Abstract
Context:
Most tumors in Carney complex (CNC) are benign, including primary pigmented nodular adrenocortical disease (PPNAD), the main endocrine tumor in CNC. Adrenocortical cancer (AC) has never been observed in the syndrome. Herein, we describe a large Azorean family with CNC caused by a point mutation in the PRKAR1A gene coding for type 1-α (RIα) regulatory subunit of the cAMP-dependent protein kinase A, in which the index patient presented with AC.
Objective:
We studied the genotype-phenotype correlation in CNC.
Design and Setting:
We reported on case series and in vitro testing of the PRKAR1A mutation in a tertiary care referral center.
Patients:
Twenty-two members of a family were investigated for Cushing syndrome and other CNC components; their DNA was sequenced for PRKAR1A mutations.
Results:
Cushing syndrome due to PPNAD occurred in four patients, including the proposita who presented with AC and three who had Cushing syndrome and/or PPNAD. Lentigines were found in six additional patients who did not have PPNAD. A base substitution (c.439A>G/p.S147G) in PRKAR1A was identified in the proposita, in the three others with PPNAD, in the proposita's twin daughters who had lentigines but no evidence of hypercortisolism, and in five other family members, including one without lentigines or evidence of hypercortisolism. Unlike in other RIα defects, loss of heterozygosity was not observed in AC. The S147G mutation was compared to other expressed PRKAR1A mutations; it led to decreased cAMP and catalytic subunit binding by RIα and increased protein kinase A activity in vitro.
Conclusions:
In a large family with CNC, one amino acid substitution caused a spectrum of adrenal disease that ranged from lack of manifestations to cancer. PPNAD and AC were the only manifestations of CNC in these patients, in addition to lentigines. These data have implications for counseling patients with CNC and are significant in documenting the first case of AC in the context of PPNAD.
Primary pigmented nodular adrenocortical disease (PPNAD) has been described in isolated cases or, more frequently, in the context of Carney complex (CNC). CNC is a multiple neoplasia syndrome that can be inherited as an autosomal dominant trait (1, 2). PPNAD is a rare cause of Cushing syndrome (CS), but it is the most frequent endocrine manifestation of CNC (2–4). The diagnosis of CS caused by PPNAD is sometimes difficult to establish because of its frequently indolent course and occasional development over several years (4, 5).
Most patients with PPNAD or CNC have mutations of the PRKAR1A gene encoding the 1-α regulatory subunit (RIα) of the cAMP-dependent protein kinase A (PKA) (4, 6). Studies have suggested that PRKAR1A may act as a tumor suppressor gene, albeit somewhat atypical in the way it mediates inhibition of tumorigenesis (7, 8). In addition, there seems to be no direct and consistent correlation between the more than 120 PRKAR1A mutations described to date and the various CNC phenotypes. Only recently, certain associations between specific mutations and particular sets of CNC manifestations have emerged (9–15). Studying individual mutations in large families remains indispensable toward better understanding of the phenotype and tissue specificity of RIα defects.
We studied a large family on the Portuguese island of São Miguel (Azores) in which the proposita presented with adrenocortical cancer (AC). A PRKAR1A mutation that belongs to a small group of RIα defects leading to an expressed but pathogenic protein was identified in the family's DNA. This is the first case of documented AC in the context of PPNAD and CS; several other affected members showed no or little manifestation of CNC. Despite the lack of proof for a direct linkage between the development of AC and CNC, the clinial and laboratory data from this family provide important implications for both genetic counseling and understanding of the molecular action of mutant PRKAR1A.
Patients and Methods
Patients and clinical protocol
The patients described in this study were referred after the diagnosis of the proposita with AC and PPNAD. Informed consent was obtained from all participants as a part of a protocol approved by the Institutional Review Boards of the participating institutions. The index patient and available relatives were evaluated by detailed history taking and physical examination. Briefly, the patients were studied for clinical signs of CS and CNC, including dermatological examination and thyroid palpation. Ovarian or testicular, thyroid, and cardiac ultrasound scans and pituitary magnetic resonance imaging were performed. Plasma concentrations of GH, prolactin, and IGF-I were determined. All patients that were carriers of the mutation, including apparently unaffected relatives, were screened for CS by standard testing (3, 5).
Preparation of DNA and sequence analysis
DNA was extracted from paraffin-embedded tissue and peripheral blood leukocytes using the Wizard Genomic DNA Purification KIT (Promega, Madison, WI), and the 12 exons and the flanking intronic sequences of the PRKAR1A gene were amplified using the primers and the conditions described previously (9–15). Both strands of the amplified products were directly sequenced, and nucleotides were numbered in accordance with the reference sequence for PRKAR1A (GenBank accession no. NM_002734). The S147G PRKAR1A mutation has never been seen in normal controls; more than 2000 chromosomes have been tested over the last 12 yr (14, 15).
Lymphocyte culture, cycloheximide treatment, and genotyping
Lymphocyte cell lines from patients and control subjects were established and treated with 100 μg/ml cycloheximide or vehicle for 6 h, as previously published (13). Total RNA was extracted, and cDNA from lymphocytes was amplified; PCR fragments were run on polyacrylamide gel, purified, and analyzed by direct sequencing, as previously published (13, 15). For the determination of disease haplotypes in the PRKAR1A locus and loss-of-heterozygosity (LOH) studies in the AC, intragenic single-nucleotide polymorphisms (SNP) were analyzed by PCR amplification and direct sequencing (10). Three intronic SNP were identified: exon 1A 109 A/C, exon 8 IVS −24 A/G, and exon 4 IVS −9 insT. Additional SNP were selected from the public SNP database (http://www.ncbi.nlm.nih.gov/SNP) and genotyped after two different amplifications: SNP rs3785906, rs3785905, rs2302232, rs2302233, and PRKAR1A(GA)n, as previously described (10, 15). In addition, two flanking microsatellite markers within a 1-cM interval around the PRKAR1A locus (D17S940 and D17S795) were tested for LOH, as previously reported (4, 6, 9, 12, 15).
Functional studies of a PRKAR1A S147G construct
A PCR-based cloning method was used to generate both the wild-type (WT) and mutant expression constructs, and the effect of mutated RIα on PKA activity was determined as previously reported (9, 13). The point mutations were then introduced into the RIα-WT construct by the use of site-directed mutagenesis. PCR was performed using PfuTurbo DNA Polymerase (Stratagene, La Jolla, CA) and the RIα-WT as the DNA template under the following conditions: 95 C for 4 min; 95 C for 25 sec, annealing temperature for 30 sec, and 72 C for 8 min with 15-sec increases at each cycle for 18 cycles; and 72 C for 15 min. The specific primers and annealing temperatures for the point mutations are published elsewhere (13, 15). The PCR products were digested with DpnI and then purified and ligated as described above. DNA was prepared for transformation into One Shot TOP10 Competent Cells (Invitrogen, San Diego, CA) using the Wizard Plus SV Minipreps (Promega) and prepared for transfection with the HiSpeed Plasmid Midi Kit (QIAGEN, Valencia, CA). All constructs were verified by sequencing before their use in expression studies.
To determine the effect of mutated RIα on PKA activity, we first assayed kinase activity with and without the addition of cAMP and a specific PKA inhibitor (PKI). HEK293 cells were cultured in DMEM supplemented with 10% fetal bovine serum, 1% l-glutamine, 1% antibiotic-antimycotic, and 1% sodium pyruvate. The cells were plated in 10-cm dishes and transiently transfected 24 h later by using Lipofectamine 2000 (Invitrogen) following the protocol provided. Cells were transfected with 6 μg of plasmid DNA expressing either RIα-WT, a mutated form of RIα, or the empty pcDNA3.1(−) vector (“mock”) as a control. Cells were harvested 48 h after transfection and were subjected to lysis. Total PKA activity represents the difference between PKA activity before and after the addition of PKI; PKA-specific activation represents the difference between total PKA and baseline activities (the latter determined when neither cAMP nor PKI is present). A ratio was calculated to determine the factor by which PKA-specific activation differs between mutant and WT RIα: PKA ratio = PKA-specific activation MUT/PKA-specific activation WT ± se (13, 16). All determinations of PKA activity were performed in duplicate, corrected for protein content (per microgram of total protein), and an average value was calculated for each experiment; the experiment was repeated twice. Statistical analysis of comparisons between groups was undertaken using a two-sample t test; P ≤ 0.05 was considered significant, whereas P ≤ 0.10 was interpreted as showing a tendency toward significance.
Because intracellular levels of cAMP often tend to be much lower than the amount used in the aforementioned kinase activity assay, only a rough assessment of kinase activity could be obtained from our classic PKA activity assay, which used relatively large doses (5 μm) of cAMP. With this knowledge, we then proceeded to test PKA activity at various lower concentrations of cAMP to simulate more realistically the intracellular situation. The lysates were diluted in the previously mentioned lysis buffer to 12.9 μg of protein, and the assay was carried out as above with the following concentrations of cAMP: 0, 0.001, 0.005, 0.01, 0.1, 0.5, and 5.0 μm.
To detect the presence and expression level of mutant R1α protein, 20 μg protein was resolved by electrophoresis on a Novex 10% Tris-Glycine gel (Invitrogen) and then transferred to a 0.2-μm nitrocellulose membrane. Western blotting was performed with primary mouse antibodies against the main PKA subunits (PRKARIα, PRKARIIα, PRKARIIβ, 1:250; PKAC, 1:1000; BD Biosciences, Sunnyvale, CA), using primary rabbit antibody against β-actin (1:5000; Abcam, Cambridge, MA) as a control, and horseradish peroxidase conjugated secondary antibodies against mouse (1:1000; EMD Biosciences, San Diego, CA) or rabbit IgG (1:3000; Abcam). Bands were detected by enhanced chemiluminescence reagent and densitometer scanning (Molecular Dynamics, Sunnyvale, CA). Protein images were quantified with Image Quant software and normalized against the expression of β-actin. To observe whether the mutations disrupted interactions between the RIα and C subunits, coimmunoprecipitation was done on 250 μg of lysates from HEK293 cells, transfected as above, lysed in commercially available mammalian protein extraction reagent (Pierce, Rockford, IL), and precipitated with 1 μg primary rabbit antibody against Cα (Santa Cruz Biotechnology, Santa Cruz, CA). Western Blot for RIα was then carried out as described above.
To determine how mutations in RIα affected the ability of PRKAR1A to bind cAMP, a cAMP binding assay was performed as described previously (13). Levels of cAMP were measured in lysates from transfected HEK293 cells following the specifications of the Direct cAMP Correlate-EIA Kit (Assay Designs, Ann Arbor, MI). Cell count was determined by a Beckman Coulter Counter (Beckman Coulter, Fullerton, CA), and 2 × 105 cells were assayed for each sample.
Results
Clinical description of the index case and her family
The proposita (member III-7, CAR 609.01; Fig. 1) was a 28-yr-old female who was first seen at the emergency department of the Hospital Divino Espírito Santo (Ponta Delgada, Azores-Portugal). She was referred by her family doctor due to fatigue, depression, and severe hypokalemia. Hypertension had been diagnosed 6 yr before this evaluation. During the preceding 2 yr, progressive frontal baldness, weight gain, hirsutism, dorsocervical fat pad, cutaneous striae, and easy bruising had developed; she had also developed amenorrhea 6 months before the evaluation. On examination, she had intense spotty skin pigmentation of her face, including the vermilion border of the lips. She was started on iv KCl and treated with short-acting insulin and oral nifedipine. Cortisol was 39.9 and 49.5 ng/dl at 0800 and 2300 h, respectively; urinary cortisol was 2578 μg/24 h; ACTH and dehydroepiandrosterone sulfate were low; but testosterone and free testosterone were high at 908.6 and 1064 ng/dl, respectively. Abdominal magnetic resonance imaging showed a 12-cm mass occupying the right adrenal space. Right adrenalectomy with complete excision of the tumor was performed. The pathological examination confirmed the clinical diagnosis of AC, but PPNAD was also diagnosed in the surrounding adrenocortical tissue. Postoperatively, clinical and biochemical signs of CS persisted without any changes in hormonal values. After the histological diagnosis of PPNAD, a left adrenalectomy was performed; excision of the metastatic liver lesions followed. The patient died 6 months postoperatively from respiratory distress, pneumonia, and complications related to metastatic AC. Her DNA was tested both from the tumor and peripheral lymphocytes; she carried a germline S147G mutation in the PRKAR1A gene.
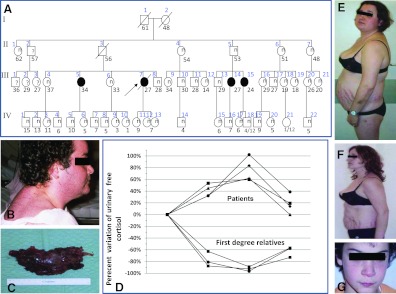
Fig. 1.
A, Pedigree of the CAR609 family. B, The proposita. C, AC from the proposita: the tumor had a maximum diameter of 12 cm. D, Family members with PPNAD had a “paradoxical” increase in their cortisol secretion in response to dexamethasone, whereas their relatives with normal PRKAR1A sequence suppressed their cortisol secretion in response to dexamethasone. E and F, Individual III-15 before (E) and after (F) bilateral adrenalectomy. G, Subject IV-12 is a carrier of the S147G mutation and has lentigines but remains healthy and eucortisolemic to date.
The family of the proposita was traced back to her grandparents [first generation (I) of the family; Fig. 1]. Her grandmother (I-1) had died at the age of 47 yr due to a “brain tumor.” From her medical records and personal photographs, it was possible to discern that she was short (less than 5 feet tall), had a full (“moon”) face with severe hirsutism, and suffered from hypertension. Her DNA, however, was not available.
The proposita's aunt (II-1), had lentigines, type 2 diabetes, hypertension, and dyslipidemia. She had the S147G mutation in PRKAR1A, and she died of complications of colon cancer. The proposita's father (II-3) died suddenly at the age of 59 yr from heart disease. Members II-2 and II-4 died of melanoma and heart disease, respectively; both were negative for PRKAR1A mutations. Family member II-5 tested positive for the S147G mutation; physical examination, imaging, and laboratory tests were all normal. Member II-6, a 55 yr old, was negative for the mutation but had a thyroid adenoma, insulin resistance, and hypertension. Member II-7, a 49 yr old, was a nonsmoker who was diagnosed with a lung adenocarcinoma and had insulin resistance, hypertension, and dyslipidemia; she was also negative for the PRKAR1A mutation.
Member III-5 (CAR 609.04), the 37-yr-old sister of the proposita, was positive for the S147G in PRKAR1A and had cutaneous striae, easy bruising, and depression for 6 yr before her diagnosis with PPNAD. She also developed aseptic necrosis of the left femoral head but was normotensive and had normal pituitary, heart, and other imaging studies. She underwent bilateral adrenalectomy and is currently well. Member III-14 (CAR 609.02), first-degree cousin of member III-5 and positive for the S147G mutation, noticed generalized acne and hirsutism after her first pregnancy, 5 yr before her diagnosis with PPNAD. She also had oligoamenorrhea for 3 yr before the diagnosis. An abdominal computed tomography scan revealed a 1.5-cm right adrenal mass. The lesion was not cancerous, and the patient underwent left adrenalectomy as well. Member III-15 (CAR 609.03), a 24 yr old, was also positive for the S147G mutation, had classic signs of CS but no other symptoms of CNC, and underwent bilateral adrenalectomy.
In this family, after PPNAD, lentigines were the most common finding among the S147G mutation carriers. These pigmented lesions were much more evident during puberty, as in members IV-12 and -13, who are twin sisters and daughters of the proposita.
Histopathology of excised lesions and β-catenin staining and sequencing
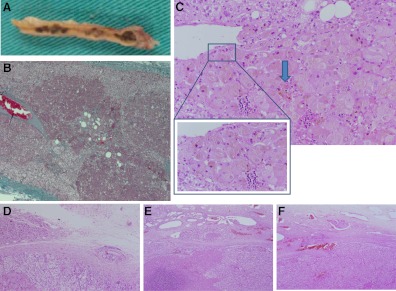
In the proposita, in addition to the excised AC, classic signs of PPNAD were seen in the remaining adrenal tissue (Fig. 2). The adrenal glands removed from the other patients had weights that ranged from 4 to 8 g, and all were affected by classic PPNAD. Immunohistochemistry of the hepatic metastatic lesions was positive for cytokeratin, vimentin, inhibin, and carcinoembryonic antigen, as was the primary AC. The hepatic lesions looked identical to the primary tumor.
Fig. 2.
A, The right adrenal removed from individual III-15 with the appearance of classic PPNAD. B, The adrenal gland excised from the proposita had characteristic features of PPNAD, including micronodules of eosinophilic cells with pigment. C, Cytoplasmic pigmentation (arrow) (magnified 40×). D–F, AC showing penetration of the capsule (D) and vascular invasion (E and F).
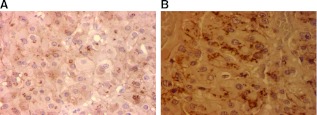
The PPNAD and the AC, both the primary and the metastatic lesions, had an intense cytoplasmic staining for β-catenin (Fig. 3). There was no nuclear staining for β-catenin. We sequenced the tumor for somatic CTNNB1 mutations in exon 3 of the gene and none were found (data not shown).
Fig. 3.
Immunostaining did not show nuclear accumulation of β-catenin in the PPNAD (A) or in the AC cells (B) from the proposita's lesions (magnified 40×).
We then applied the Weiss score. Weiss et al. (17) found that the only significant microscopic finding indicative of AC was a mitotic index of more than 20 mitoses per 50 high-power fields. We looked for mitoses in certain slides from the tumor but found none.
PRKAR1A mutation and its in vitro analysis
A single base pair substitution (c.439A>G, S147G) in PRKAR1A was identified in the proposita, in all members of the family with the histological diagnosis of PPNAD, the proposita's twin daughters (who only had lentigines and no clinical and laboratory evidence of hypercortisolism), and the other family members with CS, as described above.
We searched our continuously updated database of PRKAR1A mutations (14, 15); the c.439A>G/p.S147G mutation was also seen in a sporadic case of CS due to PPNAD. This patient had presented with CS and intense lentiginosis at the age of 9 yr. She is now 30 yr old and has had no other symptoms or signs of CNC.
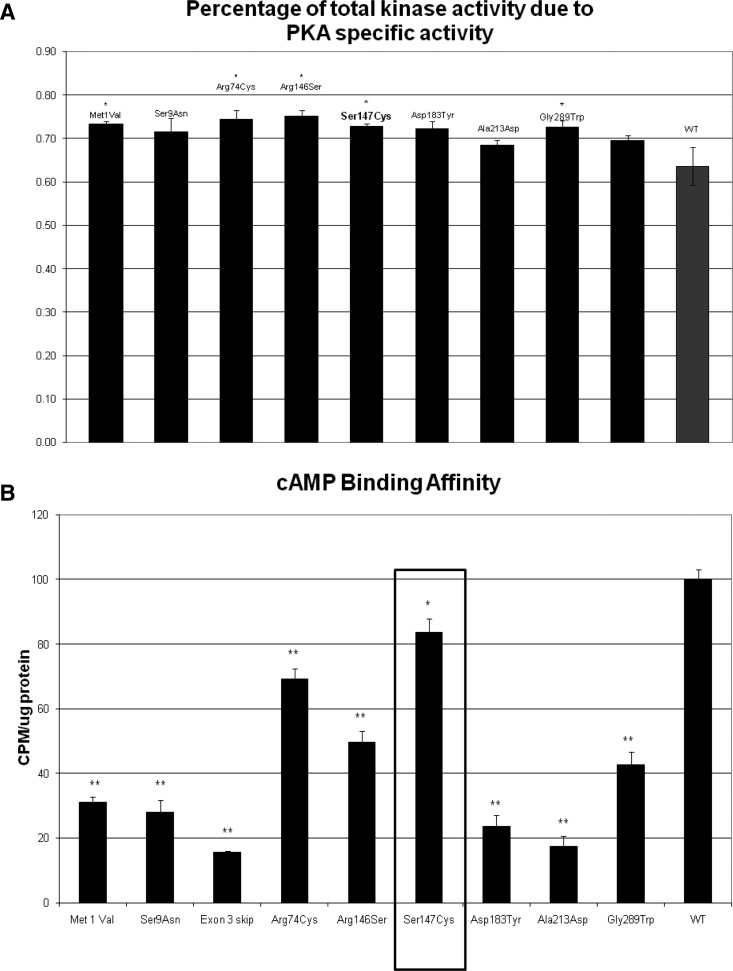
The S147G mutation led to decreased cAMP and catalytic subunit binding by RIα, and this in turn resulted in increased PKA activity and P-CREB/CREB ratio in vitro (Fig. 4); this was consistent with the effects of previously described expressed PRKAR1A mutations, which are presented in comparison.
Fig. 4.
A, PKA activity of the S147G mutation is comparable to that of other pathogenic RIα defects and greater than that of the WT molecule. B, cAMP binding affinity is lower for the S147G mutation than that of the WT RIα but generally higher than that in other expressed variants.
In brief, although baseline mutant PKA activity values were not significantly higher than WT PKA activity values (data not shown), the S147G mutant protein exhibited a significantly higher increase in activity, by comparison with the WT RIα upon exposure to PKA (Fig. 4A). At lower cAMP levels (0.01 μm), kinase activity was significantly greater than for the WT. Binding to cAMP was found to be also significantly decreased for the mutant RIα (Fig. 4B). Western blot analysis of the PKA subunits indicated the presence of the RIα, RIIα, and RIIβ regulatory subunits in normal proportion (Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org), and coimmunoprecipitation of RIα and Cα revealed decreased interaction between the mutant RIα and WT Cα (data not shown). Finally, LOH was not consistently present in the AC samples that were tested against the patient's peripheral DNA (data not shown).
Discussion
AC is a rare tumor (18), and PRKAR1A mutations have not been found in the AC screened so far (19). This is the first description of AC in a patient with familial CS and PPNAD due to a PRKAR1A mutation. One of the first reports of familial PPNAD from the island of Cuba described a member aged 15 yr with CS who succumbed to a malignant retroperitoneal tumor that may have been AC; a tissue diagnosis was not obtained, unfortunately (20). There are no other cases of AC in our database of patients with PRKAR1A defects (14, 15); it should also be noted, however, that in this family there was an extensive history of other cancers and, thus, it is possible that PPNAD was just one of the contributing factors leading to AC in the proposita.
Since the identification of PRKAR1A mutations as the cause for CNC, more than 120 such defects have been identified, with the majority leading to the absence of a mutated RIα protein by nonsense mRNA decay (NMD) (4, 6, 9, 15). In the present study, we investigated the in vitro effects of this novel PRKAR1A mutation that escapes NMD and leads to the expression of a mutated protein, and we compared its function to seven other expressed RIα variants (13). cAMP-dependent protein kinase (PKA) is a serine/threonine kinase involved in multiple steps of cell growth and differentiation (7, 8, 11). The enzyme is a tetramer composed of a pair of two regulatory subunits (RIα, RIIα RIβ, RIIβ) and a pair of catalytic subunits (Cα, Cβ, Cγ, PRKX); the enzyme dissociates in the presence of cAMP. In vitro expression of the mutant S147G RIα protein was associated with increased PKA activity; greater PKA activity was due to decreased binding of the mutant RIα to the catalytic subunit, the mechanism that appears to be the most common effect of CNC- and/or PPNAD-causing, expressed RIα variants (11, 13, 15). The S147G mutation is somewhat different from other expressed mutations in that the proportion of the type-II regulatory subunits to type-I does not change (Supplemental Fig. 1); however, it is not unique. Met1Val, Ser9Asn, Arg74Cys, Arg146Ser, and Asp183Tyr also do not change the ratio (Supplemental Fig. 1). It is possible that this may underlie, at least in part, the tissue selectivity of certain mutations like that of S147G (see discussion below also).
Experimental data have shown that binding of the regulatory to the catalytic subunits requires at least two sites to achieve high-affinity binding (21). The RIα exon 3 skipping mutation, which lacks residues 60–117, spans the primary site (residues 94–98, the inhibitor sequence), whereas the S147G mutation disrupts the secondary site (residues 138–148, 232–247) within what is known as domain “cAMP-A” (21). Coimmunoprecipitation studies confirmed the inability of the S147G mutant RIα to effectively bind Cα (data not shown). As expected, levels of unbound cAMP declined even as cAMP binding affinity fell; because the C subunit is coupled to both the inhibitor sequence and cAMP-A, it is not surprising that a mutation that affects C-subunit binding would also affect cAMP binding.
The manifestations of CNC due to S147G PRKAR1A mutation in this family covered the entire range of classic signs and symptoms of hypercortisolism caused by PPNAD but did not include myxomas, schwannomas, or any other tumors associated with CNC. We identified another patient with the same mutation de novo (CAR04.03) from a North American family who similarly had only lentigines and PPNAD. Thus, S147G mutation, the 6-bp polypyrimidine tract deletion [exon 7 IVS del (-7->-2)] (10), and the M1V start-site PRKAR1A substitution (22) should be considered RIα defects that may be primarily associated with PPNAD.
At this point, it remains unclear what makes certain RIα defects more adrenal-specific than others. One hypothesis is that the adrenal cortex is more responsive to even subtle alterations in PKA activity, such as those caused by expressed RIα variants (vs. those that cause haploinsufficiency by NMD). PPNAD is, after all, the most frequent endocrine manifestation of CNC, occurring in more than a quarter of the patients (14, 15). Gender plays a role: in this family the proposita with AC and most patients with PPNAD were females (14).
Finally, AC if caused by RIα defects may be slightly different: our patient's AC had a low Weiss score. The infrequency of mitoses in the tumor may ultimately prove to be a significant finding. The patient's AC, although microscopically (it had necrosis and vascular invasion) and clinically (it had already metastasized to the liver) a carcinoma, was somehow different from the run-of-the-mill sporadic AC. Interestingly, it was also different from other cases of single tumors forming in the background of PPNAD; immunostaining did not show nuclear accumulation of β-catenin in the AC or the PPNAD cells of the patient (Fig. 3) and the tumor did not have somatic CTNNB1 mutations. We and others have reported that nuclear accumulation of β-catenin occurs in more than 90% of cells in single adenomas that bear somatic CTNNB1 mutations in the context of PPNAD due to a germline PRKAR1A mutation (23, 24). Adrenocortical lesions with nuclear accumulation of β-catenin and somatic CTNNB1 mutations tend to be more aggressive than tumors that don't show activation of this gene (25, 26).
In conclusion, we describe a large family with PPNAD caused by a PRKAR1A mutation that escapes NMD. One family member presented with AC and, although there is no direct proof that the AC was caused by this PRKAR1A mutation, this is a significant observation for counseling of CNC patients and their families, as well as genotype-phenotype studies of RIα defects.
Supplementary Material
Acknowledgments
We thank all family members for their participation in the study. We also thank the nursing staff of Medicina III (Hospital Divino Espirito Santo) for their hard work. We are grateful to surgeons Drs. João Coutinho, António Silva Melo, and Luís Bernardo.
This work was supported in part by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, intramural National Institutes of Health Project Z01-HD-000642-04 (to C.A.S.).
Disclosure Summary: The authors have nothing to disclose.
For editorial see page 387
- AC
- Adrenocortical cancer
- CNC
- Carney complex
- LOH
- loss-of-heterozygosity
- NMD
- nonsense mRNA decay
- PKA
- protein kinase A
- PKI
- protein kinase inhibitor
- PPNAD
- primary pigmented nodular adrenocortical disease
- RIα
- 1-α regulatory subunit
- SNP
- single-nucleotide polymorphism
- WT
- wild-type.
References
- 1. Carney JA, Gordon H, Carpenter PC, Shenoy BV, Go VL. 1985. The complex of myxomas, spotty pigmentation, and endocrine overactivity. Medicine (Baltimore) 64:270–283 [DOI] [PubMed] [Google Scholar]
- 2. Stratakis CA, Carney JA, Lin JP, Papanicolaou DA, Karl M, Kastner DL, Pras E, Chrousos GP. 1996. Carney complex, a familial multiple neoplasia and lentiginosis syndrome. Analysis of 11 kindreds and linkage to short arm of chromosome 2. J Clin Invest 97:699–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stratakis CA, Kirschner LS, Carney JA. 2001. Clinical and molecular features of Carney complex: diagnostic criteria and recommendations for patient evaluation. J Clin Endocrinol Metab 86:4041–4046 [DOI] [PubMed] [Google Scholar]
- 4. Kirschner LS, Sandrini F, Monbo J, Lin JP, Carney JA, Stratakis CA. 2000. Genetic heterogeneity and spectrum of mutations of the PRKAR1A gene in patients with Carney complex. Hum Mol Genet 9:3037–3046 [DOI] [PubMed] [Google Scholar]
- 5. Stratakis CA, Sarlis N, Kirschner LS, Carney JA, Doppman JL, Nieman LK, Chrousos GP, Papanicolaou DA. 1999. Paradoxical response to dexamethasone in the diagnosis of primary pigmented nodular adrenocortical disease. Ann Intern Med 131:585–591 [DOI] [PubMed] [Google Scholar]
- 6. Kirschner LS, Carney JA, Pack SD, Taymans SE, Giatzakis C, Cho YS, Cho-Chung YS, Stratakis CA. 2000. Mutations of the gene encoding the protein kinase A type I-α regulatory subunit in patients with the Carney complex. Nat Genet 26:89–92 [DOI] [PubMed] [Google Scholar]
- 7. Robinson-White A, Meoli E, Stergiopoulos S, Horvath A, Boikos S, Bossis I, Stratakis CA. 2006. PRKAR1A mutations and protein kinase A interactions with other signalling pathways in the adrenal cortex. J Clin Endocrinol Metab 91:2380–2388 [DOI] [PubMed] [Google Scholar]
- 8. Stratakis CA. 2009. New genes and/or molecular pathways associated with adrenal hyperplasias and related adrenocortical tumors. Mol Cell Endocrinol 300:152–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Groussin L, Kirschner LS, Vincent-Dejean C, Perlemoine K, Jullian E, Delemer B, Zacharieva S, Pignatelli D, Carney JA, Luton JP, Bertagna X, Stratakis CA, Bertherat J. 2002. Molecular analysis of the cyclic AMP-dependent protein kinase A (PKA) regulatory subunit 1A (PRKAR1A) gene in patients with Carney complex and primary pigmented nodular adrenocortical disease (PPNAD) reveals novel mutations and clues for pathophysiology: augmented PKA signaling is associated with adrenal tumorigenesis in PPNAD. Am J Hum Genet 71:1433–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Groussin L, Horvath A, Jullian E, Boikos S, Rene-Corail F, Lefebvre H, Cephise-Velayoudom FL, Vantyghem MC, Chanson P, Conte-Devolx B, Lucas M, Gentil A, Malchoff CD, Tissier F, Carney JA, Bertagna X, Stratakis CA, Bertherat J. 2006. A PRKAR1A mutation associated with primary pigmented nodular adrenocortical disease in 12 kindreds. J Clin Endocrinol Metab 91:1943–1949 [DOI] [PubMed] [Google Scholar]
- 11. Meoli E, Bossis I, Cazabat L, Mavrakis M, Horvath A, Stergiopoulos S, Shiferaw ML, Fumey G, Perlemoine K, Muchow M, Robinson-White A, Weinberg F, Nesterova M, Patronas Y, Groussin L, Bertherat J, Stratakis CA. 2008. Protein kinase A (PKA) effects of an expressed PRKAR1A mutation associated with aggressive tumors. Cancer Res 68:3133–3141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Horvath A, Bossis I, Giatzakis C, Levine E, Weinberg F, Meoli E, Robinson-White A, Siegel J, Soni P, Groussin L, Matyakhina L, Verma S, Remmers E, Nesterova M, Carney JA, Bertherat J, Stratakis CA. 2008. Large deletions of the PRKAR1A gene in Carney complex. Clin Cancer Res 14:388–395 [DOI] [PubMed] [Google Scholar]
- 13. Greene EL, Horvath AD, Nesterova M, Giatzakis C, Bossis I, Stratakis CA. 2008. In vitro functional studies of naturally occurring pathogenic PRKAR1A mutations that are not subject to nonsense mRNA decay. Hum Mutat 29:633–639 [DOI] [PubMed] [Google Scholar]
- 14. Bertherat J, Horvath A, Groussin L, Grabar S, Boikos S, Cazabat L, Libe R, René-Corail F, Stergiopoulos S, Bourdeau I, Bei T, Clauser E, Calender A, Kirschner LS, Bertagna X, Carney JA, Stratakis CA. 2009. Mutations in regulatory subunit type 1A of cyclic adenosine 5′-monophosphate-dependent protein kinase (PRKAR1A): phenotype analysis in 353 patients and 80 different genotypes. J Clin Endocrinol Metab 94:2085–2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Horvath A, Bertherat J, Groussin L, Guillaud-Bataille M, Tsang K, Cazabat L, Libé R, Remmers E, René-Corail F, Faucz FR, Clauser E, Calender A, Bertagna X, Carney JA, Stratakis CA. 2010. Mutations and polymorphisms in the gene encoding regulatory subunit type 1-α of protein kinase A (PRKAR1A): an update. Hum Mutat 31:369–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nesterova MV, Sashchenko LP, Vasiliev VY, Severin ES. 1975. A cyclic adenosine 3′5′monophosphate-dependent histone kinase from pig brain. Biochim Biophys Acta 377:271–281 [DOI] [PubMed] [Google Scholar]
- 17. Weiss LM, Medeiros LJ, Vickery AL., Jr 1989. Pathologic features of prognostic significance in adrenocortical carcinoma. Am J Surg Pathol 13:202–206 [DOI] [PubMed] [Google Scholar]
- 18. Allolio B, Fassnacht M. 2006. Adrenocortical carcinoma: clinical update. J Clin Endocrinol Metab 91:2027–2037 [DOI] [PubMed] [Google Scholar]
- 19. Bertherat J, Groussin L, Sandrini F, Matyakhina L, Bei T, Stergiopoulos S, Papageorgiou T, Bourdeau I, Kirschner LS, Vincent-Dejean C, Perlemoine K, Gicquel C, Bertagna X, Stratakis CA. 2003. Molecular and functional analysis of PRKAR1A and its locus (17q22–24) in sporadic adrenocortical tumors: 17q losses, somatic mutations, and protein kinase A expression and activity. Cancer Res 63:5308–5319 [PubMed] [Google Scholar]
- 20. Arce B, Licea M, Hung S, Padrón R. 1978. Familial Cushing's syndrome. Acta Endocrinol 87:139–147 [DOI] [PubMed] [Google Scholar]
- 21. Hamuro Y, Anand GS, Kim JS, Juliano C, Stranz DD, Taylor SS, Woods VL., Jr 2004. Mapping intersubunit interactions of the regulatory subunit (RIα) in the type I holoenzyme of protein kinase A by amide hydrogen/deuterium exchange mass spectrometry (DXMS). J Mol Biol 340:1185–1196 [DOI] [PubMed] [Google Scholar]
- 22. Pereira AM, Hes FJ, Horvath A, Woortman S, Greene E, Bimpaki E, Alatsatianos A, Boikos S, Smit JW, Romijn JA, Nesterova M, Stratakis CA. 2010. Association of the M1V PRKAR1A mutation with primary pigmented nodular adrenocortical disease in two large families. J Clin Endocrinol Metab 95:338–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tadjine M, Lampron A, Ouadi L, Horvath A, Stratakis CA, Bourdeau I. 2008. Detection of somatic β-catenin mutations in primary pigmented nodular adrenocortical disease (PPNAD). Clin Endocrinol (Oxf) 69:367–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gaujoux S, Tissier F, Groussin L, Libé R, Ragazzon B, Launay P, Audebourg A, Dousset B, Bertagna X, Bertherat J. 2008. Wnt/β-catenin and 3′,5′-cyclic adenosine 5′-monophosphate/protein kinase A signaling pathways alterations and somatic β-catenin gene mutations in the progression of adrenocortical tumors. J Clin Endocrinol Metab 93:4135–4140 [DOI] [PubMed] [Google Scholar]
- 25. Gaujoux S, Grabar S, Fassnacht M, Ragazzon B, Launay P, Libé R, Chokri I, Audebourg A, Royer B, Sbiera S, Vacher-Lavenu MC, Dousset B, Bertagna X, Allolio B, Bertherat J, Tissier F. 2011. β-Catenin activation is associated with specific clinical and pathologic characteristics and a poor outcome in adrenocortical carcinoma. Clin Cancer Res 17:328–336 [DOI] [PubMed] [Google Scholar]
- 26. Bonnet S, Gaujoux S, Launay P, Baudry C, Chokri I, Ragazzon B, Libé R, René-Corail F, Audebourg A, Vacher-Lavenu MC, Groussin L, Bertagna X, Dousset B, Bertherat J, Tissier F. 2011. Wnt/β-catenin pathway activation in adrenocortical adenomas is frequently due to somatic CTNNB1-activating mutations, which are associated with larger and nonsecreting tumors: a study in cortisol-secreting and non-secreting tumors. J Clin Endocrinol Metab 96:E419–E426 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.