Abstract
Context:
The lipodystrophies (LD) are characterized by metabolic abnormalities (insulin resistance, hypertriglyceridemia, and diabetes) and a polycystic ovarian syndrome (PCOS) phenotype. Therapeutic administration of leptin improves insulin sensitivity and the metabolic features.
Objective:
The objective of the study was to investigate whether the PCOS features are corrected by increasing insulin sensitivity as a function of leptin treatment.
Design:
This was a prospective, open-label trial using leptin replacement in various forms of lipodystrophy.
Setting:
The study was performed at the Clinical Center at the National Institutes of Health.
Patients or Other Participants:
Twenty-three female patients with LD were enrolled in a leptin replacement trial from 2000 to the present. Different parameters were assessed at baseline and after 1 yr of therapy.
Intervention(s):
Patients were treated with leptin for at least 1 yr.
Main Outcome Measure(s):
We evaluated free testosterone, SHBG, and IGF-I at baseline and after 1 yr of leptin.
Results:
Testosterone levels decreased from 3.05 ±0.6 ng/ml at baseline to 1.7 ±0.3 ng/ml (P = 0.02). SHBG increased from 14.5 ±2 to 25 ±3.5 nmol/liter after 1 yr of leptin therapy. There were no significant changes in the levels of gonadotropins and ovarian size as a result of leptin replacement therapy. IGF-I increased significantly after leptin therapy from 150 ±14 to 195 ±17. There was a significant decrease in triglycerides and glycosylated hemoglobin in the context of reduced insulin requirements.
Conclusions:
In the present study, we show that LD may be a model for the common forms of PCOS and that the endocrine features are corrected by leptin therapy, which reduces insulin resistance.
Polycystic ovarian syndrome (PCOS) is a common condition characterized by menstrual irregularities, signs of androgen excess, and, in many cases, obesity. Women with PCOS have a higher incidence of glucose intolerance and diabetes compared with age and body mass index (BMI)-matched controls (1). Since 1990 the diagnosis of PCOS is based on National Institutes of Health criteria (2) that include anovulation and hyperandrogenism and were expanded in 2003 after the Rotterdam Consensus Workshop (3). The disease mechanism focuses on the role of gonadotropins vs. insulin effects on the ovary. Insulin is thought to stimulate theca cells to produce androgens and, in addition, decrease the production of SHBG by the liver, resulting in a rise in the levels of free circulating testosterone. Furthermore, insulin-sensitizing treatments such as weight loss or metformin frequently lead to improvement in ovarian function.
Animal models could be useful in understanding the complexity of PCOS, but human models are even more desirable. We have previously reported patients with lipodystrophy as a human model for the diverse metabolic features of the metabolic syndrome (4). In the present study, we have considered the simplest human model for the potentially complex PCOS. In this model, leptin deficiency represents a common etiological basis for the diverse metabolic components and, more importantly, a common therapeutic target. Phenotypic features of PCOS are common in patients with extreme forms of insulin resistance (5, 6). Recently it has been possible to study patients with syndromic forms of insulin resistance who respond to therapies that decrease the degree of insulin resistance and determine the importance of insulin resistance on that particular phenotypic feature. Our study looks at the effects of improved insulin sensitivity by means of leptin therapy on the features of PCOS in patients with lipodystrophy.
Materials and Methods
Patients
We studied 23 female patients with various forms of lipodystrophy between the ages of 13 and 43 yr who were enrolled in a protocol at the Clinical Center of the National Institutes of Health designed to study the effects of leptin replacement on various metabolic and hormonal derangements. Leptin was supplied as Meterleptin (Amgen, Thousand Oaks, CA) initially and Amylin (San Diego, CA) subsequently. The study was approved by the Institutional Review Board of the National Institute of Diabetes and Digestive and Kidney Disease, and the patients or their guardians gave informed consent. The patients were maintained on stable therapies with Food and Drug Administration-approved medications for diabetes and hypertriglyceridemia for at least 6 wk before initiation of leptin. Each patient served as their own control and the results of therapy were compared with baseline. All patients received leptin for a period of at least 12 months. This study is an extension of our previously published data on 10 female patients with lipodystrophy (7). Twelve patients had congenital generalized lipodystrophy, four patients acquired generalized lipodystrophy, four congenital partial lipodystrophy, and three acquired partial lipodystrophy. These patients were selected because they were premenopausal and postpubertal. For the IGF-I study, we included 44 patients with lipodystrophy, 38 females and six males.
Study design
The study was previously described (7). All patients were receiving continuous self-administered sc leptin in one or two daily doses. The dose of leptin was calculated based on weight and ranged between 0.06 and 0.24 mg/kg · d, and the dose was adjusted to achieve metabolic control.
Hormone assays
The assays were described previously (7). Of note, the free testosterone was measured by equilibrium dialysis at Mayo Medical Laboratories until December 2003 with a normal range up to 1.9 ng/dl, afterward performed at the National Institutes of Health (Bethesda MD), calculated using the equation supplied by The Endocrine Society based on the constants derived from A. Vermeulen et al. (8). The calculator, which includes the bioactive testosterone calculation, can be found on the Internet (http://www.issam.ch/freetesto.htm). The upper limit of the assay is 2.4 ng/dl.
Imaging
Pelvic ultrasounds were performed using an ATL HDI-5000 (Siemens, Malvern, PA) (7, 9).
Statistical analyses
Measurements are presented as mean ± sem. Given the nonparametric distribution of the data, a Wilcoxon paired t test was used to compare baseline and 1-yr data with a statistically significant P < 0.05.
Results
Twenty-three female patients with lipodystrophy of menstrual age were treated with leptin for 1 yr. The baseline characteristics of the patients are presented in Table 1. Most patients were amenorrheic or oligomenorrheic, hyperandrogenic, and infertile or hypofertile at baseline, exhibiting a phenotype similar to PCOS.
Table 1.
Baseline characteristics of female lipodystrophy patients
| LD type | Patients, n | Age (yr) | BMI | Leptin | Baseline menstrual status | Postleptin menstrual status |
|---|---|---|---|---|---|---|
| CGL | 12 | 20.5 ± 9 | 22.5 ± 2.5 | 1.25 ± 1 | 5/12 amenorrhea | 12/12 regular menses |
| 5/12 oligomenorrhea | ||||||
| 1/12 regular menses | ||||||
| 1/12 histerectomy, no oophorectomy | ||||||
| AGL | 4 | 22.7 ± 6.7 | 18.6 ± 2.7 | 1 ± 0.7 | 1/4 amenorrhea | Irregular menses |
| 2/4 oligomenorrhea | 2/2 regular menses | |||||
| 1/4 regular menses | Regular menses | |||||
| CPL | 4 | 29 ± 11 | 23.6 ± 2.5 | 5 ± 3.3 | 2/4 amenorrhea | 1/4 irregular, 1/4 amenorrhea |
| 1/4 oligomenorrhea | Regular | |||||
| 1/4 regular menses | Regular | |||||
| APL | 3 | 27 ± 8.5 | 20.6 ± 1.9 | 6 ± 5.5 | 3/3 regular menses | 3/3 regular menses |
There is a trend toward slight decrease in BMI and weight after leptin therapy.
LD, Lipodystrophy; CGL, congenital generalized lipodystrophy; AGL, acquired generalized lipodystrophy; CPL, congenital partial lipodystrophy; APL, acquired partial lipodystrophy.
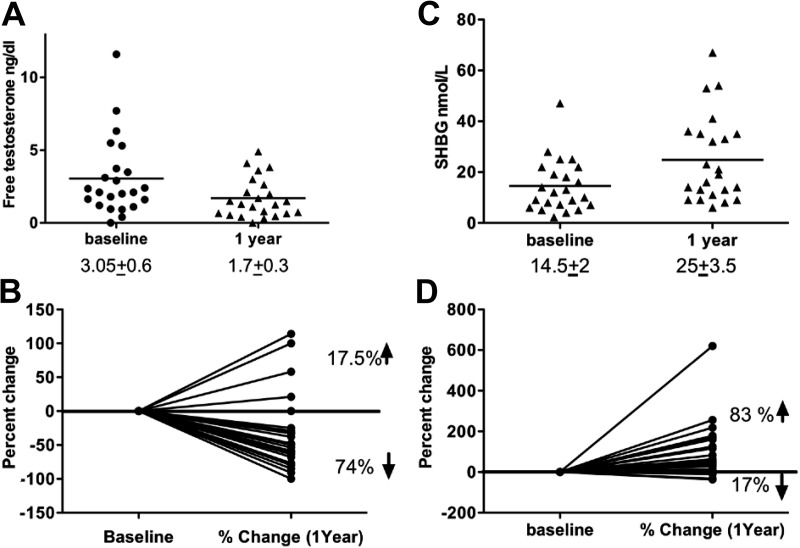
The average baseline free testosterone level was 3.05 ± 0.6 ng/dl (normal <2.4 ng/dl), and there was a significant decrease after 1 yr of therapy to 1.7 ± 0.3 ng/dl, P = 0.02 (Fig. 1A). Seventy-four percent of the patients experienced a decrease in the free testosterone level at the end of 1 yr of leptin replacement, whereas only 17.5% of patients had an increase and 8.5% experienced no change (Fig. 1B). Serum SHBG, a surrogate marker of insulin resistance, was significantly increased by leptin from 14.5 ± 2 to 25 ± 3.5 nmol/liter (Fig. 1C), and a majority of patients (83%) had an increase in SHBG after 1 yr of leptin therapy up to 6 times the baseline value, whereas only 17% had a decrease (Fig. 1D).
Fig. 1.
The effect of leptin replacement on free testosterone and SHBG in women with lipodystrophy. A, Free testosterone levels decrease significantly from 3.05 ± 0.6 to 1.7 ± 0.3 ng/ml after 1 yr of leptin replacement (P = 0.02). B, Seventy-four percent of the female patients with lipodystrophy experienced a decrease in free testosterone level at the end of 1 yr of leptin replacement, whereas only 17.5% of the patients had an increase and 8.5% remained stable. C, Serum SHBG was significantly increased by leptin from 14.5 ± 2 to 25 ± 3.5 nmol/liter. D, Eighty-three percent of patients had an increase in SHBG after 1 yr of leptin therapy.
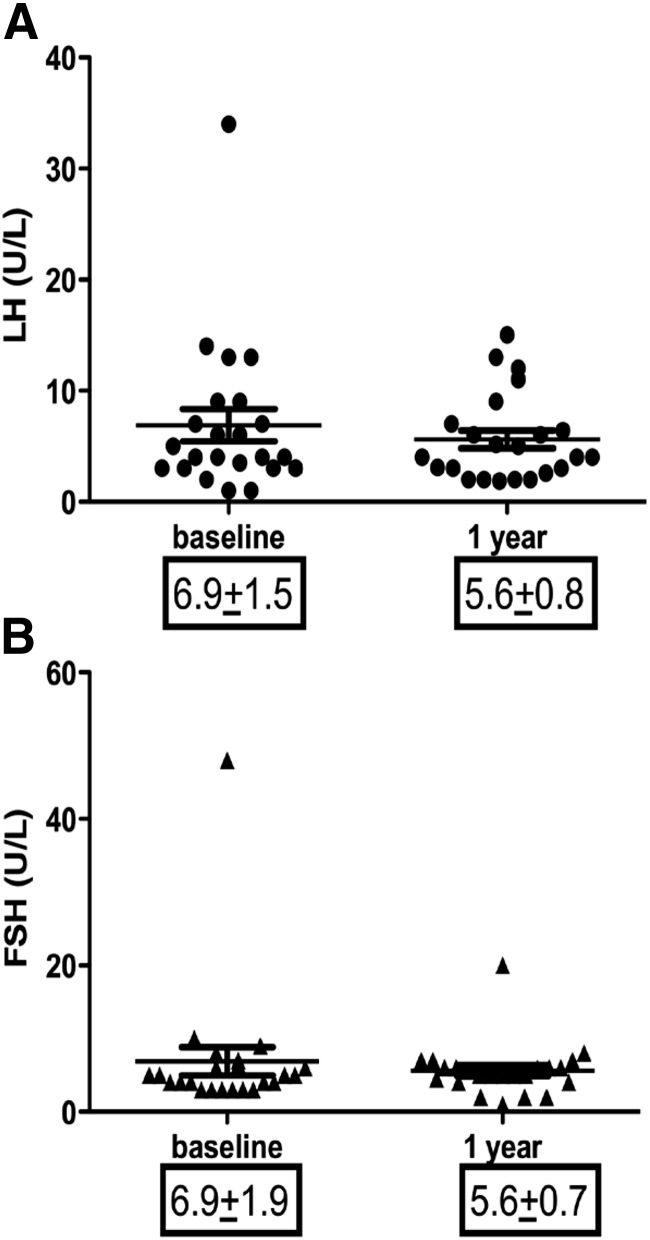
We observed no significant changes in the levels of gonadotropins and ovarian size as a result of leptin replacement therapy. The pretreatment average LH was 6.9 ± 1.5 U/liter, and there was a nonsignificant decrease to 5.6 ± 0.8 U/liter (P = 0.63) (Fig. 2A), and a similar pattern was observed for FSH (6.9 ± 1.9 to 5.6 ± 0.7 U/liter, P = 0.83, Fig. 2B). Gonadotropins were essentially unchanged, despite the fact that most of the patients had menstrual irregularities before the therapy and almost all had normal menses after therapy. Right ovary average volume was 21.3 ± 6.4 ml and left ovarian volume was 15.03 ± 2.1 ml at baseline. There was no significant change in the size of ovaries after 1 yr of leptin therapy. This is similar to our previous observation (8).
Fig. 2.
Change in LH, FSH and ovarian size following leptin administration in women with lipodystrophy. A, FSH did not change significantly after one year of leptin therapy. B, LH did not change significantly after one year of leptin therapy.
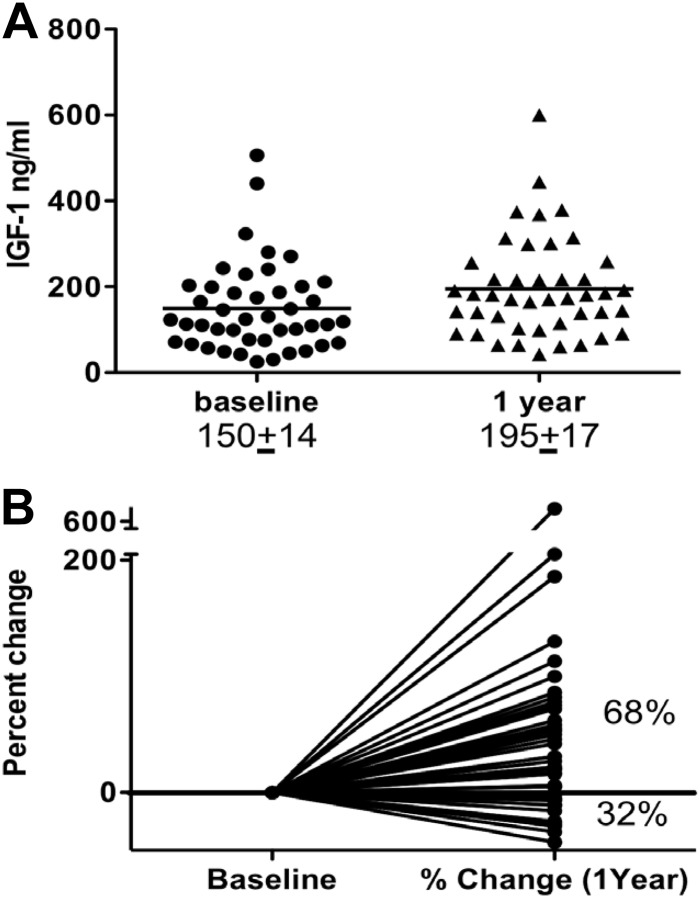
IGF-I increased significantly after leptin therapy in 44 patients with lipodystrophy of both sexes from 150 ± 14 to 195 ± 17 (Fig. 3A), with 68% of the patients experiencing an increase in IGF-I up to 6-fold after leptin therapy (Fig. 3B).
Fig. 3.
The effect of leptin replacement on the IGF-I level in patients with lipodystrophy. A, IGF-I increased significantly after leptin therapy from 150 ± 14 to 195 ± 17. B, Sixty-eight percent of the patients experienced an increase in IGF-I up to 6-fold after leptin therapy.
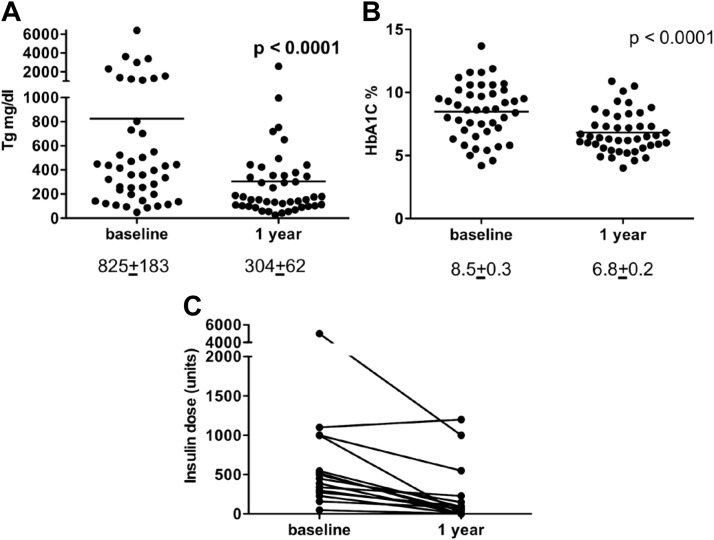
In the same patient population, triglycerides decreased significantly, from 825 ± 183 to 304 ± 62 mg/dl (Fig. 4A). Glycosylated hemoglobin (HbA1C) decreased from 8.5 to 6.8% (Fig. 1B) in the context of significantly reduced insulin requirements from an average of 774 ± 292 U to 219 ± 93 U/d at the end of 1 yr of therapy. In previous studies, we have shown that these metabolic changes occur in the context of decreased insulin resistance (9).
Fig. 4.
Metabolic effects of leptin replacement therapy. A, Triglycerides decreased significantly from 825 ± 183 to 304 ± 62 mg/dl. B, HbA1C decreased from 8.5 to 6.8%. C, Insulin requirements were significantly reduced by 1 yr of leptin therapy.
Discussion
In the present study, we have confirmed and extended our previous observations on the role of insulin resistance in PCOS (7, 9, 11). We again see a dramatic reduction of testosterone levels, rise in SHBG, and induction of menses as patients become more insulin sensitive as shown by the concomitant reduction in HbA1C and insulin requirements.
The association between insulin resistance and PCOS was first noted in syndromic forms of extreme insulin resistance (5, 6). Kahn et al. (5) reported a hyperandrogenic phenotype in premenopausal women with type A insulin resistance and insulin receptor mutations. Burghen et al. (12) in 1980 suggested that common PCOS is associated with insulin resistance and has both metabolic and reproductive morbidities.
Correcting hyperandrogenemia in patients with syndromes of extreme insulin resistance does not affect the insulin levels, suggesting that hyperinsulinemia is the cause of increased androgen production. Thus, although testosterone may have a mild effect on insulin sensitivity under some conditions, it does not appear to be relevant to our patients (13, 14). Indeed, in vitro studies show that insulin can stimulate estrogen, androgen, and progesterone production by the ovarian stroma cells (15). Brief physiological levels of insulin infusions in nondiabetic women resulted in increase in serum androgen levels (16). The signal transduction pathway is unclear. The human ovary has both insulin and IGF-I receptors, and it was proposed that high levels of insulin can bind to IGF-I receptors and induce increased androgen production by the ovary. However, patients with acromegaly have elevated IGF-I levels, and they do not exhibit a hyperandrogenic phenotype. Furthermore, patients treated with therapies known to decrease insulin levels such as metformin, troglitazone, diazoxide, and octreotide had a decrease in androgen levels and an increase in SHBG (15). Metformin was also shown to improve menstrual cyclicity in patients with PCOS (17).
Our patients exhibit a PCOS phenotype with menstrual irregularities, hyperandrogenism, which is reversed by moving from an insulin-resistant to an insulin-sensitive state. Even more dramatic changes in androgen secretion and ovarian size are seen in young women with autoantibodies to the insulin receptor and extreme insulin resistance. When they remit from the insulin-resistant to an insulin-sensitive state, there is a marked decrease in testosterone levels and ovarian size (18, 19). Furthermore, in four of our patients with mutations in the insulin receptor and one with lipodystrophy (before the advent of leptin), their ovaries were removed to control the extreme androgen excess and hypertrophy (Gorden P. and E. Cochran, unpublished observation). Taken together, these three insulin-resistant states suggest that hyperinsulinemia is the driving mechanism of the PCOS. Our focus on hyperinsulinemia in syndromic forms of insulin resistance, however, does not rule out a more heterogeneous ovarian and gonadotropic disorder in the common forms of PCOS.
In lipodystrophic patients, the features of PCOS are corrected in the absence of any quantitative change in gonadotropins. Although there is no quantitative change in gonadotropins, there could be a qualitative change in pulsatility. It is known that leptin affects the gonadotropins ultradian rhythm under certain conditions (20–22). This effect in human is unrelated to insulin sensitivity and does not appear to be directly relevant to androgen levels. In addition, complex effects have been seen in rodent and human ovarian cells exposed to leptin in vitro (23–25), but there is no evidence that these effects have relevance to in vivo effects in human. It is important to note, however, in prepubertal children with extreme forms of insulin resistance that ovarian enlargement occurs under hyperinsulinemic conditions when the gonadotropins are not measurable in plasma (7). Thus, gonadotropins represent a physiological steroidogenic and growth factor for the ovary at puberty, whereas insulin represents a pathological steroidogenic and growth factor throughout the pre- and postpubertal period.
In conclusion, using lipodystrophy as a human model of PCOS, we have shown that improved insulin sensitivity leads to decrease in androgen production and increase in SHBG in the absence of any quantitative changes in gonadotropin levels.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- Body mass index
- HbA1C
- glycosylated hemoglobin
- PCOS
- polycystic ovarian syndrome.
References
- 1. Legro RS, Kunselman AR, Dodson WC, Dunaif A. 1999. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab 84:165–169 [DOI] [PubMed] [Google Scholar]
- 2. Zawadzki JK, Dunaif A DA. 1992. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Polycystic ovary syndrome. Oxford, UK: Blackwell; 59–69 [Google Scholar]
- 3. 2004. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Hum Reprod (Oxford, England) 19:41–47 [DOI] [PubMed] [Google Scholar]
- 4. Gorden P, Lupsa BC, Chong AY, Lungu AO. 2010. Is there a human model for the ‘metabolic syndrome’ with a defined aetiology? Diabetologia 53:1534–1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kahn CR, Flier JS, Bar RS, Archer JA, Gorden P, Martin MM, Roth J. 1976. The syndromes of insulin resistance and acanthosis nigricans. Insulin-receptor disorders in man. N Engl J Med 294:739–745 [DOI] [PubMed] [Google Scholar]
- 6. Givens JR, Kerber IJ, Wiser WL, Andersen RN, Coleman SA, Fish SA. 1974. Remission of acanthosis nigricans associated with polycystic ovarian disease and a stromal luteoma. J Clin Endocrinol Metab 38:347–355 [DOI] [PubMed] [Google Scholar]
- 7. Musso C, Cochran E, Javor E, Young J, Depaoli AM, Gorden P. 2005. The long-term effect of recombinant methionyl human leptin therapy on hyperandrogenism and menstrual function in female and pituitary function in male and female hypoleptinemic lipodystrophic patients. Metabolism 54:255–263 [DOI] [PubMed] [Google Scholar]
- 8. Vermeulen A, Verdonck L, Kaufman JM. 1999. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 84:3666–3672 [DOI] [PubMed] [Google Scholar]
- 9. Musso C, Shawker T, Cochran E, Javor ED, Young J, Gorden P. 2005. Clinical evidence that hyperinsulinaemia independent of gonadotropins stimulates ovarian growth. Clin Endocrinol 63:73–78 [DOI] [PubMed] [Google Scholar]
- 10. Javor ED, Cochran EK, Musso C, Young JR, Depaoli AM, Gorden P. 2005. Long-term efficacy of leptin replacement in patients with generalized lipodystrophy. Diabetes 54:1994–2002 [DOI] [PubMed] [Google Scholar]
- 11. Oral EA, Ruiz E, Andewelt A, Sebring N, Wagner AJ, Depaoli AM, Gorden P. 2002. Effect of leptin replacement on pituitary hormone regulation in patients with severe lipodystrophy. J Clin Endocrinol Metab 87:3110–3117 [DOI] [PubMed] [Google Scholar]
- 12. Burghen GA, Givens JR, Kitabchi AE. 1980. Correlation of hyperandrogenism with hyperinsulinism in polycystic ovarian disease. J Clin Endocrinol Metab 50:113–116 [DOI] [PubMed] [Google Scholar]
- 13. Diamond MP, Grainger D, Diamond MC, Sherwin RS, Defronzo RA. 1998. Effects of methyltestosterone on insulin secretion and sensitivity in women. J Clin Endocrinol Metab 83:4420–4425 [DOI] [PubMed] [Google Scholar]
- 14. Polderman KH, Gooren LJ, Asscheman H, Bakker A, Heine RJ. 1994. Induction of insulin resistance by androgens and estrogens. J Clin Endocrinol Metab 79:265–271 [DOI] [PubMed] [Google Scholar]
- 15. Dunaif A. 1997. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev 18:774–800 [DOI] [PubMed] [Google Scholar]
- 16. Nestler JE. 1997. Role of hyperinsulinemia in the pathogenesis of the polycystic ovary syndrome, and its clinical implications. Semin Reprod Endocrinol 15:111–122 [DOI] [PubMed] [Google Scholar]
- 17. Haas DA, Carr BR, Attia GR. 2003. Effects of metformin on body mass index, menstrual cyclicity, and ovulation induction in women with polycystic ovary syndrome. Fertil Steril 79:469–481 [DOI] [PubMed] [Google Scholar]
- 18. Malek R, Chong AY, Lupsa BC, Lungu AO, Cochran EK, Soos MA, Semple RK, Balow JE, Gorden P. 2010. Treatment of type B insulin resistance: a novel approach to reduce insulin receptor autoantibodies. J Clin Endocrinol Metab 95:3641–3647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Taylor SI, Arioglu E. 1998. Syndromes associated with insulin resistance and acanthosis nigricans. J Basic Clin Physiol Pharmacol 9:419–439 [DOI] [PubMed] [Google Scholar]
- 20. Chan JL, Mantzoros CS. 2005. Role of leptin in energy-deprivation states: normal human physiology and clinical implications for hypothalamic amenorrhoea and anorexia nervosa. Lancet 366:74–85 [DOI] [PubMed] [Google Scholar]
- 21. Welt CK, Chan JL, Bullen J, Murphy R, Smith P, DePaoli AM, Karalis A, Mantzoros CS. 2004. Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med 351:987–997 [DOI] [PubMed] [Google Scholar]
- 22. Farooqi IS, Jebb SA, Langmack G, Lawrence E, Cheetham CH, Prentice AM, Hughes IA, McCamish MA, O'Rahilly S. 1999. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med 341:879–884 [DOI] [PubMed] [Google Scholar]
- 23. Karlsson C, Lindell K, Svensson E, Bergh C, Lind P, Billig H, Carlsson LM, Carlsson B. 1997. Expression of functional leptin receptors in the human ovary. J Clin Endocrinol Metab 82:4144–4148 [DOI] [PubMed] [Google Scholar]
- 24. Zachow RJ, Magoffin DA. 1997. Direct intraovarian effects of leptin: impairment of the synergistic action of insulin-like growth factor-I on follicle-stimulating hormone-dependent estradiol-17 β production by rat ovarian granulosa cells. Endocrinology 138:847–850 [DOI] [PubMed] [Google Scholar]
- 25. Spicer LJ, Francisco CC. 1998. Adipose obese gene product, leptin, inhibits bovine ovarian thecal cell steroidogenesis. Biol Reprod 58:207–212 [DOI] [PubMed] [Google Scholar]