Abstract
Context:
Idiopathic infantile hypercalcemia (IIH) is a disorder the genetic etiology and physiological basis of which are not well understood.
Objective:
The objective of the study was to describe the underlying physiology and genetic cause of hypercalcemia in an infant with severe IIH and to extend these genetic findings into an additional cohort of children with IIH.
Design:
This was an inpatient study of a single patient with consanguineous parents at an academic medical center with follow-up in a specialty clinic cohort.
Patients:
The patient population was one patient with severe IIH for gene discovery and physiological testing and 27 patients with idiopathic infantile hypercalcemia in the replication cohort.
Interventions:
Interventions included a calcium isotopic absorption study as well as homozygosity mapping and whole-exome sequencing in a single patient followed up by gene sequencing in replication cohort.
Main Outcome Measure:
Fractional absorption of calcium and genetic variants causing hypercalcemia were measured.
Results:
Intestinal calcium absorption was extremely elevated (∼90%). A rare homozygous deletion in the CYP24A1 gene was found, leading to the loss of a single highly conserved amino acid. In vivo functional studies confirmed decreased 24-hydroxylase activity because the subject had undetectable levels of 24,25-dihydroxyvitamin D. No coding variants in CYP24A1 were found in the 27 additional patients with IIH.
Conclusions:
Our study confirms that CYP24A1 plays a causal role in some but not all cases of IIH via markedly increased intestinal absorption of calcium, suggesting that genetic diagnosis could be helpful in a subset of IIH patients. This case demonstrates the power of an unbiased, genome-wide approach accompanied by informative physiological studies to provide new insights into human biology.
Idiopathic infantile hypercalcemia is a rare entity in childhood the pathophysiology of which is not well understood. Studies have suggested that the hypercalcemia is primarily due to increased absorption of calcium from the intestines (1–3), but this has not been shown definitively. Vitamin D plays a critical role in the regulation of intestinal calcium absorption and has been implicated in a host of other biological processes (4). Vitamin D is activated by sequential hydroxylation reactions in the liver and kidney. The 1α-hydroxylase reaction in the kidney produces the final active form, 1, 25-dihydroxyvitamin D [1,25(OH)2D], and is tightly regulated by PTH, calcium, and 1,25(OH)2D itself (5). Elevated levels of 1,25(OH)2D have been reported in cases of idiopathic infantile hypercalcemia, but this is not a consistent finding (3, 6–8). Active vitamin D is inactivated by the vitamin D 24-hydroxylase enzyme, which is encoded by the gene CYP24A1. Recently Schlingmann et al. (9) described four families with children with idiopathic infantile hypercalcemia with variants in CYP24A1, indicating that this gene plays a causative role in this disorder. Before that publication, we investigated the case of an infant born to consanguineous parents with severe idiopathic infantile hypercalcemia. We hypothesized that the infant would have increased calcium absorption and thus performed calcium absorption studies using stable isotopes of calcium. We then performed an unbiased genome-wide search for a genetic defect underlying his hypercalcemia. Additionally, we sought to replicate our genetic findings in a larger cohort of children with idiopathic infantile hypercalcemia.
Subjects and Methods
Subjects
The proband initially presented at age 10 months with a diagnosis of failure to thrive after failing to gain any weight in the preceding 4 months. He had no significant past medical history, and a review of systems was notable for mild irritability, increased urination, and the refusal of solid foods. He was consuming 4 oz of a standard infant formula every 2 h during the day and feeding twice overnight. Screening laboratory studies showed a serum calcium level of 15.3 mg/dl (3.8 mmol/liter). On admission to the hospital, hypercalcemia was confirmed and an extensive evaluation did not reveal a clear etiology for his hypercalcemia (Table 1). Additionally, he had severe hypercalciuria and bilateral nephrocalcinosis. X-ray studies of the knee did not show evidence of rickets or bone demineralization. The family is of European ancestry with a history notable for consanguinity (Fig. 1) and his mother subsequently developed a kidney stone. Serum calcium and urinary calcium excretion were normal in both parents (Table 1). After acute management with fluids and diuretics, he was placed on a low-calcium formula (Calcilo XD; Abbott Nutrition, Abbott Park, IL) with no supplemental vitamin D with gradual improvement of the hypercalcemia and hypercalciuria. At age 21 months, he was started on a supplement of 400 IU of vitamin D daily. After age 2 yr, his calcium intake was slowly liberalized, and by 3 yr of age, he was on a regular diet without recurrence of hypercalcemia. This proband was investigated with calcium absorption studies, homozygosity mapping, whole-exome sequencing, and measurement of 24,25-dihydroxyvitamin D [24,25(OH)2D] levels as described below.
Table 1.
Proband's laboratory evaluation
| Admission at 10 months | 15 months | 17 months | 21 months | 25 months | 31 months | 39 months | Mother | Father | Normal range | |
|---|---|---|---|---|---|---|---|---|---|---|
| Sodium (mmol/liter) | 133 | 136 | 136 | 136 | 139 | 137 | 140 | 135–148 | ||
| Potassium (mmol/liter) | 4.3 | 4.1 | 4.6 | 4 | 4.5 | 4.7 | 4.4 | 3.2–4.5 | ||
| Chloride (mmol/liter) | 99 | 100 | 101 | 100 | 103 | 101 | 104 | 99–111 | ||
| CO2 (mmol/liter) | 24 | 24 | 22 | 23 | 24 | 22 | 22 | 17–29 | ||
| BUN (mg/dl) | 18 | 19 | 15 | 13 | 10 | 16 | 21 | 4–19 | ||
| Creatinine (mg/dl) | 0.4 | 0.2 | 0.2 | 0.3 | 0.3 | 0.3 | 0.3 | 0.7 | 1.2 | 0.–0.4 |
| Calcium (mg/dl) | 16.3 | 10.7 | 10.7 | 10.6 | 10.5 | 10.4 | 10.2 | 9.9 | 9.6 | 8–10.5 |
| Phosphorus (mg/dl) | 4.4 | 5.6 | 5.3 | 5.7 | 6.7 | 5.7 | 5.8 | 3.9 | 4.5 | <1 yr: 3.5–6.6; > 1 yr: 3.0–5.7 |
| Magnesium (mg/dl) | 2.4 | 2.2 | 2 | 2.1 | 2.1 | 2 | 1.9 | 1.5–2.2 | ||
| PTH (pg/ml) | <3 | 8.9 | 10.6 | 3.4 | 5 | 6 | <3 | 10–65 | ||
| 25-Hydroxyvitamin D (ng/ml) | 43 | 20 | 13 | 21 | 37.1 | 37.3 | 36.8 | 30–80 | ||
| 1,25(OH)2D (pg/ml) | 33 | 83 | 69 | 87 | 52 | 20 | 15–75 | |||
| 24,25(OH)2D (ng/ml) | <0.2 | <0.2 | See Fig. 1 | |||||||
| Alkaline phosphatase (unit/liter) | 150 | 201 | 232 | 110–400 | ||||||
| Albumin (g/dl) | 4.2 | 4.7 | 2.5–4 | |||||||
| Urine calcium (mg/dl) | 16.1 | 3.1 | 1.2 | 4 | 6.5 | 6.3 | 13.8 | |||
| Urine creatinine (mg/dl) | 12.8 | 49.3 | 33.1 | 42.6 | 67.4 | 49.4 | 150.4 | |||
| Urine calcium to creatinine ratio | 1.26 | 0.06 | 0.04 | 0.09 | 0.1 | 0.13 | 0.09 | |||
| TSH (μU/ml) | 4.29 | 0.8–8.2 | ||||||||
| Free T4 (ng/dl) | 1.3 | 0.8–1.8 | ||||||||
| PTHrP (pmol/liter) | <1.5 | 0–4 | ||||||||
| FISH for Williams syndrome | Negative | Negative |
BUN, Blood urea nitrogen; FISH, fluorescence in situ hybridization.
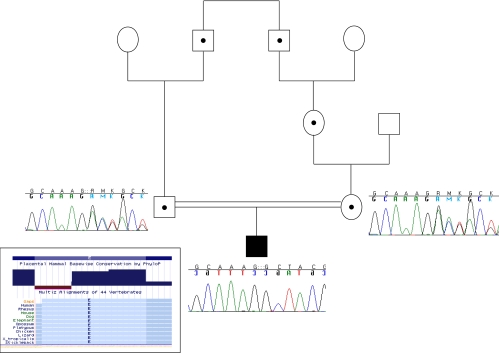
Fig. 1.
Subject's pedigree demonstrating parental consanguinity. Dots represent obligate heterozygotes. Sanger sequencing chromatograms demonstrate heterozygous deletions in both parents and homozygous deletion in the proband. Inset, Chart demonstrating evolutionary conservation of the deleted glutamate in all species.
Our replication cohort consisted of an additional 27 children who presented to a pediatric calcium disorder specialty clinic with infantile hypercalcemia (8). Laboratory characteristics of those patients are described in Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org. Control samples for the measurement of 24,25(OH)2D were obtained at primary care sites in New Haven, CT, from eight healthy children between 6 months and 3 yr of age as part of a larger study of vitamin D metabolism based at Yale.
Calcium absorption studies
The proband was admitted to the inpatient clinical research center at the age of 17 months while on a low-calcium diet (∼140 mg of calcium per day, ∼12 mg/kg of body weight, based on a 3 d dietary analysis). Twenty micrograms of 46Ca were given iv immediately before the first morning feeding, which contained 1 mg of 48Ca mixed in 90 ml of Calcilo XD formula (Abbott Nutrition), which contains 6 mg of calcium. For the second feeding, which occurred 4 h later on the same day, 1.5 mg of 42Ca was given mixed in 30 ml of whole milk, which contains 38 mg of calcium. Urine was collected for 24 h after the second feeding and then twice daily for the subsequent 4 d. Calcium isotope recovery for each isotope was measured as previously described using thermal ionization magnetic sector mass spectrometry after purification by oxalate precipitation (10). Fractional calcium absorption was calculated from the relative recovery of oral and iv calcium isotopes in the 24-h urine collection (11).
Genetic studies
The proband and his parents had genome-wide single-nucleotide polymorphism genotyping performed using an Affymetrix 6.0 chip (Affymetrix, Santa Clara, CA). Extended regions of homozygosity due to identity by descent were determined using PLINK (12). Whole-exome sequencing of the proband was performed as previously described (13). Sanger sequencing of the coding exons of CYP24A1 (NM_000782.4) as well as Sequenom iPLEX (San Diego, CA) genotyping of the ΔE143 variant was performed using standard techniques in the proband, his parents, and an additional 27 children who presented with infantile hypercalcemia (8) (Supplemental Table 1). Taqman quantitative PCR was performed to assess for whole-gene deletions using probes for exons 2 and 8 using previously described methods (14). Primer and probe sequences for all assays are available on request.
24,25(OH)2D determination
The 24,25(OH)2D was measured by RIA after separation of the fraction using a silica column followed by HPLC (Heartland Assays, Ames, IA) (15). The 24,25(OH)2D levels were measured in the proband as well as in the eight control children.
The proband's study was approved by the Institutional Review Board at Children's Hospital Boston (Boston, MA). The replication cohort study was approved by the Institutional Review Board at the Hospital for Sick Children (Toronto, Canada), and the control subject vitamin D study was approved by the Institutional Review Board at Yale New Haven Medical Center (New Haven, CT). For all three protocols, all subjects' parents provided written informed consent.
Results
Calcium absorption studies
Calcium absorption studies were performed with the use of stable isotopes of calcium. The subject absorbed 95% of the calcium from a low-calcium feed and 85% from a normal-calcium feed [normal for age 45.6 ± 2.5% (16)]. Calcium absorption from any intake is rarely greater than 80% in children, regardless of the type of feeding or calcium content (17).
Genetic studies
Given the history of consanguinity, a pathological variant leading to the proband's hypercalcemia was presumed to be inherited in an autosomal recessive fashion. Homozygosity mapping in the proband revealed three large (>15 Mb) regions of homozygosity on chromosomes 7, 16, and 20 (Supplemental Table 2), which included 363 genes. Whole-exome sequencing revealed a total of 19,048 single-nucleotide variants (Supplemental Fig. 1), 1,277 of which were novel (not in dbSNP build 129), and an additional 367 novel short insertions or deletions (indels). Of the novel variants, there were seven missense variants and seven indels present within the large regions of homozygosity. Of these variants, there was one 3-bp deletion of CTT at chromosome 20:52222874-6 (coordinates in NCBI36/hg18) in CYP24A1, the gene that encodes the vitamin D 24-hydroxylase enzyme (Supplemental Fig. 2). This deletion results in the loss of a highly evolutionary conserved glutamate at position 143 (ΔE143) (Fig. 1, inset) and was of particular interest because recessive loss-of-function mutations in the mouse ortholog, Cyp24a1, cause hypercalciuria and hypercalcemia. This variant was present in the heterozygous state in seven of 1646 subjects of mostly European descent who underwent whole-exome sequencing using the same methods indicating that the variant is quite rare, with a minor allele frequency of 0.2%. This frequency implies the presence of the homozygous deletion genotype in 1:250,000 individuals, consistent with a rare recessive disorder. We performed independent genotyping and sequencing and the proband was confirmed to be homozygous and his parents were confirmed to be heterozygous for this deletion (Fig. 1). Sequencing of the coding region of CYP24A1 and quantitative PCR did not reveal any additional nonsynonymous variants or whole-gene deletions in 27 other children with infantile hypercalcemia (Supplemental Table 1).
24,25(OH)2D levels
If this mutation leads to a loss of function in vivo, then our patient should have low levels of 24,25(OH)2D. To test this possibility, we measured these metabolite levels in our patient. The 24,25(OH)2D was undetectable in the proband at ages 17 and 39 months. Age-matched control subjects all had detectable levels with a mean 24,25(OH)2D level of 1.60 ng/ml (range 0.41–3.20) (Supplemental Fig. 3). There is a strong correlation between 25-hydroxyvitamin D and 24,25(OH)2D levels in control subjects (r = 0.86), making it extremely unusual for our subject to have an undetectable 24,25(OH)2D level at age 39 months despite a normal 25-hydroxyvitamin D of 36.8 ng/ml. Thus, our subject has genetic and in vivo biochemical evidence of a defect in vitamin D 24-hydroxylase.
Discussion
Infantile hypercalcemia has been observed for decades, and early reports associated the finding with the potential of excess maternal vitamin D exposure (18). Causality in this regard had not been consistently confirmed, and other potential etiologies for the condition have been entertained, including impaired detoxification of vitamin D (19). While in late stages of this manuscript's preparation, Schlingmann et al. (9) described additional subjects with idiopathic infantile hypercalcemia with variants in CYP24A1, confirming this gene's causative role in this disorder. Our subject presented with severe idiopathic infantile hypercalcemia and extremely elevated intestinal calcium absorption. Based on our calcium isotope absorption studies, our subject absorbed approximately 90% of ingested calcium, which is twice the typical absorption level and one of the highest recorded calcium absorption rates in a human. Our subject has a single amino acid deletion, ΔE143, in a highly evolutionarily conserved residue in CYP24A1. Based on population data, this is a very rare variant consistent with an extremely rare recessive disorder such as idiopathic infantile hypercalcemia. To determine whether this variant causes a functional defect in our subject, we measured 24,25(OH)2D levels in his serum as well as in age-matched controls. Our subject had undetectable levels of 24,25(OH)2D at two time points including one time point when his 25-hydroxyvitamin D and 1,25(OH)2D levels were normal. These supporting in vivo functional data provide strong evidence that this variant is pathogenic. Two of the subjects reported by Schlingmann et al. (9) had the same genetic variant as our patient, and they provide in vitro evidence that this variant leads to complete loss of function of the CYP24A1 enzyme activity. This complements our in vivo data demonstrating the physiological consequences of this genetic defect.
A homozygous knockout mouse for Cyp24a1 exists and has a phenotype that is strikingly similar to that of our subject. Approximately half of the homozygous knockout mice die before weaning with severe hypercalcemia and hypercalciuria (20, 21). The mice that continued to receive vitamin D supplementation developed nephrocalcinosis. Interestingly, homozygote mutants who survived past weaning had lower baseline circulating 1,25(OH)2D levels than wild-type controls but had abnormally decreased clearance of 1,25(OH)2D (21). Finally, homozygous knockout mice that survive have decreased expression of Cyp27b1, which encodes the 1α-hydroxylase enzyme, in kidney as measured by RT-PCR (22). This long-term compensatory down-regulation may explain the lower circulating levels of baseline 1,25(OH)2D and, if a similar mechanism is present in humans, may explain why the hypercalcemia in idiopathic infantile hypercalcemia appears to improve over time (7). Interestingly, our patient's 1,25(OH)2D levels were lower at 31 and 39 months despite higher levels of 25-hydroxyvitamin D, indicating that this mechanism may exist in humans and could explain the resolution of our patient's hypercalcemia. However, unlike the knockout mouse, the 1,25(OH)2D levels in our subject as well as the subjects reported by Schlingmann et al. (9) were not consistently elevated. We hypothesize that serum levels of 1,25(OH)2D may not adequately reflect tissue levels and that our subject possibly had increased intestinal levels of 1,25(OH)2D. This hypothesis requires further investigation.
It is plausible that children with idiopathic infantile hypercalcemia generally have a decreased clearance and/or increased sensitivity to exogenous vitamin D, as seen in the Cyp24a1 mouse model. A prior study demonstrated that children with idiopathic infantile hypercalcemia have an exaggerated and persistent increase in 1,25(OH)2D levels after administration of PTH (4). Our data, and that of Schlingmann et al. (9) suggest that this finding could reflect an inability to metabolize the active form of vitamin D in these patients. Nguyen et al. (23) demonstrated decreased 24-hydroxylase activity in the fibroblasts of one patient with idiopathic infantile hypercalcemia, supporting impaired inactivation of vitamin D as a common mechanism in this disorder. Furthermore, a small cohort of subjects with idiopathic absorptive hypercalciuria, some of whom had hypercalcemia, also had lower 24,25(OH)2D levels compared with subjects with renal leak hypercalciuria, although their levels were in the low-normal range (24).
However, we have now shown that, although genetic defects in CYP24A1 clearly can cause idiopathic infantile hypercalcemia, protein-coding mutations in this gene do not account for most cases of idiopathic infantile hypercalcemia. Specifically, we were unable to find nonsynonymous coding variants or whole gene deletions in CYP24A1 in an additional 27 subjects referred to a pediatric specialty clinic with infantile hypercalcemia. This is in contrast to the report by Schlingmann et al. (9) in which all four families investigated had mutations in CYP24A1. There are a number of possible explanations for these results. First, the other subjects may have pathological variants outside the coding region of CYP24A1 such as in the highly conserved vitamin D response elements in the promoter region. Second, subjects with infantile hypercalcemia may have variants in other genes in the same pathway leading to similar pathophysiology, such as activating mutations in CYP27B1. Finally, it is quite likely that the cohort of children with infantile hypercalcemia represent a heterogeneous group of individuals. Indeed, some may not manifest a distinct genetic disorder in the metabolism of vitamin D but rather represent developmental changes in vitamin D metabolism, intestinal calcium absorption, or secondary increases in skeletal mineral resorption. Consistent with the likely heterogeneity within idiopathic infantile hypercalcemia, many of these subjects did not have suppressed PTH levels as were seen in our patient. Suppression of PTH excretion is the expected physiological response to excess calcium absorption, so these patients may have a different underlying pathophysiology. To clarify this issue further, additional prospective research recruiting a cohort of children with idiopathic infantile hypercalcemia is needed, which will comprehensively examine vitamin D metabolites and PTH levels as well as sequence CYP24A1 and possibly other genes involved in vitamin D metabolism. For clinicians currently faced with infants with idiopathic hypercalcemia, our data suggest that not all of the subjects will have defects in CYP24A1. We suggest considering measurement of 24,25(OH)2D levels in those patients with low PTH levels and then sequencing of CYP24A1 in subjects with low 24,25(OH)2D levels.
It is interesting to note that our subject's mother developed a kidney stone. It is not known whether this was a calcium stone because she declined further medical workup. She had normal urinary calcium excretion based on a random spot measurement and a 24-h urine collection (98.7 mg per 24 h). However, it is possible that she has a subtle abnormality in calcium absorption, which is evident only after increased calcium or vitamin D intake. McTaggart et al. (25) described a family with two siblings with idiopathic infantile hypercalcemia and unaffected parents, suggesting an autosomal recessive mode of inheritance. Additional family members were found to have idiopathic hypercalciuria and nephrolithiasis without hypercalcemia, which may suggest that individuals who are heterozygous for pathological variants may be at increased risk of hypercalciuria. If this were the case, heterozygous carriers of variants such as we describe in our subject may contribute appreciably to the population risk of kidney stones. We hypothesize that this risk could take the form of a gene-environment interaction, in which carriers would be at increased risk only in the setting of higher levels of vitamin D intake or synthesis. Furthermore, it is possible that common variants in CYP24A1 may play a more subtle role in regulating calcium absorption and perhaps increase risk of absorptive hypercalciuria and subsequent kidney stones. Interestingly, a common single-nucleotide polymorphism close to CYP24A1 was recently identified in a genome-wide association study as being associated with vitamin D levels (26).
In conclusion, we describe an infant with hypercalcemia due to increased intestinal absorption of calcium with a pathological deletion in the vitamin D 24-hydroxylase gene. This deletion or additional variants in CYP24A1 may contribute to disorders such as idiopathic hypercalciuria and nephrolithiasis and may modulate vitamin D's diverse effects. This finding has significant implications for our understanding of human calcium absorption and its regulation by vitamin D metabolites. This case demonstrates the power of an unbiased, genome-wide approach accompanied by informative physiological studies to identify the underlying defect for rare genetic conditions and to provide new insights into human physiology.
Supplementary Material
Acknowledgments
We thank the subjects and their families for participating in this research project. We also thank the entire staff of the Clinical and Translational Study Unit at Children's Hospital Boston for their assistance with this protocol. We also thank Rocky Anzaldi for the preparation of the calcium isotopes; Zhensheng Chen, Ph.D., for mass spectrometric analyses; Rigel Chan, Cindy Liu, and Steve McCarroll for their assistance with the quantitative PCR; and Mark Daly, Ron Do, Benjamin Neale, and Sekar Kathiresan for providing allele frequency information.
This work was supported by Harvard Catalyst and The Harvard Clinical and Translational Science Center (National Institutes of Health Award UL1 RR 025758 and financial contributions from Harvard University and its affiliated academic health care centers). This work was also supported in part by National Institutes of Health Grant RC1 HD063562 (to T.O.C.) and by the March of Dimes 6-FY09-507 Program (to J.N.H.). Web sites include the following: PLINK, http://pngu.mgh.harvard.edu/purcell/plink/ and http://www.broadinstitute.org/software/igv/
The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, the National Center for Research Resources, or the National Institutes of Health.
Disclosure Summary: R.H. is the founder and holds an equity interest in Heartland Assays Inc. The remaining authors have no conflicts of interest to disclose.
For editorial see page 384
- 1,25(OH)2D
- 1,25-Dihydroxyvitamin D
- 24,25(OH)2D
- 24,25-dihydroxyvitamin D.
References
- 1. Morgan HG, Mitchell RG, Stowers JM, Thomson J. 1956. Metabolic studies on two infants with idiopathic hypercalcaemia. Lancet 270:925–931 [DOI] [PubMed] [Google Scholar]
- 2. Barr DG, Forfar JO. 1969. Oral calcium-loading test in infancy, with particular reference to idiopathic hypercalcaemia. Br Med J 1:477–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pronicka E, Rowińska E, Kulczycka H, Lukaszkiewicz J, Lorenc R, Janas R. 1997. Persistent hypercalciuria and elevated 25-hydroxyvitamin D3 in children with infantile hypercalcaemia. Pediatr Nephrol (Berlin, Germany) 11:2–6 [DOI] [PubMed] [Google Scholar]
- 4. Holick MF. 2010. Vitamin D: extraskeletal health. Endocrinol Metab Clin North Am 39:381–400, Table of Contents [DOI] [PubMed] [Google Scholar]
- 5. Ohyama Y, Yamasaki T. 2004. Eight cytochrome P450s catalyze vitamin D metabolism. Front Biosci 9:3007–3018 [DOI] [PubMed] [Google Scholar]
- 6. Pronicka E, Kulczycka H, Lorenc R, Prószyńska K, Gradzka J, Rowińska E. 1988. Increased serum level of 1,25-dihydroxyvitamin D3 after parathyroid hormone in the normocalcemic phase of idiopathic hypercalcemia. J Pediatr 112:930–933 [DOI] [PubMed] [Google Scholar]
- 7. Huang J, Coman D, McTaggart SJ, Burke JR. 2006. Long-term follow-up of patients with idiopathic infantile hypercalcaemia. Pediatr Nephrol (Berlin, Germany) 21:1676–1680 [DOI] [PubMed] [Google Scholar]
- 8. Koltin D, Rachmiel M, Wong BY, Cole DE, Harvey E, Sochett E. 2011. Mild infantile hypercalcemia: diagnostic tests and outcomes. J Pediatr 159:215–221.e1 [DOI] [PubMed] [Google Scholar]
- 9. Schlingmann KP, Kaufmann M, Weber S, Irwin A, Goos C, John U, Misselwitz J, Klaus G, Kuwertz-Bröking E, Fehrenbach H, Wingen AM, Güran T, Hoenderop JG, Bindels RJ, Prosser DE, Jones G, Konrad M. 2011. Mutations in CYP24A1 and idiopathic infantile hypercalcemia. N Engl J Med 365:410–421 [DOI] [PubMed] [Google Scholar]
- 10. Abrams SA. 1999. Using stable isotopes to assess mineral absorption and utilization by children. Am J Clin Nutr 70:955–964 [DOI] [PubMed] [Google Scholar]
- 11. Abrams SA, Griffin IJ, Hicks PD, Gunn SK. 2004. Pubertal girls only partially adapt to low dietary calcium intakes. J Bone Miner Res 19:759–763 [DOI] [PubMed] [Google Scholar]
- 12. Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. 2007. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81:559–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Musunuru K, Pirruccello JP, Do R, Peloso GM, Guiducci C, Sougnez C, Garimella KV, Fisher S, Abreu J, Barry AJ, Fennell T, Banks E, Ambrogio L, Cibulskis K, Kernytsky A, Gonzalez E, Rudzicz N, Engert JC, DePristo MA, Daly MJ, Cohen JC, Hobbs HH, Altshuler D, Schonfeld G, Gabriel SB, Yue P, Kathiresan S. 2010. Exome sequencing, ANGPTL3 mutations, and familial combined hypolipidemia. N Engl J Med 363:2220–2227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McCarroll SA, Bradner JE, Turpeinen H, Volin L, Martin PJ, Chilewski SD, Antin JH, Lee SJ, Ruutu T, Storer B, Warren EH, Zhang B, Zhao LP, Ginsburg D, Soiffer RJ, Partanen J, Hansen JA, Ritz J, Palotie A, Altshuler D. 2009. Donor-recipient mismatch for common gene deletion polymorphisms in graft-versus-host disease. Nat Genet 41:1341–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Horst RL, Littledike ET, Riley JL, Napoli JL. 1981. Quantitation of vitamin D and its metabolites and their plasma concentrations in five species of animals. Anal Biochem 116:189–203 [DOI] [PubMed] [Google Scholar]
- 16. Lynch MF, Griffin IJ, Hawthorne KM, Chen Z, Hamzo M, Abrams SA. 2007. Calcium balance in 1–4-y-old children. Am J Clin Nutr 85:750–754 [DOI] [PubMed] [Google Scholar]
- 17. Abrams SA. 2010. Calcium absorption in infants and small children: methods of determination and recent findings. Nutrients 2:474–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fellers FX, Schwartz R. 1958. Etiology of the severe form of idiopathic hypercalcemia of infancy: a defect in vitamin D metabolism. N Engl J Med 259:1050–1058 [DOI] [PubMed] [Google Scholar]
- 19. Weisman Y, Harell A, Edelstein S. 1979. Infantile hypercalcemia: a defect in the esterification of 1,25-dihydroxyvitamin D? Med Hypotheses 5:379–382 [DOI] [PubMed] [Google Scholar]
- 20. St. Arnaud R. 1999. Targeted inactivation of vitamin D hydroxylases in mice. Bone 25:127–129 [DOI] [PubMed] [Google Scholar]
- 21. St-Arnaud R, Arabian A, Travers R, Barletta F, Raval-Pandya M, Chapin K, Depovere J, Mathieu C, Christakos S, Demay MB, Glorieux FH. 2000. Deficient mineralization of intramembranous bone in vitamin D-24-hydroxylase-ablated mice is due to elevated 1,25-dihydroxyvitamin D and not to the absence of 24,25-dihydroxyvitamin D. Endocrinology 141:2658–2666 [DOI] [PubMed] [Google Scholar]
- 22. Masuda S, Byford V, Arabian A, Sakai Y, Demay MB, St. Arnaud R, Jones G. 2005. Altered pharmacokinetics of 1α,25-dihydroxyvitamin D3 and 25-hydroxyvitamin D3 in the blood and tissues of the 25-hydroxyvitamin D-24-hydroxylase (Cyp24a1) null mouse. Endocrinology 146:825–834 [DOI] [PubMed] [Google Scholar]
- 23. Nguyen M, Boutignon H, Mallet E, Linglart A, Guillozo H, Jehan F, Garabedian M. 2010. Infantile hypercalcemia and hypercalciuria: new insights into a vitamin D-dependent mechanism and response to ketoconazole treatment. J Pediatr 157:296–302 [DOI] [PubMed] [Google Scholar]
- 24. Misselwitz J, Hesse V, Markestad T. 1990. Nephrocalcinosis, hypercalciuria and elevated serum levels of 1,25-dihydroxyvitamin D in children. Possible link to vitamin D toxicity. Acta Paediatr Scand 79:637–643 [DOI] [PubMed] [Google Scholar]
- 25. McTaggart SJ, Craig J, MacMillan J, Burke JR. 1999. Familial occurrence of idiopathic infantile hypercalcemia. Pediatric Nephrology (Berlin, Germany) 13:668–671 [DOI] [PubMed] [Google Scholar]
- 26. Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D, Kiel DP, Streeten EA, Ohlsson C, Koller DL, Peltonen L, Cooper JD, O'Reilly PF, Houston DK, Glazer NL, Vandenput L, Peacock M, Shi J, Rivadeneira F, McCarthy MI, Anneli P, de Boer IH, Mangino M, Kato B, Smyth DJ, et al. 2010. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet 376:180–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.