Abstract
Very low-density lipoproteins (VLDL) are a class of large lipoprotein synthesized in the liver. The key function of VLDL, in vivo, is to carry triglyceride from the liver to adipose tissue. As a steroidogenic organ, the adrenal gland mainly uses lipoproteins as sources of cholesterol. Although VLDL receptors have been detected in the human adrenal, the function of VLDL in the adrenal gland remains unknown. Herein, we used primary cultures of human and bovine adrenal cells and the adrenocortical cell line H295R as models to determine the effects of VLDL on adrenal steroidogenesis. Our studies revealed that VLDL significantly increased aldosterone synthesis in all of the models tested. This increase was largely due to VLDL's stimulation of the expression of steroidogenic acute regulatory (StAR) protein and aldosterone synthase (CYP11B2). VLDL increased CYP11B2 mRNA expression in a concentration-dependent manner. Effects of VLDL on CYP11B2 transcript levels were not additive with angiotensin II or potassium but were additive with the cAMP pathway agonists ACTH and forskolin. Nifedipine completely inhibited the effects of VLDL on CYP11B2 mRNA, suggesting that calcium is the main signal transduction pathway used by VLDL in adrenal cells. Indeed, VLDL increased cytosolic free calcium levels. An in vivo study conducted in sucrose-fed rats showed a positive correlation between elevated triglyceride (VLDL) levels in plasma and CYP11B2 expression in the adrenal. In conclusion, we have shown that VLDL can stimulate aldosterone synthesis in adrenocortical cells by increasing StAR and CYP11B2 expression, an event likely mediated by a calcium-initiated signaling cascade.
Aldosterone, as the major mineralocorticoid produced by the adrenal gland, is normally under the tight control of the renin/angiotensin II (AngII)/aldosterone system (RAAS). There are two key regulatory steps in the control of adrenal cell aldosterone synthesis, termed the early and late rate-limiting steps. The first is controlled by AngII and serum potassium (K+) levels through the regulation of steroidogenic acute regulatory (StAR) protein expression and activity, whereas the second represents the conversion of deoxycorticosterone to aldosterone by the mitochondrial enzyme aldosterone synthase (CYP11B2) (1, 2). CYP11B2 is expressed almost exclusively within the zona glomerulosa (ZG) of the adrenal cortex (3–5) and is regulated by multiple physiological agonists, including AngII and K+. Both agonists increase intracellular free calcium levels and activate calmodulin and calmodulin kinases (5, 6), which leads to increased CYP11B2 gene transcription (7–10). However, there is mounting evidence implicating the existence of other fine-tuning mechanisms as well as alternative pathways in adrenal diseases (11–17).
Very-low-density lipoprotein (VLDL) is a low-density lipoprotein with a high triglyceride (TG) content (about 50%). Despite the well-known function of transporting fatty acids and TG to peripheral tissues, VLDL has been suggested to function in a tissue-specific manner in vivo. Previous studies have shown that VLDL can itself regulate signaling cascades in several tissue types. For example, incubating vascular smooth muscle cells with VLDL inhibits Src-dependent assembly of fibronectin and type I collagen (18). In PC-3 prostate cancer cells, VLDL can stimulate cell proliferation and activate MAPK and Akt signaling pathways via a G protein-coupled receptor (19). VLDL also up-regulates expression of the plasminogen activator inhibitor-1 gene and plasminogen activator inhibitor-1 antigen, a process leading to platelet aggregation and clot formation (20). VLDL has been proposed to stimulate ERK1/2 activity in a protein kinase C-dependent manner leading to increased expression of VLDL receptor (21, 22). Additionally, VLDL appears to be a negative regulator of the Wnt pathway in endothelial cells, where selective knockdown of its receptor results in up-regulation of low-density lipoprotein receptor-related protein 5/6 (LRP5/6) expression and activation of β-catenin (23). However, in contrast to the other members of the lipoprotein family [e.g. high-density lipoprotein (HDL) and low-density lipoprotein (LDL)], VLDL does not appear to serve as a cholesterol carrier for steroidogenic glands, and its function in the adrenal gland remains unknown.
In the present study, we focused on the steroidogenic effects of VLDL in the adrenal gland. We used both primary adrenal cell cultures and an adrenocortical cell line as in vitro model systems as well as an in vivo rat model of hypertriglyceridemia. The current data suggest that VLDL stimulates aldosterone production by triggering a series of signal transduction cascades, leading to increased expression and synthesis of CYP11B2. This finding indicates that VLDL can act as a signaling particle in the adrenal gland to regulate steroid production.
Materials and Methods
Cell culture and reagents
Adult adrenal glands were obtained with informed consent from cadaveric organ donors from the Georgia Health Sciences University (Augusta, GA). The use of these tissues was approved by the Institutional Review Board of the Georgia Health Sciences University. Primary human adrenal cell cultures were prepared from normal adrenal glands using previously described methods (24). Briefly, adrenal glands were decapsulated and minced into small pieces. The tissue was then incubated in DMEM/F12 (Life Technologies, Inc., Carlsbad, CA), containing 0.1% collagenase/0.01% deoxyribonuclease (F. Hoffmann-La Roche Ltd., Switzerland) and allowed to digest with mechanical dispersion for 1 h at 37 C. Digestion was repeated three times. After isolation, cells were either grown in DMEM/F12 supplemented with 10% Cosmic calf serum (vol/vol) (Hyclone, Logan, UT) and antibiotics at a density of 300,000 per well of a 24-well Falcon culture plate or frozen as aliquots for future use.
The bovine adrenal glomerulosa cells were isolated from near-term fetal calves and cultured overnight in Falcon Primaria dishes (Becton Dickinson Labware, Lincoln Park, NJ) in DMEM/F12 containing 10% horse serum, 2% fetal bovine serum, 100 μm ascorbate, 1.2 μm α-tocopherol, 0.05 μm Na2SeO3, 50 μm butylated hydroxyanisole, 5 μm metyrapone, 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.25 μg/ml amphotericin B as described previously (25). After replacement of the serum-containing medium with serum-free medium (with 0.2% BSA), the cells were incubated for an additional 20–24 h before use in experiments. All preparations included for analysis exhibited an AngII-stimulated aldosterone response of at least 10-fold.
The human adrenocortical cell line H295R was routinely cultured in DMEM/F12 supplemented with 10% Cosmic calf serum and antibiotics including 1% penicillin/streptomycin (Life Technologies) and 0.1% gentamicin (Sigma-Aldrich, St. Louis, MO). Cells were plated in 24-well Falcon plates at a density of 200,000 per well and incubated at 37 C for 2 d. One day before the experiment, cells were changed to a low-serum experimental medium (DMEM/F12 supplemented with 0.1% Cosmic calf serum and antibiotics). The next morning, cells were treated in the same low-serum experimental medium for the indicated times. For inhibitor studies, cells were preincubated with inhibitors for 20–30 min before any agonists were added. Reagents were obtained from Sigma-Aldrich unless indicated otherwise.
Lipoproteins
Native human VLDL were purchased from Calbiochem (Darmstadt, Germany) with three independent lots tested, Kalen Biochemical LLC (Montgomery Village, MD) with three independent lots tested, and Millipore (Billerica, MA) with two independent lots tested. In addition, two lots of VLDL prepared by our collaborators were also included in this study (26). All the figures represent results obtained using more than one source of VLDL.
RNA isolation and quantitative real-time RT-PCR (qPCR)
Total RNA was extracted from cells using RNeasy mini kits (QIAGEN, Valencia, CA) following the manufacturer's recommendations. The purity and integrity of the RNA were checked spectroscopically using a NanoDrop spectrometer (NanoDrop Technologies, Wilmington, DE). Deoxyribonuclease I-treated total RNA (2 μg) (Ambion Inc., Austin, TX) was reverse transcribed using the High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA) following the manufacturer's recommendations and stored at −80 C until further use.
The primer and probe sets used to detect transcripts for human StAR, cholesterol side-chain cleavage (CYP11A1), 3β-hydroxysteroid dehydrogenase-2 (HSD3B2), 21-hydroxylase (CYP21), CYP11B1, and CYP11B2 were designed using Primer Express 3.0 (Applied Biosystems) and purchased from Integrated DNA Technologies (Coralville, IA) as published previously (8). VLDLR and LRP5 gene expression assays were obtained from Applied Biosystems.
The qPCR analyses were performed in the ABI Prism 7000 Sequence Detection System (Applied Biosystems) in a total volume of 20 μl following the reaction parameters recommended by the manufacturer. Briefly, the TaqMan 2× Master Mix (Applied Biosystems), 900 nm of each primer, 250 nm of probe, and 5 μl of each first-strand cDNA sample were combined in each reaction vessel for gene detection. Negative controls contained water instead of first-strand cDNA. Relative quantification of mRNA levels between different tissues was performed using the comparative cycle threshold value as described previously (27), and 18S rRNA was used as an internal control. The quantification of the 18S RNA in each sample was performed using a TaqMan rRNA reagent kit (Applied Biosystems) following the manufacturer's recommendations.
Protein extraction and protein assay
Cells were lysed in 100 μl 1× mammalian protein extraction reagent (Pierce Chemical Co., Rockford, IL). The protein content of samples was then determined by the bicinchoninic acid protein assay using the micro-bicinchoninic acid protocol (Pierce).
Steroid assay
Medium was collected after cell treatment and stored at −20 C. The aldosterone content of the medium was analyzed using an RIA kit from Siemens (Los Angeles, CA), and radioactivity was determined using a multicrystal γ-counter (γ-C 12; DPC, Berthold, Germany). The cortisol and dehydroepiandrosterone (DHEA) contents of the experimental medium were determined using EIA kits (Diagnostic System Laboratories, Webster, TX). Assays were conducted following the manufacturer's recommendation except that standard curves were prepared in the experimental cell culture medium. The results were normalized to protein concentration and shown as fold change over basal conditions.
Western analysis
Immunoblotting analysis was performed using the XCell SureLock system (Invitrogen, Carlsbad, CA) following the manufacturer's recommendation. Briefly, samples were lysed with Western buffer (2% sodium dodecyl sulfate, 62.5 mm Tris, 0.04% bromophenol blue and 0.2 m dithiothreitol) and boiled at 95 C for 5 min. Equal amounts of proteins were separated on 10% Bis-Tris gels before transfer to polyvinylidene difluoride membranes. After blocking with 5% milk for 1 h, the membrane was incubated with StAR antibody (rabbit, 1:5000; kindly provided by Dr. D. B. Hales, Southern Illinois University School of Medicine, Springfield, IL), NURR1 antibody (rabbit antihuman, 1:1000; Santa Cruz Biotechnology Inc., Santa Cruz, CA), phospho-ATF1 and phospho-ATF2 antibodies (rabbit antihuman, 1:3000; Cell Signaling Technology, Inc., Danvers, MA), or β-actin antibody (mouse antihuman, 1:10,000; Santa Cruz) followed by secondary antibody (goat antirabbit, 1:7000 or goat antimouse, 1:4000; Invitrogen). A Pierce enhanced chemiluminescence kit was used for signal development.
Intracellular free calcium assay
Intracellular free calcium levels were monitored using a Fluo-4 NW calcium assay kit (Invitrogen). Briefly, H295R cells were cultured to 80–90% confluence in 96-well plates and preloaded with fluo-4 dye for 30 min at 37 C and then measured at the same temperature in the FLUOstar OPTIMA system (BMG LabTech, Offenburg, Germany) using an excitation wavelength of 494 nm and an emission wavelength of 516 nm. Basal levels were measured for 20 sec before injection, followed by 2 sec shaking with or without agonist and 100 sec of measurement. Inhibitors (losartan and 1,2-bis (2-aminophenoxy) ethane-N,N,N′,N′-tetraacetic acid tetrakis (acetoxymethyl ester) or BAPTA-AM) were added to loading dye buffer for a 30-min incubation before experimentation.
Transfection and luciferase assay
The 5′-flanking cDNA from the human CYP11B2 gene (−1521/+2) was inserted upstream of the firefly luciferase gene in the reporter vector pGL3 Basic (Promega, Madison, WI). Transient transfection assays were performed using Transfast (Promega) reagents in a ratio of 4 μl/μg plasmid DNA. Luciferase activity was determined using the dual-luciferase assay system (Promega), and values are expressed as arbitrary light units normalized to the β-galactosidase activity resulting from cotransfection of a LacZ plasmid with the CYP11B2 promoter construct. Experimental conditions were compared with the basal condition to determine the fold change after treatment.
Animal protocol
Male Sprague Dawley (SD) rats (weight about 250–275 g) obtained from Harlan (Indianapolis, IN) were maintained on a 12-h light, 12-h dark cycle in a climate-controlled room and individually housed in hanging wire mesh cages. Rats were allowed access to food and water ad libitum and acclimated to the environment 4 d before experimentation. Sixteen rats were divided into two weight-matched groups and offered ad libitum access to either chow (Harlan Teklad Rodent Diet 8604) or chow and a 30% sucrose solution (wt/vol) for 3 wk. Body weights were measured daily for the duration of the study. Serum total TG levels were measured (Wako Chemicals, Richmond, VA) in blood samples obtained every 7 d by tail bleeding. At the end of the experiment, rats were killed by rapid decapitation to avoid changes in stress-related steroids. Trunk blood samples were collected to measure total TG, whereas adrenal glands were harvested for RNA isolation, flash frozen in liquid nitrogen, and stored at −80 C. All animal procedures were approved by the Institutional Animal Care and Use Committee of the Georgia Health Sciences University and followed the recommendations of the National Institutes of Health Intramural Animal Care and Use program.
Statistical analysis
Results are given as means ± sem. Individual experiments were repeated at least three times. Statistical analysis of one-way ANOVA (including one-way ANOVA on rank) and Student's paired t tests with a confidence interval of 95% were performed using GraphPad Prism version 3.0 (GraphPad Software, Inc., San Diego, CA). Paired ANOVA followed by a Dunnett's post hoc test was performed using GraphPad Instat software.
Results
VLDL stimulates aldosterone production in primary cultures of bovine ZG cells
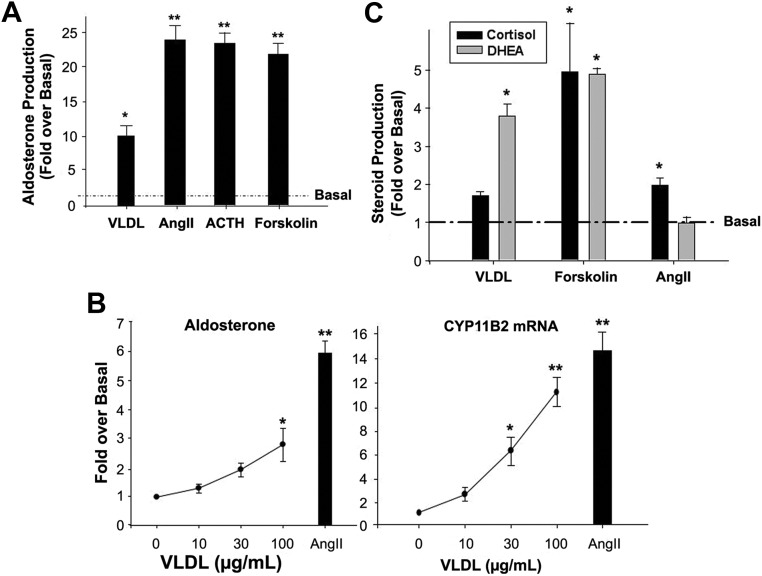
After incubation in serum-free medium for 24 h, bovine glomerulosa cells were treated with VLDL or other ligands for 6 h in low-serum medium. As expected, known agonists of adrenal cell aldosterone production, including AngII (10 nm), ACTH (10 nm), and forskolin (10 μm) stimulated aldosterone production by 20- to 25-fold. Incubation with 100 μg/ml VLDL also increased glomerulosa cell aldosterone production by approximately 10-fold (Fig. 1A).
Fig. 1.
VLDL stimulated aldosterone production in different adrenal cell model systems. A, VLDL stimulates primary bovine ZG cell aldosterone production. Bovine ZG cells were collected, as described in Materials and Methods, and incubated in serum-free medium for 20–24 h before experimentation. Cells were treated with VLDL (100 μg/ml), ACTH (10 nm), AngII (10 nm), or forskolin (10 μm) for 6 h, and medium was collected for measuring aldosterone concentration. B, VLDL dose-dependently increased aldosterone production (left) and CYP11B2 expression (right) in H295R cells. H295R cells were treated with the indicated concentrations of VLDL for 24 h. Media content of aldosterone was measured by RIA, and qPCR was used to quantify cellular CYP11B2 mRNA. qPCR data were normalized to 18S rRNA. C, VLDL stimulated DHEA production but exerted minimal effects on cortisol production in H295R cells. Cells were treated with VLDL (100 μg/ml), AngII (10 nm), or forskolin (10 μm) for 24 h, and the media content of DHEA or cortisol quantified by enzyme immunoassay. Results represent the means ± sem of data from at least three independent experiments. Statistical analyses were performed using a paired one-way ANOVA followed by a Dunnett's post hoc test. *, P < 0.05; **, P < 0.01 compared with basal condition.
VLDL stimulates aldosterone production and CYP11B2 mRNA expression in H295R cells
Incubation of H295R human adrenocortical carcinoma cells with human VLDL for 24 h increased both aldosterone production and CYP11B2 mRNA expression in a concentration-dependent manner. A maximal response for the doses of VLDL tested was detected at a concentration of 100 μg/ml (approximately 3-fold for aldosterone and 11-fold for CYP11B2) (Fig. 1B).
VLDL effects on the production of other steroids in H295R
The effects of VLDL on adrenal cell production of cortisol and DHEA were also examined. In contrast to the other two agonists (AngII and K+), VLDL significantly increased H295R DHEA production (about 4-fold) but did not increase cortisol production (Fig. 1C).
VLDL stimulates aldosterone production in primary cultures of human adrenocortical cells
Treatment of primary cultures of human adrenal cortex cells with 100 μg/ml VLDL for 48 h significantly stimulated aldosterone synthesis (∼2-fold) (Table 1). In the human cell primary cultures, maximal concentrations of ACTH and forskolin had greater effects on aldosterone production than a maximal dose of AngII (9- vs. 3-fold), which may be due to the mixed population of human adrenocortical cells. Cortisol production by these cells was tested and no significant changes were detected after a 48 h treatment with VLDL (data not shown).
Table 1.
Agonist-stimulated aldosterone production from primary cultures of human adrenocortical cells
| Aldosterone production (fold over control)† | |
|---|---|
| Control | 1.0 ± 0.1 |
| VLDL (100 μg/ml) | 2.1 ± 0.1a |
| AngII (10 nm) | 3.3 ± 0.6a |
| ACTH (10 nm) | 9.7 ± 0.9b |
| Forskolin (10 μm) | 9.3 ± 1.3b |
Steroid data were normalized to protein and expressed as fold change over basal (control). Values represent the means ± sem of six values from three separate experiments.
P < 0.01 vs. the control by an unpaired t test.
P < 0.001 vs. the control by an unpaired t test.
VLDL effects on steroidogenic enzyme expression
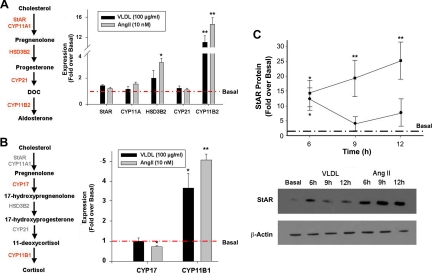
To further characterize the effects of VLDL on aldosterone production in H295R cells, we examined the expression level of mRNA encoding the proteins involved in aldosterone biosynthesis, using AngII as a positive control. As reported previously, AngII significantly increased expression of HSD3B2 and CYP11B2 (28–30), whereas in our study, VLDL treatment significantly up-regulated only CYP11B2 (Fig. 2A). Transcripts related to cortisol and DHEA production (CYP17 and CYP21) were also examined, and VLDL had no effect on CYP17 or CYP21 expression, while increasing CYP11B1 3.5-fold (Fig. 2B). As previously shown, expression of CYP11B1 is also increased by AngII (9, 31). Even though StAR mRNA was unaltered by 24 h treatment with VLDL, we measured StAR protein levels at earlier time points (Fig. 2C). Unlike AngII, which causes an increase in StAR protein synthesis that lasts up to 12 h, VLDL stimulated StAR protein levels to a peak at 6 h, followed by a return to basal levels.
Fig. 2.
VLDL effects on steroidogenic enzyme expression in H295R cells. A and B, Cells were treated with AngII (10 nm) or VLDL (100 μg/ml) for 24 h (A and B) or for the indicated time (C). RNA was isolated and reverse transcribed for qPCR quantification. qPCR data were normalized to 18S rRNA and shown as fold change compared with basal. C, Cells were lysed, and Western analysis was performed for StAR protein quantification. Western data were normalized to β-actin protein levels. Results represent the means ± sem of data from at least three independent experiments. Statistical analyses were performed using paired ANOVA followed by Dunnett's post hoc test. *, P < 0.05; **, P < 0.01, compared with the basal condition.
Time-dependent effects of VLDL on aldosterone production and CYP11B2 expression
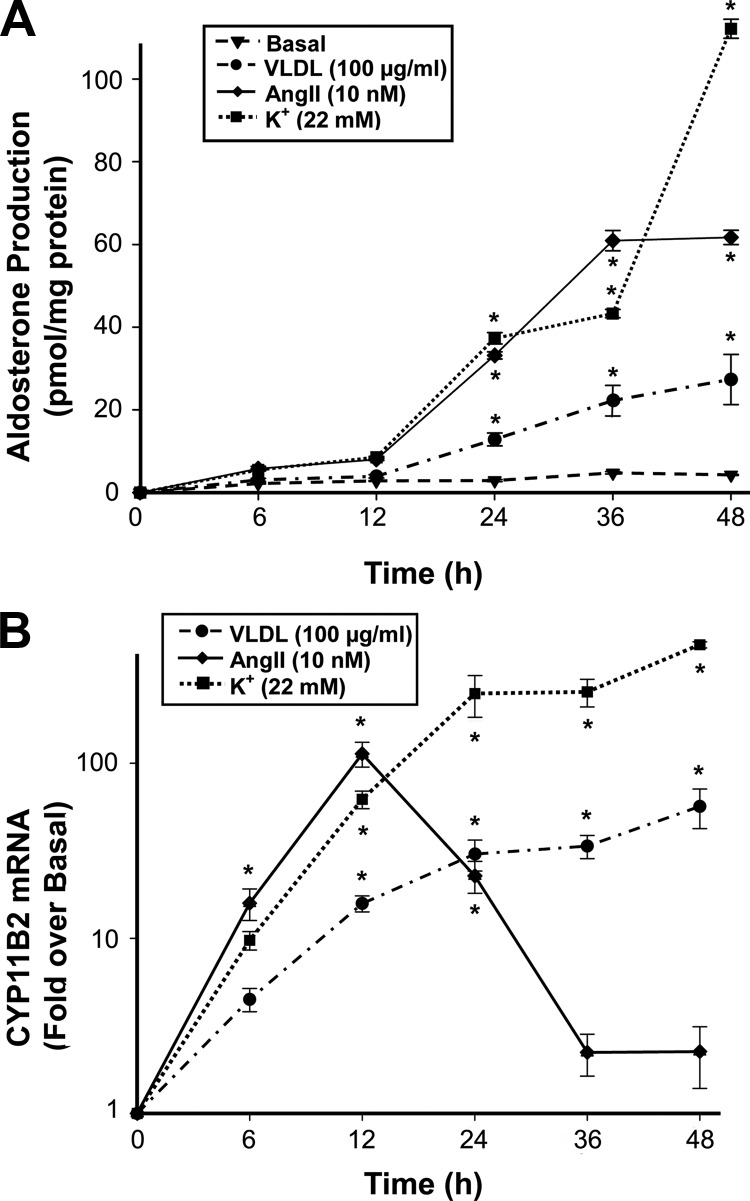
The time-dependent effects of VLDL on aldosterone production and CYP11B2 mRNA expression were also examined in H295R cells (Fig. 3) and compared with the positive controls, AngII and elevated extracellular K+. All three agonists significantly increased aldosterone production in a time-dependent manner. Whereas AngII effects on aldosterone production plateaued after 36 h, VLDL and K+ continuously increased H295R cell aldosterone synthesis (Fig. 3A), suggesting that the latter two agonists induce a nondesensitizing response compared with AngII. In agreement with the aldosterone response, AngII caused a significant time-dependent increase in CYP11B2 mRNA that peaked at 12 h. This increase was short-lived, and CYP11B2 transcript levels were returned almost to basal levels by 48 h. In contrast, K+ stimulated CYP11B2 transcript levels by approximately 100-fold over basal, and this effect reached an approximate plateau after 12 h. Similar to the effects of K+ treatment, VLDL (100 μg/ml) produced a slow increase in CYP11B2 transcript levels that essentially plateaued after 24 h at 30-fold over those observed in untreated cells (Fig. 3B).
Fig. 3.
VLDL affects aldosterone production and CYP11B2 expression in a time-dependent manner. H295R cells were treated with VLDL (100 μg/ml), AngII (10 nm), or K+ (22 mm) for the indicated times. A, Media content of aldosterone was measured by RIA and normalized to protein concentration. B, CYP11B2 expression level was quantified by qPCR and normalized to 18S rRNA. Data are shown as the fold change compared with basal. Results represent the mean ± sem from at least three independent experiments. Statistical analyses were performed using one-way ANOVA followed by a Dunnett's post hoc test. *, P < 0.05 vs. the basal condition.
Effects of VLDL alone or combined with aldosterone-stimulating agonists on CYP11B2 expression
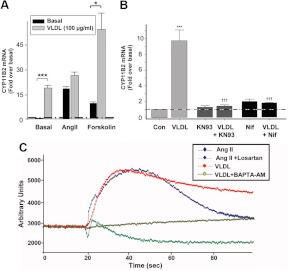
H295R cells were treated with VLDL (100 μg/ml) alone or together with AngII (10 nm) or forskolin (10 μm) for 24 h (Fig. 4A). Although VLDL alone stimulated CYP11B2 transcript levels by approximately 15-fold, this effect was not additive with AngII. Similarly, VLDL did not influence K+ stimulation of CYP11B2 transcript levels (data not shown). However, when combined with forskolin (a cAMP pathway agonist), VLDL increased CYP11B2 transcription from approximately 15-fold in the presence of forskolin alone to more than 35-fold above basal levels with the combination. This result strongly suggests that VLDL uses a signaling pathway different from cAMP, but one shared by AngII.
Fig. 4.
Calcium signaling pathways mediated the VLDL-induced stimulation of adrenal cell aldosterone production. A, The effects of VLDL on CYP11B2 expression was additive with the aldosterone-stimulating agonist forskolin but not with AngII. H295R cells were treated with AngII (10 nm) or forskolin (10 μm) with/without VLDL for 24 h, and CYP11B2 expression measured by qPCR. Statistical analyses were performed using ANOVA on rank followed by a Dunnett's post hoc test. *, P < 0.05; ***, P < 0.001 vs. the indicated condition. B, Calcium signaling antagonists inhibited VLDL stimulation of CYP11B2. H295R cells were preincubated with nifedipine (10 μm) or KN93 (3 μm) for 20 min followed by treatment with agonists (24 h). CYP11B2 qPCR data were normalized to 18S rRNA and shown as fold change compared with untreated basal cells. Results represent the means ± sem of data from at least three independent experiments. Statistical analyses were performed using ANOVA followed by a Student-Newman-Keuls post hoc test. ***, P < 0.001 vs. the control; †††, P < 0.001, compared with VLDL treatment alone. C, VLDL increased the intracellular free calcium concentration in H295R cells. Cells were allowed to grow to 80–90% confluence on a 96-well plate and incubated with fluorescent dye (100 μl) for 40 min before use. Cells were preincubated with and without BAPTA-AM (20 μm) or losartan (10 μm) for 30 min before intracellular free calcium measurement. Fluorescence intensity was recorded for the 20 sec before and 80 sec after agonist addition.
VLDL stimulates adrenal cell CYP11B2 through calcium signaling pathways
The role of calcium signaling in VLDL-stimulated CYP11B2 expression was studied using the calcium channel blocker nifedipine and the calcium/calmodulin kinase inhibitor KN93 (Fig. 4B). Preincubation of H295R cells with 10 μm nifedipine or 3 μm KN93 completely blocked VLDL effects on CYP11B2. These concentrations of KN93 and nifedipine were previously found to exert little or a minimal inhibitory effect on dibutyryl-cAMP-induced CYP11B2 expression (6, 32). This inhibition suggests that VLDL signals mainly through calcium. In agreement with previous results, both nifedipine and KN93 partially decreased the stimulating effects of AngII (5, 6, 30, 32), indicating the involvement of other signaling cascades in the effects of AngII (data not shown).
Our results were confirmed by examination of intracellular free calcium concentrations in H295R cells (Fig. 4C). As published previously (33–35), AngII caused a significant, but transient, increase in intracellular free calcium concentration that was completely blocked by the AT1 receptor antagonist losartan. VLDL induced a calcium peak similar in magnitude to that caused by AngII, but this increase was maintained for more than 100 sec. The calcium chelator BAPTA-AM blocked this increase in intracellular free calcium concentration, whereas incubation with losartan had no effect on VLDL-induced calcium changes (data not shown).
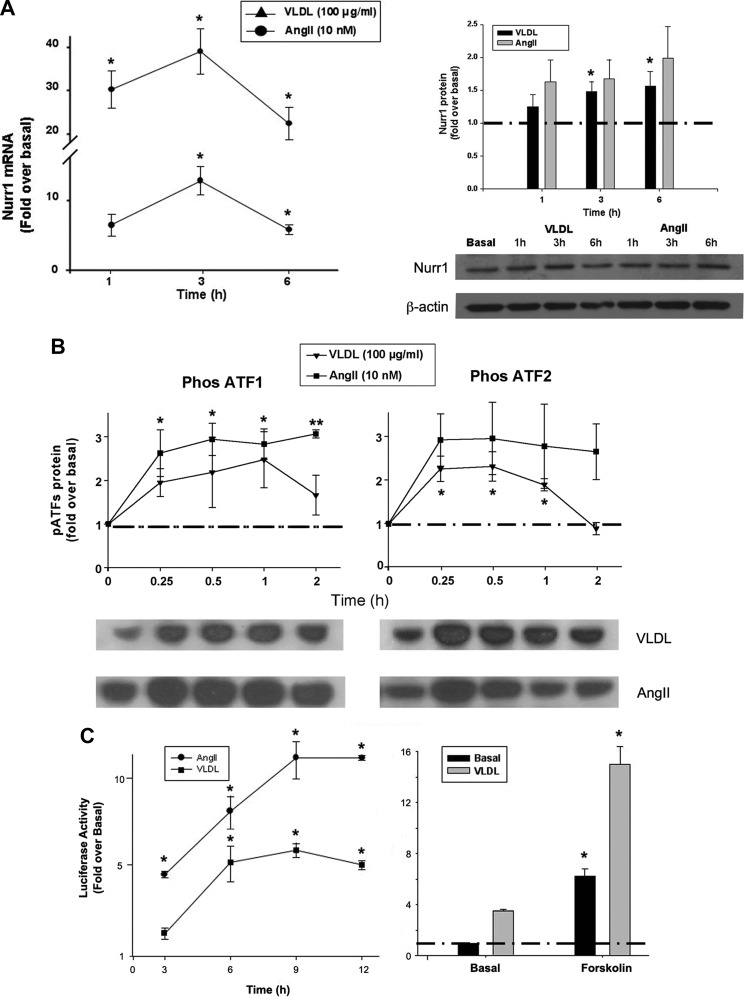
We have previously demonstrated a role for the transcription factor nuclear receptor related protein 1 (NURR1) in the transcription of CYP11B2 (9, 36). VLDL caused a time-dependent increase in both NURR1 transcript and protein expression (approximately 5-fold at 3 h for mRNA and 2-fold at 6 h for protein) (Fig. 5A) and phosphorylation of the activating transcription factor (ATF) family member ATF2 (Fig. 5B), suggesting that a VLDL-triggered signaling cascade activates the expression of NURR1 and the phosphorylation of ATF2, resulting in increased CYP11B2 transcription. VLDL exhibited a trend toward enhancing ATF1 phosphorylation as well, but this increase did not reach statistical significance (Fig. 5B).
Fig. 5.
VLDL stimulated the activities of the transcription factors NURR1 and ATF2 in a time-dependent manner. A, VLDL stimulated mRNA and protein expression of NURR1 in a time-dependent manner. H295R cells were treated with AngII (10 nm) or VLDL (100 μg/ml) for the indicated time before harvesting the cells. qPCR was used for mRNA transcript detection, and Western blotting was used for protein quantification. B, VLDL stimulated phosphorylation of ATF2 in a time-dependent manner. H295R cells were treated with AngII (10 nm) or VLDL (100 μg/ml) for the indicated time before harvesting the cells, and Western analysis was used for protein quantification. Results represent the means ± sem of four separate experiments analyzed (separately for each agonist) by paired ANOVA followed by a Dunnett's post hoc test. *, P < 0.05; **, P < 0.01 vs. time 0. C, VLDL stimulated CYP11B2 promoter activity, and this effect was additive with forskolin. H295R cells were transfected with the CYP11B2 promoter construct (1 μg/ml) and allowed to recover overnight. Transfected cells were treated with or without VLDL (100 μg/ml) or AngII (10 nm) for the indicated time (left panel) or VLDL (100 μg/ml) with or without forskolin (10 nm) for 9 h (right panel). Data were normalized to a cotransfected β-galactosidase vector and shown as the fold induction over the untreated group. Results represent the means ± sem from at least three independent experiments, each performed in triplicate. Statistical analyses were performed using one-way ANOVA followed by a Dunnett's post hoc test (left panel) or Student's t test (right panel), *, P < 0.05 vs. time 0 or the basal value.
VLDL, alone or in combination with forskolin, stimulates CYP11B2 promoter activity
H295R cells were transfected with a vector possessing the 5′ promoter region of CYP11B2 and treated with VLDL or AngII for 3–12 h. VLDL significantly stimulated promoter activity in a time-dependent manner, starting from 6 h and peaking around 9 h, with a maximal stimulation of 5-fold (Fig. 5C, left panel). Similar to VLDL, AngII also stimulated CYP11B2 promoter activity over a shorter time period, but to a greater degree. In combination with forskolin, VLDL exhibited additive effects to those observed with the cAMP agonist alone at 9 h (Fig. 5C, right panel).
VLDL levels correlate with CYP11B2 mRNA in vivo
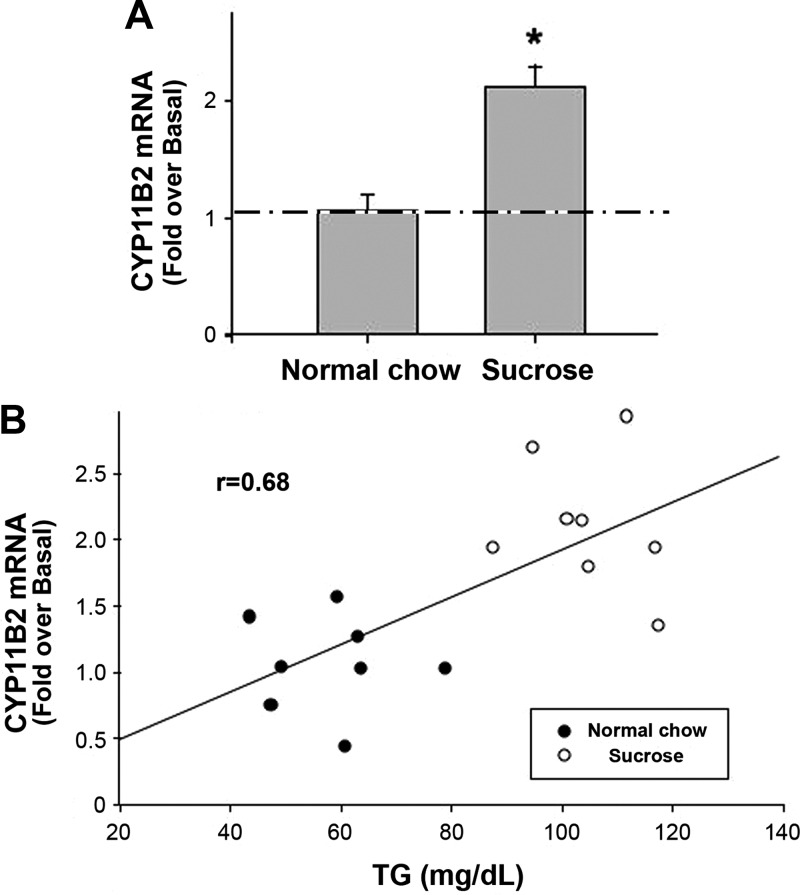
To study the possible role of VLDL on aldosterone secretory capacity in vivo, SD rats were fed with normal chow or chow and a 30% sucrose solution for 3 wk before they were killed. As shown in Fig. 6A, a diet of chow and 30% sucrose solution increased CYP11B2 mRNA levels 2-fold in the adrenal gland of SD rats compared with chow-fed controls.
Fig. 6.
Rat adrenal CYP11B2 transcript levels were increased by a TG-elevating diet in vivo. A, Consumption of sucrose solution in addition to chow increased CYP11B2 mRNA level in rats. Male SD rats were fed with a diet of normal chow or chow with a sucrose solution for 3 wk before they were killed. Blood was collected for TG concentration determination, and RNA was extracted from the whole adrenal gland for quantification of CYP11B2 transcripts. qPCR data were normalized to 18S rRNA and shown as fold change compared with control (normal chow-fed rats). Results represent the means ± sem of data from eight rats per group. Statistical analyses were performed using a Student's t test. *, P < 0.05, compared with normal chow-diet group. B, TG levels correlated with CYP11B2 mRNA in sucrose- and normal chow-fed rats. The control (normal chow) value for TG levels was 55 ± 4 mg/dl.
The major transporters of TG in serum are chylomicrons and VLDL, and TG comprise a large percentage of the weight of these particles. Therefore, in a fasted state when few chylomicrons circulate in the serum, TG levels should predominantly reflect the contribution of VLDL. Because in these experiments, at the time of killing, the rats were in a postabsorptive state (3–4 h fasting), TG levels are an indirect measure of VLDL levels. Interestingly, TG levels correlated nicely with CYP11B2 mRNA transcript levels in the normal- and sucrose-diet groups (Fig. 6B).
Discussion
VLDL is a class of large lipoproteins with a density ranging between 0.95 and 1.006 g/ml. VLDL is synthesized in the liver and transports fatty acids and TG to peripheral tissues (37). Upon binding to its receptor, VLDL particles are taken up through the endocytotic process, during which the VLDL/receptor complex fuses with lysosomes to release the loaded TG, fatty acids, and phospholipids. Besides its traditional role in lipid/TG transport, VLDL has also been proposed to activate signaling pathways, including the MAPK and ERK1/2 as well as Wnt and Src in different tissues (18, 19, 21, 23). The results presented here suggest that adrenocortical cells respond to VLDL by increasing intracellular free calcium levels via calcium influx through voltage-dependent calcium channels, leading to stimulated expression of CYP11B2 and StAR protein. The normal, circulating concentration of VLDL in human plasma is approximately 100 μg/ml, which is similar to the effective doses used in the studies mentioned above and in our current study on adrenocortical cells. The ability of physiological concentrations of VLDL to act on adrenal cells suggests that VLDL may influence aldosterone production in vivo.
On the other hand, some studies have found no correlation between VLDL and aldosterone levels. Lopes et al. (38) examined correlations among various metabolic and cardiovascular parameters in normotensive, normal-weight offspring with a family history of hypertension vs. those with no family history of this disorder. VLDL levels were slightly, but significantly, elevated in the offspring with a family history of hypertension, although they were still in the normal range; however, there was no significant difference in aldosterone levels. Nonetheless, the group size was small, with 20 individuals with a family history of hypertension and 22 without such a history, and it seems possible that the small sample size might obscure any potential differences in serum aldosterone levels. There are also concerns that variation in physiological aldosterone levels are greatly impacted by dietary sodium, which was not controlled in this study. In addition, Campbell (39) demonstrated no effect or an inhibitory action of VLDL on basal and/or K+-stimulated aldosterone secretion from rat ZG cells. However, this author used rat VLDL, which has a slightly different composition from the human VLDL (40) used in our study.
Obesity is recognized as a major health problem throughout the world. Obese patients typically have increased lipoprotein (VLDL, LDL, and intermediate-density lipoprotein) levels and an elevated risk of hypertension and cardiovascular disease (41, 42). Although excess body fat (especially visceral fat) has been determined to be a major cause of increased blood pressure in most patients with essential hypertension, it remains unclear as to how excess fat deposits result in increased blood pressure. Several mechanisms have been proposed including the activation of the sympathetic system and RAAS, oversecretion of adipokines, and physical compression of the kidneys, especially when visceral obesity is present (43–45). Although several reports have shown that aldosterone levels are a major link between obesity and hypertension (45–50), an activated RAAS may not be the sole cause of increased aldosterone levels, because aldosterone levels in obese patients are higher than would be predicted by renin concentrations. Additionally, aldosterone/renin ratios are normally elevated in obese patients (51–53). This association is more obvious in obese patients on a high-salt diet, in which renin activity is suppressed (54). These observations raise the possibility that in obese patients, there is an alternative aldosterone regulatory system in addition to AngII, ACTH, and potassium.
In the rat model of diet-induced obesity reported here, the increase in VLDL levels relates to the fructose content of the diet. The majority (98%) of fructose is taken up by the liver in an insulin-independent manner. In sucrose-drinking rats, the fructose is accompanied by glucose, which induces insulin secretion and the promotion of glycolysis, glycogen synthesis, and activity of the pentose phosphate pathway, but in conditions of high-energy intake, glucose entry into the glycolytic pathway is limited by feedback inhibition of phosphofructokinase. Fructose enters glycolysis independent of this enzyme, thereby increasing pyruvate and acetyl-coenzyme A production. Under conditions of high-energy intake, acetyl-coenzyme A will be shunted toward lipid synthesis rather than to the tricarboxylic acid cycle. Therefore, it is not necessarily obesity that is promoting TG synthesis, but the combination of a high-energy intake with high fructose ingestion. These synthesized TG are then transported via VLDL throughout the body.
Our results suggest that in addition to contributing to atherosclerotic progression, VLDL may also act to directly influence basal and ACTH-stimulated aldosterone biosynthesis by the adrenal gland. It should be noted that the primary cultures of bovine adrenal glomerulosa cells are unlikely to be completely pure; similarly, the primary human adrenocortical cells are clearly a mixed population. Nevertheless, the ability of VLDL to stimulate aldosterone secretion from both of these primary cultures as well as the human adrenocortical carcinoma cell line H295R indicates the universality of this response (Fig. 1 and Table 1). Furthermore, VLDL's enhancement of CYP11B2 expression in the human H295R cells (Fig. 1B) suggests that the lipoprotein can increase the capacity of the adrenal gland to synthesize aldosterone.
On the other hand, although VLDL addition did not significantly stimulate cortisol production, the lipoprotein increased CYP11B1 expression (Fig. 1C). In addition, VLDL increased DHEA production but not CYP17 expression. These seeming disparities are likely the result of some differences in the production of these two adrenocortical steroids in comparison with aldosterone. Thus, the H295R cells basally synthesize large amounts of cortisol (much greater absolute quantities than those of aldosterone); the high basal background limits the ability to detect small increases in cortisol synthesis. Furthermore, the expression/levels of CYP11B1 are not rate limiting in the synthesis of cortisol, whereas CYP11B2 is the late rate-limiting step for aldosterone production. Similarly, the expression/levels of CYP17 are not rate limiting for the production of DHEA; rather, for both of these steroids, the rate-limiting step is StAR, the expression of which is increased by VLDL (Fig. 2).
In a previous study, we have shown that another lipoprotein, HDL, can stimulate aldosterone production mainly by activating CYP11B2 expression (55). However, compared with HDL, VLDL is more effective at the same concentration and further affects StAR expression, which makes VLDL a better aldosterone stimulator in adrenal cells. Preincubating the cells with a VLDL receptor blocker did not influence VLDL's activity (data not shown), suggesting that in contrast to HDL, VLDL functions in a receptor-independent manner to stimulate CYP11B2 expression.
In summary, we have shown for the first time that physiological concentrations of VLDL, a member of the lipoprotein family, can stimulate aldosterone production in adrenocortical cells. The expression of both rate-limiting proteins involved in aldosterone production (StAR and CYP11B2) were up-regulated by this lipoprotein. The activity of VLDL was mediated by increased intracellular free calcium levels and the expression/activation of the transcription factors NURR1 and ATF2 (and perhaps ATF1). Our results suggest that VLDL may be a potential mediator of obesity-induced hypertension.
Acknowledgments
The authors were supported by an American Heart Association Predoctoral Fellowship (to Y.X.), National Institutes of Health Awards DK43140 and DK69950 (to W.E.R.), DK53903 (to R.B.S.H.), and Veterans Affairs Merit and Georgia Health Sciences University Diabetes and Obesity Discovery Institute Synergy Awards (to W.B.B.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AngII
- Angiotensin II
- ATF
- activating transcription factor
- BAPTA-AM
- 1,2-bis (2-aminophenoxy) ethane-N,N,N′,N′-tetraacetic acid tetrakis (acetoxymethyl ester)
- CYP11B2
- aldosterone synthase
- DHEA
- dehydroepiandrosterone
- HDL
- high-density lipoprotein
- NURR1
- nuclear receptor related protein 1
- qPCR
- quantitative real-time RT-PCR
- RAAS
- renin/angiotensin II/aldosterone system
- SD
- Sprague Dawley
- StAR
- steroidogenic acute regulatory
- TG
- triglyceride
- VLDL
- very-low-density lipoprotein
- ZG
- zona glomerulosa.
References
- 1. Mornet E, Dupont J, Vitek A, White PC. 1989. Characterization of two genes encoding human steroid 11β-hydroxylase (P-45011β). J Biol Chem 264:20961–20967 [PubMed] [Google Scholar]
- 2. Kater CE, Biglieri EG, Rost CR, Schambelan M, Hirai J, Chang BC, Brust N. 1985. The constant plasma 18-hydroxycorticosterone to aldosterone ratio: an expression of the efficacy of corticosterone methyloxidase type II activity in disorders with variable aldosterone production. J Clin Endocrinol Metab 60:225–228 [DOI] [PubMed] [Google Scholar]
- 3. Ogishima T, Suzuki H, Hata J, Mitani F, Ishimura Y. 1992. Zone-specific expression of aldosterone synthase cytochrome P-450 and cytochrome P-45011β in rat adrenal cortex: histochemical basis for the functional zonation. Endocrinology 130:2971–2977 [DOI] [PubMed] [Google Scholar]
- 4. Wotus C, Levay-Young BK, Rogers LM, Gomez-Sanchez CE, Engeland WC. 1998. Development of adrenal zonation in fetal rats defined by expression of aldosterone synthase and 11β-hydroxylase. Endocrinology 139:4397–4403 [DOI] [PubMed] [Google Scholar]
- 5. Condon JC, Pezzi V, Drummond BM, Yin S, Rainey WE. 2002. Calmodulin-dependent kinase I regulates adrenal cell expression of aldosterone synthase. Endocrinology 143:3651–3657 [DOI] [PubMed] [Google Scholar]
- 6. Pezzi V, Clyne CD, Ando S, Mathis JM, Rainey WE. 1997. Ca(2+)-regulated expression of aldosterone synthase is mediated by calmodulin and calmodulin-dependent protein kinases. Endocrinology 138:835–838 [DOI] [PubMed] [Google Scholar]
- 7. Lu L, Suzuki T, Yoshikawa Y, Murakami O, Miki Y, Moriya T, Bassett MH, Rainey WE, Hayashi Y, Sasano H. 2004. Nur-related factor 1 and nerve growth factor-induced clone B in human adrenal cortex and its disorders. J Clin Endocrinol Metab 89:4113–4118 [DOI] [PubMed] [Google Scholar]
- 8. Bassett MH, Mayhew B, Rehman K, White PC, Mantero F, Arnaldi G, Stewart PM, Bujalska I, Rainey WE. 2005. Expression profiles for steroidogenic enzymes in adrenocortical diseases. J Clin Endocrinol Metab 90:5446–5455 [DOI] [PubMed] [Google Scholar]
- 9. Bassett MH, Suzuki T, Sasano H, White PC, Rainey WE. 2004. The orphan nuclear receptors NURR1 and NGFIB regulate adrenal aldosterone production. Mol Endocrinol 18:279–290 [DOI] [PubMed] [Google Scholar]
- 10. Bassett MH, White PC, Rainey WE. 2004. A role for the NGFI-B family in adrenal zonation and adrenocortical disease. Endocr Res 30:567–574 [DOI] [PubMed] [Google Scholar]
- 11. Kagerer SM, Eichholz C, Jöhren O. 2011. Orexins/hypocretins increase the promoter activity of selective steroidogenic enzymes. Peptides 32:839–843 [DOI] [PubMed] [Google Scholar]
- 12. Otani H, Otsuka F, Inagaki K, Suzuki J, Makino H. 2010. Roles of bone morphogenetic protein-6 in aldosterone regulation by adrenocortical cells. Acta Med Okayama 64:213–218 [DOI] [PubMed] [Google Scholar]
- 13. Ko T, Kakizoe Y, Wakida N, Hayata M, Uchimura K, Shiraishi N, Miyoshi T, Adachi M, Aritomi S, Konda T, Tomita K, Kitamura K. 2010. Regulation of adrenal aldosterone production by serine protease prostasin. J Biomed Biotechnol 2010:793843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kageyama K, Hanada K, Suda T. 2010. Differential regulation and roles of urocortins in human adrenal H295R cells. Regul Pept 162:18–25 [DOI] [PubMed] [Google Scholar]
- 15. Somekawa S, Imagawa K, Naya N, Takemoto Y, Onoue K, Okayama S, Takeda Y, Kawata H, Horii M, Nakajima T, Uemura S, Mochizuki N, Saito Y. 2009. Regulation of aldosterone and cortisol production by the transcriptional repressor neuron restrictive silencer factor. Endocrinology 150:3110–3117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mikhaylova IV, Jääskeläinen T, Jääskeläinen J, Palvimo JJ, Voutilainen R. 2008. Leukemia inhibitory factor as a regulator of steroidogenesis in human NCI-H295R adrenocortical cells. J Endocrinol 199:435–444 [DOI] [PubMed] [Google Scholar]
- 17. Mikhaylova IV, Kuulasmaa T, Jääskeläinen J, Voutilainen R. 2007. Tumor necrosis factor-α regulates steroidogenesis, apoptosis, and cell viability in the human adrenocortical cell line NCI-H295R. Endocrinology 148:386–392 [DOI] [PubMed] [Google Scholar]
- 18. Frontini MJ, Nong Z, Gros R, Drangova M, O'Neil C, Rahman MN, Akawi O, Yin H, Ellis CG, Pickering JG. 2011. Fibroblast growth factor 9 delivery during angiogenesis produces durable, vasoresponsive microvessels wrapped by smooth muscle cells. Nat Biotechnol 29:421–427 [DOI] [PubMed] [Google Scholar]
- 19. Sekine Y, Koike H, Nakano T, Nakajima K, Suzuki K. 2007. Remnant lipoproteins stimulate proliferation and activate MAPK and Akt signaling pathways via G protein-coupled receptor in PC-3 prostate cancer cells. Clin Chim Acta 383:78–84 [DOI] [PubMed] [Google Scholar]
- 20. Olufadi R, Byrne CD. 2006. Effects of VLDL and remnant particles on platelets. Pathophysiol Haemost Thromb 35:281–291 [DOI] [PubMed] [Google Scholar]
- 21. Liu Z, Li H, Li Y, Wang Y, Zong Y, Feng Y, Feng Z, Deng Y, Qu S. 2009. Up-regulation of VLDL receptor expression and its signaling pathway induced by VLDL and β-VLDL. J Huazhong Univ Sci Technolog Med Sci 29:1–7 [DOI] [PubMed] [Google Scholar]
- 22. Banfi C, Mussoni L, Risé P, Cattaneo MG, Vicentini L, Battaini F, Galli C, Tremoli E. 1999. Very low density lipoprotein-mediated signal transduction and plasminogen activator inhibitor type 1 in cultured HepG2 cells. Circ Res 85:208–217 [DOI] [PubMed] [Google Scholar]
- 23. Chen Y, Hu Y, Lu K, Flannery JG, Ma JX. 2007. Very low density lipoprotein receptor, a negative regulator of the wnt signaling pathway and choroidal neovascularization. J Biol Chem 282:34420–34428 [DOI] [PubMed] [Google Scholar]
- 24. Rehman KS, Sirianni R, Parker CR, Jr, Rainey WE, Carr BR. 2007. The regulation of adrenocorticotrophic hormone receptor by corticotropin-releasing hormone in human fetal adrenal definitive/transitional zone cells. Reprod Sci 14:578–587 [DOI] [PubMed] [Google Scholar]
- 25. Qin H, Frohman MA, Bollag WB. 2010. Phospholipase D2 mediates acute aldosterone secretion in response to angiotensin II in adrenal glomerulosa cells. Endocrinology 151:2162–2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yancey PG, de la Llera-Moya M, Swarnakar S, Monzo P, Klein SM, Connelly MA, Johnson WJ, Williams DL, Rothblat GH. 2000. High density lipoprotein phospholipid composition is a major determinant of the bi-directional flux and net movement of cellular free cholesterol mediated by scavenger receptor BI. J Biol Chem 275:36596–36604 [DOI] [PubMed] [Google Scholar]
- 27. Ye P, Mariniello B, Mantero F, Shibata H, Rainey WE. 2007. G-protein-coupled receptors in aldosterone-producing adenomas: a potential cause of hyperaldosteronism. J Endocrinol 195:39–48 [DOI] [PubMed] [Google Scholar]
- 28. Ye P, Kenyon CJ, MacKenzie SM, Seckl JR, Fraser R, Connell JM, Davies E. 2003. Regulation of aldosterone synthase gene expression in the rat adrenal gland and central nervous system by sodium and angiotensin II. Endocrinology 144:3321–3328 [DOI] [PubMed] [Google Scholar]
- 29. Rainey WE, Naville D, Mason JI. 1991. Regulation of 3 β-hydroxysteroid dehydrogenase in adrenocortical cells: effects of angiotensin-II and transforming growth factor-β. Endocr Res 17:281–296 [DOI] [PubMed] [Google Scholar]
- 30. Szekeres M, Turu G, Orient A, Szalai B, Süpeki K, Cserzo M, Várnai P, Hunyady L. 2009. Mechanisms of angiotensin II-mediated regulation of aldosterone synthase expression in H295R human adrenocortical and rat adrenal glomerulosa cells. Mol Cell Endocrinol 302:244–253 [DOI] [PubMed] [Google Scholar]
- 31. Ye P, Nakamura Y, Lalli E, Rainey WE. 2009. Differential effects of high and low steroidogenic factor-1 expression on CYP11B2 expression and aldosterone production in adrenocortical cells. Endocrinology 150:1303–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Denner K, Rainey WE, Pezzi V, Bird IM, Bernhardt R, Mathis JM. 1996. Differential regulation of 11β-hydroxylase and aldosterone synthase in human adrenocortical H295R cells. Mol Cell Endocrinol 121:87–91 [DOI] [PubMed] [Google Scholar]
- 33. Burnay MM, Python CP, Vallotton MB, Capponi AM, Rossier MF. 1994. Role of the capacitative calcium influx in the activation of steroidogenesis by angiotensin-II in adrenal glomerulosa cells. Endocrinology 135:751–758 [DOI] [PubMed] [Google Scholar]
- 34. Quinn SJ, Williams GH, Tillotson DL. 1988. Calcium oscillations in single adrenal glomerulosa cells stimulated by angiotensin II. Proc Natl Acad Sci USA 85:5754–5758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Startchik I, Morabito D, Lang U, Rossier MF. 2002. Control of calcium homeostasis by angiotensin II in adrenal glomerulosa cells through activation of p38 MAPK. J Biol Chem 277:24265–24273 [DOI] [PubMed] [Google Scholar]
- 36. Nogueira EF, Xing Y, Morris CA, Rainey WE. 2009. Role of angiotensin II-induced rapid response genes in the regulation of enzymes needed for aldosterone synthesis. J Mol Endocrinol 42:319–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dallinga-Thie GM, Franssen R, Mooij HL, Visser ME, Hassing HC, Peelman F, Kastelein JJ, Péterfy M, Nieuwdorp M. 2010. The metabolism of triglyceride-rich lipoproteins revisited: new players, new insight. Atherosclerosis 211:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lopes HF, Silva HB, Soares JA, Filho B, Consolim-Colombo FM, Giorgi DM, Krieger EM. 1997. Lipid metabolism alteraton in normotensive subjects with positive family history of hypertension. Hypertension 30:629–631 [DOI] [PubMed] [Google Scholar]
- 39. Campbell DJ. 1982. Effect of rat plasma lipoproteins on aldosterone production by rat zona glomerulosa cells in vitro. J Steroid Biochem 17:709–711 [DOI] [PubMed] [Google Scholar]
- 40. Tso P, Ragland JB, Sabesin SM. 1983. Isolation and characterization of lipoprotein of density less than 1.006 g/ml from rat hepatic lymph. J Lipid Res 24:810–820 [PubMed] [Google Scholar]
- 41. Martins IJ, Redgrave TG. 2004. Obesity and post-prandial lipid metabolism. Feast or famine? J Nutr Biochem 15:130–141 [DOI] [PubMed] [Google Scholar]
- 42. Yoshino G, Hirano T, Kazumi T. 1996. Dyslipidemia in diabetes mellitus. Diabetes Res Clin Pract 33:1–14 [DOI] [PubMed] [Google Scholar]
- 43. da Silva AA, do Carmo J, Dubinion J, Hall JE. 2009. The role of the sympathetic nervous system in obesity-related hypertension. Curr Hypertens Rep 11:206–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bogaert YE, Linas S. 2009. The role of obesity in the pathogenesis of hypertension. Nat Clin Pract Nephrol 5:101–111 [DOI] [PubMed] [Google Scholar]
- 45. Dall'Asta C, Vedani P, Manunta P, Pizzocri P, Marchi M, Paganelli M, Folli F, Pontiroli AE. 2009. Effect of weight loss through laparoscopic gastric banding on blood pressure, plasma renin activity and aldosterone levels in morbid obesity. Nutr Metab Cardiovasc Dis 19:110–114 [DOI] [PubMed] [Google Scholar]
- 46. Kidambi S, Kotchen JM, Krishnaswami S, Grim CE, Kotchen TA. 2009. Aldosterone contributes to blood pressure variance and to likelihood of hypertension in normal-weight and overweight African Americans. Am J Hypertens 22:1303–1308 [DOI] [PubMed] [Google Scholar]
- 47. Fallo F, Dalla Pozza A, Tecchio M, Tona F, Sonino N, Ermani M, Catena C, Bertello C, Mulatero P, Sabato N, Fabris B, Sechi LA. 2010. Nonalcoholic fatty liver disease in primary aldosteronism: a pilot study. Am J Hypertens 23:2–5 [DOI] [PubMed] [Google Scholar]
- 48. Nagase M, Fujita T. 2009. Mineralocorticoid receptor activation in obesity hypertension. Hypertens Res 32:649–657 [DOI] [PubMed] [Google Scholar]
- 49. Goodfriend TL, Calhoun DA. 2004. Resistant hypertension, obesity, sleep apnea, and aldosterone: theory and therapy. Hypertension 43:518–524 [DOI] [PubMed] [Google Scholar]
- 50. Egan BM, Stepniakowski K, Goodfriend TL. 1994. Renin and aldosterone are higher and the hyperinsulinemic effect of salt restriction greater in subjects with risk factors clustering. Am J Hypertens 7:886–893 [DOI] [PubMed] [Google Scholar]
- 51. Hiramatsu K, Yamada T, Ichikawa K, Izumiyama T, Nagata H. 1981. Changes in endocrine activities relative to obesity in patients with essential hypertension. J Am Geriatr Soc 29:25–30 [DOI] [PubMed] [Google Scholar]
- 52. Rocchini AP, Katch VL, Grekin R, Moorehead C, Anderson J. 1986. Role for aldosterone in blood pressure regulation of obese adolescents. Am J Cardiol 57:613–618 [DOI] [PubMed] [Google Scholar]
- 53. Goodfriend TL, Egan BM, Kelley DE. 1999. Plasma aldosterone, plasma lipoproteins, obesity and insulin resistance in humans. Prostaglandins Leukot Essent Fatty Acids 60:401–405 [DOI] [PubMed] [Google Scholar]
- 54. Goodfriend TL, Egan BM, Kelley DE. 1998. Aldosterone in obesity. Endocr Res 24:789–796 [DOI] [PubMed] [Google Scholar]
- 55. Xing Y, Cohen A, Rothblat G, Sankaranarayanan S, Weibel G, Royer L, Francone OL, Rainey WE. 2011. Aldosterone production in human adrenocortical cells is stimulated by high-density lipoprotein 2 (HDL2) through increased expression of aldosterone synthase (CYP11B2). Endocrinology 152:751–763 [DOI] [PMC free article] [PubMed] [Google Scholar]