KT, Xu X, Nathanson KL, Nolop K. 2010. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature 467:596–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Salerno P, De Falco V, Tamburrino A, Nappi TC, Vecchio G, Schweppe RE, Bollag G, Santoro M, Salvatore G. 2010. Cytostatic activity of adenosine triphosphate-competitive kinase inhibitors in BRAF mutant thyroid carcinoma cells. J Clin Endocrinol Metab 95:450–455 [DOI] [PubMed] [Google Scholar]
- 18. Xing J, Liu R, Xing M, Trink B. 2011. The BRAF(T1799A) mutation confers sensitivity of thyroid cancer cells to the BRAF(V600E) inhibitor PLX4032 (RG7204). Biochem Biophys Res Commun 404:958–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Adjei AA, Cohen RB, Franklin W, Morris C, Wilson D, Molina JR, Hanson LJ, Gore L, Chow L, Leong S, Maloney L, Gordon G, Simmons H, Marlow A, Litwiler K, Brown S, Poch G, Kane K, Haney J, Eckhardt SG. 2008. Phase I pharmacokinetic and pharmacodynamic study of the oral, small-molecule mitogen-activated protein kinase kinase 1/2 inhibitor AZD6244 (ARRY-142886) in patients with advanced cancers. J Clin Oncol 26:2139–2146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Elrod HA, Lin YD, Yue P, Wang X, Lonial S, Khuri FR, Sun SY. 2007. The alkylphospholipid perifosine induces apoptosis of human lung cancer cells requiring inhibition of Akt and activation of the extrinsic apoptotic pathway. Mol Cancer Ther 6:2029–2038 [DOI] [PubMed] [Google Scholar]
- 21. Liu D, Hou P, Liu Z, Wu G, Xing M. 2009. Genetic alterations in the phosphoinositide 3-kinase/Akt signaling pathway confer sensitivity of thyroid cancer cells to therapeutic targeting of Akt and mammalian target of rapamycin. Cancer Res 69:7311–7319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hirai H, Sootome H, Nakatsuru Y, Miyama K, Taguchi S, Tsujioka K, Ueno Y, Hatch H, Majumder PK, Pan BS, Kotani H. 2010. MK-2206, an allosteric Akt inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Mol Cancer Ther 9:1956–1967 [DOI] [PubMed] [Google Scholar]
- 23. Liu R, Liu D, Trink E, Bojdani E, Ning G, Xing M. 2011. The Akt-specific inhibitor MK2206 selectively inhibits thyroid cancer cells harboring mutations that can activate the PI3K/Akt pathway. J Clin Endocrinol Metab 96:E577–E585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gills JJ, Dennis PA. 2009. Perifosine: update on a novel Akt inhibitor. Curr Oncol Rep 11:102–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tolcher AW, Yap TA, Fearen I, Taylor A, Carpenter C, Brunetto AT, Beeram M, Papadopoulos K, Yan L, de Bono J. 2009. A phase I study of MK-2206, an oral potent allosteric Akt inhibitor (Akti), in patients (pts) with advanced solid tumor. J Clin Oncol 27:15s [Google Scholar]
- 26. Kondapaka SB, Singh SS, Dasmahapatra GP, Sausville EA, Roy KK. 2003. Perifosine, a novel alkylphospholipid, inhibits protein kinase B activation. Mol Cancer Ther 2:1093–1103 [PubMed] [Google Scholar]
- 27. Chou TC, Talalay P. 1983. Analysis of combined drug effects: a new look at a very old problem. Trends Pharmacol Sci 4:450–454 [Google Scholar]
- 28. Liu D, Xing J, Trink B, Xing M. 2010. BRAF mutation-selective inhibition of thyroid cancer cells by the novel MEK inhibitor RDEA119 and genetic-potentiated synergism with the mTOR inhibitor temsirolimus. Int J Cancer 127:2965–2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Meng J, Dai B, Fang B, Bekele BN, Bornmann WG, Sun D, Peng Z, Herbst RS, Papadimitrakopoulou V, Minna JD, Peyton M, Roth JA. 2010. Combination treatment with MEK and AKT inhibitors is more effective than each drug alone in human non-small cell lung cancer in vitro and in vivo. PLoS One 5:e14124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gopal YN, Deng W, Woodman SE, Komurov K, Ram P, Smith PD, Davies MA. 2010. Basal and treatment-induced activation of AKT mediates resistance to cell death by AZD6244 (ARRY-142886) in Braf-mutant human cutaneous melanoma cells. Cancer Res 70:8736–8747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sgambato A, Cittadini A, Faraglia B, Weinstein IB. 2000. Multiple functions of p27(Kip1) and its alterations in tumor cells: a review. J Cell Physiol 183:18–27 [DOI] [PubMed] [Google Scholar]
- 32. Kumar A, Fillmore HL, Kadian R, Broaddus WC, Tye GW, Van Meter TE. 2009. The alkylphospholipid perifosine induces apoptosis and p21-mediated cell cycle arrest in medulloblastoma. Mol Cancer Res 7:1813–1821 [DOI] [PubMed] [Google Scholar]
- 33. Patel V, Lahusen T, Sy T, Sausville EA, Gutkind JS, Senderowicz AM. 2002. Perifosine, a novel alkylphospholipid, induces p21(WAF1) expression in squamous carcinoma cells through a p53-independent pathway, leading to loss in cyclin-dependent kinase activity and cell cycle arrest. Cancer Res 62:1401–1409 [PubMed] [Google Scholar]
- 34. Rahmani M, Anderson A, Habibi JR, Crabtree TR, Mayo M, Harada H, Ferreira-Gonzalez A, Dent P, Grant S. 2009. The BH3-only protein Bim plays a critical role in leukemia cell death triggered by concomitant inhibition of the PI3K/Akt and MEK/ERK1/2 pathways. Blood 114:4507–4516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Friday BB, Adjei AA. 2008. Advances in targeting the Ras/Raf/MEK/Erk mitogen-activated protein kinase cascade with MEK inhibitors for cancer therapy. Clin Cancer Res 14:342–346 [DOI] [PubMed] [Google Scholar]
- 36. Ball DW, Jin N, Rosen DM, Dackiw A, Sidransky D, Xing M, Nelkin BD. 2007. Selective growth inhibition in BRAF mutant thyroid cancer by the mitogen-activated protein kinase kinase 1/2 inhibitor AZD6244. J Clin Endocrinol Metab 92:4712–4718 [DOI] [PubMed] [Google Scholar]
- 37. Hou P, Liu D, Ji M, Liu Z, Engles JM, Wahl RL, Xing M. 2009. Induction of thyroid gene expression and radioiodine uptake in melanoma cells: novel therapeutic implications. PLoS One 4:e6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pal SK, Reckamp K, Yu H, Figlin RA. 2010. Akt inhibitors in clinical development for the treatment of cancer. Expert Opin Investig Drugs 19:1355–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hideshima T, Catley L, Yasui H, Ishitsuka K, Raje N, Mitsiades C, Podar K, Munshi NC, Chauhan D, Richardson PG, Anderson KC. 2006. Perifosine, an oral bioactive novel alkylphospholipid, inhibits Akt and induces in vitro and in vivo cytotoxicity in human multiple myeloma cells. Blood 107:4053–4062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fu L, Kim YA, Wang X, Wu X, Yue P, Lonial S, Khuri FR, Sun SY. 2009. Perifosine inhibits mammalian target of rapamycin signaling through facilitating degradation of major components in the mTOR axis and induces autophagy. Cancer Res 69:8967–8976 [DOI] [PMC free article] [PubMed] [Google Scholar]