Abstract
Previously we showed that 17β-estradiol (E2) and/or the xenoestrogen bisphenol A (BPA) alter ventricular myocyte Ca2+ handing, resulting in increased cardiac arrhythmias in a female-specific manner. In the present study, the roles of estrogen receptors (ER) in mediating the rapid contractile and arrhythmogenic effects of estrogens were examined. Contractility was used as an index to assess the impact of E2 or BPA on Ca2+ handling in rodent ventricular myocytes. The concentration-response curve for the stimulatory effects of BPA and E2 on female myocyte was inverted-U shaped. Detectable effects for each compound were observed at 10−12 m, and the most efficacious concentrations for each were at 10−9 m. Sensitivity to E2 and BPA was not observed in male myocytes and was abolished in myocytes from ovariectomized females. Analysis using protein-conjugated E2 suggests that these rapid actions are induced by membrane-associated receptors. Analysis using selective ER agonists and antagonists and a genetic ERβ knockout mouse model showed that ERα and ERβ have opposing actions in myocytes and that the balance between ERβ and ERα signaling is the prime regulator of the sex-specific sensitivity toward estrogens. The response of female myocytes to E2 and BPA is dominated by the stimulatory ERβ-mediated signaling, and the absence of BPA and E2 responsiveness in males is due to a counterbalancing-suppressive action of ERα. We conclude that the sex-specific sensitivity of myocytes to estrogens and the rapid arrhythmogenic effects of BPA and estradiol in the female heart are regulated by the balance between ERα and ERβ signaling.
Cardiovascular (CV) disease is the leading cause of death in the United Stated for both men and women. There are well-known sex-related differences in baseline CV physiology and susceptibility to CV diseases. Endogenous gonadal hormones play major roles in determining sexual-dimorphism of the normal function of the heart. Estrogens, particularly 17β-estradiol (E2), have favorable effects on cholesterol and lipid metabolism, and contribute to the generally improved CV function and vascular homeostasis observed in premenopausal women (1, 2). It is also well established that the levels of circulating E2 influence the response of the myocardium to pathophysiological conditions including hypertrophy, heart failure, ischemic injury, and arrhythmia (1, 3, 4). Whereas E2 is generally cardioprotective, increased concentrations of circulating E2 are associated with increased arrhythmia incidence in females (4, 5). A clear understanding of the CV effects of endogenous estrogens, estrogenic endocrine-disrupting chemical (EDC), and their interactions is important for determining the unique vulnerability and responsiveness of each sex to CV insults and for the development of optimal sex-specific therapeutic interventions that would improve outcomes for both sexes and might eliminate the disparity of treatment benefits that exist for women with CV diseases.
Accumulating experimental evidence suggests a possible association between increased bisphenol A (BPA; an endocrine disrupting chemical with some estrogen-like activity) and a variety of adverse health outcomes including obesity, diabetes, and CV diseases (6). BPA is used primarily in the manufacturing of polycarbonate plastic and epoxy resins (7) and is present in a wide range of consumer products such as reusable drinking water bottles, baby bottles, food containers, and the linings of food and beverage cans. There is well-documented and widespread human exposure to BPA, with detectable levels of BPA found in the majority of the US population (8–10). However, the effect of BPA on the heart, an estrogen-sensitive system, remained unknown until recently. We have demonstrated that exposure to nanomolar BPA and/or E2 rapidly induced arrhythmogenic triggered activities and promoted the development of ventricular arrhythmias in female rat hearts (11). The proarrhythmic effect of BPA was more pronounced in the presence of physiological concentrations of E2 and was augmented by β-adrenergic stress. The cardiac sensitivity to those estrogens was female specific; neither myocytes nor hearts from male rats were affected. We further demonstrated that the arrhythmogenic effects of BPA and E2 were mediated by alterations of myocyte Ca2+ handling properties, particularly increased spontaneous Ca2+ leak from the sarcoplasmic reticulum (SR). Those findings revealed that BPA has potentially harmful impacts on the female heart resulting from its female-specific proarrhythmic rapid actions.
Upstream, endogenous and exogenous estrogens exert their biological effects by activation of the nuclear hormone receptors ERα and ERβ. The activated receptors may act as nuclear ERs to regulate estrogen-responsive gene expression and influence a wide range of physiological functions. Estradiol also directly activates intracellular signaling pathways via membrane or cytoplasmic localized ER-like receptors (12, 13). Whereas the nuclear hormone receptor effects of E2 in the heart have been well studied, the nature and mechanism of the rapid estrogen signaling in the heart and its integrated contribution to the cardiac actions of E2 are not fully understood. Multiple studies have demonstrated the expression of functional ERα and ERβ in cardiac tissues and myocytes in animals, including rodents, and humans (14–17). The integral membrane protein G protein-coupled ER 1 (GPER1 or GPR30) has also been localized to isolated rat cardiac myocytes (18). In this study, we investigated the role of ER signaling in mediating the rapid cardiac effects of BPA and 17β-estradiol and the mechanism underlying the sexually-dimorphic responses of rodent cardiac myocytes to those estrogens.
Materials and Methods
Reagents
All reagents and solvents used were of the highest purity available. Aqueous solutions were prepared using BPA-free water (18 mΩ; <6 ppb total oxidizable organics; Millipore A10 system; Millipore Corp., Milford, MA). Dimethyl sulfoxide (Chromasolv Plus, HPLC ≤99.7%; batch no. 00451HE), BPA, CAS 80-05-7, >99%, 23,965-8; lot Cl03105ES, isoproterenol hydrochloride, CAS 5984-95-2, BSA, CAS 9048-46-8, >98% (batch no. 078K0730), and Nω-nitro-l-arginine methyl ester hydrochloride (l-NAME), CAS 51298-62-5, >98% were from Sigma-Aldrich (St. Louis, MO). 1,3,5 (10)-estratriene-3,17β-diol (17β-estradiol, 17β-E2) catalog no. E0959, batch B0356; 1,3,5 (10)-estratriene-3,17β-diol 17-hemisuccinate:BSA (17β-E2-BSA) conjugate, E-8750; lot 111k4083 were from Steraloids (Newport RI). 4,4′,4′′-(4-propyl-[1H]-pyrazole-1,3,5-triyl) trisphenol (PPT), CAS 263717-53-9; 2,3-bis(4-hydroxyphenyl)-propionitrile diarylpropionitrile (DPN), CAS 1428-67-7; methyl-piperidino-pyrazole, 1,3-bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy)phenol]-1H-pyrazole dihydrochloride (MPP), CAS 289726-02-9; 4-[2-phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-a]pyrimidin-3-yl]phenol (PHTPP), CAS 805239-56-9 and (±)-1-[(3aR*,4S*,9bS*)-4-(6-bromo-1,3-benzodioxol-5-yl)-3a,4,5,9b-tetrahydro-3H-cyclopenta[c]quinolin-8-yl]-ethanone (G1), CAS 881639–98-1 were from Tocris Cookson (Ellisville, MO).
Animals
All animal procedures were done in accordance with protocols approved by the University of Cincinnati Institutional Animal Care and Use Committee and followed recommendations of the Panel on Euthanasia of the American Veterinary Medical Association.
Adult Sprague Dawley rats (200–250 g, both sexes; Harlan; Indianapolis, IN) and ERβ (Esr2) knockout mice (ERβ−/−, female; The Jackson Laboratory; Bar Harbor, ME) were used as nonsurviving sources of ventricular tissue. Animals were anesthetized with sodium pentobarbital (80 mg/kg, ip) and heart rapidly dissected for myocyte dissociation. The ERβ−/− mice and age-matched wild-type littermates were reared at the University of Cincinnati laboratory animal facility and used at 6–8 wk of age. Gonadectomy surgery was performed by the vendor, and gonadectomized rats were received from the vendor and used 2 wk after surgery. Animals were housed as previously described (11). Sani-chip bedding (Irradiated Aspen Sani-chip; P.J. Murphy Forest Products Corp., Montville, NJ) was used to eliminate possible corn cob-based mycoestrogen exposure. All animals were fed ad libitum Teklad diet 2020 (Harlan), which lacks soybean meal, alfalfa, or animal products to limit introduction of estrogenic compounds and were provided with BPA-free drinking water (Millipore Rios 16 with ELIX UV/Progard 2). Concentrations of BPA in drinking water and all experimental reagents were confirmed below the level of detection of a BPA-specific and extremely sensitive ELISA-based assay with a minimum quantitative detection limits of 0.05 ng/ml (19).
Myocyte contraction analysis, imaging of myocyte Ca2+ spark, and Ca2+ after transient
Ventricular myocytes from rodent hearts were enzymatically dissociated as previously described (11). Myocytes were placed in a Plexiglass cell chamber filled with Tyrode solution containing 1.8 mm CaCl2 at room temperature (24 C). The solution also contained either vehicle or various treatment compounds that were serially diluted from appropriate stock solutions. Myocyte contraction was recorded between 2 and 7 min after treatment. Myocytes were excited with field stimulation (Grass S48 stimulator; Grass Instruments, Quincy, MA) with 2 msec 1.5 × threshold pulses at a rate of 0.5 Hz. Steady-state myocyte shortening was imaged with a charge-coupled device camera and examined using a video-edge detector (Crescent Electronics, Sandy, UT). Data were sampled through an Axon Digidata 1322A board using the PCLAMP 9 software (both by Molecular Devices, Sunnyvale, CA). Isolated ventricular myocytes were loaded with fluo-4 acetoxymethyl ester (5 μm; Molecular Probes, Eugene, OR) and imaged for calcium transients and sparks as previously described (11).
Statistical analysis
All experiments were independently repeated using myocytes isolated from at least four hearts. All measurements were performed with the experimenter blinded to treatment. Statistical analysis was conducted using a one-way or two-way (e.g. sex vs. treatment) ANOVA differences between treatment groups assessed using a multiple comparison posttest. Values measured in the same cells before and after treatment (e.g. Ca2+ spark frequency before and after BPA exposure) were analyzed using a paired Student's t test. Frequency of events (e.g. percentage of myocyte with triggered activities) was analyzed using a χ2 test. Minimal level of statistical significance for differences in values is considered to be P < 0.05 (*). Data were analyzed with SigmaPlot 11.0, Excel, and GraphPad Prism version 5.0 (GraphPad Software, Inc., San Diego, CA) and expressed as average ± sem.
Results
Concentration-response analysis of BPA and E2 in cardiac myocytes
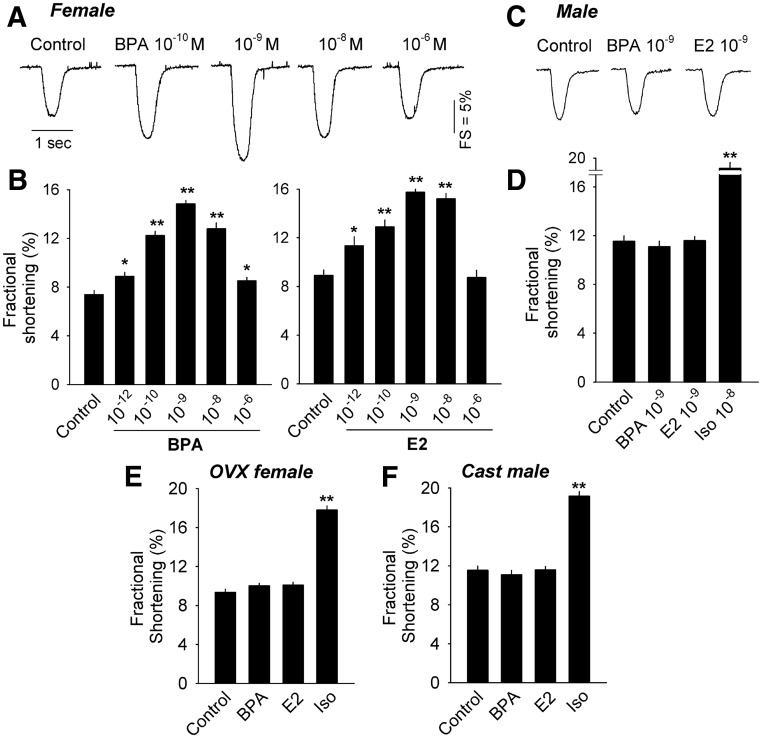
Previous results demonstrated that exposure to BPA or E2 rapidly promoted arrhythmogenesis in cardiac myocytes, and that those actions were mediated by alteration of myocyte Ca2+ cycling (11). Calcium cycling in isolated cardiac myocytes is manifested as contraction and plays a central role in determining the contractility of myocytes (20). Because contractility is a close proxy of myocyte Ca2+ cycling and a parameter that can be measured quantitatively with relatively high throughput using video edge detection, it was used in the present study as an index to quantify the rapid effects of BPA and E2. Exposure to E2 or BPA rapidly (2–7 min) enhanced contractility of ventricular myocytes from female rat hearts in a concentration-dependent manner (Fig. 1, A and B). An inverted U-shaped concentration-response curve was observed for both E2 and BPA; for each compound, a stimulatory effect on contractility was observed at doses as low as 10−12 m, with 10−9 m being the most efficacious concentration (Fig. 1B). The effects of each compound on contractility were female specific; contractility of myocytes from male rat hearts was not affected by either BPA or E2 (Fig. 1, C and D). The β-adrenergic agonist isoproterenol (Iso) was used to demonstrate the responsiveness of the estrogen-insensitive male myocytes. Contractility was significantly enhanced in the presence of 10−8 m Iso (Fig. 1D).
Fig. 1.
A, Representative contraction traces of female rat myocytes exposed to indicated concentrations of BPA containing or control solution (2–7 min exposures). FS, Fractional shortening. B, Concentration-response of the mean fractional shortening effects of BPA (n = 32–46) and E2 (n = 32–40) on female rat myocytes. C, Representative contraction traces of male rat myocytes exposed to each indicated concentration of BPA, E2, or control (2–7 min exposures). D, Mean fractional shortening effects in male myocytes induced by 10−9 m BPA, E2, or Iso (10−8 m) as positive control; n = 30–42. E and F, Effects of BPA or E2 (10−9 m) on contractility (mean fractional shortening) in myocytes from OVX female rat (n = 34–42) or castrated (Cast) male rat (n = 33–35). Iso (10−8 m) served as positive control. Error bars are sem *, P < 0.05; **, P < 0.01 vs. control in a one-way ANOVA.
Sex-specific effects of BPA and E2 in cardiac myocytes
To determine the role of sex hormones in maintaining the sex-specific sensitivity of myocytes to estrogens, we examined the responsiveness of myocytes from adult rats that were ovariectomized (OVX) or castrated 2 wk before experimentation. In contrast to myocytes from intact females, myocytes from OVX females were unresponsive to E2 or BPA (Fig. 1E). Similar to intact males, E2 or BPA did not impact contractility of myocytes isolated from hearts of castrated males (Fig. 1F). In each case, 10−8 m Iso produced a robust stimulation in myocyte contractility. These results suggest that female ovarian hormones play a key role in maintaining estrogen sensitivity of female myocytes.
Rapid actions of E2 in cardiac myocytes are initiated by membrane-associated receptor
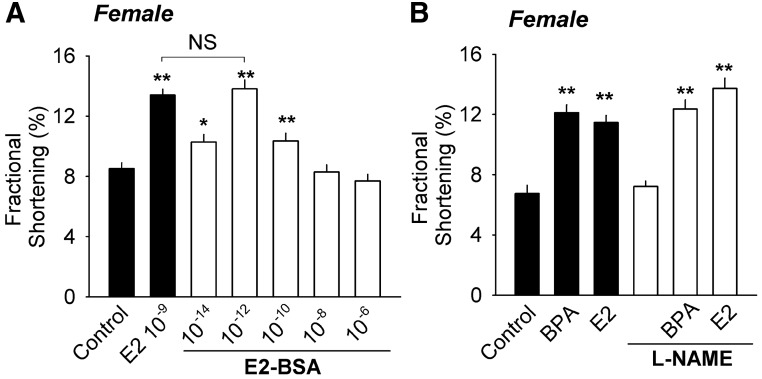
Membrane-impermeable, protein-conjugated E2 (E2-BSA) also rapidly stimulated contractility in female myocytes. Concentration response analyses showed that the concentration-response curve for E2-BSA was also in the shape of an inverted U and that E2-BSA was a more potent stimulator of contractility than E2 (Fig. 2A). The observation of increased potency related to the multiple constrained ligands per mole of BSA is consistent with previous findings investigating the role of membrane receptors in mediating the rapid estrogen responses in neuronal cells (21, 22). These results support the interpretation that rapid actions of E2 in female myocytes are mediated by membrane-associated receptor systems.
Fig. 2.
A, Dose-dependent effect of membrane-impermeable protein-conjugated E2 (E2-BSA) on contractility of female myocytes (n = 25–26). A maximal equivalent concentration of BSA served as control (n = 26), and 10−9 m unconjugated E2 plus maximal equivalent concentration of BSA served as positive control (n = 27). B, Effects of BPA or E2 (10−9 m) on contractility of female myocytes in the absence and presence of 50 μm l-NAME (n = 19–25). There is no statistically significant difference (P > 0.1) between groups without l-NAME and their respective l-NAME-treated groups. Error bars are sem. *, P < 0.05; **, P < 0.01 vs. control in a one-way ANOVA. NS (not significant), P > 0.1.
In vascular endothelial cells E2 can rapidly activate nitric oxide synthase (NOS) through membrane-associated ER-mediated pathways to stimulate synthesis of the nitric oxide (NO). NO has been shown to be a key regulator of myocardial function, including myocyte excitation-contraction coupling and contractility (23). The possibility that estradiol's or BPA's effect on contractility was mediated through paracrine or autocrine actions of NO was examined. The presence of the nonselective NOS inhibitor l-NAME did not impact the effect of BPA or E2 (Fig. 2B), demonstrating that rapid actions of estrogens on myocyte contractility are independent of endothelial cell-like rapid increases in NOS-stimulated generation of NO.
Defining the roles of ERβ and ERα in Mediating Sex-Specific Estrogen Sensitivity
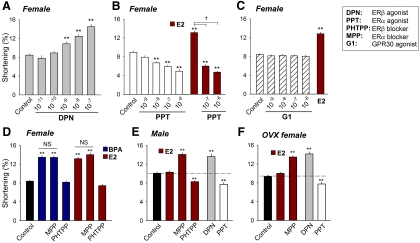
The role of ERβ and ERα receptors as mediators of sex-specific estrogen sensitivity was investigated using selective ER agonists and antagonists. In myocytes from females, the selective ERβ agonist DPN mimicked the effect of E2 and BPA by enhancing contractility in a concentration-dependent manner (Fig. 3A). In contrast, the selective ERα agonist PPT suppressed contractility in a dose-dependent manner and completely inhibited the effects of the nonselective agonist E2 (Fig. 3B). Although the orphan G protein-coupled receptor GPR30 has previously been suggested to act as a mediator of rapid estrogen signaling in some cell types (24, 25), the GPR30 agonist G1 had no effect on myocyte contractility (Fig. 3C).
Fig. 3.
Role of ER in mediating the sex-specific response of myocytes to estrogens. A, Average dose-dependent effects of DPN on contractility of female rat myocytes (n = 22–30). B, Average dose-dependent effects of PPT on contractility of female rat myocytes and effect of 10−9 m E2 alone and in the presence of PPT (n = 32–42). C, Average dose-dependent effects of G1 on contractility of female rat myocytes. E2 (10−9 m) served as positive control (n = 27–32). D, Effects of MPP (10−6 m) and PHTPP (5 × 10−6 m) on BPA- or E2-induced stimulation of contractility in female rat myocytes (n = 35–57). Concentrations of BPA and E2 were both 10−9 m. E, Effects of E2 (10−9 m), E2 + MPP (10−6 m), E2 + PHTPP (5 × 10−6 m), DPN (10−7 m), and PPT (10−7 m) on contractility of male rat myocytes (n = 40–48). F, Effects of E2 (10−9 m), E2 + MPP (10−6 m), DPN (10−7 m), and PPT (10−7 m) on contractility of OVX female rat myocytes (n = 25–34). **, P < 0.01 vs. control in a one-way ANOVA; NS (not significant), P > 0.1; †, P < 0.01.
In myocytes from females, blockade of ERβ with PHTPP abolished the stimulatory effects of BPA and E2 on contractility; by contrast, the selective ERα antagonist MPP did not influence the effect of BPA or E2 (Fig. 3D). In myocytes from male hearts, where E2 had no detectable effect on contractility, blockade of ERα with MPP revealed an E2-induced contractile stimulation, whereas blockade of ERβ resulted in a suppression of contractility in response to E2 (Fig. 3E). In male myocytes, selective activation of ERβ with 10−7 m DPN induced a female-like stimulation of contractility, whereas activation of ERα with 10−7 m PPT suppressed contractility (Fig. 3E). Myocytes isolated from OVX female exhibited male-like contractile responses to E2 (insensitivity) and to MPP and each of the ER-selective agonists (Fig. 3F).
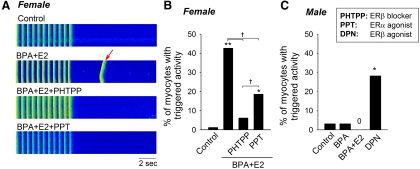
Previously we reported that exposure to BPA rapidly induced arrhythmogenic triggered activities in female myocytes, and this effect was particularly pronounced in the presence of physiologically relevant concentrations of E2 (11). Here we examined whether or not blockade of ERβ signaling inhibited E2/BPA-induced triggered activities. PHTPP blockade of ERβ abrogated the development of triggered activities induced by 10−9 m BPA and E2 in female rat myocytes (Fig. 4, A and B), further supporting a determinant role of ERβ signaling in mediating sensitivity of female myocytes to these estrogens. Consistent with a suppressive role of ERα that opposes ERβ signaling, activation of ERα with 1 μm PPT also reduced the frequency of estrogen-induced triggered activities (Fig. 4, A and B). Of interest, PHTPP was significantly more effective in blocking the arrhythmogenic actions of estrogens than PPT (Fig. 4B). Additionally, a female-like stimulatory effect on contractility by the ERβ agonist DPN was observed in male myocytes (Fig. 3E). Here treatment of male myocytes with DPN (10−7 m) rapidly induced triggered activities in approximately 30% of myocytes, a response similar to those elicited by the BPA or E2 in female myocytes (Fig. 4C).
Fig. 4.
Role of ER in mediating the proarrhythmic effect of estrogens. A, Representative recordings of pacing-elicited Ca2+ transients under control condition from female rat myocytes, spontaneous Ca2+ transient (i.e. triggered activity; arrow) after pacing in the presence of BPA plus E2 (both 10−9 m) and blockade of estrogen-induced spontaneous Ca2+ transients by PHTPP (5 × 10−6 m) or PPT (10−7 m). Myocytes were loaded with fluo-4 and imaged using a confocal microscope; vertical fluorescent signals indicated elevations of intracellular Ca2+ levels (i.e. Ca2+ transients) as a result of pacing-induced or spontaneous excitation of the myocyte. B, Percentages of female myocytes with triggered activities under various treatments (n = 59–85). C, Percentages of male myocytes with triggered activities under various treatments (n = 32). *, P < 0.05; **, P < 0.01 vs. control in a χ2 test; †, P < 0.01.
Genetic ablation of ERβ eliminates rapid effects of BPA and E2
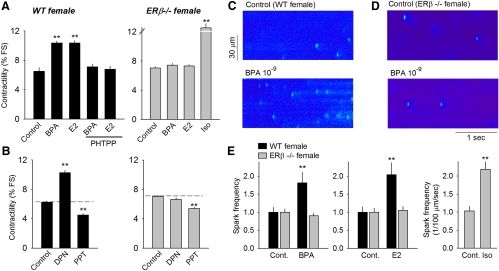
The role of ERβ in mediating the sensitivity of female myocytes to E2 and BPA was further examined using an ERβ knockout (ERβ−/−) mouse model (26). The responses of myocytes isolated from wild-type female mice to BPA, E2, the ERβ agonist PHTPP, and each of the selective ER agonists closely resembled the response profiles observed for myocytes from female rats (Fig. 5, A and B, left panels). By contrast, myocytes from ERβ−/− female heart were insensitive to BPA, E2, and the ERβ agonist DPN (Fig. 5, A and B, right panels). However, the decreased contractility induced by the ERα agonist PPT was preserved (Fig. 5, A and B, right panels), suggesting that the inhibitory actions of ERα remain intact and do not require ERβ activities.
Fig. 5.
A, Effects of BPA (10−9 m), E2 (10−9 ]scap]m), and estrogen in the presence of PHTPP (5 × 10−6 m) on contractility of myocytes from female wild-type (WT) mice (left), and effects of estrogens on myocyte contractility of female ERβ knockout mice (right). Iso (10−8 m) served as positive control (Cont) for the ERβ−/− group. B, Effects of DPN (10−7 m) and PPT (10−7 m) on contractility of myocytes from female WT (left) and ERβ knockout (right) mice (n = 38–63 for WT and 38–81 for ERβ−/−. C and D, Ca2+ sparks recorded from a WT myocyte and ERβ−/− myocyte, respectively, before and after rapid exposure to BPA (2–3 min). Myocytes were loaded with fluo-4 and imaged using a confocal microscope; spot-shaped fluorescent signals indicated local elevations of intracellular Ca2+ levels as a result of spontaneous release of Ca2+ from the SR (i.e. Ca2+ sparks). E, Effect of 10−9 m BPA (left) and E2 (center) on Ca2+ spark frequency (normalized to control) in myocytes from female WT and ERβ−/− mice; right, effect of 10−8 m Iso on Ca2+ spark frequency in myocytes from female ERβ−/− mice. Data are averages from seven myocytes for each group. **, P < 0.01 vs. control in a one-way ANOVA or paired t test.
We previously demonstrated that the cellular mechanism of the proarrhythmic effects of BPA and E2 involved aberrant SR Ca2+ leak (11), a central factor in the development of triggered activities and lethal ventricular arrhythmias under disease conditions such as heart failure (27, 28). SR Ca2+ leak was examined in ERβ knockout female myocytes through analysis of Ca2+ sparks, which represent quantal release of SR Ca2+ through clusters of ryanodine receptors (29). Similar to findings in female rat myocytes, 10−9 m BPA rapidly increased the Ca2+ spark frequency without affecting spark amplitude in wild-type female myocytes (Fig. 5, C and E). Ablation of ERβ completely abolished the stimulatory effect by BPA on spark frequency in ERβ−/− female mouse myocytes (Fig. 5, D and E). Likewise, the stimulatory effect of E2 on spark frequency was also abolished in knockout myocytes (Fig. 5E; center panel). As a control for detectable responsiveness in ERβ−/− myocytes, Iso markedly increased Ca2+ spark frequency (Fig. 5E, right panel). These results are in agreement with previous findings showing that genetic ablation of ERβ completely abolished triggered activities induced by BPA and E2 in female mouse myocytes (11).
Discussion
The physiology and pathophysiology of the heart have been extensively studied; however, the impact of environmental chemicals, including EDCs, on heart health has received very limited attention. Recent cross-sectional epidemiological studies suggest a possible link between BPA exposure and CV disease in the US population (30). The focus of the studies presented here was to determine the pharmacodynamic profile of the rapid cardiac effects of BPA and E2 and to elucidate the mechanistic function of individual ER in mediating the sex-specific sensitivity of cardiac myocytes to these estrogens.
Using myocyte contractility as an endpoint, we demonstrate that the responsiveness of female rat myocytes to estrogens is maintained by female sex hormones and likely involves membrane-associated receptor signaling. Over the dose range of 10−12 to 10−6 m, the dose-response curves for both BPA and E2 in female myocytes have an inverted U shape. Importantly, efficacious concentrations of BPA (10−12 to 10−9 m) are within the range of expected free E2 and BPA based on exposure estimates for humans (10). Similar inverted U-shaped, or nonmonotonic dose responses have been described for the effects of hormones and EDC in various in vitro and in vivo systems (for review, see Refs. 31 and 32). The mechanisms mediating most nonmonotonic concentration responses are unknown and probably involve a diversity of molecular mechanisms. Receptor down-regulation at high hormone concentrations has been reported as one possible mechanism (33). However this mechanism is highly unlikely to play any major roles in the nonmonotonic cardiac effects of BPA or E2 described here due to the rapid nature of the effects. It is considered likely that the combination of receptor-specific responses (e.g. inhibitory vs. stimulatory) and differences in ligand-binding properties at specific receptors play a role in defining the observed concentration-response characteristics. Additionally, superphysiological concentrations of E2 (>1 μm) can inhibit the L-type Ca2+ current in cardiac myocytes in several species including rat and human (34, 35). Such inhibition can reduce Ca2+ influx and suppress myocyte contraction and may contribute to the inhibitory phase of the inverted U-shaped dose-response curves at high concentrations of estrogens. Regardless of the exact mechanism, the nonmonotonic cardiac effect of BPA strongly supports the view that prediction of toxicity at low doses using linear extrapolation from high-dose effects is not adequate for assessing the endocrine effects of low concentrations EDCs such as BPA.
We demonstrate that ERα and ERβ have opposing actions on myocyte contractility and arrhythmogenesis and that the balance of ERβ to ERα signaling is the prime regulator of sex-specific estrogen sensitivity of rodent cardiac myocytes. We showed that, in both sexes, activation of ERβ signaling increased contractility, whereas ERα inhibited contractility. In female rodents, the responses of myocytes to BPA and E2 were dominated by the stimulatory ERβ-mediated signaling. Thus, blockade of ERβ, but not ERα, as well as ablation of ERβ abolished the rapid response of female myocytes to estrogens both in terms of contractility change and development of arrhythmias (i.e. SR Ca2+ leak and triggered activities). Interestingly, although an ERα-mediated response appeared to be silent and overshadowed by the dominant ERβ signaling in female myocytes, activation of ERα with PPT was able to markedly inhibit the contractile and proarrhythmic effects of E2 or E2 plus BPA. This finding demonstrates a potentially negative modulatory role for ERα signaling in female cardiac function that could serve to stabilize cardiac function in response to normal circadian, reproductive, and lifetime fluctuations in hormonal levels.
Our results suggest that the actions of BPA and E2 in myocytes are independent of GPR30 activation. BPA has been shown to bind to the estrogen-related receptors, or ERR (36). The role of ERR in mediating the response of female hearts to estrogens remains to be tested; however, given the ability of blockade or genetic ablation of ERβ to completely and independently abolish the actions of E2 and BPA, the contribution of ERR to the rapid estrogen effects in myocyte is likely to be limited. This is further supported by the ability of selective activation of ERβ to elicit full response in female myocytes and to feminize the responsiveness of myocytes from males. Thus the complete pharmacological profile of the rapid effects of E2 and BPA strongly support the notion that the actions of ER-initiated signaling control the sex-specific sensitivity of myoctyes to estrogens.
In myocytes from male rat hearts there was no observable response to the nonselective estrogens BPA and E2, yet the stimulatory effect of ERβ and the inhibitory effect of ERα were both intact. Indeed, the ERβ agonist DPN elicited a female-like response and rapidly promoted the development of triggered activities in male myocytes. Further, an E2-mediated contractile response (either stimulatory or inhibitory) was unmasked when each individual ER was selectively blocked. These results demonstrate that in male rat myocytes, the lack of an observable estrogen response is at least partially due to the suppressive actions of ERα and a counterbalance between ERα and ERβ. To the best of our knowledge, these results are the first to demonstrate that the counterbalancing actions of ERα and ERβ control a cellular or physiological function via rapid signaling and the first to suggest that the opposing actions of ER determine the sex specificity of estrogen sensitivity.
Opposing actions of ERα and ERβ have been described at various levels of organization. Such counterbalancing interaction may provide a regulatory mechanism that allows fine turning of the biological actions of estrogen and cell-specific responses. ERβ has been shown to oppose the transcriptional activity of ERα in heterologous expression systems (37) and in mouse bone tissue (38). The two ERs have also been shown to have antagonistic neural/behavioral influences in animal models. Studies suggest that ERβ activation has favorable effect on spatial learning whereas ERα has a detrimental influence (39). In addition, ERα appears to increase aggressive behavior, fear and anxiety, whereas ERβ activation has the opposite effect (40). The molecular mechanism of the antagonism between the ERs and its fine turning is not clear. It has been shown that the relative abundance of heterologous expressed recombinant ERα and ERβ determines the cellular responses to ERs agonists in cell lines (37). Whether or not differential expression of ER, and/or coupling to specific intracellular signaling pathways plays a role in determining the balance between ERα/ERβ signaling in cardiac myocytes remains to be determined.
Estrogen plays a key role in influencing physiology and disease of the female heart. Clear understanding of the cardiac-specific actions of estrogen and individual ERs is necessary for improving treatment of cardiac disease in women and likely also men. Currently, it is generally believed that both ERα and ERβ have cardioprotective effects in the female heart. Activation of either ERα or ERβ has been shown to protect again ischemia-reperfusion injury in female hearts (41). In addition, ERβ, but not ERα, has been shown to mediate the estrogen-dependent protection against ventricular hypertrophy in female animal models (42, 43). Highlighting the complex and multifaceted actions of ER in the heart, we show in the present study that ERβ plays a central role in mediating the proarrhythmic cardiac effects of BPA and estrogen in female animals. Such proarrhythmic action of ERβ (as well as the ability of ERα to antagonize the estrogen-induced arrhythmia) needs to be taken into consideration when assessing the cardiac risks/benefits of other estrogenic EDCs, such as the ERβ-selective phytoestrogens. The cellular and receptor mechanisms of the proarrhythmic effect of BPA elucidated in our studies may be of value for the development of therapeutic measures to protect women against arrhythmia risks associated with estrogenic EDC exposure.
Acknowledgments
We thank Dr. Richard H. Kennedy at Loyola University Chicago for his enthusiasm and support for this research.
This work was supported by National Institute of Health Grants RO1 ES017262 and HL084539 (to H.S.W.) and R01-ES015145 and RC2 ES018765 (to S.M.B.), and University of Cincinnati Center for Environmental Genetics (P30-ES06096).
Current address for S.Y.: Department of Cardiology, Second Affiliated Hospital of Nanchang University, Nanchang, Jiangxi, 330006 China.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BPA
- Bisphenol A
- CV
- cardiovascular
- DPN
- 2,3-bis(4-hydroxyphenyl)-propionitrile diarylpropionitrile
- E2
- 17β-estradiol
- EDC
- endocrine-disrupting chemical
- ER
- estrogen receptor
- ERR
- estrogen-related receptors
- GPER
- protein G protein-coupled ER
- Iso
- isoproterenol
- l-NAME
- Nω-nitro-l-arginine methyl ester hydrochloride
- MPP
- methyl-piperidino-pyrazole, 1,3-Bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy)phenol]-1H-pyrazole dihydrochloride
- OVX
- ovariectomized
- PHTPP
- 4-[2-phenyl-5,7-bis(trifluoromethyl)pyrazolo[1, 5-a]pyrimidin-3-yl]phenol
- PPT
- 4,4′,4′′-(4-propyl-[1H]-pyrazole-1,3,5-triyl) trisphenol
- SR
- sarcoplasmic reticulum.
References
- 1. Babiker FA, De Windt LJ, van Eickels M, Grohe C, Meyer R, Doevendans PA. 2002. Estrogenic hormone action in the heart: regulatory network and function. Cardiovasc Res 53:709–719 [DOI] [PubMed] [Google Scholar]
- 2. Mendelsohn ME, Karas RH. 2005. Molecular and cellular basis of cardiovascular gender differences. Science 308:1583–1587 [DOI] [PubMed] [Google Scholar]
- 3. Du XJ, Fang L, Kiriazis H. 2006. Sex dimorphism in cardiac pathophysiology: experimental findings, hormonal mechanisms, and molecular mechanisms. Pharmacol Ther 111:434–475 [DOI] [PubMed] [Google Scholar]
- 4. Bhupathy P, Haines CD, Leinwand LA. 2010. Influence of sex hormones and phytoestrogens on heart disease in men and women. Womens Health (Lond Engl) 6:77–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wolbrette D, Naccarelli G, Curtis A, Lehmann M, Kadish A. 2002. Gender differences in arrhythmias. Clin Cardiol 25:49–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC. 2009. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev 30:293–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Welshons WV, Nagel SC, vom Saal FS. 2006. Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology 147:S56–S69 [DOI] [PubMed] [Google Scholar]
- 8. Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Ekong J, Needham LL. 2005. Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ Health Perspect 113:391–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. 2008. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ Health Perspect 116:39–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJ, Schoenfelder G. 2010. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ Health Perspect 118:1055–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yan S, Chen Y, Dong M, Song W, Belcher SM, Wang HS. 2011. Bisphenol A and 17β-estradiol promote arrhythmia in the female heart via alteration of calcium handling. PLoS One 6:e25455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Belcher SM. 2008. Rapid signaling mechanisms of estrogens in the developing cerebellum. Brain Res Rev 57:481–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Levin ER. 2008. Rapid signaling by steroid receptors. Am J Physiol Regul Integr Comp Physiol 295:R1425–R1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grohé C, Kahlert S, Löbbert K, Vetter H. 1998. Expression of oestrogen receptor α and β in rat heart: role of local oestrogen synthesis. J Endocrinol 156:R1–R7 [DOI] [PubMed] [Google Scholar]
- 15. Mendelsohn ME, Karas RH. 1999. The protective effects of estrogen on the cardiovascular system. N Engl J Med 340:1801–1811 [DOI] [PubMed] [Google Scholar]
- 16. Mahmoodzadeh S, Eder S, Nordmeyer J, Ehler E, Huber O, Martus P, Weiske J, Pregla R, Hetzer R, Regitz-Zagrosek V. 2006. Estrogen receptor α up-regulation and redistribution in human heart failure. FASEB J 20:926–934 [DOI] [PubMed] [Google Scholar]
- 17. Paquette A, Wang D, Gauthier MS, Prud'homme D, Jankowski M, Gutkowska J, Lavoie JM. 2007. Specific adaptations of estrogen receptor α and β transcripts in liver and heart after endurance training in rats. Mol Cell Biochem 306:179–187 [DOI] [PubMed] [Google Scholar]
- 18. Deschamps AM, Murphy E. 2009. Activation of a novel estrogen receptor, GPER, is cardioprotective in male and female rats. Am J Physiol Heart Circ Physiol 297:H1806–H1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Le HH, Carlson EM, Chua JP, Belcher SM. 2008. Bisphenol A is released from polycarbonate drinking bottles and mimics the neurotoxic actions of estrogen in developing cerebellar neurons. Toxicol Lett 176:149–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Eisner DA, Isenberg G, Sipido KR. 2003. Normal and pathological excitation-contraction coupling in the heart—an overview. J Physiol 546:3–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wong JK, Le HH, Zsarnovszky A, Belcher SM. 2003. Estrogens and ICI182,780 (Faslodex) modulate mitosis and cell death in immature cerebellar neurons via rapid activation of p44/p42 mitogen-activated protein kinase. J Neurosci 23:4984–4995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zsarnovszky A, Le HH, Wang HS, Belcher SM. 2005. Ontogeny of rapid estrogen-mediated extracellular signal-regulated kinase signaling in the rat cerebellar cortex: potent nongenomic agonist and endocrine disrupting activity of the xenoestrogen bisphenol A. Endocrinology 146:5388–5396 [DOI] [PubMed] [Google Scholar]
- 23. Ziolo MT, Kohr MJ, Wang H. 2008. Nitric oxide signaling and the regulation of myocardial function. J Mol Cell Cardiol 45:625–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr 2000. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol 14:1649–1660 [DOI] [PubMed] [Google Scholar]
- 25. Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. 2005. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 307:1625–1630 [DOI] [PubMed] [Google Scholar]
- 26. Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. 1998. Generation and reproductive phenotypes of mice lacking estrogen receptor β. Proc Natl Acad Sci USA 95:15677–15682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wehrens XH, Lehnart SE, Marks AR. 2005. Ryanodine receptor-targeted anti-arrhythmic therapy. Ann NY Acad Sci 1047:366–375 [DOI] [PubMed] [Google Scholar]
- 28. Marks AR. 2006. Novel therapy for heart failure and exercise-induced ventricular tachycardia based on ‘fixing’ the leak in ryanodine receptors. Novartis Found Symp 274:132–147; discussion 147–155; review 272–276 [PubMed] [Google Scholar]
- 29. Cheng H, Lederer WJ, Cannell MB. 1993. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science 262:740–744 [DOI] [PubMed] [Google Scholar]
- 30. Lang IA, Galloway TS, Scarlett A, Henley WE, Depledge M, Wallace RB, Melzer D. 2008. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA 300:1303–1310 [DOI] [PubMed] [Google Scholar]
- 31. Welshons WV, Thayer KA, Judy BM, Taylor JA, Curran EM, vom Saal FS. 2003. Large effects from small exposures. I. Mechanisms for endocrine-disrupting chemicals with estrogenic activity. Environ Health Perspect 111:994–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM. 2009. Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr Rev 30:75–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Medlock KL, Lyttle CR, Kelepouris N, Newman ED, Sheehan DM. 1991. Estradiol down-regulation of the rat uterine estrogen receptor. Proc Soc Exp Biol Med 196:293–300 [DOI] [PubMed] [Google Scholar]
- 34. Jiang C, Poole-Wilson PA, Sarrel PM, Mochizuki S, Collins P, MacLeod KT. 1992. Effect of 17 β-oestradiol on contraction, Ca2+ current and intracellular free Ca2+ in guinea-pig isolated cardiac myocytes. Br J Pharmacol 106:739–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Meyer R, Linz KW, Surges R, Meinardus S, Vees J, Hoffmann A, Windholz O, Grohé C. 1998. Rapid modulation of L-type calcium current by acutely applied oestrogens in isolated cardiac myocytes from human, guinea-pig and rat. Exp Physiol 83:305–321 [DOI] [PubMed] [Google Scholar]
- 36. Takayanagi S, Tokunaga T, Liu X, Okada H, Matsushima A, Shimohigashi Y. 2006. Endocrine disruptor bisphenol A strongly binds to human estrogen-related receptor γ (ERRγ) with high constitutive activity. Toxicol Lett 167:95–105 [DOI] [PubMed] [Google Scholar]
- 37. Hall JM, McDonnell DP. 1999. The estrogen receptor β-isoform (ERβ) of the human estrogen receptor modulates ERα transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology 140:5566–5578 [DOI] [PubMed] [Google Scholar]
- 38. Lindberg MK, Movérare S, Skrtic S, Gao H, Dahlman-Wright K, Gustafsson JA, Ohlsson C. 2003. Estrogen receptor (ER)-β reduces ERα-regulated gene transcription, supporting a “ying yang” relationship between ERα and ERβ in mice. Mol Endocrinol 17:203–208 [DOI] [PubMed] [Google Scholar]
- 39. Rissman EF. 2008. Roles of oestrogen receptors α and β in behavioural neuroendocrinology: beyond Yin/Yang. J Neuroendocrinol 20:873–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sugiyama N, Barros RP, Warner M, Gustafsson JA. 2010. ERβ: recent understanding of estrogen signaling. Trends Endocrinol Metab 21:545–552 [DOI] [PubMed] [Google Scholar]
- 41. Murphy E, Steenbergen C. 2007. Gender-based differences in mechanisms of protection in myocardial ischemia-reperfusion injury. Cardiovasc Res 75:478–486 [DOI] [PubMed] [Google Scholar]
- 42. Pedram A, Razandi M, Aitkenhead M, Levin ER. 2005. Estrogen inhibits cardiomyocyte hypertrophy in vitro. Antagonism of calcineurin-related hypertrophy through induction of MCIP1. J Biol Chem 280:26339–26348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pedram A, Razandi M, Lubahn D, Liu J, Vannan M, Levin ER. 2008. Estrogen inhibits cardiac hypertrophy: role of estrogen receptor-β to inhibit calcineurin. Endocrinology 149:3361–3369 [DOI] [PMC free article] [PubMed] [Google Scholar]