Abstract
Context:
Most medullary thyroid cancers (MTC) express somatostatin receptors; therefore, 111In-octreotide somatostatin receptor scintigraphy (SRS) may be useful in detecting sites of metastases in children with MTC.
Objective:
The aim of the study was to evaluate tumor metastases in children and adolescents with MTC using SRS in comparison to conventional imaging.
Design and Setting:
A case series was conducted as part of baseline evaluation for cancer treatment protocol at the National Institutes of Health Clinical Center.
Patients:
Eleven patients with a median age of 15 (range, 9–17) yr participated in the study, 10 with histologically proven, metastatic MTC due to the M918T mutation of the RET protooncogene, and one with a known RET polymorphism.
Intervention:
After receiving 0.086 mCi/kg 111Indium-pentreotide, patients were examined with a single photon emission computed tomography scan 4 and 24 h after injection. Baseline conventional imaging, including computed tomography (neck, chest, abdomen, ± pelvis, adrenals), magnetic resonance imaging (neck), and bone scan, was performed on all patients.
Main Outcome Measures:
SRS results were compared with conventional imaging.
Results:
Five of the 11 patients had abnormal findings on SRS. Of the 53 total target lesions present in the patients, only 24.5% were accurately identified through SRS.
Conclusions:
SRS appears to be less sensitive than conventional imaging at detecting the full extent of metastatic disease in children and adolescents with hereditary MTC. SRS incompletely identified sites of tumor and failed to visualize small sites of tumor or liver and lung metastases, and it has a limited role in the evaluation of metastatic disease in pediatric MTC patients.
Effective and reliable imaging technology for the identification of metastatic disease in medullary thyroid carcinoma (MTC) is essential. Somatostatin receptor scintigraphy (SRS) is widely used to visualize neuroendocrine tumors; however, the use of this imaging technology has not been previously studied for its effectiveness in children with MTC. The goal of our study was to evaluate tumor metastases in children with histologically proven hereditary MTC using SRS compared with conventional imaging.
MTC is a rare neuroendocrine tumor arising from the parafollicular C cells of the thyroid. Somatostatin is a regulatory peptide hormone with an inhibitory role in hormone release, as well as a neurotransmitter modulatory role (1). MTC secretes and stores somatostatin and expresses its receptors, which are also thought to play a role in the growth of neuroendocrine tumors (1, 2). Scintigraphy using the radiolabeled somatostatin analog octreotide is one radiological method of neuroendocrine tumor localization, and it has been used for imaging of gastroenteropancreatic tumors, pheochromocytoma, as well as MTC (3–6). The role of SRS in the detection of metastatic disease in pediatric patients with MTC remains unclear. In adults, the sensitivity rate of SRS for detecting MTC ranges from 37–90% (3, 5, 7–9). Immunohistochemical analysis of MTC in adults has shown that 43–57% of MTC express the somatostatin subtypes 2 or 5 that bind to octreotide (10).
MTC represents approximately 4–7% of all thyroid cancer cases (11, 12). MTC may occur as a sporadic tumor, or within the context of a hereditary syndrome, including multiple endocrine neoplasia 2A and 2B, as well as familial MTC. Hereditary MTC is inherited in an autosomal dominant fashion, and it has a nearly 100% level of penetrance (13). MTC is relatively unresponsive to radiation therapy and to standard chemotherapeutic regimens (14, 15). Early results show that targeted therapy with tyrosine kinase inhibitors may present an effective therapeutic option in patients with advanced hereditary MTC (16, 17). At present, the only cure for patients with MTC is total thyroidectomy performed at an early stage when the disease is confined to the thyroid gland. Once MTC metastasizes, it has a tendency to spread to local and regional lymph nodes, and more distantly to lung, liver, brain, and bone (14). In the United States, the incidence of MTC in children and adolescents is 0.5 per year per million (18). The low incidence of MTC in children presents a challenge toward gaining clinical expertise and definitive recommendations in clinical care. In this study, we evaluated how SRS compares to conventional imaging techniques in visualizing metastatic MTC in children and adolescents.
Patients and Methods
Ten patients with metastatic hereditary MTC and one patient with MTC and a known RET polymorphism (six males, five females; median age, 15 yr) were seen at the National Institutes of Health (NIH) Clinical Center; their initial evaluation took place between June 2007 and September 2010. All patients had histologically proven disease, elevated carcinoembryonic antigen (CEA) and basal calcitonin. All patients had measurable disease—defined by the response evaluation criteria in solid tumors as the presence of at least one lesion with longest diameter greater than 1 cm. Sites of metastases were evaluated by conventional diagnostic imaging using computed tomography (CT) (neck, chest, abdomen, ± pelvis, adrenals), magnetic resonance imaging (MRI) (neck), and bone scan in all subjects. Additional anatomic sites were evaluated by CT/MRI as clinically indicated. Target metastatic tumors on CT and/or MRI were identified using the response evaluation criteria in solid tumors in which up to five lesions with the longest diameter greater than 1 cm per anatomic site were measured. Lesions on bone scan were also enumerated. Planar whole body and single photon emission CT imaging was performed at 4 and 24 h after iv injection of 0.086 mCi/kg 111In-pentreotide, with a minimum of 3 mCi up to a maximum of 6 mCi. Ten of 11 patients had 4-h SRS imaging performed, whereas eight of 11 had 24-h imaging performed. Beginning in 2008, single photon emission CT/CT was added as an additional imaging modality for attenuation correction and coregistration purposes. A single designated nuclear medicine physician, blinded to the conventional imaging findings, scored each lesion on SRS from a scale of 1 to 5, with 1 representing absence of MTC and 5 representing definitive presence of MTC (1 = not MTC, 2 = doubtful, 3 = equivocal, 4 = probable, 5 = definite MTC). Lesions with a score of at least 4 were considered positive. For the purposes of this study, conventional imaging was used as a “gold standard” for identification of metastases because biopsy of all lesions was not feasible.
All study tests were performed as part of the baseline evaluation on a treatment study for children and adolescents with MTC (www.ClinicalTrials.gov identifier, NCT00514046). Study tests were approved by the National Cancer Institute Institutional Review Board, and consent from parents was obtained under protocol 07-C-0189, which was conducted at the NIH Clinical Center.
Statistical analysis
Data were described using frequency distributions and simple descriptive statistics; continuous data are reported as median (range). A two-sample t test, or its nonparametric Wilcoxon rank sum test, was used for comparing continuous data from distinct groups. Dichotomous data from independent groups were compared by Fisher's exact tests. Pearson correlation coefficients or Spearman's ρ, as appropriate, were used for correlation analyses. Logistic regression analysis assessed the relationship between continuous explanatory variables and the dichotomous outcome of SRS scan positivity. Data were analyzed using SAS system software version 9.1 (SAS Institute, Cary, NC). A two-sided P value of ≤0.05 was considered to be statistically significant.
Results
Patient description
Baseline patient characteristics are presented in Table 1. The median (range) age of subjects was 15 yr (9–17). Sequencing of the RET protooncogene via evaluation of germline DNA revealed 10 patients with M918T mutations and one patient with the G691S polymorphism (Table 1); thus, the majority of patients had the characteristic RET mutation for multiple endocrine neoplasia 2B. Nine of the patients had undergone prior thyroidectomy, two patients had prior radiation therapy, whereas two patients had no prior therapy. One patient had received imatinib, interferon α, pamidronate, and octreotide therapies in the past. Target lesions detected by conventional imaging and SRS are presented in Table 2. SRS was positive at any site in five of 11 patients (45.5%).
Table 1.
Baseline patient characteristics
| Age (yr), median (range) | 15 (9–17) |
| Males:females | 6:5 |
| RET M918T mutation | 10 |
| RET G691S polymorphism | 1 |
| Size (cm) of individual target lesions, median (range) | 1.7 (1.0–9.9) |
| No. of target lesions per patient, median (range) | 3 (2–10) |
| Sum (cm) of total size of target lesions per patient, median (range) | 8.2 (2.6–23.8) |
| CEA (μg/liter), median (range) | 103.8 (5.2–768.5) |
| Calcitonin (pg/ml), median (range) | 13,281 (498–67,056) |
Normal range of CEA, 0–2.5 μ g/liter. Normal range of calcitonin, 0–8 pg/ml.
Table 2.
Imaging modalities in MTC
| Anatomic site of metastases | No. of target lesions on conventional imaging (CT, MRI, bone scan) | Size of target lesions on conventional imaging (longest diameter in cm), median (range) | No. of target lesions on SRS | Scoring of target lesions on SRS by radiologist |
|
|---|---|---|---|---|---|
| 5 = definite | 4 = probable | ||||
| Lymph node | 31 | 1.6 (1.0–4.0) | 9 | 1 | 8 |
| Liver | 12 | 1.9 (1.1–6.2) | 0 | 0 | 0 |
| Lung | 5 | 1.7 (1.0–2.1) | 0 | 0 | 0 |
| Bone | 2 | 3.6, 9.9 | 2 | 2 | 0 |
| Thyroid | 3 | 1.5 (1.5–1.7) | 2 | 0 | 2 |
Site of lesions
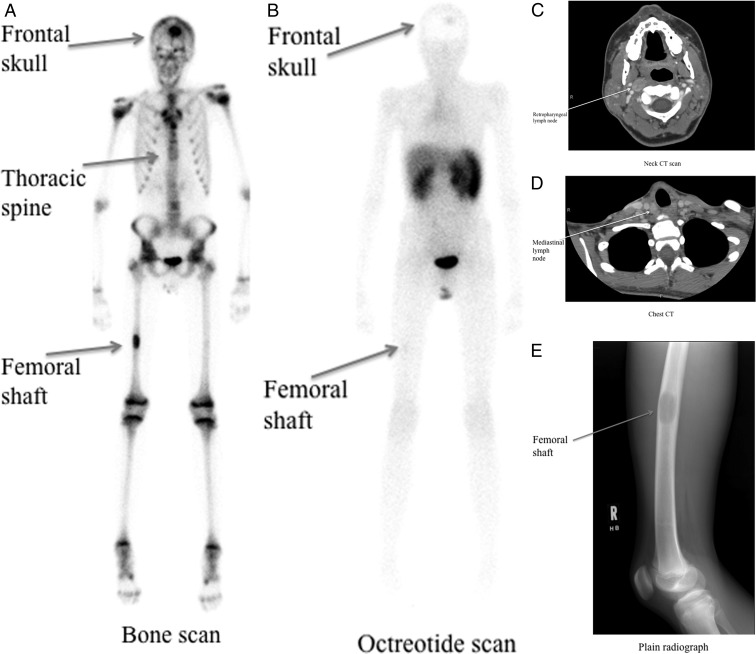
In the 11 patients studied, the cumulative number of MTC target lesions measured was 53. The overall sensitivity of SRS to correctly detect MTC lesions identified by conventional imaging was 13 of 53, or 24.5%. An organ-by-organ analysis was performed to determine whether the proportion of lesions detected by SRS scan varied according to type of lesion. Of the 31 lesions in the lymph nodes visible on conventional imaging, SRS correctly identified nine lesions, with a sensitivity of 29.0%. Only one of 17 head and neck lymph node target lesions was detected by SRS, whereas seven of the 13 mediastinal lymph node target lesions were identified by SRS, and the one axillary target lymph node was identified on SRS. None of the 12 hepatic lesions, or the five pulmonary lesions was detectable by SRS; however, two of two target bone lesions were detected by SRS. Two of the three target lesions within the thyroid gland (66.7%) were identified by SRS. There were no statistically significant differences in the proportion of lesions detected by SRS when compared with the reference site of the lymph nodes. No new tumor sites were detected by the SRS scan results. Figure 1 illustrates the lesions detected on SRS scan compared with the conventional imaging modalities in patient 2 in our series.
Fig. 1.
Imaging modalities to detect metastatic disease in patient 2 at baseline. A, Bone scan; B, 111In-octreotide scan (4 h); C, neck CT; D, chest CT; E, plain radiograph.
Size of lesions
The median (range) size of the 13 target metastatic lesions that were detected by both conventional imaging and SRS was 2.1 (1.3–9.9) cm, and the median size of the 40 lesions detected only on conventional imaging was 1.6 (1.0–6.2) cm (P = 0.0386); thus, it appears that the SRS may detect larger lesions better than smaller lesions.
Tumor markers
We hypothesized that higher levels of baseline tumor markers would correlate with a larger tumor burden (sum of longest diameters of target lesions) at baseline. The median number of target lesions per patient was three, with a range of two to 10. The median total size of tumor burden as measured by target lesions was 8.2 cm, with a range of 2.6 to 23.8 cm. There were no statistically significant correlations between baseline CEA or calcitonin and baseline tumor burden of patients. Neither level of tumor markers (CEA and calcitonin) nor total size of tumor burden affected the likelihood of a patient having a positive SRS result.
Discussion
Our study showed that SRS identified fewer metastases than conventional imaging; no metastases were uniquely identified on SRS. SRS appears to be less sensitive than other imaging techniques at detecting the full extent of metastatic disease in children and adolescents with hereditary MTC; SRS incompletely identified sites of tumor and failed to visualize small sites of tumor or liver metastases. Thus, based on this preliminary observation, SRS may have a limited role in the management of metastatic disease in pediatric MTC patients.
Although calcitonin levels have been shown to be useful as a tumor marker and to indicate postoperative tumor size, interestingly, our study did not show a correlation between calcitonin level and either tumor burden or likelihood of positive SRS result (19). However, we noted that SRS may be more likely to detect larger lesions compared with smaller lesions. Limitations of our study included the low numbers of lesions in certain anatomic sites, as well as the lack of a true “gold standard,” given that biopsy of every lesion was not feasible. Differences may exist in the pathophysiology of MTC between adults and children that could possibly explain the low sensitivity of SRS in this population. Although up to 57% of MTC in adults has been shown to express octreotide-sensitive somatostatin receptor subtypes (10), pediatric MTC may have a lower level of expression of the somatostatin receptor. Sufficient tumor material was not available to allow us to perform somatostatin receptor immunohistochemistry in this study. The evaluation of pediatric MTC tissue for expression of somatostatin receptor subtypes remains an important area for future research. In addition, it is important to point out that children with M918T mutations are classified as having the most aggressive form of MTC (20). As MTC proliferates and metastasizes, somatostatin receptor expression may be lost. In vitro studies have shown increased distribution of somatostatin receptor labeling on highly differentiated MTC tumors (21). Therefore, one possible explanation for the lower sensitivity of SRS in children with the M918T mutation may be that more aggressive, less differentiated MTC is less likely to express somatostatin receptors.
In 2009, the American Thyroid Association (ATA) published management guidelines for MTC (22). In these guidelines, evidence-based recommendations were created, including those for the utility of diagnostic and follow-up imaging in patients with metastatic MTC. Fluorodeoxyglucose positron emission tomography (PET) and somatostatin receptor imaging are not recommended for routine initial screening for MTC metastases in patients when fine-needle aspiration and/or calcitonin level is diagnostic or suspicious for MTC (22). In patients with elevations of serum calcitonin after thyroidectomy, optional imaging techniques for localization of disease include neck ultrasound, neck and chest CT, MRI, bone scintigraphy, and fluorine-18 dihydroxyphenylalanine PET as well as fluorodeoxyglucose PET scanning. The sensitivity for these imaging modalities ranges between 50 and 80% at localizing disease; however, SRS appears to be less sensitive (7, 8, 23). In addition, SRS exposes children to a relatively high radiation dose; the average effective whole body radiation doses for 10 and 15 yr olds undergoing SRS are 10 and 11 mSv, respectively.
Of the 53 total lesions present in our patients, only 24.5% were accurately identified through SRS. The low yield in lesions detected calls into question the added benefit of this imaging technology. Based on our clinical experience with the initial 11 patients, and the recommendations of the ATA that were published in the interval, our institution has stopped performing SRS as part of our protocol for evaluation of metastatic MTC in children and adolescents as of November 2010. The present study reports our findings up until the conclusion of this arm of the protocol.
Our data thus far provide the first published experience with SRS in children with MTC. Based on these results, we agree with the ATA and do not recommend using SRS for the localization of metastatic MTC in children because it does not appear to improve detection of disease and its potential harms outweigh the benefits in growing children, where avoiding unnecessary radiation exposure is critical.
Acknowledgments
This work was supported by the Intramural programs of the National Cancer Institute and the Eunice Kennedy Shriver National Institute of Child Health & Human Development.
Disclosure Summary: The authors have no conflicts of interest to declare.
Footnotes
- CEA
- Carcinoembryonic antigen
- CT
- computed tomography
- MRI
- magnetic resonance imaging
- MTC
- medullary thyroid carcinoma
- PET
- positron emission tomography
- SRS
- somatostatin receptor scintigraphy.
References
- 1. Christian JA, Cook GJ, Harmer C. 2003. Indium-111-labelled octreotide scintigraphy in the diagnosis and management of non-iodine avid metastatic carcinoma of the thyroid. Br J Cancer 89:258–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mato E, Matías-Guiu X, Chico A, Webb SM, Cabezas R, Berná L, De Leiva A. 1998. Somatostatin and somatostatin receptor subtype gene expression in medullary thyroid carcinoma. J Clin Endocrinol Metab 83:2417–2420 [DOI] [PubMed] [Google Scholar]
- 3. Tisell LE, Ahlman H, Wängberg B, Hansson G, Mölne J, Nilsson O, Lindstedt G, Fjälling M, Forssell-Aronsson E. 1997. Somatostatin receptor scintigraphy in medullary thyroid carcinoma. Br J Surg 84:543–547 [PubMed] [Google Scholar]
- 4. Wängberg B, Nilsson O, Johanson V V, Kölby L, Forssell-Aronsson E, Andersson P, Fjälling M, Tisell L, Ahlman H. 1997. Somatostatin receptors in the diagnosis and therapy of neuroendocrine tumor. Oncologist 2:50–58 [PubMed] [Google Scholar]
- 5. Kaltsas G, Rockall A, Papadogias D, Reznek R, Grossman AB. 2004. Recent advances in radiological and radionuclide imaging and therapy of neuroendocrine tumours. Eur J Endocrinol 151:15–27 [DOI] [PubMed] [Google Scholar]
- 6. Ilias I, Chen CC, Carrasquillo JA, Whatley M, Ling A, Lazúrová I, Adams KT, Perera S, Pacak K. 2008. Comparison of 6–18F-fluorodopamine PET with 123I-metaiodobenzylguanidine and 111In-pentetreotide scintigraphy in localization of nonmetastatic and metastatic pheochromocytoma. J Nucl Med 49:1613–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baudin E, Lumbroso J, Schlumberger M, Leclere J, Giammarile F, Gardet P, Roche A, Travagli JP, Parmentier C. 1996. Comparison of octreotide scintigraphy and conventional imaging in medullary thyroid carcinoma. J Nucl Med 37:912–916 [PubMed] [Google Scholar]
- 8. Kwekkeboom DJ, Reubi JC, Lamberts SW, Bruining HA, Mulder AH, Oei HY, Krenning EP. 1993. In vivo somatostatin receptor imaging in medullary thyroid carcinoma. J Clin Endocrinol Metab 76:1413–1417 [DOI] [PubMed] [Google Scholar]
- 9. Dörr U, Würstlin S, Frank-Raue K, Raue F, Hehrmann R, Iser G, Scholz M, Guhl L, Buhr HJ, Bihl H. 1993. Somatostatin receptor scintigraphy and magnetic resonance imaging in recurrent medullary thyroid carcinoma: a comparative study. Horm Metab Res Suppl 27:48–55 [PubMed] [Google Scholar]
- 10. Papotti M, Kumar U, Volante M, Pecchioni C, Patel YC. 2001. Immunohistochemical detection of somatostatin receptor types 1–5 in medullary carcinoma of the thyroid. Clin Endocrinol (Oxf) 54:641–649 [DOI] [PubMed] [Google Scholar]
- 11. Hundahl SA, Fleming ID, Fremgen AM, Menck HR. 1998. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985–1995 [see comments]. Cancer 83:2638–2648 [DOI] [PubMed] [Google Scholar]
- 12. Kebebew E, Clark OH. 2000. Medullary thyroid cancer. Curr Treat Options Oncol 1:359–367 [DOI] [PubMed] [Google Scholar]
- 13. Ponder BA, Ponder MA, Coffey R, Pembrey ME, Gagel RF, Telenius-Berg M, Semple P, Easton DF. 1988. Risk estimation and screening in families of patients with medullary thyroid carcinoma. Lancet 1:397–401 [DOI] [PubMed] [Google Scholar]
- 14. Kebebew E, Ituarte PH, Siperstein AE, Duh QY, Clark OH. 2000. Medullary thyroid carcinoma: clinical characteristics, treatment, prognostic factors, and a comparison of staging systems. Cancer 88:1139–1148 [DOI] [PubMed] [Google Scholar]
- 15. Quayle FJ, Moley JF. 2005. Medullary thyroid carcinoma: including MEN 2A and MEN 2B syndromes. J Surg Oncol 89:122–129 [DOI] [PubMed] [Google Scholar]
- 16. Wells SA, Jr, Gosnell JE, Gagel RF, Moley J, Pfister D, Sosa JA, Skinner M, Krebs A, Vasselli J, Schlumberger M. 2010. Vandetanib for the treatment of patients with locally advanced or metastatic hereditary medullary thyroid cancer. J Clin Oncol 28:767–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lam ET, Ringel MD, Kloos RT, Prior TW, Knopp MV, Liang J, Sammet S, Hall NC, Wakely PE, Jr, Vasko VV, Saji M, Snyder PJ, Wei L, Arbogast D, Collamore M, Wright JJ, Moley JF, Villalona-Calero MA, Shah MH. 2010. Phase II clinical trial of sorafenib in metastatic medullary thyroid cancer. J Clin Oncol 28:2323–2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Waguespack S, Wells S, Ross J, Bleyer A. 2006. Thyroid cancer. In: Bleyer A, O'Leary M, Barr R, Ries LAG, eds. Cancer epidemiology in older adolescents and young adults 15 to 29 years of age, including SEER incidence and survival: 1975–2000. Bethesda MD: National Cancer Institute; 143–154 [Google Scholar]
- 19. Cohen R, Campos JM, Salaün C, Heshmati HM, Kraimps JL, Proye C, Sarfati E, Henry JF, Niccoli-Sire P, Modigliani E. 2000. Preoperative calcitonin levels are predictive of tumor size and postoperative calcitonin normalization in medullary thyroid carcinoma. Groupe d'Etudes des Tumeurs a Calcitonine (GETC). J Clin Endocrinol Metab 85:919–922 [DOI] [PubMed] [Google Scholar]
- 20. Brandi ML, Gagel RF, Angeli A, Bilezikian JP, Beck-Peccoz P, Bordi C, Conte-Devolx B, Falchetti A, Gheri RG, Libroia A, Lips CJ, Lombardi G, Mannelli M, Pacini F, Ponder BA, Raue F, Skogseid B, Tamburrano G, Thakker RV, Thompson NW, Tomassetti P, Tonelli F, Wells SA, Jr, Marx SJ. 2001. Guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab 86:5658–5671 [DOI] [PubMed] [Google Scholar]
- 21. Reubi JC, Chayvialle JA, Franc B, Cohen R, Calmettes C, Modigliani E. 1991. Somatostatin receptors and somatostatin content in medullary thyroid carcinomas. Lab Invest 64:567–573 [PubMed] [Google Scholar]
- 22. Kloos RT, Eng C, Evans DB, Francis GL, Gagel RF, Gharib H, Moley JF, Pacini F, Ringel MD, Schlumberger M, Wells SA., Jr 2009. Medullary thyroid cancer: management guidelines of the American Thyroid Association. Thyroid 19:565–612 [DOI] [PubMed] [Google Scholar]
- 23. Frank-Raue K, Bihl H, Dörr U, Buhr H, Ziegler R, Raue F. 1995. Somatostatin receptor imaging in persistent medullary thyroid carcinoma. Clin Endocrinol (Oxf) 42:31–37 [DOI] [PubMed] [Google Scholar]