Abstract
Context:
There has been much speculation as to whether defects in glucagon-like peptide-1 (GLP-1) secretion play a role in the pathogenesis of type 2 diabetes and the progression from normal glucose tolerance to prediabetes and diabetes.
Objective:
Our objective was to determine whether fasting and postchallenge concentrations of active and total GLP-1 decrease as glucose tolerance and insulin secretion worsen across the spectrum of prediabetes.
Design:
This was a cross-sectional study.
Setting:
The study was performed in the clinical research unit of an academic medical center.
Participants:
Participants included 165 subjects with a fasting glucose below 7.0 mmol/liter and not taking medications known to affect gastrointestinal motility or glucose metabolism.
Intervention:
Intervention included a 2-h, 75-g oral glucose tolerance test with insulin, C-peptide, glucagon, and GLP-1 measurements at seven time points.
Main Outcome Measure:
We evaluated the association of integrated, incremental active, and total GLP-1 concentrations with integrated, incremental glucose response to 75 g oral glucose.
Results:
After accounting for covariates, there was no evidence of a relationship of incremental glucose concentrations after oral glucose tolerance test with active and total GLP-1 (rs = −0.16 and P = 0.14, and rs = 0.00 and P > 0.9, respectively). There also was no association of GLP-1 concentrations with insulin secretion and action.
Conclusions:
The lack of association of GLP-1 concentrations with glucose tolerance status and with insulin secretion and action in a cohort encompassing the full spectrum of prediabetes strongly argues against a significant contribution of defects in GLP-1 secretion to the pathogenesis of prediabetes.
Glucagon-like peptide-1 (GLP-1) is an incretin hormone that arises out of differential posttranslational processing of proglucagon in intestinal enteroendocrine L cells dispersed throughout the intestine (1). It is secreted into the portal circulation in response to meal ingestion and the presence of endoluminal nutrients (2). GLP-1 is a powerful insulin secretagogue, stimulating insulin secretion and suppressing glucagon secretion in a glucose-dependent manner (3). Pharmacological manipulation of the GLP-1 pathway has enabled effective therapy for type 2 diabetes, either by direct agonism of the GLP-1 receptor or by inhibition of dipeptidyl peptidase-4 (DPP-4), the enzyme mediating degradation of active GLP-1 in the circulation. Such therapies are effective at stimulating insulin secretion and lowering fasting and postprandial glucose concentrations in people with type 2 diabetes (4).
Because the development of type 2 diabetes is characterized by defects in insulin secretion and glucagon suppression (5), both of which can be ameliorated by GLP-1, there has been speculation that defects in GLP-1 secretion contribute to the pathogenesis of type 2 diabetes. Overt type 2 diabetes is preceded by a state of impaired fasting glucose (IFG) and/or impaired glucose tolerance (IGT), both of which are characterized by a high rate of progression to type 2 diabetes (6). Previous studies have examined this question with conflicting results, suggesting the presence of decreased (7–9), normal (10, 11), or increased (12) GLP-1 concentrations in subjects with IFG, IGT, and type 2 diabetes. There has also been a suggestion that insulin-resistant states such as obesity may impair GLP-1 secretion (13). Indeed, Vollmer et al. (11) described an inverse relationship of GLP-1 concentrations with nonesterified fatty acids, although a relationship with body mass index (BMI) was not apparent (3).
In a recent meta-analysis, Nauck et al. (14) reanalyzed the data from the Vollmer study as well as data from other studies comparing active and total GLP-1 in people with diabetes and weight-matched controls. They concluded that after an oral glucose challenge or after a mixed meal, the integrated, incremental concentrations of GLP-1 did not differ between patients with type 2 diabetes and controls. GLP-1 concentrations were unaffected by weight or age. However, somewhat surprisingly, two studies noted a relationship of fasting and postprandial glucagon concentrations (9, 11) with GLP-1.
Most of the reported studies have been performed in relatively small numbers of subjects and have not examined the full spectrum of prediabetes. In addition, not all of these studies examined total and active GLP-1 concentrations, leaving unanswered the possibility that differences in GLP-1 inactivation may be present in prediabetes. We therefore examined the relationship, if any, of GLP-1 secretion with the defects in insulin secretion and action present in prediabetes. We measured total and active GLP-1 concentrations in response to an oral glucose tolerance test (OGTT) across a wide-range of fasting (and postchallenge) glucose concentrations. Multiple variable analyses were used to identify covariates that might be associated with GLP-1 secretion. We report a weak relationship of GLP-1 with fasting and postchallenge glucagon concentrations. However, the integrated GLP-1 concentrations were not significantly associated with glucose tolerance or with indices of insulin secretion and action. These results suggest that defects in GLP-1 secretion do not play a significant role in the pathogenesis of prediabetes.
Subjects and Methods
Subjects
After approval by the Mayo Institutional Review Board, subjects who previously participated in a population-based, cross-sectional study (15) were contacted by means of a letter and invited to participate. Subjects with a previous diagnosis of diabetes or a fasting glucose of at least 126 mg/dl (≥7 mmol/liter) at the time of screening were excluded, as were those taking medications that could affect glucose metabolism. A total of 165 subjects (87 women, 78 men) gave informed written consent to participate in the study. All subjects were in good health and were at a stable weight and did not engage in regular vigorous exercise. They were instructed to follow a weight-maintenance diet containing 55% carbohydrate, 30% fat, and 15% protein for at least 3 d before the study.
Experimental design
Body composition was measured using dual-energy x-ray absorptiometry (DPX scanner; Lunar, Madison, WI) at the time of screening. This allowed determination of lean body mass and percent body fat. Participating subjects were admitted to the Mayo Clinic Clinical Research Unit at 0630 h after an overnight fast. An 18-gauge cannula was inserted in a retrograde fashion into a dorsal hand vein of the nondominant arm. The hand was placed in a heated box (55 C) to enable sampling of arterialized venous blood. At 0700 h (time 0), subjects ingested 75 g glucose over a period of 5 min. Blood was drawn for glucose and hormone measurements at 0, 10, 20, 30, 60, 90, and 120 min.
Analytical techniques
Arterialized plasma samples were placed in ice, centrifuged at 4 C, separated, and stored at −20 C until assay. Plasma glucose concentrations were measured using a glucose oxidase method (Analox Instruments Inc., Lunenburg, MA). Plasma insulin concentrations were measured using a chemiluminescence assay with reagents obtained from Beckman (Access Assay; Beckman, Chaska, MN). Plasma C-peptide concentrations were measured by RIA (Linco Research, St. Louis, MO). Samples tubes used for the measurement of GLP-1 had 100 μm DPP-4 inhibitor (Linco Research, St. Louis, MO) added.
Active GLP-1 and inactive GLP-1 in serum samples were measured off-site using the Theranos System (Theranos, Inc., Palo Alto, CA). The system is a fully automated, chemiluminescence ELISA. Duplicate measurements of each analyte were made and averaged. The antibodies selected for the assay were directed to the N terminus (active) and to an epitope in the middle of the peptide, respectively, so that the two forms of the analyte gave equal assay responses. For measurement of inactive GLP-1, the selected antibodies were directed to the N terminus of inactive GLP-1 and to an epitope in the middle of the peptide. Total GLP-1 is reported as the sum of active and inactive GLP-1.
Calculations
Net insulin action (Si), which measures the overall effect of insulin to stimulate glucose disposal and suppress endogenous glucose production was estimated from plasma insulin and glucose concentrations using the unlabeled oral minimal model (16). β-Cell responsivity indices (φ) were estimated from the plasma glucose and C-peptide concentrations observed during the experiment by using the oral C-peptide minimal model, incorporating age-associated changes in C-peptide kinetics as measured by Van Cauter et al. (17). Disposition indices (DI) were calculated, as previously described (18), to evaluate the appropriateness of insulin secretion for the prevailing degree of insulin action.
Statistical analysis
The univariate associations of basal and integrated (area over baseline) active and total GLP-1 concentrations with fasting and postprandial glucose levels, indices of insulin secretion and action, and fasting and integrated postmeal glucagon concentrations were assessed using Spearman's correlation coefficients (rs) due to the skewed distributions of GLP-1 concentrations, and similar skewness in the distributions of the other responses measured (e.g. fasting glucagon). In addition, the associations with glucose were also assessed after adjusting for the relevant covariates of age, gender, body weight, and glucagon. In these analyses, a rank transformation of active, and separately total, GLP-1, basal and integrated concentrations, were examined in four separate multiple linear regression models (GLP-1 as the response or dependent variable) incorporating the above covariates along with the corresponding glucagon responses (e.g. the rank transformed fasting glucagon levels as a predictor of rank transformed GLP-1 basal levels).
Results
A total of 165 subjects (87 women, 78 men) participated in the study. Mean age was 66.2 ± 0.6 yr, BMI was 27.2 ± 0.3 kg/m2 (mean ± sem). Subjects were categorized according to fasting glucose values [normal fasting glucose (NFG) <5.56 mmol/liter; IFG >5.56 and <7.0 mmol/liter] and 120-min glucose values [normal glucose tolerance (NGT) <7.78 mmol/liter; IGT >7.78 and <11.1 mmol/liter; and diabetes mellitus (DM) >11.1 mmol/liter]. Please see Table 1 for other demographic characteristics across groups.
Table 1.
Indices of insulin secretion and action according to categorization
| NFG/NGT | IFG/NGT | NFG/IGT | IFG/IGT | IFG/DM | Pa | |
|---|---|---|---|---|---|---|
| n | 36 | 18 | 36 | 57 | 18 | |
| Glucose0 (mmol/liter) | 5.22 ± 0.04 | 5.90 ± 0.06 | 5.22 ± 0.06 | 5.90 ± 0.04 | 6.20 ± 0.21 | <0.01 |
| Pb | <1.0 × 10−5 | 0.70 | <1.0 × 10−5 | <1.0 × 10−5 | ||
| Glucose120 (mmol/liter) | 6.83 ± 0.11 | 6.47 ± 0.17 | 8.98 ± 0.16 | 8.94 ± 0.12 | 13.12 ± 0.27 | <0.01 |
| Pb | 0.63 | <1.0 × 10−5 | <1.0 × 10−5 | <1.0 × 10−5 | ||
| % body fat | 38.3 ± 1.8 | 39.5 ± 2.3 | 40.3 ± 1.6 | 42.2 ± 1.2 | 42.7 ± 2.1 | >0.10 |
| BMI (kg/m2) | 25.9 ± 0.7 | 28.1 ± 0.8 | 25.3 ± 0.6 | 29.0 ± 0.6 | 27.4 ± 0.9 | <0.01 |
| Pb | 0.06 | 0.46 | 0.002 | 0.10 | ||
| Age (yr) | 63.9 ± 1.1 | 62.3 ± 1.9 | 67.1 ± 1.3 | 67.4 ± 0.9 | 69.0 ± 1.6 | <0.01 |
| Pb | 0.48 | 0.03 | 0.02 | 0.03 | ||
| Si (10−4 dl/kg · min per μU/ml) | 22.0 ± 2.5 | 17.8 ± 2.2 | 12.4 ± 1.1 | 8.9 ± 1.0 | 3.1 ± 0.7 | <0.01 |
| Pb | 0.31 | 3.6 × 10−4 | 3.9 × 10−7 | 3.0 × 10−5 | ||
| φ (10−9 min−1) | 54.9 ± 3.1 | 64.8 ± 7.1 | 48.0 ± 4.2 | 55.0 ± 3.0 | 41.6 ± 2.7 | <0.01 |
| Pb | 0.17 | 0.15 | 0.98 | 0.009 | ||
| DI (10−14 dl/kg/min−2 per pmol/liter) | 1807 ± 189 | 1874 ± 306 | 940 ± 113 | 681 ± 59 | 209 ± 45 | <0.01 |
| Pb | 0.82 | 7.6 × 10−5 | 3.4 × 10−9 | 3.4 × 10−6 |
Data represent mean ± sem.
P value calculated by ANOVA.
P value calculated by two-tailed t test in comparison with NFG/NGT.
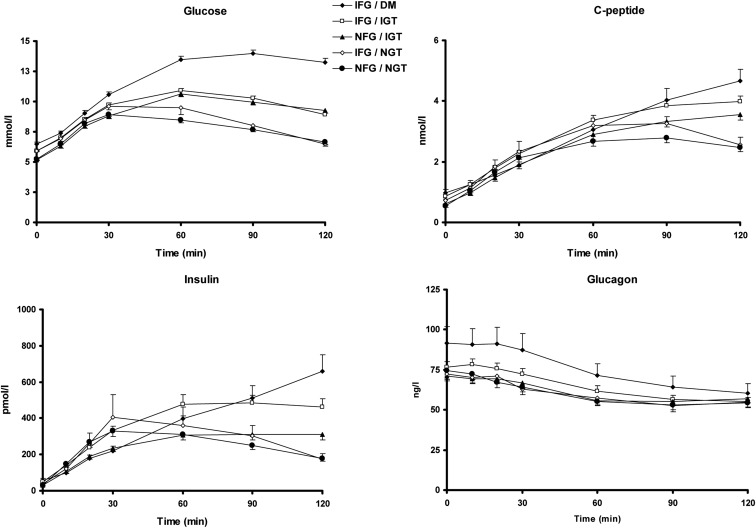
Glucose, insulin, and C-peptide concentrations in response to glucose challenge (Fig. 1)
Fig. 1.
Glucose (top, left panel), insulin (bottom, left panel), C-peptide (top, right panel) and glucagon (bottom, right panel) concentrations in response to a 75-g glucose challenge. Responses are categorized by fasting and postchallenge glucose concentrations (NFG <5.56 mmol/liter; IFG >5.56 and <7.0 mmol/liter; NGT, i.e. 2-h post-OGTT glucose, <7.78 mmol/liter; IGT >7.78 and <11.1 mmol/liter; DM >11.1 mmol/liter).
By definition, fasting glucose concentrations differed between groups, as did 120-min glucose concentrations (see Table 1). Peak glucose concentrations were achieved at 30 min in the NFG/NGT (9.4 ± 0.2 mmol/liter) and IFG/NGT (10.2 ± 0.4 mmol/liter, P < 0.03 compared with NFG/NGT) groups. In contrast, peak concentrations in the NFG/IGT (10.9 ± 0.2 mmol/liter, P < 0.001) and IFG/IGT (11.2 ± 0.2 mmol/liter, P < 0.001) groups were achieved 60 min after glucose ingestion. In the IFG/DM group (14.3 ± 0.2 mmol/liter, P < 0.001), peak concentrations occurred at 90 min.
Fasting insulin concentrations were significantly associated with group status with differences between people with NFG/NGT (29.5 ± 2.6 pmol/liter) vs. the group with IFG/DM (57.9 ± 10.6 pmol/liter, P < 0.0001) and IFG/IGT (51.1 ± 5.0 pmol/liter, P < 0.002). Peak insulin concentrations occurred at 30 min in the NFG/NGT (398 ± 36 pmol/liter) and IFG/NGT (439 ± 49 pmol/liter, P = 0.13 compared with NFG/NGT) groups but were delayed in the other groups; peak concentrations occurred at 60 min for the NFG/IGT (371 ± 28 pmol/liter, P = 0.56) and IFG/IGT (581 ± 58 pmol/liter, P = 0.03) groups. Peak insulin concentrations were observed at 120 min in the IFG/DM group (666 ± 91 pmol/liter, P = 0.002). As expected, differences in C-peptide concentrations followed the observed differences in insulin concentrations across groups.
The oral C-peptide and insulin minimal models were used to calculate φ and Si, respectively. Although φ was significantly lower from that observed in NFG/NGT, only in subjects with IFG/DM, when expressed as a function of prevailing insulin action as a DI, were defects apparent in subjects with NFG/IGT, IFG/IGT, and IFG/DM (see Table 1).
Although fasting glucagon concentrations were slightly higher in IFG/DM, these differences were not significantly associated with group status (see also Table 2). Nadir concentrations of glucagon were not significantly associated with group status (Table 2).
Table 2.
Glucagon and GLP-1 concentrations according to categorization of fasting glucose and glucose tolerance status
| NFG/NGT | IFG/NGT | NFG/IGT | IFG/IGT | IFG/DM | Pa | |
|---|---|---|---|---|---|---|
| Fasting active GLP-1 | 4.5 ± 0.5 | 5.7 ± 0.7 | 4.6 ± 0.5 | 5.7 ± 0.5 | 5.7 ± 0.9 | 0.30 |
| Maximum active GLP-1 | 25.1 ± 4.0 | 20.2 ± 2.6 | 23.8 ± 3.0 | 19.6 ± 2.1 | 18.4 ± 2.5 | 0.54 |
| Active GLP-1 AUC | 1623 ± 162 | 1456 ± 143 | 1671 ± 176 | 1466 ± 103 | 1435 ± 164 | 0.74 |
| Active GLP-1 AAB | 1085 ± 146 | 774 ± 137 | 1117 ± 159 | 785 ± 82 | 755 ± 144 | 0.13 |
| Pb | 0.18 | 0.88 | 0.06 | 0.18 | ||
| Fasting total GLP-1 | 48.7 ± 7.5 | 38.6 ± 7.5 | 32.5 ± 6.9 | 41.2 ± 4.5 | 37.4 ± 6.1 | 0.48 |
| Max total GLP-1 | 84.5 ± 18.1 | 73.6 ± 11.7 | 93.7 ± 18.3 | 80.4 ± 10.3 | 91.7 ± 24.1 | 0.90 |
| Total GLP-1 AUC | 7324 ± 736 | 6980 ± 1160 | 7328 ± 936 | 7058 ± 603 | 8605 ± 2166 | 0.90 |
| Total GLP-1 AAB | 1480 ± 623 | 2346 ± 901 | 3427 ± 457 | 2112 ± 421 | 2116 ± 655 | 0.10 |
| Pb | 0.43 | 0.009 | 0.39 | 0.57 | ||
| Fasting glucagon | 74.6 ± 3.5 | 72.5 ± 3.6 | 71.2 ± 3.2 | 76.6 ± 3.3 | 91.5 ± 10.4 | 0.08 |
| Pb | 0.71 | 0.48 | 0.69 | 0.06 | ||
| Glucagon nadir | 53.2 ± 3.0 | 52.7 ± 3.8 | 55.4 ± 2.9 | 55.3 ± 2.4 | 60.5 ± 5.9 | 0.73 |
Data represent mean ± sem. AUC, Area under the curve.
P value calculated by ANOVA.
P value calculated by two-tailed t test in comparison with NFG/NGT.
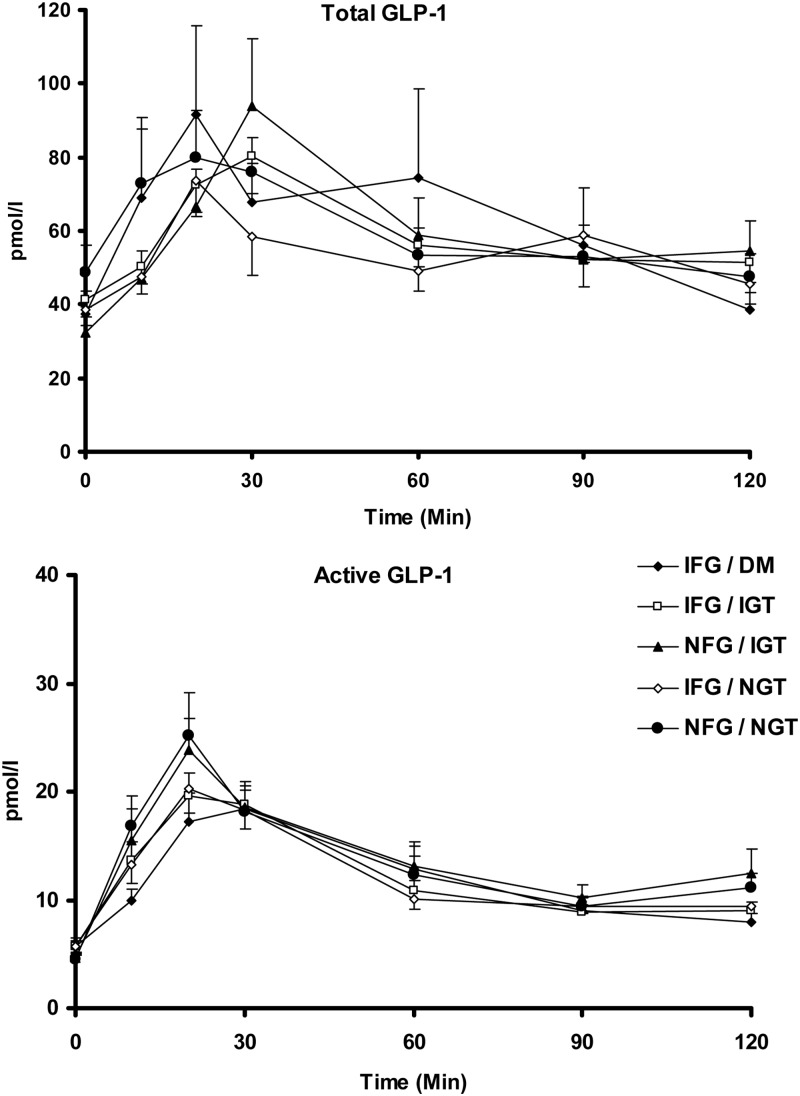
Total and active GLP-1 concentrations in response to a glucose challenge (Fig. 2)
Fig. 2.
Total (top panel) and active (bottom panel) GLP-1 concentrations in response to 75-g glucose challenge with responses categorized by fasting and postchallenge glucose concentrations.
Fasting total GLP-1 concentrations were not significantly associated with group status (Table 2). Peak concentrations were observed at 30 min after glucose challenge in the NFG/IGT and IFG/IGT group, compared with 20 min in the other groups. However, peak and integrated concentrations over the first 30 min after meal challenge were also not significantly associated with group status (Table 2 and Supplemental Appendix, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). Integrated incremental concentrations [area above basal (AAB)] of total GLP-1 were higher in the NFG/IGT group compared with the NFG/NGT group, but otherwise no significant differences were observed. Examination of area under the curve concentrations of total GLP-1 indicated no significant association with group status (Table 2). No significant association for active GLP-1 concentrations with group status was observed.
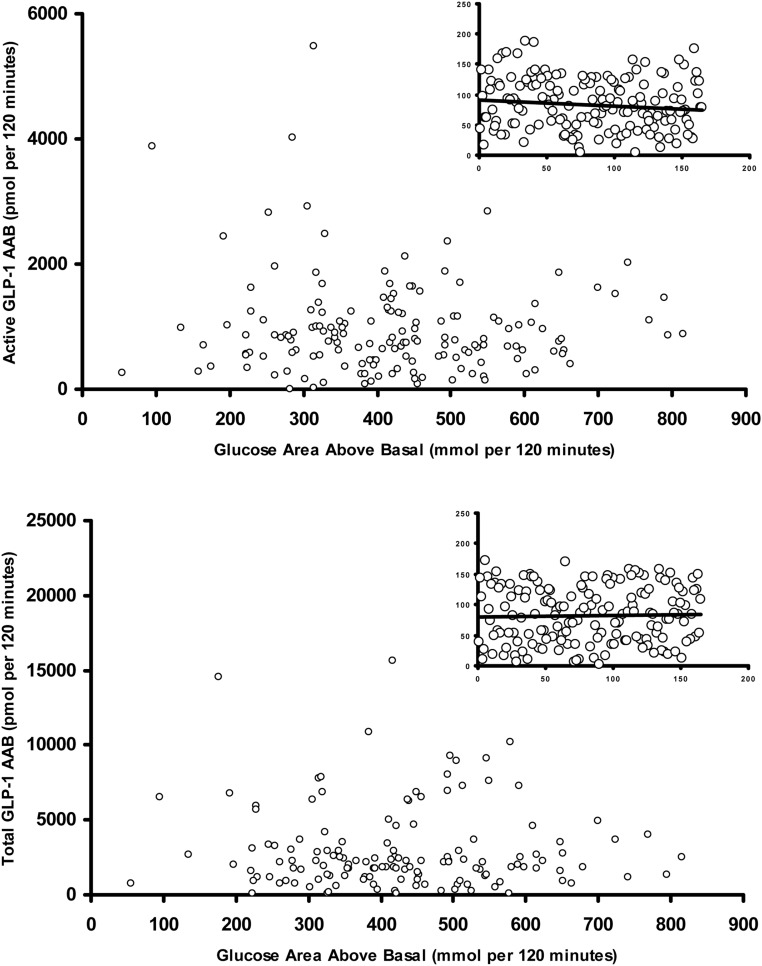
Integrated active and total GLP-1 concentrations after a glucose challenge in relation to integrated glucose concentrations (Fig. 3)
Fig. 3.
Relationship of integrated total GLP-1 concentrations, calculated as AAB with integrated postchallenge (top panel) glucose concentrations and relationship of fasting integrated active GLP-1 concentrations with postchallenge (bottom panel) glucose concentrations. The inset panels represent the rank transformed values of active and total GLP-1 concentrations plotted against AAB glucose values.
Given the potential variability associated with classification of glucose tolerance status using an OGTT (19) and differences in age and BMI between groups, we subsequently examined the relationship of incremental, integrated active and total GLP-1 concentrations (top and bottom panels respectively) with integrated postprandial plasma glucose concentrations (as a continuous variable), after adjusting for relevant covariates via multiple linear regression. Covariates included in these models were basal glucagon and fasting glucose (when basal GLP-1 was the response variable) and integrated postchallenge glucagon and glucose concentrations (when AAB GLP-1 was the response variable) along with gender, age, and weight. Additional multiple variable models for AAB GLP-1 also included AAB insulin (0–120 min). The multiple-variable analyses were used to test the hypothesis that GLP-1 secretion varies with postchallenge glucose concentrations after adjusting for relevant covariates. Given the lack of a strong univariate association of AAB insulin (0–120 min) with AAB active GLP-1 (0–120 min) and AAB total GLP-1 (0–120 min) (both rs values <0.1; P > 0.8), it was not surprising the multiple-variable models were not qualitatively altered by inclusion or exclusion of AAB insulin.
The integrated AAB levels of active GLP-1 were univariately associated with AAB glucagon levels (rs = 0.26; P < 0.001) but not integrated postprandial plasma glucose concentrations (rs = −0.05; P = 0.5). No univariate association between total AAB GLP-1 and AAB glucagon was observed (rs = 0.00; P > 0.9) or with integrated postprandial glucose concentrations (rs = 0.06; P = 0.5). Active GLP-1 concentrations were also weakly associated with weight (P = 0.02; partial r2 = 3.2%). Basal glucagon concentrations were associated with basal active GLP-1 (P = 0.007; partial r2 = 4.6%), and basal total GLP-1 (P = 0.006; partial r2 = 4.6%). Both active and total GLP-1 concentrations were weakly associated with fasting glucose concentrations and with incremental glucose concentrations immediately after the glucose challenge. Please refer to Supplemental Appendix for additional details.
After accounting for the relevant covariates weakly associated with GLP-1 concentrations, there was no evidence of a relationship of incremental glucose concentrations after OGTT with active and total GLP-1 (rs = −0.16 and P = 0.14, and rs = 0.00 and P > 0.9, respectively).
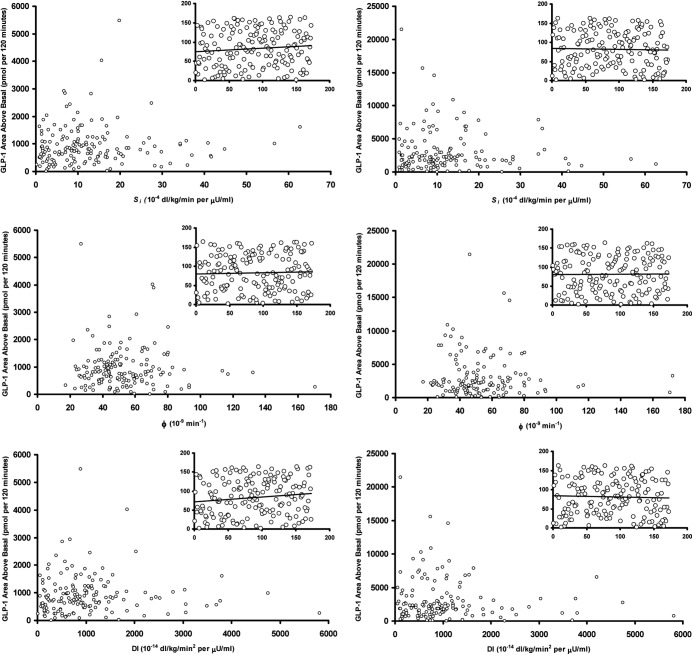
Indices of insulin secretion and action and their relationship with integrated active and total GLP-1 concentrations after a glucose challenge (Fig. 4)
Fig. 4.
Relationship of Si (upper panel), φ (middle panel), and DI (lower panel) with integrated postchallenge active (left column) and total (right column) integrated GLP-1 concentrations. The inset panels represent the rank transformed values of these indices plotted against AAB active and total GLP-1 values.
Active and total GLP-1 concentrations (left and right upper panels, respectively) were not correlated with Si (rs = 0.09 and P = 0.24, and rs = −0.03 and P = 0.73, respectively). Similarly, active and total GLP-1 concentrations (left and right middle panels respectively) were not correlated with φTotal (rs = 0.04 and P = 0.60, and rs = 0.00 and P > 0.9, respectively). There was also no correlation of active and total GLP-1 concentrations with the static and dynamic components of φTotal (data not shown).
DI were not significantly correlated (rs = 0.13 and P = 0.08, and rs = −0.04 and P = 0.64, respectively) with active and total GLP-1 concentrations (left and right lower panels, respectively). The inset panels represent the rank transformed values of the respective x- and y-axis values.
Discussion
Previous studies have demonstrated a variable relationship of postprandial GLP-1 concentrations with diabetes and prediabetes (7–12). Such studies have, in general, been hampered by the small numbers of study subjects, thereby not examining GLP-1 secretion in the full spectrum of prediabetes. In the current study, fasting and integrated postprandial concentrations of total and active GLP-1 did not change significantly among subjects with NGT or IGT; indeed, in some subsets, incremental GLP-1 concentrations were increased compared with subjects with NGT (Table 2). Moreover, in this study, changes in observed insulin secretion (quantified by φ) and insulin action (quantified by Si) could not be explained by changes in endogenous GLP-1 secretion. After adjusting for multiple covariates and accounting for the skewed distribution of GLP-1 concentrations, no significant differences in total and active GLP-1 concentrations occurred across the spectrum of postprandial glucose concentrations observed in nondiabetic individuals. Therefore, these data would argue that deficiencies in circulating GLP-1 cannot explain the pathogenesis of IGT and diabetes.
GLP-1 is secreted into the portal circulation by the enteroendocrine L cells (widely distributed in the proximal and distal intestine) in response to nutrient appearance in the duodenum. Active GLP-1 is rapidly degraded by DPP-4, which is widely distributed throughout the vasculature so that active GLP-1 concentrations represent the net result of GLP-1 secretion as well as DPP-4-mediated clearance. For this reason, total GLP-1 concentrations may be a better measure of endogenous GLP-1 secretion than are active GLP-1 concentrations. Although there are no data to suggest that DPP-4-mediated inactivation of GLP-1 is altered in prediabetes, we examined both active and total GLP-1 concentrations in this study. Both measures of GLP-1 secretion exhibited similar relationships with glucose concentrations, implying that there are no differences in DPP-4 activity across the spectrum of prediabetes.
Of note, GLP-1 (7-36) has a half-life of 1 min (20), whereas GLP-1 (9-36) has a half-life of approximately 3.5 min in pigs (21) and humans (22). This in part explains the difference between active and total GLP-1 concentrations during fasting and after a meal stimulus. It must be remembered that peripheral concentrations represent the net sum of secretion and clearance, and it is possible that disease states produce equivalent but opposing effects on these parameters so that there is no net difference in concentrations. Given that DPP-4 activity does not seem to be affected by type 2 diabetes (23), this does not seem likely. Model-based measures of GLP-1 secretion will need to be developed to further address this question.
Under these experimental conditions, GLP-1 acts as an insulin secretagogue and is unlikely to affect insulin action (24). However, it is important to consider insulin secretion as a function of the prevailing insulin action, hence the use of DI as a measure of β-cell function. GLP-1 concentrations exhibited no relationship with DI and also separately with its constituents: insulin secretion (φ) or action (Si). Given that DI is decreased in subjects with IGT compared with those with NGT (25), and this difference is not explained by altered (decreased) GLP-1 secretion, it is possible that β-cell responsiveness to GLP-1 is decreased in prediabetes. Indeed, this has previously been directly examined using a hyperglycemic clamp to measure insulin secretion in the presence and absence of GLP-1 infusion (26). Subjects with IGT exhibited decreased insulin secretion in response to glucose alone and to GLP-1 in the presence of hyperglycemia. These results are congruent with our data, although the (sustained) concentrations of circulating active GLP-1 that resulted from infusion of the hormone were higher than those observed in the current experiment. Indeed, in individuals with decreased β-cell responsiveness, defective response to a secretagogue may be less apparent at pharmacological, as opposed to physiological, concentrations of GLP-1.
A previous study has suggested that fasting free fatty acid concentrations are related to GLP-1 secretion (11). Free fatty acid concentrations reflect the ability of insulin to suppress production and are an indirect marker of insulin action (27). Although free fatty acid concentrations were not measured in this study, there was no relationship between directly measured insulin action and GLP-1 concentrations (Table 1). Intriguingly, Hansen et al. (28) showed that in the presence of steroid-induced insulin resistance, the incretin effect is impaired, although GLP-1 secretion is unchanged compatible with decreased β-cell responsiveness to GLP-1. In obese, hyperglycemic mice, metformin increases β-cell expression of the GLP-1 receptor, again supporting a link between insulin action and incretin responsiveness (29). However, in this experiment, direct measurement of insulin action did not correlate with GLP-1 concentrations.
Vollmer et al. (11) previously suggested that circulating metabolite concentrations during fasting could alter L cell secretion in response to nutrient ingestion. This hypothesis is as yet untested, although in our cohort, fasting glucose concentrations were weakly associated with GLP-1 response (see Supplemental Appendix). Fasting glucagon concentrations have also been identified as a predictor of GLP-1 concentrations after an OGTT and a mixed meal (11). Fasting and postchallenge glucagon concentrations exhibited a weak but significant relationship to GLP-1. Compatible with previous observations (11), fasting glucagon concentrations were weakly related to fasting active and total GLP-1 concentrations. Furthermore, integrated postchallenge glucagon concentrations were also related to integrated active GLP-1 concentrations. The significance of this is unclear given that administration of iv GLP-1 is associated with suppression of glucagon (albeit at higher circulating concentrations of GLP-1). One potential explanation that has been proposed is that because GLP-2 is secreted in equimolar concentrations with GLP-1, the glucagonotropic effects of GLP-2 outweigh the glucagonostatic effects of GLP-1 at least under physiological conditions (30). Despite these observations, no firm conclusion on the (small) effects of α-cell secretion on L cell secretion or vice versa can be reached from these data and will require further study.
In this experiment, we measured only peripheral GLP-1 concentrations. Although GLP-1 is an important mediator of the incretin effect, it is not the sole component and mediator of this effect; other incretin hormones such as GIP may also play a role. Direct examination of the contribution of oral nutrient delivery to postprandial insulin secretion has shown that net incretin effect is unchanged in diabetes (31). A final limitation of our study is that the nutrient challenge used in this experiment was glucose alone, raising the possibility of a differential GLP-1 response to a mixed meal across the spectrum of glucose tolerance. However, other studies that have used a mixed meal also failed to show a difference in GLP-1 response between IGT and NGT (9) and did not demonstrate a qualitative difference between the GLP-1 response to a mixed meal or to an OGTT (11). GLP-1 secretion partly occurs in response to the appearance of nutrients in the duodenum so that differences in gastric emptying could ostensibly impact incretin secretion after a meal challenge. However, to date, there is no evidence that gastric emptying is affected by glucose intolerance or prediabetes (32).
In conclusion, we report that after adjusting for potential covariates, active and total GLP-1 concentrations observed after an oral glucose challenge did not change across the spectrum of prediabetes. Although active and total GLP-1 concentrations after glucose challenge were weakly associated with fasting glucose concentrations and initial incremental glucose concentrations, defects in GLP-1 secretion cannot explain the decline in insulin secretion or the decline in insulin action observed with progressive impairment of fasting and postprandial glucose concentrations. Therefore, defects in GLP-1 secretion do not seem to contribute significantly to the pathogenesis of IGT.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health/National Institute of NIDDK Grant RO1-DK78646. We acknowledge the support of the Mayo Clinic Center for Translational Science Activities Grant (RR24150), Minnesota Obesity Center Grant (DK50456), the Mayo Clinic CR20 Program (grant to A.V.). This work was supported by grants from The National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (A.V. by DK78646 and DK82396, R.A.R. by DK29953, and M.C. by DK 67071). The original cohort development for the Olmsted County Heart Function Study was funded by HL-55502.
G.S., C.D.M., A.R.Z., and A.V. researched data. A.S., C.D.M., A.R.Z., M.C., R.A.R., C.C., and A.V. contributed to the discussion. A.V. wrote the manuscript. G.S., A.R.Z., M.C., R.A.R., and A.V. reviewed and edited the manuscript.
Disclosure Summary: The authors have no relevant conflict of interest to disclose.
Footnotes
- φ
- β-Cell responsivity index
- AAB
- area above basal
- BMI
- body mass index
- DI
- disposition index
- DM
- diabetes mellitus
- DPP-4
- dipeptidyl peptidase-4
- GLP-1
- glucagon-like peptide-1
- IFG
- impaired fasting glucose
- IGT
- impaired glucose tolerance
- NFG
- normal fasting glucose
- NGT
- normal glucose tolerance
- OGTT
- oral glucose tolerance test Si, insulin action.
References
- 1. Drucker DJ. 2001. The glucagon-like peptides. Endocrinology 142:521–527 [DOI] [PubMed] [Google Scholar]
- 2. Gribble FM, Williams L, Simpson AK, Reimann F. 2003. A novel glucose-sensing mechanism contributing to glucagon-like peptide-1 secretion from the GLUTag cell line. Diabetes 52:1147–1154 [DOI] [PubMed] [Google Scholar]
- 3. Nauck MA, Kleine N, Orskov C, Holst JJ, Willms B, Creutzfeldt W. 1993. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7-36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia 36:741–744 [DOI] [PubMed] [Google Scholar]
- 4. Drucker DJ, Nauck MA. 2006. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 368:1696–1705 [DOI] [PubMed] [Google Scholar]
- 5. Dinneen SF. 1995. Mechanism of postprandial hyperglycaemia in diabetes mellitus. Eur J Gastroenterol Hepatol 7:724–729 [PubMed] [Google Scholar]
- 6. Dinneen SF, Maldonado D, 3rd, Leibson CL, Klee GG, Li H, Melton LJ, 3rd, Rizza RA. 1998. Effects of changing diagnostic criteria on the risk of developing diabetes. Diabetes Care 21:1408–1413 [DOI] [PubMed] [Google Scholar]
- 7. Laakso M, Zilinskaite J, Hansen T, Boesgaard TW, Vänttinen M, Stancáková A, Jansson PA, Pellmé F, Holst JJ, Kuulasmaa T, Hribal ML, Sesti G, Stefan N, Fritsche A, Häring H, Pedersen O, Smith U. 2008. Insulin sensitivity, insulin release and glucagon-like peptide-1 levels in persons with impaired fasting glucose and/or impaired glucose tolerance in the EUGENE2 study. Diabetologia 51:502–511 [DOI] [PubMed] [Google Scholar]
- 8. Muscelli E, Mari A, Natali A, Astiarraga BD, Camastra S, Frascerra S, Holst JJ, Ferrannini E. 2006. Impact of incretin hormones on beta-cell function in subjects with normal or impaired glucose tolerance. Am J Physiol Endocrinol Metab 291:E1144–E1150 [DOI] [PubMed] [Google Scholar]
- 9. Toft-Nielsen MB, Damholt MB, Madsbad S, Hilsted LM, Hughes TE, Michelsen BK, Holst JJ. 2001. Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab 86:3717-3723 [DOI] [PubMed] [Google Scholar]
- 10. Ahrén B, Larsson H, Holst JJ. 1997. Reduced gastric inhibitory polypeptide but normal glucagon-like peptide 1 response to oral glucose in postmenopausal women with impaired glucose tolerance. Eur J Endocrinol 137:127–131 [DOI] [PubMed] [Google Scholar]
- 11. Vollmer K, Holst JJ, Baller B, Ellrichmann M, Nauck MA, Schmidt WE, Meier JJ. 2008. Predictors of incretin concentrations in subjects with normal, impaired, and diabetic glucose tolerance. Diabetes 57:678–687 [DOI] [PubMed] [Google Scholar]
- 12. Faerch K, Vaag A, Holst JJ, Glümer C, Pedersen O, Borch-Johnsen K. 2008. Impaired fasting glycaemia vs impaired glucose tolerance: similar impairment of pancreatic alpha and beta cell function but differential roles of incretin hormones and insulin action. Diabetologia 51:853–861 [DOI] [PubMed] [Google Scholar]
- 13. Carr RD, Larsen MO, Jelic K, Lindgren O, Vikman J, Holst JJ, Deacon CF, Ahrén B. 2010. Secretion and dipeptidyl peptidase-4-mediated metabolism of incretin hormones after a mixed meal or glucose ingestion in obese compared to lean, nondiabetic men. J Clin Endocrinol Metab 95:872–878 [DOI] [PubMed] [Google Scholar]
- 14. Nauck MA, Vardarli I, Deacon CF, Holst JJ, Meier JJ. 2011. Secretion of glucagon-like peptide-1 (GLP-1) in type 2 diabetes: what is up, what is down? Diabetologia 54:10–18 [DOI] [PubMed] [Google Scholar]
- 15. Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. 2003. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA 289:194–202 [DOI] [PubMed] [Google Scholar]
- 16. Dalla Man C, Caumo A, Basu R, Rizza R, Toffolo G, Cobelli C. 2004. Minimal model estimation of glucose absorption and insulin sensitivity from oral test: validation with a tracer method. Am J Physiol Endocrinol Metab 287:E637–E643 [DOI] [PubMed] [Google Scholar]
- 17. Van Cauter E, Mestrez F, Sturis J, Polonsky KS. 1992. Estimation of insulin secretion rates from C-peptide levels. Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes 41:368–377 [DOI] [PubMed] [Google Scholar]
- 18. Dalla Man C, Bock G, Giesler PD, Serra DB, Ligueros Saylan M, Foley JE, Camilleri M, Toffolo G, Cobelli C, Rizza RA, Vella A. 2009. Dipeptidyl peptidase-4 inhibition by vildagliptin and the effect on insulin secretion and action in response to meal ingestion in type 2 diabetes. Diabetes Care 32:14–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Utzschneider KM, Prigeon RL, Tong J, Gerchman F, Carr DB, Zraika S, Udayasankar J, Montgomery B, Mari A, Kahn SE. 2007. Within-subject variability of measures of β-cell function derived from a 2 h OGTT: implications for research studies. Diabetologia 50:2516–2525 [DOI] [PubMed] [Google Scholar]
- 20. Deacon CF, Nauck MA, Toft-Nielsen M, Pridal L, Willms B, Holst JJ. 1995. Both subcutaneously and intravenously administered glucagon-like peptide I are rapidly degraded from the NH2-terminus in type II diabetic patients and in healthy subjects. Diabetes 44:1126–1131 [DOI] [PubMed] [Google Scholar]
- 21. Deacon CF, Danielsen P, Klarskov L, Olesen M, Holst JJ. 2001. Dipeptidyl peptidase IV inhibition reduces the degradation and clearance of GIP and potentiates its insulinotropic and antihyperglycemic effects in anesthetized pigs. Diabetes 50:1588–1597 [DOI] [PubMed] [Google Scholar]
- 22. Vahl TP, Paty BW, Fuller BD, Prigeon RL, D'Alessio DA. 2003. Effects of GLP-1-(7-36)NH2, GLP-1-(7-37), and GLP-1- (9-36)NH2 on intravenous glucose tolerance and glucose-induced insulin secretion in healthy humans. J Clin Endocrinol Metab 88:1772–1779 [DOI] [PubMed] [Google Scholar]
- 23. Pala L, Ciani S, Dicembrini I, Bardini G, Cresci B, Pezzatini A, Giannini S, Mannucci E, Rotella CM. 2010. Relationship between GLP-1 levels and dipeptidyl peptidase-4 activity in different glucose tolerance conditions. Diabet Med 27:691–695 [DOI] [PubMed] [Google Scholar]
- 24. Vella A, Shah P, Basu R, Basu A, Holst JJ, Rizza RA. 2000. Effect of glucagon-like peptide 1(7-36) amide on glucose effectiveness and insulin action in people with type 2 diabetes. Diabetes 49:611–617 [DOI] [PubMed] [Google Scholar]
- 25. Bock G, Dalla Man C, Campioni M, Chittilapilly E, Basu R, Toffolo G, Cobelli C, Rizza R. 2006. Pathogenesis of pre-diabetes: mechanisms of fasting and postprandial hyperglycemia in people with impaired fasting glucose and/or impaired glucose tolerance. Diabetes 55:3536–3549 [DOI] [PubMed] [Google Scholar]
- 26. Fritsche A, Stefan N, Hardt E, Häring H, Stumvoll M. 2000. Characterisation of β-cell dysfunction of impaired glucose tolerance: evidence for impairment of incretin-induced insulin secretion. Diabetologia 43:852–858 [DOI] [PubMed] [Google Scholar]
- 27. Basu A, Basu R, Shah P, Vella A, Rizza RA, Jensen MD. 2001. Systemic and regional free fatty acid metabolism in type 2 diabetes. Am J Physiol Endocrinol Metab 280:E1000–E1006 [DOI] [PubMed] [Google Scholar]
- 28. Hansen KB, Vilsbøll T, Bagger JI, Holst JJ, Knop FK. 2010. Reduced glucose tolerance and insulin resistance induced by steroid treatment, relative physical inactivity, and high-calorie diet impairs the incretin effect in healthy subjects. J Clin Endocrinol Metab 95:3309–3317 [DOI] [PubMed] [Google Scholar]
- 29. Maida A, Lamont BJ, Cao X, Drucker DJ. 2011. Metformin regulates the incretin receptor axis via a pathway dependent on peroxisome proliferator-activated receptor-alpha in mice. Diabetologia 54:339–349 [DOI] [PubMed] [Google Scholar]
- 30. Meier JJ, Nauck MA, Pott A, Heinze K, Goetze O, Bulut K, Schmidt WE, Gallwitz B, Holst JJ. 2006. Glucagon-like peptide 2 stimulates glucagon secretion, enhances lipid absorption, and inhibits gastric acid secretion in humans. Gastroenterology 130:44–54 [DOI] [PubMed] [Google Scholar]
- 31. Salehi M, Aulinger B, Prigeon RL, D'Alessio DA. 2010. Effect of endogenous GLP-1 on insulin secretion in type 2 diabetes. Diabetes 59:1330–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vella A, Camilleri M, Rizza RA. 2004. The gastrointestinal tract and glucose tolerance. Curr Opin Clin Nutr Metab Care 7:479–484 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.