Abstract
Progesterone (P4) and estradiol (E2) modulate neurogenesis and synaptic remodeling in the hippocampus during the rat estrous cycle and in response to deafferenting lesions, but little is known about the steroidal regulation of hippocampal progesterone receptors associated with these processes. We examined the neuronal expression of progesterone receptor membrane component-1 (Pgrmc1) and the classical progesterone receptor (Pgr), by in situ hybridization and immunohistochemistry. Pgr, a transcription factor, has been associated with synaptic remodeling and other major actions of P4, whereas Pgrmc1 is implicated in P4-dependent proliferation of adult neuroprogenitor cells and with rapid P4 effects on membranes. Ovariectomized adult rats were given E2, P4, or E2+P4 on two schedules: a 4-d model of the rodent estrous cycle and a 30-d model of postmenopausal hormone therapy. Pgr was hormonally responsive only in CA1 pyramidal neurons, and the induction of Pgr by E2 was partly antagonized by P4 only on the 30-d schedule. In CA3 pyramidal and dentate gyrus (DG) neurons, Pgr was largely unresponsive to all hormone treatments. In contrast to Pgr, Pgrmc1 was generally induced by E2 and/or P4 throughout the hippocampus in CA1, CA3, and DG neurons. In neuroprogenitor cells of the DG (immunopositive for bromodeoxyuridine and doublecortin), both Pgrmc1 and Pgr were detected. The differential regulation of hippocampal Pgrmc1 and Pgr by E2 and P4 may guide drug development in hormonal therapy for support of neurogenesis and synaptic regeneration.
Interactions of estradiol (E2) and progesterone (P4) drive reproductive organ remodeling during ovulatory cycles (1, 2). In anticipation of implantation, uterine cell growth is stimulated by blood elevations of E2 during the follicular phase. During the luteal phase in the absence of implantation, endometrial cell death (apoptosis) is promoted by the cyclic elevation and decrease of P4 (1, 2). Moreover, during rodent ovulatory cycles, there is cyclic synaptic remodeling in the hippocampus (3, 4), a brain region critical for memory. In the follicular phase, hippocampal CA1 pyramidal neurons grow additional dendritic spines and synapses, which then regress rapidly after ovulation when E2 falls and P4 rises (3–5). These ovarian-driven processes have been further resolved in ovariectomized (OVX) rats as independent actions of E2 and P4: after induction by E2 treatment, the decline of CA1 spines is dependent on the presence of elevated plasma P4 (4).
In these examples, E2 and P4 appear to act independently at different phases of cyclical remodeling processes. However, in several animal models, cotreatment with P4 attenuated E2-induced synaptic growth. In the OVX macaque, the induction by E2 of the synaptic proteins syntaxin, synaptophysin, and spinophilin in CA1 neurons was attenuated by coadministration of P4 (6). Similarly, in the rat entorhinal cortex lesion model of Alzheimer disease, we showed that cotreatment with P4 attenuated E2-induced neurite outgrowth in the dentate gyrus (DG) (7). In contrast to many examples of P4-E2 cross talk throughout the reproductive system, the proliferation of neural progenitor cells (NPC) derived from adult rat DG was induced by both E2 and P4, in vitro (8) and in vivo (9). Because combined concurrent E2+P4 is commonly used for menopausal hormone therapy (HT) (10–12), we sought to clarify E2-P4 interactions in neuronal expression of Pgr, a transcription factor, and in progesterone receptor membrane component-1(Pgrmc1), a putative progesterone receptor (13, 14). Both Pgr and Pgrmc1 have high-affinity P4 binding: Pgr, dissociation constant (Kd) = 0.38 nm (15); Pgrmc1, Kd = 11 nm (16).
Neuronal responses to P4 have been associated with both Pgrmc1 and Pgr. The decline of hippocampal CA1 spines by P4 in OVX rats described above was blocked by RU486, a specific antagonist of Pgr (4). We also observed antagonism of neurite outgrowth by RU486 in an in vitro model (7). However, Pgrmc1 mediated in vitro proliferation of rat NPC, in which Pgr was not detected (8). Based on these findings, we hypothesized that Pgrmc1 will be more responsive than Pgr to ovarian steroids in DG neurons, whereas Pgr regulation will be more responsive in CA1 neurons than CA3 and DG neurons.
Pgrmc1 has been associated with diverse functions across the reproductive system that are less understood relative to Pgr. In rat ovarian granulosa cells, which lack Pgr, Pgrmc1 mediates the antiapoptotic effects of P4 (17). Rapid membrane effects of P4 are also mediated by Pgrmc1, independently of Pgr, e.g. in the rapid P4-induced Ca2+ influx of the acrosome reaction (18, 19). Pgrmc1 sequences are associated with a remarkable variety of cell functions under yet other names (13, 20, 21). During development, Pgrmc1 mediates neuronal guidance under the names: Vema (mouse) and VEM-1 (nematode) (22). We verified that Vema and Pgrmc1 in GenBank share amino acid sequences. In adult rodents, Pgrmc1 was detected in the hippocampus, hypothalamus, and cerebellum (23, 24). Both E2 and P4 induced Pgrmc1 in the sexually dimorphic nucleus of the preoptic area and the ventromedial nucleus of the hypothalamus (25). However, these reports did not describe its cell level expression.
We extend to the cellular level prior findings of steroid regulation of Pgrmc1 and Pgr. In whole hippocampal extracts and hypothalamic subregions, Pgr was induced by E2 (25, 26). Whereas some studies have shown P4 antagonism of E2 induction of Pgr, the P4 antagonism may be only transient (27). Moreover, in hypothalamus and posterior pituitary from chick embryos, P4 can induce Pgr (28). Thus, P4 regulation of Pgr is physiologically complex and may vary widely between cell types. Less is known about Pgrmc1, which showed induction by both E2 and P4 in hypothalamic subregions (sexually dimorphic nucleus of the preoptic area and ventromedial nucleus) (25). The hippocampal regulation of Pgrmc1and Pgr by E2 and P4 is undefined.
Two hormone treatment schedules were used: a 4-d model of rodent ovulatory cycles (4, 26, 29) and a 30-d model of the KEEPS trial of postmenopausal HT (10, 30). We show differential regulation of Pgrmc1 and Pgr in hippocampal neurons by E2 and P4 and discuss the potential relevance to optimization of postmenopausal HT for maintaining cognitive functions (31–33).
Materials and Methods
Animals and steroid replacement
Experiments conformed with standards of humane animal care in the National Institutes of Health Ethical Guidelines. Adult female Sprague Dawley rats (3 months old, 250–300 g; nulliparous; 44 rats total − six rats per group for the 4-d replacement schedule and five rats per group for 30-d schedule) were used throughout. All animal procedures were performed under anesthesia with ketamine (80 mg/kg) plus xylazine (10 mg/kg). Experiments for the two hormone replacement schedules were run separately with different cohorts of animals. Nonetheless, the in situ hybridization (ISH) grain densities for both receptors in control OVX tissues were very similar in each experiment (Table 1).
Table 1.
Pgrmc1 and Pgr ISH grain densities in hippocampal neurons on 4-d and 30-d hormone schedules
| OVX | E2 | P4 | E2+P4 | |
|---|---|---|---|---|
| Pgrmc1 | ||||
| CA1 | ||||
| 4–d | 8.7 ± 0.6 | 12.5 ± 1.6a | 14.9 ± 1.8a | 13.3 ± 1.3a |
| 30–d | 10.7 ± 1.4 | 13.8 ± 0.7a | 16.6 ± 0.6a | 14.4 ± 0.4a |
| CA3 | ||||
| 4–d | 9.7 ± 0.6 | 13.5 ± 1.1a | 15.5 ± 1.3a | 15.9 ± 1.7a |
| 30–d | 10.1 ± 1.0 | 13.1 ± 1.1a | 13.4 ± 0.5a | 13.4 ± 0.5a |
| DG | ||||
| 4–d | 5.1 ± 0.3 | 7.7 ± 0.9a | 9.3 ± 0.8a | 8.7 ± 0.8a |
| 30–d | 5.1 ± 0.5 | 6.6 ± 0.6a | 7.4 ± 0.6a | 7.1 ± 0.1a |
| Pgr | ||||
| CA1 | ||||
| 4–d | 9.2 ± 1.0 | 14.4 ± 1.9a | 17.6 ± 0.7a | 16.5 ± 1.1a |
| 30–d | 11.6 ± 1.4 | 16.2 ± 1.3a | 15.7 ± 1.3a | 13.2 ± 1.0 |
| CA3 | ||||
| 4–d | 23.2 ± 2.0 | 25.8 ± 2.1 | 28.3 ± 0.7a | 28.9 ± 1.8a |
| 30–d | 23.9 ± 0.9 | 27.0 ± 2.5 | 27.3 ± 0.8 | 26.3 ± 1.1 |
| DG | ||||
| 4–d | 4.1 ± 0.1 | 4.2 ± 0.2 | 4.4 ± 0.2 | 4.6 ± 0.2 |
| 30–d | 3.0 ± 0.4 | 3.1 ± 0.4 | 3.5 ± 0.2 | 3.2 ± 0.4 |
Pgrmc1 and Pgr grain densities after hormone treatment. Emulsion-dipped slides for Pgrmc1 and Pgr were exposed for different times to achieve similar grain densities. Statistical comparisons were made within treatment groups for each receptor. Grain densities are means ± sem of six rats per hormone group for 4-d and five rats per group for 30-d schedules; 100 cells per brain in CA1 and CA3, and 120 cells per brain in DG. Pgrmc1:a, P < 0.03 vs. respective OVX group. Pgr: a, P < 0.05, vs. respective OVX group.
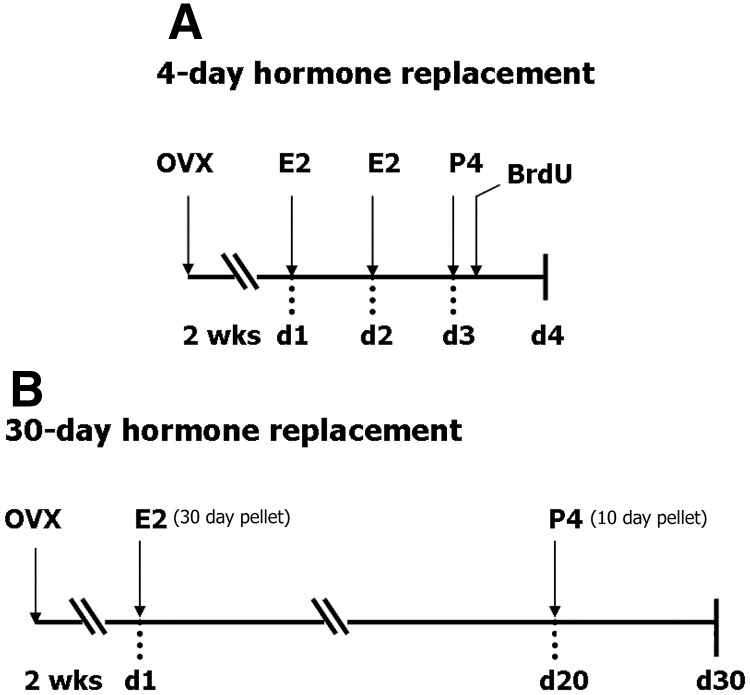
Four-day replacement (Fig. 1A).
Fig. 1.
Two hormone replacement schedules were studied. A, For 4-d hormone replacement, rats (n = 6 rats per group) were given two injections of E2 (10 μg, sc), 24 h apart, followed by single P4 injection (4 mg/kg, sc) 24 h after last E2 injection. A single injection of BrdU (100 mg/kg, ip) was given 1 h after P4; tissue collection, 30 h after BrdU. B, For 30-d hormone replacement, rats (n = 5 rats per group) were implanted with E2 pellet (0.72 mg/30 d release) 2 wk after OVX, followed by P4 pellet (50 mg/15 d release) in the last 10 d; tissue collection on d 30.
Rats were bilaterally ovariectomized (OVX) 2 wk before hormone replacement and treated in four groups (n = 6 per group): 1) Vehicle, 2) E2 alone, 3) P4 alone, and 4) E2+P4. The E2 alone and E2+P4 groups received two injections of E2 benzoate (10 μg, sc in 100 μl sesame oil) 24 h apart; other groups received only vehicle (100 μl sesame oil) injections. On d 3, P4 alone and E2+P4 groups received P4 to simulate the luteal phase P4 elevation (single injection, 4 mg/kg, sc in 100 μl sesame oil), the remaining two groups (E2 and vehicle) received vehicle injections. For evaluation of NPC, all groups were given a single injection of bromodeoxyuridine (BrdU) (100 mg/kg, ip) 1 h after last steroid injection, and killed 30 h after BrdU injection (Fig. 1A).
Thirty-day replacement (Fig. 1B).
Fourteen days after OVX, rats were implanted sc with E2 pellets (0.72 mg/30 d release; Innovative Research of America, Sarasota, FL) or sham pellets (Innovative Research of America) for a total of 30 d. P4 pellets were administered to P4 alone and E2+P4 groups (50 mg/15 d release; Innovative Research of America) starting on d 21 for a total of 10 d. The E2 alone group received E2 pellet, then sham implant for the last 10 d. The P4-only group received a sham pellet for the first 20 d, followed by the P4 pellet for the last 10 d. OVX controls received sham implants (Fig. 1B). Uterine weights showed expected doubling of wet weight in response to E2; the P4-only group was equivalent to OVX (data not shown). The 30-d hormone schedule with these implants yielded physiological levels of plasma E2 and P4 in our prior study (7).
Tissue collection
After lethal anesthesia, rats were cardiac perfused with 0.9% saline, and brains were removed from the skull. One brain hemisphere was frozen on dry ice for ISH; the other was fixed in 4% paraformaldehyde, followed by sucrose cryoprotection (30% sucrose in 0.1 m phosphate buffer, pH 7.4) for immunohistochemistry (IHC).
Quantitative RT-PCR
Hippocampal tissue was obtained from intact female Sprague Dawley rats at defined stages of the estrous cycle (estrus; proestrus) and from OVX females. Total cellular RNA was extracted (Tri Reagent), and cDNA was prepared (2 ug RNA; Superscript III kit, Invitrogen, Carlsbad, CA). RT-PCR was performed with SYBR Green I and used the following primers: rPgrmc1 (forward, 5′-GCCTCAAGCCGCGTGACTTC-3′; reverse, 5′-CTGGGCAGGAGTGAGGTCAG-3′); rPgr (forward, 5′-GTCAGTGGACAGATGCTA-3′; reverse, 5′-AGCTGTTTCACAAGATCA-3′). Standard curves were constructed from serial dilutions of Pgrmc1 and Pgr plasmid controls and used the same primers.
ISH
Frozen brain hemispheres were sectioned sagitally (18-μm) on a cryostat and stored at −80 C until use. For ISH, [35S]UTP-labeled sense- and antisense riboprobes were generated by in vitro transcription using 1 μg linearized plasmid from the following sequences: for Pgrmc1, nucleotides 1012–1374 of rat Pgrmc1 mRNA (34); for Pgr, nucleotides 1–548 of the steroid-binding domain of both rat progesterone receptor isoforms (PR-A and PR-B) [kindly provided by Dr. S.L. Petersen (35)]. Both Pgrmc1 and Pgr cRNA probes had the same specific activity (4.9 × 106 cpm/μl) and concentration in hybridization buffer (0.3 ng/μl/kb). Labeled probe (1 ng) was used per slide and hybridized at 55 C. Posthybridization washes were performed in 50% formamide/2 × saline-sodium citrate (SSC), 0.5 × SSC, and 0.1 × SSC at 60 C. Slides were then dehydrated in graded 0.3 m ammonium acetate-alcohol series and exposed to x-ray film for 18–48 h. Slides were emulsion dipped (NTB2, Eastman Kodak, Rochester, NY), developed, and counterstained in Harris modified hematoxylin according to standard procedures (36). Based on x-ray film density, the emulsion-dipped slides were exposed for different times to reach equivalent grain densities needed for accurate comparison: Pgrmc1, 4 d; Pgr, 21 d. Grain density in emulsion-dipped, developed slides was analyzed from bright-field images (Supplemental Figs. 1 and 2 published on The Endocrine Society's Journals Online web site at http://end.endojournals.org) and counted manually around individual neurons (perikarya) that did not overlap. For CA1 and CA3 neurons, 100 cells were analyzed across six to eight images per brain; for DG neurons, 120 cells across 10 images per brain. Cells for analyses were chosen randomly to ensure uniform sampling. The frequency distributions of grain densities showed negligible overlap of sense and antisense strand grain densities (Supplemental Fig. 2). Cells with at least two grains per cell were classified as positive for mRNA with antisense probes; for sense-strand background probes, few cells (<3%) had at least two grains per cell. The data shown here were calculated from cells with at least two grains per cell. Pgr grain cluster development took 5 times longer than for Pgrmc1. Based on frequency distributions of grain densities for antisense and sense cRNA probes (Supplemental Figs. 2–6), the sense-strand background controls for all probes and regions showed no grains in most cells and few cells showed more than two grains, yielding an average background, 0.3 ± 0.03 grains per cell. We only analyzed perikarya showing at least two grains per cell with antisense cRNA probes.
Antibodies
Primary antibodies used were: polyclonal rabbit anti-Pgrmc1 (1:300, HPA002877, Sigma-Aldrich, St. Louis, MO); polyclonal rabbit anti-Pgr (1:50, sc-538, Santa Cruz Biotechnology, Inc., Santa Cruz, CA); polyclonal goat anti-doublecortin (1:100, sc-8066, Santa Cruz Biotechnology; Refs. 37 and 38); monoclonal rat anti-BrdU (1:100, MCA2060, AbDSerotec, Raleigh, NC; Refs. 39–41). Secondary antibodies used were goat antirabbit biotinylated antibody (1:200, Vector Laboratories,), goat antirat conjugated to Alexa Fluor 594 (1:400, Molecular Probes, Inc., Eugene, OR; Invitrogen), donkey antigoat conjugated to Alexa Fluor 594 (1:400, Molecular Probes, Invitrogen). The specificity of the Pgrmc1 antibody was confirmed by Western blotting of rat whole hippocampal and cortical lysates. Two bands were detected, a strong band at 25 kDa and a weak band at 50 kDa corresponding to Pgrmc1 monomer and dimmer, respectively (42, 43). The Santa Cruz Pgr antibody detected both PR-A and B isoforms. Western blot bands corresponded to PR-A and B at approximately 95 kDa and 110 kDa (Supplemental Fig. 7A). Specificity of the antibody was confirmed by preadsorbing the antibody with 10-fold excess of immunizing peptide (sc-538P, Santa Cruz Biotechnology), which depleted the Pgr bands on Western blots (Supplemental Fig. 7B) and parikaryal staining after IHC (Supplemental Fig. 7, C and D). We did not detect cellular staining with the rabbit PR antibody (1:100, A0098, DAKO Corp., Carpinteria, CA) in adult rat hippocampus, confirming the results of Waters et al. (44).
Immunohistochemistry (IHC)
Perfused brain hemispheres were fixed in 4% paraformaldehyde at 4 C for 24 h followed by cryoprotection in 30% sucrose/PB before sagittal sectioning as above. IHC was performed on whole hemisphere sagittal sections according to Morgan et al. (36), and the dorsal hippocampus was analyzed. Briefly, sections were fixed in 4% paraformaldehyde and permeabilized in 1% Nonidet P-40, followed by blocking in 5% normal serum. Sections were then incubated in primary antibodies overnight at room temperature for Pgrmc1 and Pgr and at 4 C for others. Secondary antibody incubation was performed for 1 h at room temperature. The Pgrmc1 epitope was visualized by fluorescence using goat antirabbit secondary antibody conjugated to Alexa Fluor 488 (1:400, Molecular Probes). For Pgr, sections were treated with biotinylated secondary antibody, followed by incubation with ABC reagent (Avidin-Biotin-horseradish peroxidase complex, Vector Laboratories). Pgr signal was visualized after signal amplification using tyramide-fluorescein as the horseradish peroxidase substrate (Tyramide Signal Amplification-Fluorescein, PerkinElmer). For BrdU IHC, sections were treated with 2 n HCl (to denature DNA) at 37 C for 45 min followed by neutralization in 0.1 m boric acid for 10 min before blocking in normal serum. BrdU+ cells were quantified in seven sections per animal.
Data analysis
Data are shown as means ± sem. Grain densities in Fig. 3 were calculated as the percentage of OVX controls. Statistical comparisons are based on ANOVA followed by Fisher post hoc analysis, with significance at P < 0.05.
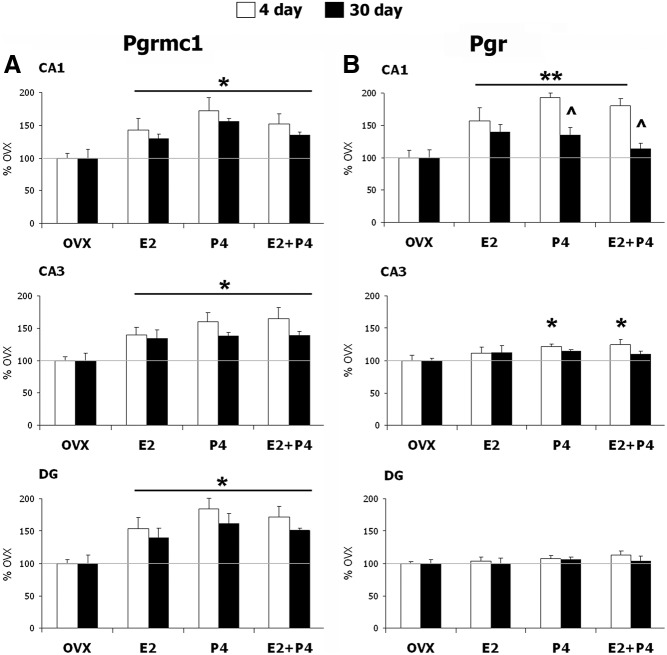
Fig. 3.
Regulation of Pgrmc1 and Pgr mRNA by E2 and P4. A, Pgrmc1 mRNA was increased by E2, P4, and E2+P4 in CA1, CA3, and DG neurons after both 4-d (n = 6 rats/group) and 30-d hormone replacement schedules (n = 5 rats per group). *, P < 0.03 compared with respective OVX. B, In CA1 neurons Pgr mRNA was increased by E2, P4, and E2+P4 on the 4-d schedule, and by E2 or P4 alone on the 30-d schedule. Modest increase in Pgr mRNA was also seen in CA3 neurons by P4 (P4 alone and E2+P4 group) only on the 4-d schedule. Pgr mRNA did not respond to either the 4-d or 30-d schedule in DG neurons. **, P < 0.01 vs. OVX in CA1. ^, P < 0.01 compared with 4-d schedule in CA1. *, P < 0.05, vs. OVX in CA3 neurons.
Results
Distribution of progesterone receptors in hippocampal subregions
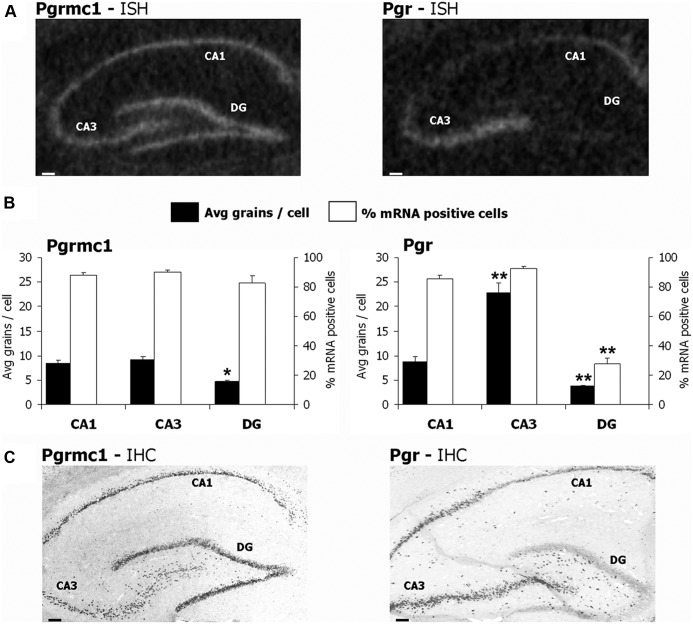
First, we determined the relative prevalence of Pgrmc1 and Pgr mRNA in whole hippocampus. By RT-PCR, Pgrmc1 mRNA prevalence was 4-fold above Pgr mRNA in OVX rats. At proestrus and estrus stages, Pgrmc1 was 2-fold above Pgr (Supplemental Fig. 8). The ISH exposure times to reach equivalent grain density differed correspondingly for cRNA probes of the same concentration and specific activity (see Materials and Methods). Hippocampal regions differed in neuronal expression of Pgrmc1 and Pgr. Pgrmc1 mRNA per cell was 2-fold higher in CA1 and CA3 pyramidal neurons than in DG neurons (Fig. 2, A and B, and Table 1). However, CA1 and CA3 neurons had similar levels of Pgrmc1 mRNA. Within hippocampal regions, neuronal Pgr mRNA prevalence was highest in CA3, followed by CA1 and DG, in ratios of 4:2:1 (Fig. 2, A and B, and Table 1). These regional differences in Pgrmc1 and Pgr expression extend semiquantitative analyses of Intlekofer and Petersen (23).
Fig. 2.
Expression of Pgrmc1 and Pgr in hippocampal neuronal layers. A, Autoradiographic film images showed differential distribution of mRNA for Pgrmc1 (left) and Pgr (right) in hippocampal neuronal layers. Pgrmc1 was prevalent across all three neuronal layers. By contrast, Pgr mRNA was prevalent in CA1 and CA3 pyramidal neurons, but it was barely detected in adjacent DG neurons. B, Average grain density for Pgrmc1 and Pgr in individual neurons of CA1 (100 cells per rat), CA3 (100 cells per rat), and DG (120 cells per rat). Pgrmc1 grain density was similar in CA1 and CA3 neuronal perikarya and far below in DG neurons. *, P < 0.01 vs. CA1 and CA3. More than 80% neurons in CA1, CA3, and DG were positive for Pgrmc1 mRNA. CA3 neurons had 2-fold more Pgr mRNA/perikaryon than CA1 and DG neurons. CA1 neurons had 2-fold Pgr over DG neurons. More than 80% CA1 and CA3 neurons, but only approximately 25% DG neurons were positive for Pgr mRNA. **, P < 0.0001 vs. other groups. C, Immunohistochemistry for Pgrmc1 and Pgr showed similar protein expression of both receptors as mRNA. Scale bars, 100 μm. Avg., Average.
A minority of DG neurons expressed Pgr mRNA (27 ± 4.2% cells had ≥2 grains per cell for Pgr), whereas, Pgrmc1 was detected in most DG neurons (82 ± 5.1% cells had ≥2 grains per cell; P < 0.0001) (Fig. 2B). Thus, 3-fold more DG neurons had significant signal for Pgrmc1 than for Pgr, in contrast to CA1 and CA3 pyramidal neurons, in which both receptors were detected in more than 80% neurons (Fig. 2B).
Immunostaining for cell body protein levels of Pgrmc1 and Pgr (Fig. 2C) corresponded to RNA levels by ISH grain densities (Fig. 2A). Again, Pgr protein was detected in a minority of DG neurons. A caveat is that protein levels of Pgrmc1 and Pgr cannot be directly compared due to the different techniques used to visualize the signals from both receptors (Methods; Pgr detection required two rounds of signal amplification, whereas Pgrmc1 did not require amplification). Whereas Pgrmc1 immunostaining had similar intensity throughout the hippocampal cell layers (Fig. 2C), Pgr immunostaining was stronger in CA3 than in CA1 and DG neurons (Fig. 2C).
E2 and P4 differentially regulate Pgrmc1 and Pgr mRNA in hippocampal subregions
Two schedules of steroid replacements (E2, P4, and E2+P4) of OVX young rats were compared: a 4-d model of the ovulatory cycle and a 30-d model of HT (Fig. 1). By ISH, Pgrmc1 mRNA was broadly induced above OVX controls by 44–92% with E2 and/or P4 on both schedules in all neuronal layers of the hippocampus (Fig. 3A and Table 1). In contrast, Pgr induction was regionally restricted on both hormone schedules. Pgr was induced only in CA1 neurons, with minimal response in CA3 and DG neurons (Fig. 3B and Table 1). All steroid treatments in the 4-d schedule induced Pgr mRNA by at least 50% in CA1 neurons. However, the 30-d schedule showed more modest effects, with Pgr induction in CA1 only by E2 or P4 alone (∼35%, P < 0.05). The absence of induction by E2+P4 suggests antagonism. In CA3 neurons, only the 4-d schedule induced Pgr mRNA, and very modestly (22% by P4, P < 0.05; 24% by E2+P4, P < 0.04; no response to E2 alone), whereas the 30-d schedule had no effect. Moreover, Pgr in DG neurons was unresponsive to all hormone schedules.
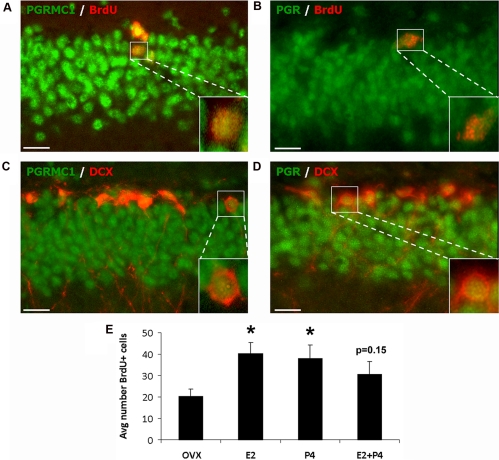
Progesterone receptor expression in NPC
Because of conflicting reports on the expression of Pgr in NPC (see Introduction), we evaluated Pgrmc1 and Pgr expression in the endogenous NPC of the DG subgranular zone. Double IHC colocalized both Pgrmc1 and Pgr in more than 90% of the BrdU-labeled progenitor cells (Fig. 4, A and B). Furthermore, the Doublecortin marker for NPC destined to be neurons also colocalized with both Pgrmc1 and Pgr (Fig. 4, C and D). The 4-d steroid treatments increased BrdU-labeled cells in the DG subgranular zone by 50–100% (Fig. 4E), consistent with the findings of Liu et al. (9). Separately, E2 or P4 treatment doubled the number of proliferating NPC (P < 0.03), wherease the combination of E2+ P4 was not significant (Fig. 4E).
Fig. 4.
Expression of Pgrmc1 and Pgr in NPC. A and B, Colocalization of Pgrmc1 and Pgr in replicating (BrdU) NPC by double immunohistochemistry for Pgrmc1 with BrdU (A) and Pgr with BrdU (B); individual colabeled cell magnified in inset. C and D, Double immunohistochemistry for Pgrmc1 (C) with Doublecortin (DCX), a marker of newly generated neurons in SGZ of DG and Pgr (D) with DCX showed colocalization of both in DCX-positive newly generated neurons; individual colabeled cell magnified in inset. Scale bars, 20 μm. E, Hormonal regulation of neurogenesis by E2 and P4 in the DG subgranular zone. Both E2 and P4 (4-d schedule; n = 6 rats per group) increased the number of BrdU+ cells in SGZ; response to E2+P4 was slightly lower (P = 0.15; not significant). *, P < 0.03, vs. OVX. Avg., Average.
Discussion
We show that Pgrmc1 and Pgr differ markedly in expression and in regulation by E2 and P4 in neurons of the adult rat hippocampus, and discuss implications for hormonal regulation of synaptic plasticity and NPC proliferation. Overall, Pgrmc1 was widely expressed throughout hippocampal neurons and was induced by both E2 and P4. In contrast, Pgr showed limited expression and regulation by steroids that differed widely among neuronal subpopulations. In those responding neurons, both acute and chronic E2 and P4 replacements gave equivalent induction of Pgrmc1 and Pgr, with little or no mutual antagonism. We hypothesize that Pgrmc1 and Pgr mediate selective hippocampal P4 effects in different neuron subtypes based on their different regional expression and responses to E2 and P4. These findings are relevant to cognitive functions in postmenopausal HT.
Differential expression of Pgrmc1 and Pgr in hippocampal subregions
First we discuss the neuroanatomical differences in expression of these receptors in the hippocampus, a key site of learning and memory. Pgrmc1 was equally prevalent in CA1 and CA3 neurons, with lower expression in DG neurons. Pgr, on the other hand, was most prevalent in CA3 neurons and much less in DG neurons. CA1 neurons had intermediate levels of Pgr mRNA. These findings at the cellular level confirm the regional distributions of Pgrmc1 and Pgr reported by Intlekofer et al. (23). With electron microscopy, Waters et al. (44) localized extranuclear Pgr in axons of hippocampal neurons, with greater detection in CA3 axons than in other hippocampal neurons. However, detection of Pgr protein by immunohistochemistry required secondary signal amplification in the present study. We confirmed the neuronal cell type-restricted expression of Pgr by RNA and protein content (in situ hybridization and immunohistochemistry, respectively). In contrast, Pgrmc1 was expressed throughout the hippocampal neuron layers. Expression in the hippocampal hilus of Pgrmc1 and Pgr (Fig. 2C) could represent interneurons, as well as glia. Although this study was focused on Pgrmc1 and Pgr expression in different hippocampal neurons, we also observed glial expression of both receptors both in vivo, and in cultured primary glia (data not shown; our unpublished results, Bali N., T. E. Morgan, C. E. Finch).
Differential regulation of Pgrmc1 and Pgr mRNA
In addition to the regional differences in levels of expression between hippocampal neuron types, both Pgrmc1 and Pgr responded differently to E2 and P4. Two hormone replacement schedules, 4-d and 30-d (Fig. 1) induced similar responses in expression. The 4-day schedule models the normal rodent ovulatory cycle and has been widely used in studies of hippocampal sprouting (4, 29), as well as in a few studies of neurogenesis (45). The 30-d schedule of continual E2 with P4 in the last 10 d is a model of the ongoing KEEPS trial of postmenopausal HT (10). We have used this 30-d replacement schedule to show P4 antagonism of E2-dependent synaptic sprouting in response to deafferenting lesions of the hippocampus (7). Clinical trials for postmenopausal HT for age-associated cognitive decline and Alzheimer disease are controversial. The Women's Health Initiative study, one of the largest randomized controlled clinical trial of HT, found increased breast cancer incidence in the group receiving equine estrogens plus a progestin, and no cognitive benefits (11, 12). Thus, it is imperative to study the combined effects of E2 and P4 on cognition, cardiovascular disease, and other health outcomes. The KEEPS trial addresses the effects of delayed initiation of HT (critical window hypothesis); KEEPS also evaluates cyclic vs. continuous P4. In a triple transgenic mouse model of Alzheimer disease, three consecutive 30-d cycles of E2 and P4 (present model) were neuroprotective (30). Most animal models of long-term HT have evaluated E2 alone (46–48). The present studies also used a 30-d hormone schedule, as well as a 4-d schedule to model the normal estrous cycle.
On both the 4- and 30-d replacement schedules, Pgr was induced by both E2 and P4, but only in CA1 neurons and not in CA3 or DG neurons. In contrast, Pgrmc1 was more broadly responsive to E2 and P4, across all neurons examined. About 40–80% induction was seen after both 4- and 30-d schedules, with similar responses in the CA1, CA3, and DG neurons. The similar induction in CA1 neurons of both Pgrmc1 and Pgr by E2 and P4 is relevant to synaptic remodeling. CA1 neurons are notable for synaptic remodeling during the rodent estrous cycle, not observed in CA3 or DG neurons (Introduction). Moreover, in OVX macaques, E2 replacement for 28 d alone induced synaptogenesis in CA1 neurons (6). Introduction of P4 to the last 14 d of E2 treatment antagonized the E2-mediated increase in pre- and postsynaptic proteins, syntaxin, synaptophysin, and spinophilin, whereas P4 alone treatment increased synaptophysin in CA1 neurons. This report gives an unusual example in which P4 alone can induce synaptic proteins and with mutual antagonism of E2+P4. We found similar responses of Pgr in CA1 neurons during the 30-d schedule. However, on a 4-d schedule, there was no indication of mutual antagonism. The differences in P4 actions when acting alone vs. E2+ P4 on a 30-d schedule are relevant to HT strategies.
The equal induction of Pgrmc1 and Pgr by E2 and P4 raises interesting questions about transcriptional regulation, particularly their autoinduction by P4. We did not find full consensus progesterone response elements (PRE) in the Pgr gene. However, Pgr has multiple half-PRE sites, which, in other genes, can bind the Pgr peptides in synergy with other cofactors (49, 50). The autoinduction by P4 is consistent with the elevation of Pgr protein in the hypothalamus during pregnancy, which peaks at d 19 of rat pregnancy when plasma P4 is maximal, with much lower E2 (51). The transcriptional regulation of Pgrmc1 has not been studied. We did not find consensus PRE in the Pgrmc1 upstream promoter, but did find multiple half-PRE sites. Thus, the P4 autoinduction of Pgrmc1 observed in the hippocampus here and in the hypothalamus (25) could be mediated indirectly by Pgr through binding of Pgr peptides to half-PRE sites.
The observed E2 regulation of Pgrmc1 and Pgr could be mediated by estrogen receptors (ER). We can dismiss a mechanism of cross talk at the ligand-binding level, because E2 does not compete with P4 for binding to either Pgrmc1 (16) or Pgr (53); nor does P4 compete with E2 at physiological levels for binding to ER (54). The Pgr promoter contains multiple ERE (estrogen response element) sequences that could mediate induction by E2 (55, 56, 57). Although we did not find consensus ERE in the Pgrmc1 upstream promoter, there are multiple half-ERE sites. Both ERα and ERβ are expressed in hippocampal neurons (58–60), though ERβ was more prevalent in CA1 and CA3 pyramidal neurons than in DG neurons (59, 60), resembling the expression of Pgrmc1 and Pgr (Fig. 2). The importance of Erα to Pgr regulation is shown by the absence of induction by E2 in the hippocampus of ERKOα mice (61).
The pharmacological specificity of Pgrmc1 and Pgr also needs further study. The antiprogestin RU486 blocked the decrease in CA1 dendritic spines during the estrus (4) and also blocked P4 antagonism of E2-induced neurite outgrowth in an in vitro lesion model (7). Although RU486 does act on recombinant Pgr (62, 63), it is not known whether it also acts on Pgrmc1. In addition to Pgrmc1, three other membrane PR are recognized: the G protein-coupled receptors mPRα, mPRβ, and mPRγ. Little is known of their expression patterns in brain cell types, P4 binding characteristics, and steroidal regulation, or P4-specific functions (23, 25, 64–67).
CA1 neurons were the only hippocampal subregion that showed similar hormonal responses of both Pgrmc1 and Pgr (Fig. 3). This regional restriction is interesting because CA1 neurons are more vulnerable to Alzheimer disease (68, 69), postischemic damage (70). and hypoxia-induced seizure activity, relative to CA3 and DG neurons (71). The responsiveness of progesterone receptors to E2 and P4 is relevant to stroke because CA1 neurons are protected in rodent models of ischemia by both E2 (72, 73) and P4 (74). The P4 neuroprotection of CA1 could be mediated by either Pgrmc1 or Pgr. The receptor involved in CA1 neuroprotection could be identified using Pgr knockout mice (PRKO). However, Pgrmc1 knockout mice have not been reported so far.
Pgrmc1 and Pgr expression in NPC of the subgranular zone of DG
The DG was examined for expression of Pgrmc1 and Pgr in its P4-sensitive NPC, which have not been characterized in detail. In adult female rats (9) and male mice (75), P4 promoted the generation of nascent neurons in the DG subgranular zone. Both E2 and P4 stimulated NPC proliferation in cells obtained by whole hippocampus cell sorting (9) and in an established adult rat NPC line derived from the DG subgranular zone (8, 76), as confirmed in vivo here. The in vitro proliferative effects of P4 involve Pgrmc1 through P4-induced kinase signaling (8). The present study detected both Pgrmc1 and Pgr in newly formed immature neurons (Doublecortin immunopositive) in the subgranular zone. However, most DG mature neurons lack Pgr (Fig. 2B), which may be consequent to DG neuronal maturation, e.g. Pgr expression in the DG peaked by postnatal d 7 and was undetectable by d 28 (77, 78). Lastly, we note the divergence in Pgr expression between NPC lines. Whereas a rat NPC line derived from the DG subgranular zone did not have Pgr by PCR (8), Pgr protein was detected in NPC originated from the subventricular zone, another site of adult neurogenesis (52). This difference could be outcomes of continued in vitro propagation of the DG subgranular cell line or to the different sites of NPC origination.
The responses of Pgrmc1 and Pgr to P4 ± E2 were very similar for both 4-d and 30-d hormone replacement schedules. Pgrmc1 was increased in all hippocampal neuronal layers on both schedules in which E2 alone, P4 alone, or combined gave similar induction. Whereas Pgr responses were restricted to CA1 neurons, responses to E2 and P4 alone or together were again equivalent on the 4-d schedule. However, the weaker increase of Pgr in CA1 on the 30-d schedule of combined E2+P4 suggests possible antagonism. Others have reported selective induction. In the hypothalamus (25) only select nuclei showed Pgrmc1 elevations with P4 alone or E2+P4; unlike hippocampal responses, there was no effect of E2 alone. In contrast, Pgrmc1 (cited as 25-Dx) was increased in the hypothalamus of E2-primed OVX females, but this increase was attenuated by P4 (34). Reports on Pgr are also divergent. As we observed in CA1 neurons with both hormone schedules, P4 alone increased Pgr in chick embryo hypothalamus and posterior pituitary (28). However, Intlekofer and Petersen (25) observed that P4 alone did not increase hypothalamic Pgr mRNA. A more detailed study of the time course is warranted because Turgeon et al. (27) showed the transiency of P4 down-regulation of Pgr, with receptor levels returning to the level of E2-treated controls by 12 h after treatment.
Conclusions
Pgrmc1 is widely expressed in neuronal layers of all hippocampal regions and is induced by both E2 and P4. In contrast, Pgr shows restricted regional expression and regulation by E2 and P4. The shared induction of both Pgrmc1 and Pgr by E2-P4 in CA1 neurons may be relevant to their capacity for E2-dependent synaptic remodeling and to CA1 sensitivity to neurodegeneration from Alzheimer disease and ischemia. The differential regulation of hippocampal Pgrmc1 and Pgr gives a rationale for development of drugs in hormonal therapy to target multiple receptors in the support of neurogenesis, neuroprotection, and synaptic regeneration.
Supplementary Material
Acknowledgements
This work was supported by National Institute on Aging Grants 1PO1 AG026572 (to R.D.B.); Project 4 (to C.E.F. and T.E.M.), Animal Core A (to T.E.M.), and Analytic Core C (to L.Z.).
Disclosure Summary: All authors declare no conflicts.
Footnotes
- BrdU
- Bromodeoxyuridine
- DG
- dentate gyrus
- E2
- estradiol
- ER
- estrogen receptor
- ERE
- estrogen response element
- HT
- hormone therapy
- ISH
- in situ hybridization
- NPC
- neural progenitor cell
- OVX
- ovariectomized
- P4
- progesterone
- SSC
- saline-sodium citrate.
References
- 1. Graham JD, Clarke CL. 1997. Physiological action of progesterone in target tissues. Endocr Rev 18:502–519 [DOI] [PubMed] [Google Scholar]
- 2. Rosario G, Sachdeva G, Okulicz WC, Ace CI, Katkam RR, Puri CP. 2003. Role of progesterone in structural and biochemical remodeling of endometrium. Front Biosci 8:s924–s935 [DOI] [PubMed] [Google Scholar]
- 3. Woolley CS, McEwen BS. 1992. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci 12:2549–2554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Woolley CS, McEwen BS. 1993. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol 336:293–306 [DOI] [PubMed] [Google Scholar]
- 5. Cooke BM, Woolley CS. 2005. Gonadal hormone modulation of dendrites in the mammalian CNS. J Neurobiol 64:34–46 [DOI] [PubMed] [Google Scholar]
- 6. Choi JM, Romeo RD, Brake WG, Bethea CL, Rosenwaks Z, McEwen BS. 2003. Estradiol increases pre- and post-synaptic proteins in the CA1 region of the hippocampus in female rhesus macaques (Macaca mulatta). Endocrinology 144:4734–4738 [DOI] [PubMed] [Google Scholar]
- 7. Wong AM, Rozovsky I, Arimoto JM, Du Y, Wei M, Morgan TE, Finch CE. 2009. Progesterone influence on neurite outgrowth involves microglia. Endocrinology 150:324–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu L, Wang J, Zhao L, Nilsen J, McClure K, Wong K, Brinton RD. 2009. Progesterone increases rat neural progenitor cell cycle gene expression and proliferation via extracellularly regulated kinase and progesterone receptor membrane components 1 and 2. Endocrinology 150:3186–3196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu L, Zhao L, She H, Chen S, Wang JM, Wong C, McClure K, Sitruk-Ware R, Brinton RD. 2010. Clinically relevant progestins regulate neurogenic and neuroprotective responses in vitro and in vivo. Endocrinology 151:5782–5794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Harman SM, Brinton EA, Cedars M, Lobo R, Manson JE, Merriam GR, Miller VM, Naftolin F, Santoro N. 2005. KEEPS: The Kronos Early Estrogen Prevention Study. Climacteric 8:3–12 [DOI] [PubMed] [Google Scholar]
- 11. Rapp SR, Espeland MA, Shumaker SA, Henderson VW, Brunner RL, Manson JE, Gass ML, Stefanick ML, Lane DS, Hays J, Johnson KC, Coker LH, Dailey M, Bowen D. 2003. Effect of estrogen plus progestin on global cognitive function in postmenopausal women. The Women's Health Initiative Memory Study: a randomized controlled trial. JAMA 289:2663–2672 [DOI] [PubMed] [Google Scholar]
- 12. Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, 3rd, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J. 2003. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women. The Women's Health Initiative Memory Study: a randomized controlled trial. JAMA 289:2651–2662 [DOI] [PubMed] [Google Scholar]
- 13. Brinton RD, Thompson RF, Foy MR, Baudry M, Wang J, Finch CE, Morgan TE, Pike CJ, Mack WJ, Stanczyk FZ, Nilsen J. 2008. Progesterone receptors: form and function in brain. Front Neuroendocrinol 29:313–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thomas P. 2008. Characteristics of membrane progestin receptor alpha (mPRα) and progesterone membrane receptor component 1 (PGMRC1) and their roles in mediating rapid progestin actions. Front Neuroendocrinol 29:292–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Keightley DD. 1979. The binding of progesterone, R-5020 and ORG-2058 to progesterone receptor. Eur J Cancer 15:785–790 [DOI] [PubMed] [Google Scholar]
- 16. Meyer C, Schmid R, Scriba PC, Wehling M. 1996. Purification and partial sequencing of high-affinity progesterone-binding site(s) from porcine liver membranes. Eur J Biochem 239:726–731 [DOI] [PubMed] [Google Scholar]
- 17. Peluso JJ, Romak J, Liu X. 2008. Progesterone receptor membrane component-1 (PGRMC1) is the mediator of progesterone's antiapoptotic action in spontaneously immortalized granulosa cells as revealed by PGRMC1 small interfering ribonucleic acid treatment and functional analysis of PGRMC1 mutations. Endocrinology 149:534–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Buddhikot M, Falkenstein E, Wehling M, Meizel S. 1999. Recognition of a human sperm surface protein involved in the progesterone-initiated acrosome reaction by antisera against an endomembrane progesterone binding protein from porcine liver. Mol Cell Endocrinol 158:187–193 [DOI] [PubMed] [Google Scholar]
- 19. Falkenstein E, Heck M, Gerdes D, Grube D, Christ M, Weigel M, Buddhikot M, Meizel S, Wehling M. 1999. Specific progesterone binding to a membrane protein and related nongenomic effects on Ca2+-fluxes in sperm. Endocrinology 140:5999–6002 [DOI] [PubMed] [Google Scholar]
- 20. Cahill MA. 2007. Progesterone receptor membrane component 1: an integrative review. J Steroid Biochem Mol Biol 105:16–36 [DOI] [PubMed] [Google Scholar]
- 21. Rohe HJ, Ahmed IS, Twist KE, Craven RJ. 2009. PGRMC1 (progesterone receptor membrane component 1): a targetable protein with multiple functions in steroid signaling, P450 activation and drug binding. Pharmacol Ther 121:14–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Runko E, Kaprielian Z. 2004. Caenorhabditis elegans VEM-1, a novel membrane protein, regulates the guidance of ventral nerve cord-associated axons. J Neurosci 24:9015–9026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Intlekofer KA, Petersen SL. 2011. 17β-Estradiol and progesterone regulate multiple progestin signaling molecules in the anteroventral periventricular nucleus, ventromedial nucleus and sexually dimorphic nucleus of the preoptic area in female rats. Neuroscience 176:86–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sakamoto H, Ukena K, Takemori H, Okamoto M, Kawata M, Tsutsui K. 2004. Expression and localization of 25-Dx, a membrane-associated putative progesterone-binding protein, in the developing purkinje cell. Neuroscience 126:325–334 [DOI] [PubMed] [Google Scholar]
- 25. Intlekofer KA, Petersen SL. 2011. Distribution of mRNAs encoding classical progestin receptor, progesterone membrane components 1 and 2, serpine mRNA binding protein 1, and progestin and ADIPOQ receptor family members 7 and 8 in rat forebrain. Neuroscience 172:55–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guerra-Araiza C, Villamar-Cruz O, González-Arenas A, Chavira R, Camacho-Arroyo I. 2003. Changes in progesterone receptor isoforms content in the rat brain during the oestrous cycle and after oestradiol and progesterone treatments. J Neuroendocrinol 15:984–990 [DOI] [PubMed] [Google Scholar]
- 27. Turgeon JL, Van Patten SM, Shyamala G, Waring DW. 1999. Steroid regulation of progesterone receptor expression in cultured rat gonadotropes. Endocrinology 140:2318–2325 [DOI] [PubMed] [Google Scholar]
- 28. Guennoun R, Gasc JM. 1990. Estrogen-independent and estrogen-induced progesterone receptors, and their regulation by progestins in the hypothalamus and pituitary of the chick embryo: an immunohistochemical study. Brain Res Dev Brain Res 55:151–159 [DOI] [PubMed] [Google Scholar]
- 29. Gould E, Woolley CS, Frankfurt M, McEwen BS. 1990. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci 10:1286–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Carroll JC, Rosario ER, Villamagna A, Pike CJ. 2010. Continuous and cyclic progesterone differentially interact with estradiol in the regulation of Alzheimer-like pathology in female 3x transgenic-Alzheimer's disease mice. Endocrinology 151:2713–2722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Practice Committee of American Society for Reproductive Medicine 2008. Estrogen and progestogen therapy in postmenopausal women. Fertil Steril 90:S88–102 [DOI] [PubMed] [Google Scholar]
- 32. Asthana S, Brinton RD, Henderson VW, McEwen BS, Morrison JH, Schmidt PJ; Frontiers Proposal for Estrogen Work Group; Cognitive Aging Work Group 2009. Frontiers proposal. National Institute on Aging “bench to bedside: estrogen as a case study.” Age (Dordr) 31:199–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sherwin BB. 2009. Estrogen therapy: is time of initiation critical for neuroprotection? Nat Rev Endocrinol 5:620–627 [DOI] [PubMed] [Google Scholar]
- 34. Krebs CJ, Jarvis ED, Chan J, Lydon JP, Ogawa S, Pfaff DW. 2000. A membrane-associated progesterone-binding protein, 25-Dx, is regulated by progesterone in brain regions involved in female reproductive behaviors. Proc Natl Acad Sci USA 97:12816–12821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Park OK, Mayo KE. 1991. Transient expression of progesterone receptor messenger RNA in ovarian granulosa cells after the preovulatory luteinizing hormone surge. Mol Endocrinol 5:967–978 [DOI] [PubMed] [Google Scholar]
- 36. Morgan TE, Xie Z, Goldsmith S, Yoshida T, Lanzrein AS, Stone D, Rozovsky I, Perry G, Smith MA, Finch CE. 1999. The mosaic of brain glial hyperactivity during normal ageing and its attenuation by food restriction. Neuroscience 89:687–699 [DOI] [PubMed] [Google Scholar]
- 37. Snyder JS, Choe JS, Clifford MA, Jeurling SI, Hurley P, Brown A, Kamhi JF, Cameron HA. 2009. Adult-born hippocampal neurons are more numerous, faster maturing, and more involved in behavior in rats than in mice. J Neurosci 29:14484–14495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hodge RD, Kowalczyk TD, Wolf SA, Encinas JM, Rippey C, Enikolopov G, Kempermann G, Hevner RF. 2008. Intermediate progenitors in adult hippocampal neurogenesis: Tbr2 expression and coordinate regulation of neuronal output. J Neurosci 28:3707–3717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Farioli-Vecchioli S, Saraulli D, Costanzi M, Pacioni S, Cinà I, Aceti M, Micheli L, Bacci A, Cestari V, Tirone F. 2008. The timing of differentiation of adult hippocampal neurons is crucial for spatial memory. PLoS Biol 6:e246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Farioli-Vecchioli S, Saraulli D, Costanzi M, Leonardi L, Cinà I, Micheli L, Nutini M, Longone P, Oh SP, Cestari V, Tirone F. 2009. Impaired terminal differentiation of hippocampal granule neurons and defective contextual memory in PC3/Tis21 knockout mice. PLoS One 4:e8339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang JM, Singh C, Liu L, Irwin RW, Chen S, Chung EJ, Thompson RF, Brinton RD. 2010. Allopregnanolone reverses neurogenic and cognitive deficits in mouse model of Alzheimer's disease. Proc Natl Acad Sci USA 107:6498–6503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Peluso JJ, Liu X, Gawkowska A, Lodde V, Wu CA. 2010. Progesterone inhibits apoptosis in part by PGRMC1-regulated gene expression. Mol Cell Endocrinol 320:153–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Falkenstein E, Eisen C, Schmieding K, Krautkrämer M, Stein C, Lösel R, Wehling M. 2001. Chemical modification and structural analysis of the progesterone membrane binding protein from porcine liver membranes. Mol Cell Biochem 218:71–79 [DOI] [PubMed] [Google Scholar]
- 44. Waters EM, Torres-Reveron A, McEwen BS, Milner TA. 2008. Ultrastructural localization of extranuclear progestin receptors in the rat hippocampal formation. J Comp Neurol 511:34–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tanapat P, Hastings NB, Gould E. 2005. Ovarian steroids influence cell proliferation in the dentate gyrus of the adult female rat in a dose- and time-dependent manner. J Comp Neurol 481:252–265 [DOI] [PubMed] [Google Scholar]
- 46. Markowska AL, Savonenko AV. 2002. Effectiveness of estrogen replacement in restoration of cognitive function after long-term estrogen withdrawal in aging rats. J Neurosci 22:10985–10995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Marriott LK, Hauss-Wegrzyniak B, Benton RS, Vraniak PD, Wenk GL. 2002. Long-term estrogen therapy worsens the behavioral and neuropathological consequences of chronic brain inflammation. Behav Neurosci 116:902–911 [DOI] [PubMed] [Google Scholar]
- 48. Rusa R, Alkayed NJ, Crain BJ, Traystman RJ, Kimes AS, London ED, Klaus JA, Hurn PD. 1999. 17β-Estradiol reduces stroke injury in estrogen-deficient female animals. Stroke 30:1665–1670 [DOI] [PubMed] [Google Scholar]
- 49. De Amicis F, Zupo S, Panno ML, Malivindi R, Giordano F, Barone I, Mauro L, Fuqua SA, Andò S. 2009. Progesterone receptor B recruits a repressor complex to a half-PRE site of the estrogen receptor α gene promoter. Mol Endocrinol 23:454–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lieberman BA, Bona BJ, Edwards DP, Nordeen SK. 1993. The constitution of a progesterone response element. Mol Endocrinol 7:515–527 [DOI] [PubMed] [Google Scholar]
- 51. Steyn FJ, Anderson GM, Grattan DR. 2007. Expression of ovarian steroid hormone receptors in tuberoinfundibular dopaminergic neurones during pregnancy and lactation. J Neuroendocrinol 19:788–793 [DOI] [PubMed] [Google Scholar]
- 52. Waldron J, McCourty A, Lecanu L. 2010. Aging differentially affects male and female neural stem cell neurogenic properties. Stem Cells, Cloning: Adv.& Appl. 3:119–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pichon MF, Milgrom E. 1977. Characterization and assay of progesterone receptor in human mammary carcinoma. Cancer Res 37:464–471 [PubMed] [Google Scholar]
- 54. Kuiper GG, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, Gustafsson JA. 1997. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors α and β. Endocrinology 138:863–870 [DOI] [PubMed] [Google Scholar]
- 55. Bonéy-Montoya J, Ziegler YS, Curtis CD, Montoya JA, Nardulli AM. 2010. Long-range transcriptional control of progesterone receptor gene expression. Mol Endocrinol 24:346–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Petz LN, Nardulli AM. 2000. Sp1 binding sites and an estrogen response element half-site are involved in regulation of the human progesterone receptor A promoter. Mol Endocrinol 14:972–985 [DOI] [PubMed] [Google Scholar]
- 57. Petz LN, Ziegler YS, Schultz JR, Kim H, Kemper JK, Nardulli AM. 2004. Differential regulation of the human progesterone receptor gene through an estrogen response element half site and Sp1 sites. J Steroid Biochem Mol Biol 88:113–122 [DOI] [PubMed] [Google Scholar]
- 58. Solum DT, Handa RJ. 2001. Localization of estrogen receptor alpha (ERα) in pyramidal neurons of the developing rat hippocampus. Brain Res Dev Brain Res 128:165–175 [DOI] [PubMed] [Google Scholar]
- 59. Kalita K, Szymczak S, Kaczmarek L. 2005. Non-nuclear estrogen receptor β and α in the hippocampus of male and female rats. Hippocampus 15:404–412 [DOI] [PubMed] [Google Scholar]
- 60. Shughrue PJ, Lane MV, Merchenthaler I. 1997. Comparative distribution of estrogen receptor-α and –β mRNA in the rat central nervous system. J Comp Neurol 388:507–525 [DOI] [PubMed] [Google Scholar]
- 61. Alves SE, McEwen BS, Hayashi S, Korach KS, Pfaff DW, Ogawa S. 2000. Estrogen-regulated progestin receptors are found in the midbrain raphe but not hippocampus of estrogen receptor alpha (ERα) gene-disrupted mice. J Comp Neurol 427:185–195 [DOI] [PubMed] [Google Scholar]
- 62. Skafar DF. 1991. Differences in the binding mechanism of RU486 and progesterone to the progesterone receptor. Biochemistry 30:10829–10832 [DOI] [PubMed] [Google Scholar]
- 63. Raaijmakers HC, Versteegh JE, Uitdehaag JC. 2009. The X-ray structure of RU486 bound to the progesterone receptor in a destabilized agonistic conformation. J Biol Chem 284:19572–19579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ashley RL, Arreguin-Arevalo JA, Nett TM. 2009. Binding characteristics of the ovine membrane progesterone receptor α and expression of the receptor during the estrous cycle. Reprod Biol Endocrinol 7:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Foster H, Reynolds A, Stenbeck G, Dong J, Thomas P, Karteris E. 2010. Internalisation of membrane progesterone receptor-α after treatment with progesterone: potential involvement of a clathrin-dependent pathway. Mol Med report 3:27–35 [DOI] [PubMed] [Google Scholar]
- 66. Labombarda F, Meffre D, Delespierre B, Krivokapic-Blondiaux S, Chastre A, Thomas P, Pang Y, Lydon JP, Gonzalez SL, De Nicola AF, Schumacher M, Guennoun R. 2010. Membrane progesterone receptors localization in the mouse spinal cord. Neuroscience 166:94–106 [DOI] [PubMed] [Google Scholar]
- 67. Liu B, Arbogast LA. 2009. Gene expression profiles of intracellular and membrane progesterone receptor isoforms in the mediobasal hypothalamus during pro-oestrus. J Neuroendocrinol 21:993–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Simonian NA, Hyman BT. 1995. Functional alterations in neural circuits in Alzheimer's disease. Neurobiol Aging 16:305–309 [DOI] [PubMed] [Google Scholar]
- 69. West MJ, Coleman PD, Flood DG, Troncoso JC. 1994. Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer's disease. Lancet 344:769–772 [DOI] [PubMed] [Google Scholar]
- 70. Chang HS, Sasaki T, Kassell NF. 1989. Hippocampal unit activity after transient cerebral ischemia in rats. Stroke 20:1051–1058 [DOI] [PubMed] [Google Scholar]
- 71. Kawasaki K, Traynelis SF, Dingledine R. 1990. Different responses of CA1 and CA3 regions to hypoxia in rat hippocampal slice. J Neurophysiol 63:385–394 [DOI] [PubMed] [Google Scholar]
- 72. Jover-Mengual T, Miyawaki T, Latuszek A, Alborch E, Zukin RS, Etgen AM. 2010. Acute estradiol protects CA1 neurons from ischemia-induced apoptotic cell death via the PI3K/Akt pathway. Brain Res 1321:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lebesgue D, Traub M, De Butte-Smith M, Chen C, Zukin RS, Kelly MJ, Etgen AM. 2010. Acute administration of non-classical estrogen receptor agonists attenuates ischemia-induced hippocampal neuron loss in middle-aged female rats. PLoS One 5:e8642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Moralí G, Letechipía-Vallejo G, López-Loeza E, Montes P, Hernández-Morales L, Cervantes M. 2005. Post-ischemic administration of progesterone in rats exerts neuroprotective effects on the hippocampus. Neurosci Lett 382:286–290 [DOI] [PubMed] [Google Scholar]
- 75. Zhang Z, Yang R, Zhou R, Li L, Sokabe M, Chen L. 2010. Progesterone promotes the survival of newborn neurons in the dentate gyrus of adult male mice. Hippocampus 20:402–412 [DOI] [PubMed] [Google Scholar]
- 76. Gage FH, Coates PW, Palmer TD, Kuhn HG, Fisher LJ, Suhonen JO, Peterson DA, Suhr ST, Ray J. 2005. Survival and differentiation of adult neuronal progenitor cells transplanted to the adult brain. Proc Natl Acad Sci USA. 92:11879–11883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Quadros PS, Pfau JL, Wagner CK. 2007. Distribution of progesterone receptor immunoreactivity in the fetal and neonatal rat forebrain. J Comp Neurol 504:42–56 [DOI] [PubMed] [Google Scholar]
- 78. Gonzales K, Willing J, Milner TA, McEwen BS, Wagner CK, Waters EM, Transient expression of progesterone receptors in the developing dentate gyrus may alter hippocampally dependent behaviors in adulthood. Annual Meeting of The Society for Neuroscience, Neuroscience 2011, Washington, DC Abstract 282.18 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.