Abstract
Kisspeptin (KP) and KP-1 receptor (KISS1R) have emerged as important upstream regulators in the control of puberty. However, how developmental changes in KP-KISS1R contribute to the pubertal increase in GnRH release still remains elusive. In this study, we examined the effects of the KP agonist, human KP-10 (hKP-10), and the KP antagonist, peptide 234, on in vivo GnRH release in prepubertal and pubertal ovarian-intact female rhesus monkeys using a microdialysis method. We found that direct infusion of hKP-10 into the medial basal hypothalamus and stalk-median eminence region stimulated GnRH release in a dose-responsive manner, whereas infusion of peptide 234 suppressed GnRH release in both developmental stages. Because ovarian steroid feedback on GnRH release becomes prominent after the initiation of puberty in primates, we further examined whether ovarian steroids modify the GnRH response to hKP-10. Results demonstrate that the hKP-10-induced stimulation of GnRH release was eliminated by ovariectomy in pubertal, but not prepubertal, monkeys. Furthermore, replacement of estradiol into ovariectomized pubertal monkeys resulted in a partial recovery of the hKP-10-induced GnRH release. Collectively, these results suggest that a KISS1R-mediated mechanism, in addition to the pubertal increase in KP-54 release we previously reported, contributes to the pubertal increase in GnRH release and that there is a switch from an ovarian steroid-independent to -dependent mechanism in the response of GnRH to KP.
Despite the well-established concept that puberty onset is triggered by an ovarian steroid-independent increase in GnRH release in primates (1–4), the neurobiological mechanism triggering the pubertal increase in GnRH release remains elusive. The hope for a breakthrough arrived in 2003, when groundbreaking studies proposed that kisspeptin (KP) and the KP-1 receptor (KISS1R) play a role in the mechanism of puberty. These studies identified mutations in KISS1R that result in a delay in or absence of puberty in humans (5, 6), and evidence from transgenic KISS1R-deficient mice, which exhibit hypogonadotropic hypogonadism (5), supports this concept.
Previous studies in our laboratory in primates demonstrate that 1) KP increases along with the pubertal increase in GnRH release and 2) direct infusion of the KP agonist, human KP-10 (hKP-10), into the medial basal hypothalamus (MBH)/stalk-median eminence (S-ME) region stimulates in vivo GnRH release, whereas a similar infusion of the KP antagonist, peptide 234, suppresses GnRH release in ovarian-intact pubertal female rhesus monkeys (7, 8). However, it is unclear whether the pubertal increase in KP release solely contributes to the pubertal increase in GnRH release or whether developmental changes in a KISS1R-mediated mechanism also contribute to the pubertal increase in GnRH release.
In both male and female mice, the number of KP-expressing neurons increases before puberty onset (9, 10), although there is a clear sex difference in the timing of the pubertal increase in KP expression pattern (9), which is likely due to the difference in the age when circulating gonadal steroids increase (10, 11). In primates, KP signaling also increases at the time of puberty, as indicated by an increase in KP mRNA in ovarian-intact female and agonadal male monkeys (12) and KP release in ovarian-intact female monkeys (7). However, because a direct comparison between the two sexes has not been made, it is unclear whether there are any sex differences in the timing of the pubertal increase in KP signaling or whether this timing is influenced by gonadal steroids in primates.
The number of KISS1R-expressing neurons, including the number of GnRH neurons that express KISS1R, also increases during development (13). Interestingly, however, it appears that no sex difference in the developmental pattern of KISS1R expression exists. In both male and female mice, the number of GnRH neurons expressing KISS1R reaches adult levels by postnatal d 20 (13). Moreover, the number of GnRH neurons responding to exogenous KP increase before puberty (14), and the LH response (and presumably response of GnRH neurons) in juvenile mice to a higher (0.1 nmol) dose of KP-10 is larger than that in adult (14) male mice, indicating that the GnRH response to KP-10 increases well before puberty onset and that developmental changes in a KISS1R-mediated mechanism are independent from the influence of gonadal steroid feedback. In primates, developmental changes in KISS1R also appear to be independent of the pubertal increase in gonadal steroids, because KISS1R mRNA levels do not change at puberty in agonadal males (12). However, this is not as clear in female primates, because data on a developmental change in KISS1R mRNA has been reported only from ovarian-intact, but not ovariectomized (OVX), female monkeys (12).
Clearly, there is a gap in our knowledge as to the contribution of developmental changes in KP input to GnRH neurons vs. the KISS1R-mediated mechanism in GnRH neurons. In the present study, we therefore conducted a series of studies addressing the question of whether any changes in the GnRH response to a KP agonist and antagonist occur during pubertal development in female rhesus monkeys and whether these changes are dependent upon the pubertal increase in ovarian steroids.
Materials and Methods
Animals
Thirteen prepubertal (13.0–20.2 months of age) and 15 pubertal (28.0–43.7 months of age) female rhesus monkeys (Macaca mulatta) were used in this study. All animals were born and raised at the Wisconsin National Primate Research Center and housed in pairs (cages 172 × 86 × 86 cm) with 12 h light, 12 h dark, at a controlled temperature (22 C). They were fed a standard diet of Teklad Primate Chow (Harlan, Madison, WI) twice per day, and water was available ad libitum. Fresh fruit or other enrichment was provided on a daily basis. Pubertal stages of female rhesus monkeys were defined as previously reported (15). During the prepubertal stage, circulating levels of 17β-estradiol (E2) and LH are low, no physical signs of puberty are apparent, and monkeys are typically younger that 21 months of age. During the pubertal stage, circulating levels of E2 and LH are elevated with clear nocturnal increases in LH, and physical signs of puberty (development of perineal sex-skin, larger nipple size, and vaginal bleeding) are apparent. All experiments were conducted under the National Institutes of Health and U.S. Department of Agriculture guidelines, and the protocol was approved by the Animal Care and Use Committee, University of Wisconsin-Madison.
Experimental design
Four series of experiments using an in vivo microdialysis method were conducted to compare developmental changes in female rhesus monkeys at the prepubertal and pubertal stages. In experiment 1, to determine developmental changes in KISS1R in ovarian-intact prepubertal and pubertal female monkeys, the effects of the KP agonist human KP-10 (hKP-10) [hKP10 (112–121)-amide; Phoenix, Belmont, CA] or vehicle [perfusion fluid for central nervous system (CNS); CMA, Stockholm, Sweden] on GnRH release were examined. After 60 min of control sampling, 10 nm (low dose, experiment 1a) or 100 nm (high dose, experiment 1b) hKP-10 or vehicle was infused through the microdialysis probe into the MBH/S-ME for 20 min while dialysates were continuously collected at 10-min intervals. In experiment 1a, a set of four prepubertal (13.4 ± 0.4 months) and four pubertal (31.8 ± 0.2 months) females received 10 nm hKP-10. In experiment 1b, a second set of four prepubertal (15.2 ± 0.5 months) and four pubertal (30.2 ± 1.4 months) females received 100 nm hKP-10. Control data (vehicle effects) were obtained from two prepubertal and two pubertal females from each set of animals in experiments 1a and 1b.
In experiment 2, to determine whether there are developmental changes in the response of GnRH release to the blockade of endogenous KP signal, the KP antagonist peptide 234 (8) at 10 nm or vehicle (CNS perfusion fluid) was infused through the microdialysis probe for 30 min while dialysates were continuously collected at 10-min intervals. Five prepubertal (14.4 ± 0.5 months) and four pubertal (32.3 ± 0.4 months) monkeys were used for this experiment. Vehicle data were obtained from all monkeys. Because experiments 1 and 2 were concurrently conducted, three prepubertal and all of the pubertal monkeys examined in experiment 2 were also examined in experiment 1.
In experiment 3, to determine whether the developmental changes in the GnRH response to hKP-10 observed in experiment 1 are due to the pubertal increase in circulating ovarian steroids, the effects of 10 nm hKP-10 were initially examined in six prepubertal (19.2 ± 0.7 months) and four pubertal (33.6 ± 1.9 months) OVX monkeys: three of the prepubertal and two of the pubertal females used in experiments 1 and 2 and an additional three prepubertal and two pubertal females were OVX at least 1 month before experimentation. Vehicle data were obtained from three of the monkeys at each developmental stage. Subsequently, the effects of a higher dose (100 nm) of hKP-10 or vehicle were examined in three additional OVX pubertal monkeys (29.6 ± 0.6 months). The protocol for this experiment was similar to that described in experiment 1.
In experiment 4, to determine the role of E2 on the GnRH response to hKP-10, three OVX pubertal females (one from experiment 3 and two additional monkeys, 37.0 ± 4.6 months) received a 4-cm silastic capsule containing E2 12–14 d before the microdialysis experiments, as described previously (16). The protocol for this experiment was similar to that described in experiment 1.
Cranial pedestal implantation and guide cannula insertion
All animals were well adapted to the primate chair, experimental conditions, and the researchers before experimentation. At least 1 month before microdialysis experiments, all animals were surgically implanted with a cranial pedestal under isoflurane anesthesia as previously described (17). On the day of the microdialysis experiment, the monkey was anesthetized with ketamine (10–15 mg/kg body weight) and dexmedetomidine (up to 3.0 μg/kg body weight) and placed in a stereotaxic apparatus. A microdrive unit, which allows for three-dimensional adjustment, was secured to the cranial pedestal, and a guide cannula with an inner stylet was inserted 5 mm above the S-ME with the microdrive unit. The custom-made guide cannula (CMA) consisted of a stainless steel shaft (76.0 mm in length, 0.91 mm diameter) with a removable stainless steel stylet (96.0 mm in length, 0.6 mm in diameter). Venticulographs were used to visualize and verify the x-, y-, z-coordinates of the guide cannula above the S-ME.
Microdialysis experiment
Immediately after guide cannula placement, the monkey was removed from the stereotaxic apparatus and placed in a primate chair. The inner stylet was removed, and a microdialysis probe (stainless steel shaft 96.0 mm in length, 0.6 mm in diameter) with a polyarylethersulfone membrane (20-kDa molecular mass cutoff, 5.0 mm in length, 0.5 mm in diameter) was inserted into the guide cannula so that the tip of the probe was located in the MBH/S-ME as previously described (18). CMA CNS perfusion fluid (147 mm NaCl, 2.7 mm KCl, 1.2 mm CaCl2, 0.85 mm MgCl2) containing bacitracin (4 U/ml) was infused through the influx tubing at 2 μl/min with a CMA/102 microdialysis pump (CMA). Perfusates were collected into borosilicate tubes at 10-min intervals with a fraction collector (FC203B; Gilson, Middleton, WI) and 130 μl buffer (0.1% gelatin, 0.01 m PO4, 0.15 m NaCl, and 0.1% NaN3 at pH 7.4) was added after sample collection. Samples were immediately frozen on dry ice and stored at −80 C until assayed. Experiments were conducted for up to 12 h, during which the animal was provided monkey chow, fresh fruit, other snacks, and water and seated close to a partner monkey for visualization and vocalization. After experiments, monkeys were returned to their home cage for at least 1 month before participating in a subsequent microdialysis experiment.
Radioimmunoassay
RIA for GnRH measurement was conducted using the R42 antiserum provided by Dr. Terry Nett (Colorado State University, Fort Collins, CO) with a protocol previously described (19). Assay sensitivity was 0.02 pg/tube. Intra- and interassay coefficients of variation were 8.1 and 11.3%, respectively. Circulating levels of E2 before the first experiment from each monkey were measured as described previously (20).
Statistical analysis
As described in the experimental design, three to six animals were used for each experiment. However, in some experiments, data were obtained twice in an animal at the same developmental stage. In this case, we counted the results of the two trials as an independent entry, because the data of each trial were obtained at least 2–4 months apart, and the placement of the microdialysis probe was different for each infusion. In experiment 1a, two of the four prepubertal and all four pubertal monkeys were examined twice (n = 6, prepubertal stage; n = 8, pubertal stage). In experiment 1b, two of the four prepubertal and three of the four pubertal monkeys were examined twice (n = 6, prepubertal stage; n = 7, pubertal stage). In experiment 2, two of the five prepubertal and two of the four pubertal monkeys were examined twice (n = 7, prepubertal stage; n = 6, pubertal stage). In experiment 3, one of the six prepubertal and all four pubertal monkeys were examined twice (n = 7, prepubertal stage; n = 8 pubertal stage) with 10 nm hKP-10, and one of the three pubertal monkeys was examined twice (n = 4) with 100 nm hKP-10. In experiment 4, 1 of the 3 pubertal monkeys was examined twice (n = 4.) In experiment 1, the mean of 30-min time blocks (three successive 10-min samples) from 60 min before hKP-10 or vehicle infusion (baseline GnRH levels) to 90 min after infusion (GnRH responses) was calculated in individual trials. Subsequently, the effects of 10 nm hKP-10, 100 nm hKP-10, or vehicle on the 30-min time blocks within an experimental group were examined using two-way ANOVA with repeated measure followed by Bonferroni post hoc analysis (Prism version 5.00; GraphPad Software, San Diego, CA). Next, treatment effects (vehicle vs. 10 nm hKP-10; vehicle vs. 100 nm hKP-10; 10 nm hKP-10 vs. 100 nm hKP-10) were similarly examined using two-way ANOVA with unrepeated measure followed by Bonferroni post hoc analysis. Finally, treatment between developmental stages (prepubertal vs. pubertal) was tested with two-way ANOVA and Bonferroni post hoc analysis. Overall significance is described in the text (see Results and figure legends), but only post hoc analysis is depicted on the figures. In experiment 2, analysis for 1) treatment effects over time within a developmental stage, 2) the effects of treatments (vehicle vs. 10 nm peptide 234) also within a developmental stage, and 3) treatment between developmental stages (prepubertal vs. pubertal) was conducted as described in experiment 1. In experiment 3, analysis was conducted as described in experiment 1. Additionally, the effects of gonadal status (OVX vs. ovarian-intact) on GnRH response to hKP-10 within a developmental stage were similarly tested. In experiment 4, analysis was conducted as described in experiment 1. Additionally, the effects of gonadal status (OVX+E2 vs. OVX or OVX+E2 vs. ovarian-intact) were similarly tested. Circulating E2 levels between groups were examined with Student's t test. Differences were considered significant at P < 0.05.
Results
Developmental changes in GnRH response to hKP-10 (experiment 1)
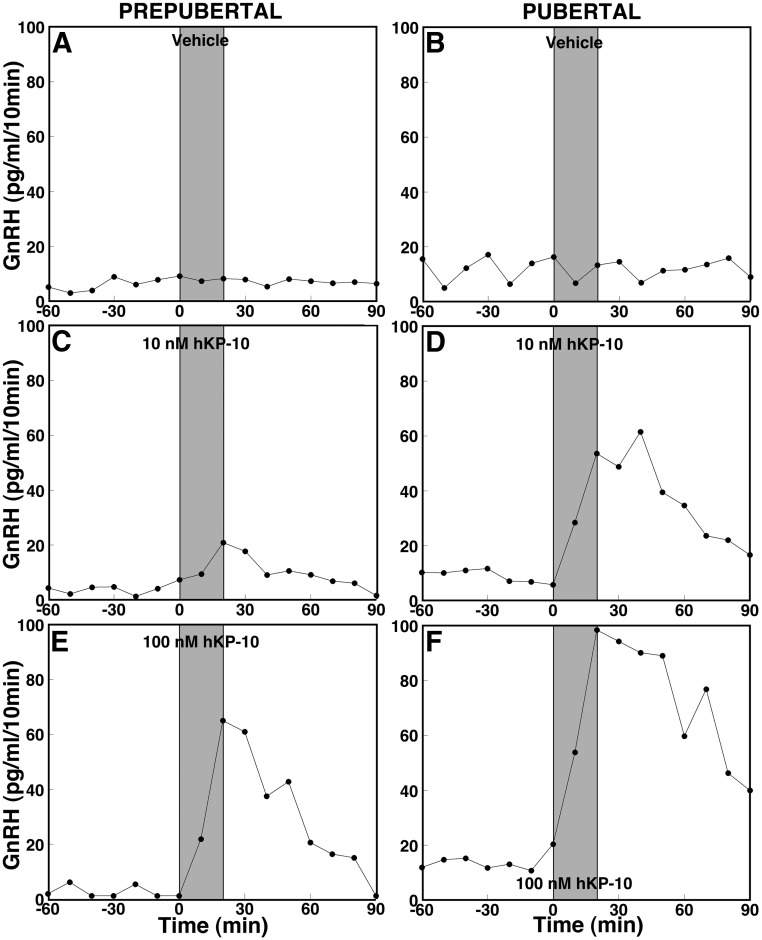
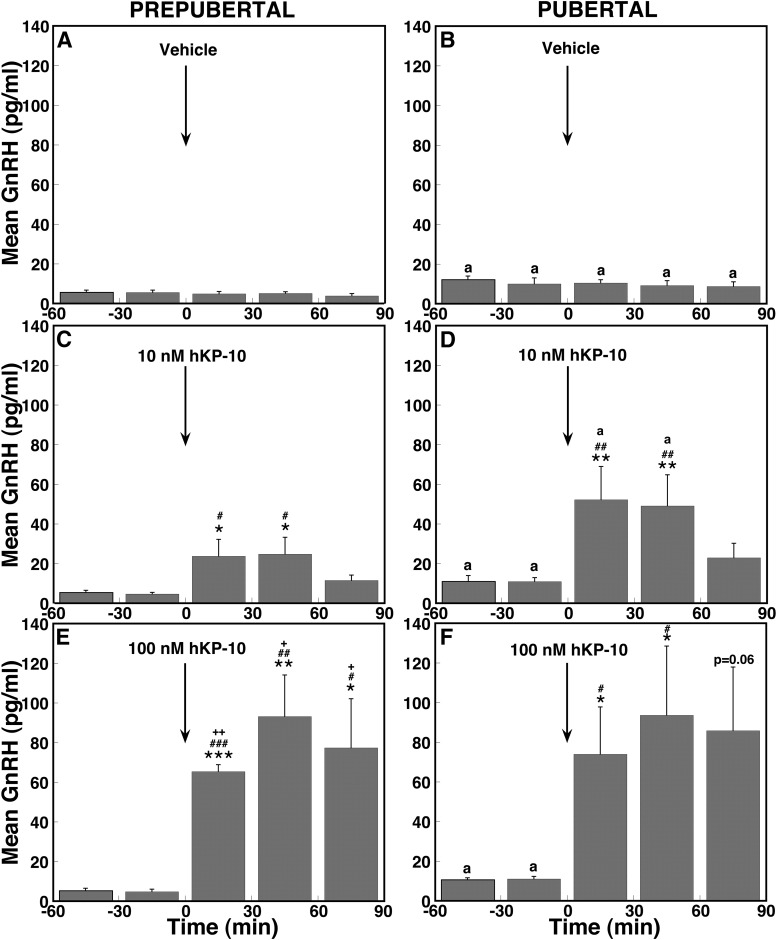
Consistent with our previous observation (1), baseline GnRH levels before hKP-10 challenges in prepubertal monkeys were lower than those in pubertal monkeys (Figs. 1 and 2). Infusion of 10 and 100 nm hKP-10 for 20 min in prepubertal monkeys stimulated GnRH release in a dose-responsive manner (Fig. 1, C and E). Statistical analysis indicated that the increases in GnRH release at both doses in prepubertal monkeys (Fig. 2, C and E) were significant (overall significance: P < 0.05 for low dose; P < 0.01 for high dose) compared with vehicle infusion (Fig. 2A). Post hoc analysis indicated that hKP-10 significantly (P < 0.05 for low dose; P < 0.001to P < 0.05 for high dose) stimulated GnRH release for up to 60 (10 nm) or 90 (100 nm) min over baseline GnRH levels as well as GnRH values with vehicle treatment at corresponding time periods (Fig. 2A). Moreover, the increase in GnRH release induced by 100 nm hKP-10 (Fig. 2E) was significantly (overall significance: P < 0.01) greater than that by 10 nm hKP-10 (Fig. 2C) and post hoc analysis indicated that all 30-min blocks of GnRH release during and up to 90 min after 100 nm hKP-10 (Fig. 2E) infusion were significantly (P < 0.05 to P < 0.01) higher than corresponding 30-min blocks with 10 nm hKP-10 (Fig. 2C).
Fig. 1.
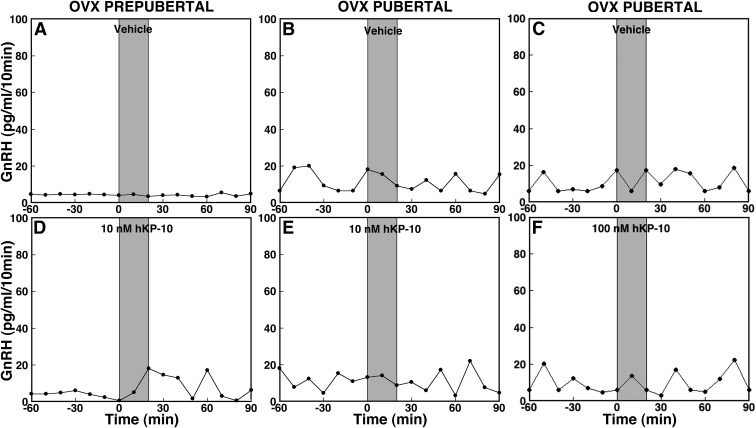
Effects of hKP-10 on GnRH release in ovarian-intact prepubertal (left panels) and pubertal (right panels) female monkeys. After 60 min control sampling with microdialysis, vehicle (top panels), 10 nm hKP-10 (middle panels), or 100 nm hKP-10 (bottom panels) was directly infused into the S-ME for 20 min as shown by shaded bars, and dialysates were continuously collected for an additional 90 min. Representative cases with each treatment are shown. At both 10 nm (C) and 100 nm (E), hKP-10 clearly stimulated GnRH release in vivo in prepubertal monkeys, whereas vehicle (A) did not. Similarly, hKP-10 infusion at both doses (10 nm in D and 100 nm in F) stimulated GnRH release in pubertal monkeys, whereas vehicle (B) failed to induce any changes. Note that baseline levels of GnRH release are higher in the pubertal monkeys than in the prepubertal monkeys.
Fig. 2.
Direct hKP-10 infusion stimulates mean GnRH release in vivo in ovarian-intact prepubertal and pubertal monkeys. Group data (mean ± sem) from prepubertal monkeys treated with vehicle (A, n = 4), 10 nm hKP-10 (C, n = 6), and 100 nm hKP-10 (E, n = 6) and from pubertal monkeys treated with vehicle (B, n = 4), 10 nm hKP-10 (D, n = 8), and 100 nm hKP-10 (F, n = 7) are shown. In prepubertal monkeys, both 10 and 100 nm hKP-10 significantly increased GnRH release over the baseline levels (P < 0.05 for 10 nm and P < 0.01 for 100 nm hKP-10), the effect of hKP-10 at both doses was significantly higher than those in vehicle (P < 0.05 for 10 nm and P < 0.01 for 100 nm hKP-10), and the effect of 100 nm hKP-10 was significantly larger than the effect of 10 nm hKP-10 (P < 0.01). Similarly, in pubertal monkeys, both 10 and 100 nm hKP-10 significantly stimulated GnRH release over the baseline levels (P < 0.01 for 10 and 100 nm hKP-10), the effects of hKP-10 at both doses were significantly higher than those in vehicle (P < 0.01 for 10 and 100 nm hKP-10), and the effect of 100 nm hKP-10 was significantly larger than the effect of 10 nm hKP-10 (P < 0.05). Moreover, the GnRH response to 10 nm hKP-10 during the first 60 min was significantly larger than that in prepubertal monkeys. *, P < 0.05; **, P < 0.01; ***, P < 0.001 vs. before 10 or 100 nm hKP-10 infusion; #, P < 0.05; ##, P < 0.01; ###, P < 0.001 vs. vehicle infusion; +, P < 0.05; ++, P < 0.01 vs. 10 nm hKP-10; a, P < 0.05 vs. corresponding time block in prepubertal monkeys.
In pubertal monkeys, 10 and 100 nm hKP-10 infusion also induced a robust increase in GnRH release (Fig. 1, D and F). Increases in GnRH release induced by hKP-10 at both doses (Fig. 2, D and F) were also significant (overall significance: P < 0.01 for both doses) compared with vehicle infusion. Post hoc analysis indicated that both 10 and 100 nm hKP-10 significantly (P < 0.01 for low dose; P < 0.05 for high dose) stimulated GnRH release for up to 60 min after the start of the infusion compared with GnRH baseline levels as well as corresponding values with vehicle treatment (Fig. 2B). Interestingly, although the GnRH response to the 100 nm hKP-10 dose was significantly (overall significance: P < 0.05) larger than that to the 10 nm hKP-10 dose in pubertal monkeys, post hoc analysis indicated only a trend toward an increase. Importantly, the GnRH response to 10 nm hKP-10 during the first 60 min was significantly greater in pubertal monkeys compared with prepubertal monkeys (P < 0.05 for both). Vehicle infusion had no effect on GnRH release in monkeys at either developmental stage (Figs. 1, A and B, and 2, A and B).
Developmental changes in GnRH response to peptide 234 (experiment 2)
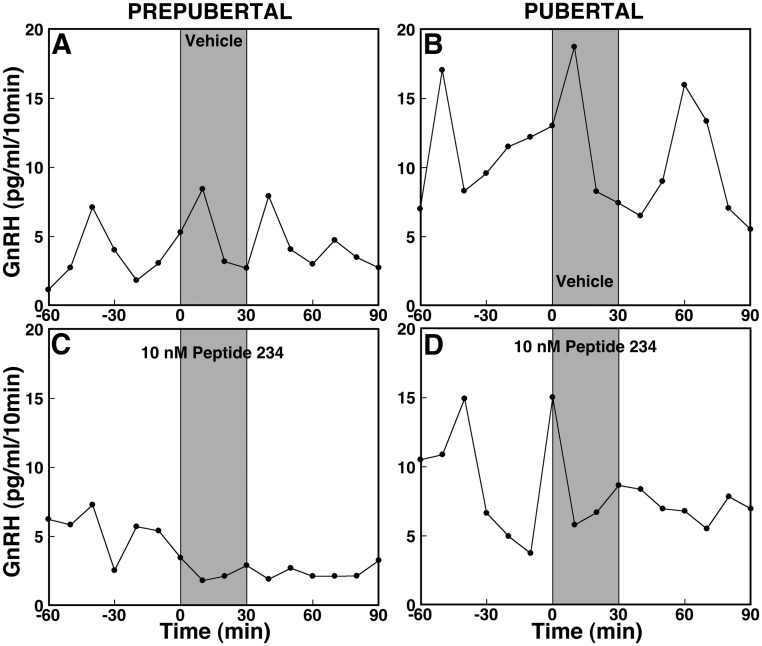
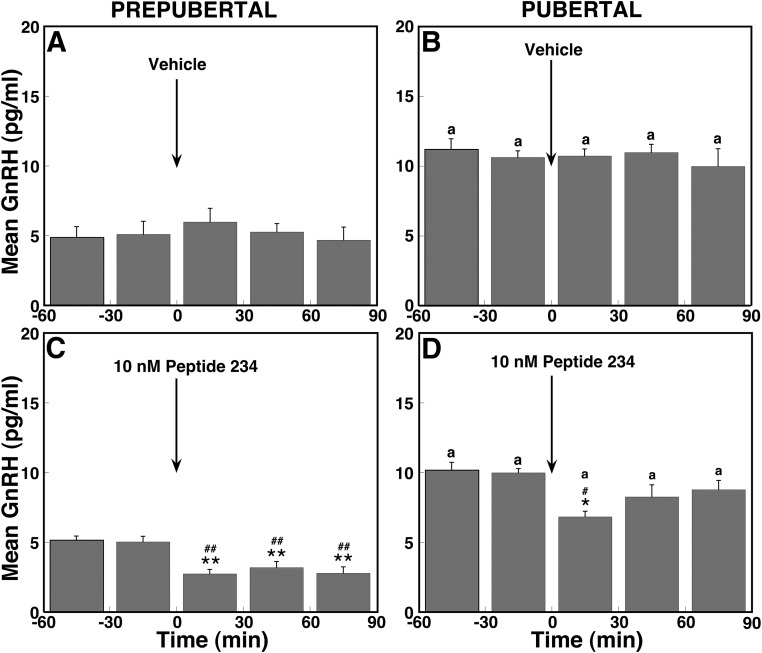
To assess the contribution of endogenous KP signaling to GnRH release across pubertal onset, we examined the effects of the novel KP antagonist, peptide 234 (10 nm), on GnRH release. Results indicate that peptide 234 suppressed GnRH release in prepubertal monkeys (Fig. 3C). The peptide 234-induced suppression of GnRH release (Fig. 4C) in prepubertal monkeys was significant (overall significance: P < 0.01) compared with vehicle infusion (Fig. 4A). Post hoc analysis indicated that peptide 234 significantly (P < 0.01) suppressed GnRH release during and up to 90 min after the start of the infusion compared with baseline GnRH levels as well as respective mean values for vehicle treatment (Fig. 4A).
Fig. 3.
Effects of peptide 234 on GnRH release in ovarian-intact prepubertal (left panels) and pubertal (right panels) female monkeys. After 60 min control sampling with microdialysis, vehicle (top panels) or 10 nm peptide 234 (bottom panels) was directly infused into the S-ME for 30 min as shown by shaded bars, and dialysates were continuously collected for an additional 90 min. Representative cases with each treatment are shown. Peptide 234 at 10 nm (C) clearly suppressed GnRH release in vivo in a prepubertal monkey, whereas vehicle (A) did not. Similarly, 10 nm peptide 234 (D) suppressed GnRH release in a pubertal monkey, whereas vehicle (B) failed to induce any changes. Note that baseline levels of GnRH release are higher in the pubertal monkeys than in the prepubertal monkeys.
Fig. 4.
Direct peptide 234 infusion suppresses mean GnRH release in vivo in ovarian-intact prepubertal and pubertal monkeys. Group data (mean ± sem) from prepubertal monkeys treated with vehicle (A, n = 5) or 10 nm peptide 234 (C, n = 7) and from pubertal monkeys treated with vehicle (B, n = 4) or 10 nm peptide 234 (D, n = 6) are shown. In prepubertal monkeys, peptide 234 at 10 nm significantly decreased GnRH release compared with baseline levels (P < 0.01), and the effect of peptide 234 was significantly lower than that in vehicle (P < 0.01). Similarly, in pubertal monkeys, 10 nm peptide 234 significantly suppressed GnRH release compared with baseline levels (P < 0.05), and the effect of peptide 234 was significantly lower than that in vehicle. *, P < 0.05; **, P < 0.01 vs. before peptide 234 infusion; #, P < 0.05; ##, P < 0.01 vs. vehicle infusion; a, P < 0.05 vs. corresponding time block in prepubertal monkeys.
In pubertal monkeys, peptide 234 similarly suppressed GnRH release (Figs. 3D and 4D), and this suppression was significant (overall significance: P < 0.05) compared with vehicle infusion (Fig. 4B). Interestingly, post hoc analysis indicated that suppression of GnRH release by peptide 234 was significant (P < 0.05) during, but not after, infusion compared with baseline GnRH levels and respective vehicle treatment means. Vehicle infusion had no effect on GnRH release in monkeys at either developmental stage (Figs. 3, A and B, and 4, A and B).
Effects of OVX on GnRH response to hKP-10 (experiment 3)
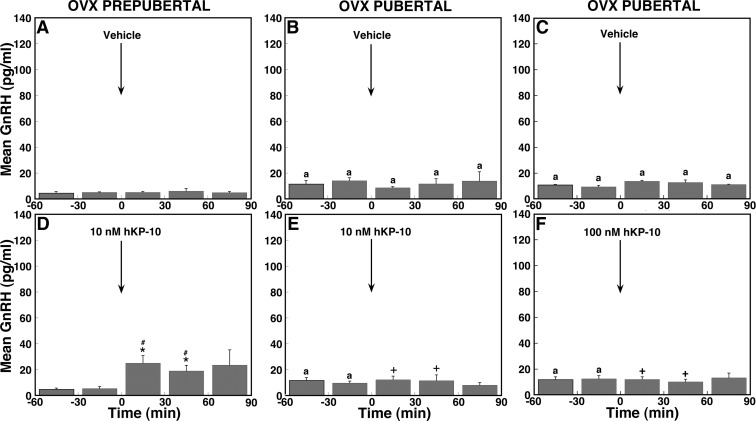
Because circulating levels of ovarian steroid hormones differ before and after pubertal onset, we next examined whether ovarian steroid feedback plays a role in the developmental difference in GnRH response to hKP-10. Infusion of 10 nm hKP-10 resulted in a small increase in GnRH release in OVX prepubertal monkeys (Fig. 5D), and this increase was significant (overall significance: P < 0.05) compared with vehicle infusion. Post hoc analysis indicated that hKP-10 significantly (P < 0.05) stimulated GnRH release (Fig. 6D) for up to 60 min after the start of infusion over GnRH baseline levels as well compared with corresponding vehicle treatment means (Fig. 6A). Comparison between OVX and ovarian-intact prepubertal monkeys indicated that there was no overall difference in the GnRH response to hKP-10 (Fig. 2C vs. 6D).
Fig. 5.
Effects of 10 nm hKP-10 (D and E), 100 nm hKP-10 (F), or vehicle (A–C) on GnRH release in vivo in prepubertal (left panels) and pubertal (middle and right panels) OVX female monkeys. Representative cases are shown. Infusion of 10 nm hKP-10 clearly stimulated GnRH release in an OVX prepubertal monkey (D), whereas infusion of either 10 (E) or 100 (F) nm hKP-10 failed to stimulate GnRH release in OVX pubertal monkeys. Vehicle infusion had no effect on GnRH release in monkeys at either developmental stage.
Fig. 6.
Effects of OVX on mean GnRH release in response to hKP-10 infusion in prepubertal and pubertal monkeys. Group data (mean ± sem) from prepubertal monkeys treated with vehicle (A, n = 4) or 10 nm hKP-10 (D, n = 7) or from pubertal monkeys treated with vehicle (B, n = 4; C, n = 3), 10 nm hKP-10 (E, n = 8), or 100 nm hKP-10 (F, n = 4) are shown. In prepubertal monkeys, hKP-10 at 10 nm significantly increased GnRH release compared with baseline levels (P < 0.05), and the effect of hKP-10 was significantly higher than that in vehicle (P < 0.05). In contrast, in pubertal monkeys, both 10 and 100 nm hKP-10 failed to induce any changes in GnRH release compared with baseline levels and vehicle infusion. Moreover, the GnRH responses to 10 or 100 nm hKP-10 were significantly different between OVX and ovarian-intact pubertal monkeys (P < 0.05 for both). *, P < 0.05 vs. before hKP-10 infusion; #, P < 0.05 vs. vehicle infusion; +, P < 0.05 vs. hKP-10 infusion in ovarian-intact pubertal monkeys; a, P < 0.05 vs. corresponding time block in prepubertal monkeys.
In contrast, both the 10 and 100 nm hKP-10 infusion in OVX pubertal monkeys failed to induce any increase in GnRH release (Figs. 5, E and F, and 6, E and F). In fact, there was no significant difference during or after either hKP-10 infusion (10 or 100 nm) compared with before infusion as well compared with vehicle infusion. Furthermore, no difference in the GnRH response was found between the low and high dose of hKP-10. Comparison between OVX and ovarian-intact pubertal monkeys in experiment 1 indicated that OVX clearly (overall significance: P < 0.05) abolished the 10 nm hKP-10-induced stimulation of GnRH release (Fig. 2D vs. 6E). Post hoc analysis further indicated that GnRH levels for up to 60 min after the start of the hKP-10 infusion were significantly different (P < 0.05) between OVX and ovarian-intact pubertal monkeys. Similarly, OVX (overall significance: P < 0.05) abolished the 100 nm hKP-10-induced stimulation of GnRH observed in ovarian-intact pubertal monkeys, and post hoc analysis indicated that GnRH levels were significantly different (P < 0.05) for up to 60 min after the start of the hKP-10 infusion between OVX and ovarian-intact pubertal monkeys (Fig. 2F vs. 6F). Vehicle infusion had no effect on GnRH release in either developmental stage (Figs. 5, A–C, and 6, A–C).
Effects of E2 replacement on GnRH response to hKP-10 (experiment 4)
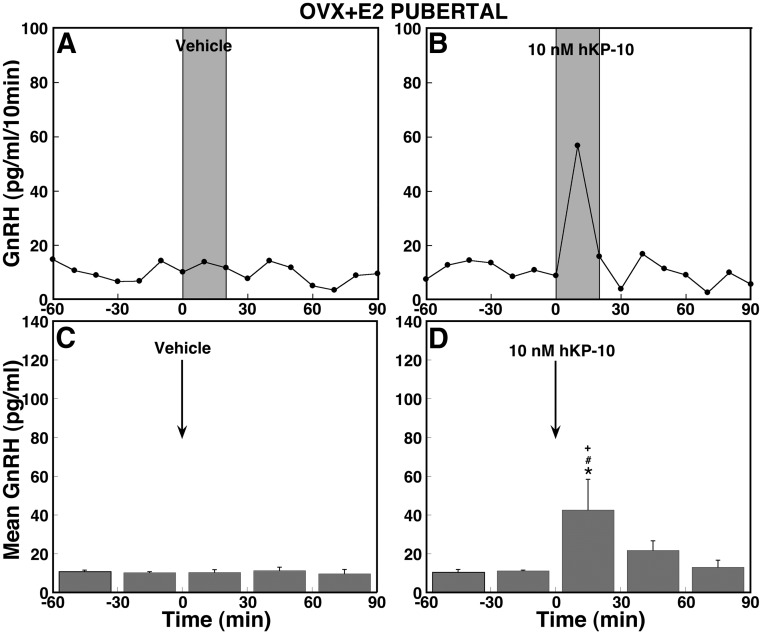
To determine whether replacement of E2 restores the absence of the hKP-10-induced GnRH release in OVX pubertal monkeys in experiment 3, OVX pubertal monkeys were implanted with a silastic capsule containing E2 benzoate (OVX+E2). Infusion of hKP-10, but not vehicle, induced an increase in GnRH release (Fig. 7, A and B) in OVX+E2 pubertal monkeys. The hKP-10-induced GnRH increase was significant (overall significance: P < 0.05) over GnRH baseline levels (Fig. 7D). Post hoc analysis indicated that the effects of hKP-10 were significant during (P < 0.05), but not after, infusion (Fig. 7D). Comparison between OVX+E2 and OVX monkeys indicated that hKP-10 significantly (overall significance: P < 0.05) stimulated GnRH release in OVX+E2, but not OVX, pubertal monkeys. Post hoc analysis indicated that only GnRH levels during, but not after, the hKP-10 infusion in OVX+E2 pubertal monkeys were significantly (P < 0.05) greater than those in OVX pubertal monkeys. Further comparison between OVX+E2 and intact monkeys indicated that the GnRH response to hKP-10 was similar (no overall significant difference) during the infusion (Fig. 7D vs. 2D). Interestingly, the duration of the GnRH stimulation in OVX+E2 monkeys was shorter than in ovarian-intact pubertal monkeys.
Fig. 7.
Replacement with E2 only partially restores the hKP-10-induced stimulation in the OVX pubertal monkey. Representative cases and group data (mean ± SEM) with vehicle infusion (A and C, n = 4) and 10 nm hKP-10 (B and D, n = 4) are shown. During the infusion, hKP-10 at 10 nm significantly stimulated mean GnRH release over baseline levels (P < 0.05), whereas vehicle infusion had no effect on GnRH release. Comparison between OVX+E2 and OVX pubertal monkeys shows that the GnRH response to hKP-10 is significantly different during the infusion (P < 0.05). *, P < 0.05 vs. before hKP-10 infusion; #, P < 0.05 vs. vehicle infusion; +, P < 0.05 vs. OVX pubertal monkeys.
Circulating estradiol levels in females in this study
Measurement of circulating E2 levels in monkeys from all experiments, shown in Table 1, indicated that E2 levels in ovarian-intact prepubertal monkeys were low and similar to their OVX counterparts. Although E2 levels in ovarian-intact pubertal monkeys were significantly (P < 0.001) higher than those in ovarian-intact prepubertal monkeys, OVX eliminated the elevated E2 in pubertal monkeys, resulting in levels as low as those seen in OVX prepubertal monkeys (P < 0.001). Finally, implantation of the E2 capsule significantly (P < 0.001) increased E2 levels in OVX pubertal monkeys, although these restored E2 levels were slightly higher than those in ovarian-intact pubertal monkeys (P < 0.05).
Table 1.
Circulating E2 levels in ovarian-intact (INT) and OVX animals with or without capsules containing E2 in this study
| Developmental stage | Gonadal status | n | E2 (pg/ml) |
|---|---|---|---|
| Prepubertal | INT | 9 | 42.8 ± 3.1 |
| Pubertal | INT | 8 | 82.6 ± 8.7a |
| Prepubertal | OVX | 7 | 38.6 ± 1.4 |
| Pubertal | OVX | 8 | 38.1 ± 2.7b |
| Pubertal | OVX+E2 | 3 | 113.6 ± 7.2c |
Values are the mean ± sem.
P < 0.001 vs. prepubertal intact.
P < 0.001 vs. pubertal intact.
P < 0.001 vs. pubertal OVX.
Discussion
In the present study, we found that 1) GnRH release was stimulated by hKP-10 in both prepubertal and pubertal monkeys in a dose-responsive manner; 2) release of GnRH in both prepubertal and pubertal monkeys was suppressed by peptide 234; 3) OVX eliminated the hKP-10-induced GnRH release in pubertal, but not prepubertal, monkeys; and 4) replacement of E2 in OVX pubertal monkeys only partially restored the hKP-10-induced GnRH release that was absent in OVX pubertal monkeys. These results indicate that there are developmental changes in the GnRH responsiveness through a KISS1R-mediated mechanism.
Nearly a decade ago, the role of KP and its receptor, KISS1R, in the mechanism of puberty was proposed (5, 6, 21). Since then, new avenues of KP-KISS1R signaling to GnRH neurons have been revealed. GnRH neurons express KISS1R (22), KP mRNA expression in the MBH increases at puberty (9, 12, 14, 23), and administration of KP-10 advances puberty onset in rodents (23, 24), whereas administration of peptide 234 delays the onset of puberty in rodents (25). Moreover, we have previously shown parallel pubertal increases of KP-54 release and GnRH release (7). These findings, together with the observations from the present study that hKP-10 stimulates GnRH release in a dose-responsive manner in both prepubertal and pubertal monkeys, allow us to conclude that the pubertal increase in GnRH release is, at least in part, a consequence of the pubertal increase in KP release.
The pubertal increase in GnRH release could also be a consequence of developmental changes in KISS1R. The results of the present study show that hKP-10 induced dose-dependent responses in both age groups. The absolute magnitude of the GnRH responses to hKP-10 at the 10 nm dose in pubertal monkeys was significantly larger than in prepubertal monkeys, whereas GnRH responses to hKP-10 at the 100 nm dose in prepubertal monkeys were similar to that in pubertal monkeys. Perhaps, with the high (100 nm) hKP-10 dose, GnRH neurons reach their limit of secretory capacity, because it has been reported that stimulation of LH release by KP-10 reaches a plateau with higher KP doses in humans (26) and in sheep (27). Although we did not assess the effect of hKP-10 at doses lower than 10 nm in this study, our results with the 10 nm dose are consistent with those described by Han et al. (14) showing that the LH response to intracerebroventricular infusion of lower (10 fmol and 0.1 pmol), not higher (0.1 nmol), KP-10 doses in male mice increases across puberty. Therefore, functional changes in KISS1R occur at the time of puberty. This view is further supported by reports that 1) there is a pubertal increase in KISS1R mRNA expression in the MBH in ovarian-intact female monkeys (12) and 2) KISS1R mRNA expression in the anteroventricular periventricular nucleus increases shortly before puberty in ovarian-intact female rats (28).
One can argue that there is no difference in the relative GnRH response to hKP-10 between prepubertal and pubertal monkeys in this study, because the baseline GnRH levels before the hKP-10 challenge differ. Initially, we had a similar viewpoint. However, the absence of a GnRH response to hKP-10 in OVX pubertal monkeys and the difference in circulating E2 levels between two developmental stages cannot be ignored. Additionally, the relationship between baseline GnRH levels vs. the GnRH response to KP-10 is currently unknown. Thus, the absolute GnRH response to hKP-10 should reflect developmental changes in KISS1R signaling in the hypothalamus. Hypothetically, studies of the response to hKP-10 in single primate GnRH neurons (if the procedure becomes available) may be helpful to tease out the two possible interpretations.
In contrast to changes with the KP agonist, peptide 234, the KP antagonist, suppressed GnRH release in both prepubertal and pubertal monkeys. However, the magnitude of the suppression (maximal suppression) by peptide 234 at the dose examined did not differ between the two age groups. Interestingly, the suppression of GnRH release by peptide 234 continued after peptide 234 infusion in prepubertal, but not pubertal, monkeys. This can be interpreted to mean that because there is more endogenous KP available in pubertal monkeys (7, 29) for competitive binding to KISS1R against peptide 234 than in prepubertal monkeys, peptide 234 is not able to continue suppressing GnRH release after infusion in pubertal monkeys. We cannot, however, exclude the possibility that there are pubertal changes in the pharmacokinetics of peptide 234 binding to KISS1R, because both age and steroid milieu can influence receptor pharmacokinetics (30, 31). Although in the present study we were not able to examine a higher concentration of peptide 234, because it occluded the microdialysis membrane, additional experiments with peptide 234 at lower doses and different steroid milieu may clarify this possibility.
In the present study, we found that in ovarian-intact female monkeys, the low dose of hKP-10 induced larger GnRH responses in pubertal monkeys compared with prepubertal monkeys. This difference is, in part, attributable to an elevated circulating E2 concentration at puberty (15), because OVX in pubertal monkeys eliminated the GnRH response to both low and high doses of hKP-10, and results of our preliminary studies show that there is no GnRH response to peptide 234 in OVX pubertal monkeys (Guerriero, K. A., and E. Terasawa, unpublished findings). Moreover, the finding in prepubertal monkeys that hKP-10 with the low dose induced a small, but significant, increase in GnRH release, regardless of the presence or absence of the ovary indicates that feedback action of E2 on GnRH release is not operative in prepubertal monkeys. This view is consistent with our previous findings that OVX increases pulsatile GnRH release in pubertal, but not prepubertal, monkeys (3) and that sc injection of E2 suppresses GnRH release in OVX pubertal monkeys, but E2 is ineffective in OVX prepubertal monkeys (4).
In this study, baseline GnRH levels in ovarian intact pubertal monkeys were not different from those in OVX counterparts. These data differ from those reported in the previous study (3). We believe that the higher variability of GnRH baseline levels in this study is due to the differences in the methods from the previous study (3). That study used a push-pull perfusion method, by which 200-μl samples were collected exclusively from the S-ME where GnRH neuroterminals are concentrated, whereas the current study used a microdialysis method, by which 20-μl samples were collected not only from the S-ME but also MBH. Therefore, the amount of GnRH in samples with microdialysis is likely to yield higher variability among individuals.
In contrast to our present findings, absence of the ovary does not eliminate the LH response to peptide 234 in sheep and rodents (8), nor does the LH response to KP-10 in rats (32). The difference in the results of the present study and those in sheep and rodents could be due to differences in species, sex, the age of gonadectomy, and/or developmental stages. Because in this study we examined the effects of hKP-10 only in OVX pubertal, but not adult, monkeys, it is unknown whether a significant change in a KISS1R-mediated mechanism observed in pubertal monkeys continues into adulthood or whether it is specific to puberty. Nonetheless, the loss of the GnRH response in OVX pubertal, but not OVX prepubertal, monkeys suggests that the GnRH responsiveness through KISS1R in primates appears to undergo a significant change after exposure to the pubertal rise in circulating E2, such that this KISS1R-mediated mechanism becomes dependent upon ovarian steroids after puberty onset. The underlying mechanism of developmental changes in responsiveness of KISS1R in the absence of the ovary remains to be elucidated.
Synaptic input onto GnRH neurons increases along with puberty. The contour of GnRH neurons increases across puberty (33), presumably due to increased synaptic innervation. In fact, an increase in the number of synapses onto GnRH neurons has been reported (34). Because KP stimulates GnRH neuronal activity directly or transsynaptically through interneurons and E2 further enhances KP-induced GnRH neuronal activity through transsynaptic networks in mice (35), it is likely that synaptic plasticity induced by E2 after puberty onset greatly amplifies KP action to GnRH neurons. Once this feed-forward mechanism is initiated at puberty, increased E2 in circulation further accelerates KP-induced GnRH release during pubertal progression.
Unexpectedly, replacement of E2 in OVX pubertal monkeys only partly restored the hKP-10-induced GnRH response. The reason for this partial recovery remains speculative. First, this is not due to the amount of circulating E2. Although E2 capsule implantation in OVX pubertal females results in E2 levels slightly higher than those in their ovarian-intact counterparts, this level of E2 is within the normal range for ovarian-intact midpubertal monkeys (15). Second, other factors from the ovary besides E2 may be involved. For example, a maximal LH response to KP-10, as observed in an estrous rat, requires replacement of both E2 and progesterone in adult OVX rats (32). However, this is an unlikely explanation, because progesterone release from the ovary remains low until first ovulation (15), and the prepubertal and pubertal monkeys examined in this study are not yet capable of ovulating, as previously characterized (15). Third, it is possible that properties of estrogen receptors are altered, because we did not replace E2 immediately after OVX. Expression of estrogen receptor-α differs depending on the time period between OVX and E2 replacement (36). Fourth, longer exposure to E2 may be necessary in pubertal monkeys. Although physiological levels of E2 (15) are achieved with similar capsule implantation (37), it is possible that short-term (∼2 wk) E2 replacement is not sufficient to fully revert the GnRH responsiveness through KISS1R back to the ovarian-intact state. In adult female OVX rabbits, a short-term E2 replacement with a physiologically equivalent E2 level, only partially restored the GnRH-induced LH release, and implantation of two times higher the level of E2 fully restored the effect of OVX on LH release (38). Possible effects of a longer exposure to E2 remain to be investigated.
In the present study, we have shown that the pubertal increase in GnRH release appears to be attributable to an increase in a KISS1R-mediated mechanism on GnRH neurons. To our knowledge, this is the first study examining developmental changes in KISS1R function by direct measurement of GnRH release. Our finding adds to a previous observation (7) indicating that an increase in KP-54 release occurs with the pubertal increase in GnRH release. Importantly, the GnRH responsiveness through KISS1R appears to undergo developmental changes, switching from an ovarian steroid-independent to -dependent state. This can be interpreted to mean that in primates, an important neurocircuitry change is required before puberty onset, and this change occurs before KP-KISS1R signaling becomes a prominent factor in the control of GnRH release. Previously, we and others have shown (39–41) that in primates the enervation of predominant central inhibition is a prerequisite for the onset of puberty. Perhaps the inability of E2 to modify stimulatory KP action on GnRH release before puberty is due to this central inhibition. After puberty onset, reduction in this central inhibition allows KP action on GnRH neurons, enhanced by E2, to be fully functional (42). The relationship between the pubertal increase in KP release and diminution of central inhibition, however, remains to be further investigated.
Acknowledgments
We express our sincere appreciation to Mr. Frederick Wegner for his great assistance for GnRH RIA and to Mr. Nicholas Shiel for his technical assistance. This study was not possible without assistance from the veterinarians and veterinary technicians in the Wisconsin National Primate Research Center.
This work was supported by National Institutes of Health (NIH) Grants R01HD11355 and R01HD15433 (to E.T.) and T32HD041921 (to K.A.G.) and was made possible to perform by NIH support (P51RR000167, RR15459, and RR020141) to the Wisconsin National Primate Research Center.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CNS
- Central nervous system
- E2
- 17β-estradiol
- hKP-10
- human KP-10
- KISS1R
- KP-1 receptor
- KP
- kisspeptin
- MBH
- medial basal hypothalamus
- OVX
- ovariectomy
- S-ME
- stalk-median eminence.
References
- 1. Watanabe G, Terasawa E. 1989. In vivo release of luteinizing hormone releasing hormone (LHRH-1) increases with puberty in the female rhesus monkey. Endocrinology 125:92–99 [DOI] [PubMed] [Google Scholar]
- 2. Wildt L, Häusler A, Marshall G, Hutchison JS, Plant TM, Belchetz PE, Knobil E. 1981. Frequency and amplitude of gonadotropin-releasing hormone stimulation and gonadotropin secretion in the rhesus monkey. Endocrinology 109:376–385 [DOI] [PubMed] [Google Scholar]
- 3. Chongthammakun S, Claypool LE, Terasawa E. 1993. Ovariectomy increases in vivo luteinizing hormone-releasing hormone release in pubertal, but not prepubertal female rhesus monkeys. J Neuroendocrinol 5:41–50 [DOI] [PubMed] [Google Scholar]
- 4. Chongthammakun S, Terasawa E. 1993. Negative feedback effects of estrogen on luteinizing hormone-releasing hormone release occur in pubertal, but not prepubertal, ovariectomized female rhesus monkeys. Endocrinology 132:735–743 [DOI] [PubMed] [Google Scholar]
- 5. Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. 2003. The GPR43 gene as a regulator of puberty. N Engl J Med 349:1614–1627 [DOI] [PubMed] [Google Scholar]
- 6. de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. 2003. Hypogonadotropic hypogonadism due to loss of function of the kiss1-derived peptide receptor GPR54. Proc Natl Acad Sci USA 100:10972–10976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Keen KL, Wegner FH, Bloom SR, Ghatei MA, Terasawa E. 2008. An increase in kisspeptin-54 release occurs with the pubertal increase in luteinizing hormone-releasing hormone-1 release in the stalk-median eminence of female rhesus monkeys in vivo. Endocrinology 149:4151–4157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roseweir AK, Kauffman AS, Smith JT, Guerriero KA, Morgan K, Pielecka-Fortuna J, Pineda R, Gottsch ML, Tena-Sempere M, Moenter SM, Terasawa E, Clarke IJ, Steiner RA, Millar RP. 2009. Discovery of potent kisspeptin antagonists delineate physiological mechanisms of gonadotropin regulation. J Neurosci 29:3920–3929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clarkson J, Herbison AE. 2006. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology 147:5817–5825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Clarkson J, Boon WC, Simpson ER, Herbison AE. 2009. Postnatal development of an estradiol-kisspeptin positive feedback mechanism implicated in puberty onset. Endocrinology 150:3214–3220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kauffman AS, Navarro VM, Kim J, Clifton DK, Steiner RA. 2009. Sex differences in the regulation of Kiss1/NKB neurons in juvenile mice: implications for the timing of puberty. Am J Physiol Endocrinol Metab 297:E1212–E1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. 2005. Increased hypothalamic GPR54 signaling: A potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci USA 102:2129–2134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Herbison AE, de Tassigny X, Doran J, Colledge WH. 2010. Distribution and postnatal development of Gpr54 gene expression in mouse brain and gonadotropin-releasing hormone neurons. Endocrinology 151:312–321 [DOI] [PubMed] [Google Scholar]
- 14. Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. 2005. Activation of gonadotropin-releasing hormone (GnRH) neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci 25:11349–11356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Terasawa E, Nass TE, Yeoman RR, Loose MD, Schultz NJ. 1983. Hypothalamic control of puberty in female rhesus macaque. In: Norman RL, ed. Neuroendocrine aspects of reproduction. New York: Academic Press; 149–182 [Google Scholar]
- 16. Terasawa E, Yeoman RR, Schultz NJ. 1984. Factors influencing the progesterone-induced luteinizing hormone surge in rhesus monkeys: diurnal influence and time of interval after estrogen. Biol Reprod 31:732–741 [DOI] [PubMed] [Google Scholar]
- 17. Terasawa E. 1994. In vivo measurement of pulsatile release of neuropeptides and neurotransmitters in rhesus monkeys using push-pull perifusion. In: Conn PM, Levine JE, eds. Methods in neurosciences. San Diego: Academic Press; 184–202 [Google Scholar]
- 18. Frost SI, Keen KL, Levine JE, Terasawa E. 2008. Microdialysis methods for in vivo neuropeptide measurement in the stalk-median eminence in the rhesus monkey. J Neurosci Methods 168:26–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gearing M, Terasawa E. 1988. Luteinizing hormone releasing hormone (LHRH) neuroterminals mapped using the push-pull perfusion method in the rhesus monkey. Brain Res Bull 21:117–121 [DOI] [PubMed] [Google Scholar]
- 20. Keen KL, Burich AJ, Mitsushima D, Kasuya E, Terasawa E. 1999. Effects of pulsatile infusion of the GABAA receptor blocker bicuculline on the onset of puberty in female rhesus monkeys. Endocrinology 140:5257–5266 [DOI] [PubMed] [Google Scholar]
- 21. Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden JM, Le Poul E, Brézillon S, Tyldesley R, Suarez-Huerta N, Vandeput F, Blanpain C, Schiffmann SN, Vassart G, Parmentier M. 2001. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem 276:34631–34636 [DOI] [PubMed] [Google Scholar]
- 22. Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, Caraty A, Aparicio SA. 2005. Kisspeptin directly stimulates hormone via G protein-coupled receptor 54. Proc Natl Acad Sci USA 102:1761–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Navarro VM, Castellano JM, Fernández-Fernández R, Barreiro ML, Roa J, Sanchez-Criado JE, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. 2004. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology 145:4565–4574 [DOI] [PubMed] [Google Scholar]
- 24. Matsui H, Takatsu Y, Kumano S, Matsumoto H, Ohtaki T. 2004. Peripheral administration of metastin induces marked gonadotropin release and ovulation in the rat. Biochem Biophys Res Commun 320:383–388 [DOI] [PubMed] [Google Scholar]
- 25. Pineda R, Garcia-Galiano D, Roseweir A, Romero M, Sanchez-Garrido MA, Ruiz-Pino F, Morgan K, Pinilla L, Millar RP, Tena-Sempere M. 2010. Critical roles of kisspeptins in female puberty and preovulatory gonadotropin surges as revealed by a novel antagonist. Endocrinology 151:722–730 [DOI] [PubMed] [Google Scholar]
- 26. Dhillo WS, Chaudhri OB, Patterson M, Thompson EL, Murphy KG, Badman MK, McGowan BM, Amber V, Patel S, Ghatei MA, Bloom SR. 2005. Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. J Clin Endocrinol Metab 90:6609–6615 [DOI] [PubMed] [Google Scholar]
- 27. Caraty A, Smith JT, Lomet D, Ben Saïd S, Morrissey A, Cognie J, Doughton B, Baril G, Briant C, Clarke IJ. 2007. Kisspeptin synchronizes preovulatory surges in cyclical ewes and causes ovulation in seasonally acyclic ewes. Endocrinology 148:5258–5267 [DOI] [PubMed] [Google Scholar]
- 28. Takase K, Uenoyama Y, Inoue N, Matsui H, Yamada S, Shimizu M, Homma T, Tomikawa J, Kanda S, Matsumoto H, Oka Y, Tsukamura H, Maeda KI. 2009. Possible role of oestrogen in pubertal increase of kiss1/kisspeptin expression in discrete hypothalamic areas of female rats. J Neuroendocrinol 21:527–537 [DOI] [PubMed] [Google Scholar]
- 29. Guerriero KA, Keen KL, Terasawa E, Striking developmental changes in kisspeptin-Gpr54 signaling to GnRH neurons in female monkeys: modification by estrogen feedback. Program of the 7th International Congress of Neuroendocrinology, Rouen, France, 2010 (Abstract P1-36) [Google Scholar]
- 30. Febo M, González-Rodríguez LA, Capó-Ramos DE, González-Segarra NY, Segarra AC. 2003. Estrogen-dependent alterations in D2/D3-induced G protein activation in cocaine-sensitized female rats. J Neurochem 86:405–412 [DOI] [PubMed] [Google Scholar]
- 31. Mahan LC, McKernan RM, Insel PA. 1987. Metabolism of α- and β-adrenergic receptors in vitro and in vivo. Annu Rev Pharmacol Toxicol 27:215–235 [DOI] [PubMed] [Google Scholar]
- 32. Roa J, Vigo E, Castellano JM, Navarro VM, Fernández-Fernández R, Casanueva FF, Dieguez C, Aguilar E, Pinilla L, Tena-Sempere M. 2006. Hypothalamic expression of KiSS-1 system and gonadotropin-releasing effects of kisspeptin in different reproductive states of the female rat. Endocrinology 147:2864–2878 [DOI] [PubMed] [Google Scholar]
- 33. Wray S, Hoffman G. 1986. Postnatal morphological changes in rat LHRH neurons correlated with sexual maturation. Neuroendocrinology 43:93–97 [DOI] [PubMed] [Google Scholar]
- 34. Cottrell EC, Campbell RE, Han SK, Herbison AE. 2006. Postnatal remodeling of dendritic structure and spine density in gonadotropin-releasing hormone neurons. Endocrinology 147:3652–3661 [DOI] [PubMed] [Google Scholar]
- 35. Pielecka-Fortuna J, Chu Z, Moenter SM. 2008. Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology 149:1979–1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cardoso CC, Ricardo VP, Frussa-Filho R, Porto CS, Abdalla FM. 2010. Effects of 17β-estradiol on expression of muscarinic acetylcholine receptor subtypes and estrogen receptor α in rat hippocampus. Eur J Pharmacol 634:192–200 [DOI] [PubMed] [Google Scholar]
- 37. Mizuno M, Terasawa E. 2005. Search for neural substrates mediating inhibitory effects of oestrogen on pulsatile luteinizing hormone-releasing hormone release in vivo in ovariectomized female Rhesus monkeys (Macaca mulatta). J Neuroendocrinol 17:238–245 [DOI] [PubMed] [Google Scholar]
- 38. Pau KY, Orstead KM, Hess DL, Spies HG. 1986. Feedback effects of ovarian steroids on the hypothalamic-hypophyseal axis in the rabbit. Biol Reprod 35:1009–1023 [DOI] [PubMed] [Google Scholar]
- 39. Grumbach MM. 2002. The neuroendocrinology of human puberty revisited. Horm Res 57:2–14 [DOI] [PubMed] [Google Scholar]
- 40. Terasawa E, Fernandez DL. 2001. Neurobiological mechanisms of the onset of puberty in primates. Endocr Rev 22:111–151 [DOI] [PubMed] [Google Scholar]
- 41. Plant TM, Witchel SF. 2006. Puberty in nonhuman primates and humans. In: Neill JD, ed. Physiology of Reproduction. Boston: Elsevier; 2177–2230 [Google Scholar]
- 42. Terasawa E, Kurian JR, Guerriero KA, Kenealy BP, Hutz ED, Keen KL. 2010. Recent discoveries on the control of gonadotropin-releasing hormone neurons in nonhuman primates. J Neuroendocrinol 22:630–638 [DOI] [PMC free article] [PubMed] [Google Scholar]