Abstract
Central glucagon-like-peptide-1 (GLP-1) receptor activation reduces food intake; however, brain nuclei and mechanism(s) mediating this effect remain poorly understood. Although central nervous system GLP-1 is produced almost exclusively in the nucleus of the solitary tract in the hindbrain, GLP-1 receptors (GLP-1R) are expressed throughout the brain, including nuclei in the mesolimbic reward system (MRS), e.g. the ventral tegmental area (VTA) and the nucleus accumbens (NAc). Here, we examine the MRS as a potential site of action for GLP-1-mediated control of food intake and body weight. Double immunohistochemistry for Fluorogold (monosynaptic retrograde tracer) and GLP-1 neuron immunoreactivity indicated that GLP-1-producing nucleus tractus solitarius neurons project directly to the VTA, the NAc core, and the NAc shell. Pharmacological data showed that GLP-1R activation in the VTA, NAc core, and NAc shell decreased food intake, especially of highly-palatable foods, and body weight. Moreover, blockade of endogenous GLP-1R signaling in the VTA and NAc core resulted in a significant increase in food intake, establishing a physiological relevance for GLP-1 signaling in the MRS. Current data highlight these nuclei within the MRS as novel sites for GLP-1R-mediated control of food intake and body weight.
The incidence of obesity and type 2 diabetes mellitus (T2DM) has risen dramatically in the United States over the past several decades. Given the associated physiological impairments that exist between T2DM and obesity, a range of reports have focused attention on the merits of the glucagon-like-peptide-1 (GLP-1) system in offering positive treatment outcomes for T2DM (1–3) and, in some cases, for obesity as well (3–5). Indeed, GLP-1 receptor (GLP-1R) agonists reduce food intake in both humans and animal models (4–12). Recent evidence indicates that the intake-suppressive effects of GLP-1R ligands are mediated, in part, through direct central GLP-1R signaling after either peripheral or central administration (12–15); however, the nuclei and mechanism(s) mediating these effects remain largely uninvestigated. GLP1-R are expressed in multiple central nervous system (CNS) nuclei including classic homeostatic feeding sites in the hypothalamus (e.g. paraventricular nucleus of the hypothalamus) and hindbrain [e.g. nucleus tractus solitarius (NTS)] (16, 17). Although food intake responses after GLP-1R ligand administration have been explored for these homeostatic feeding centers (18–22), the role of GLP-1R signaling in brain regions that are linked with the nonhomeostatic control of feeding, such as the mesolimbic reward system (MRS), have only recently begun to be investigated (23).
The ventral tegmental area (VTA) and the nucleus accumbens (NAc) of the MRS play a critical role in the control of food intake (see Refs. 24 and 25 for review). Beyond taste afferent signaling modulating dopamine and opioid processing within these nuclei (26, 27), recent attention has been devoted to understanding how peripheral neuropeptides, typically studied for their homeostatic control of energy balance through action in the hypothalamus and hindbrain (e.g. leptin and ghrelin), also regulate food intake through signaling in the MRS (23, 28–33). Should GLP-1R ligands reduce food intake via direct activation of the MRS, it is possible that these intake-inhibitory effects would occur through modulation of higher-order aspects of food acquisition, potentially affecting reward and motivation (24, 25). Here, we test the hypothesis that GLP-1R ligands reduce food intake in the CNS through MRS signaling.
Preproglucagon (PPG, which is cleaved into GLP-1)- expressing neurons are located in the CNS almost exclusively in the caudal NTS (34) and are required for normal control of food intake (14). Interestingly, NTS neurons of unknown phenotype project to many brain regions in the hindbrain, midbrain, and forebrain, including the VTA and NAc, and GLP-1 mRNA and immunopositive fibers are found in the VTA and NAc (17, 35). However, the presence of GLP-1R mRNA or immunocytochemical detection of GLP-1 fibers does not prove a direct monosynaptic NTS PPG projection to these nuclei. Thus, whether PPG-expressing neurons themselves project to the VTA is unknown, and if so, whether GLP-1 signaling via these potential projections contributes to food intake control. Similarly, GLP-1 projections to the NAc (23) also require further assessment in control of food intake. This report therefore focuses on whether 1) CNS GLP-1-producing neurons (i.e. PPG neurons) in the NTS project directly to the VTA, and NAc and 2) the intake-inhibitory effects of GLP-1 ligands involve a reduction in the rewarding value of food via activation of GLP-1R in the VTA and NAc.
Here, we employ double-immunohistochemical (IHC) and pharmacological techniques to study the neuroanatomical connections from PPG neurons in the NTS to the VTA, NAc core, and NAc shell and to examine the feeding effects of GLP-1R signaling in these nuclei. Current data indicate that GLP-1R activation in both the VTA and the NAc core and shell reduces food intake, especially intake of highly-palatable foods, and that this occurs endogenously through direct GLP-1 projections from the NTS to the VTA and NAc core.
Materials and Methods
Subjects and drugs
Male Sprague Dawley rats (Charles River Laboratories, Wilmington, MA) were housed individually in hanging, wire-bottom cages in a 12-h light, 12-h dark cycle and had ad libitum access to pelleted chow (Purina Rodent Chow, 5001; Ralston Purina Co., Battle Creek, MI) and water except when noted. All procedures conformed to the institutional standards of the University of Pennsylvania animal care and use committee.
The long-acting GLP-1R agonist Exendin-4 (American Peptide Co., Sunnyvale, CA) and the GLP-1R antagonist Exendin-9 (American Peptide Co.) were dissolved in artificial cerebrospinal fluid (aCSF). The monosynaptic retrograde tracer Fluorogold (Fluorochrome LLC, Denver, CO) was diluted to 2% in distilled water.
Surgery
Under ketamine (90 mg/kg), xylazine (2.7 mg/kg), and acepromazine (0.64 mg/kg) anesthesia and analgesia (metacam, 2 mg/kg), rats were stereotaxically implanted with a 26-gauge bilateral guide cannula (Plastics One, Inc., Roanoke, VA) directed at the VTA alone or together with a unilateral cannula directed at either the NAc core or the NAc shell according to the following coordinates. VTA guide cannulae were positioned ±0.5 mm lateral from midline, 6.8 mm posterior from bregma, and 6.6 mm ventral from skull, with the injector aimed 8.6 mm ventral from skull. NAc core guide cannulae were positioned ±1.4 mm lateral from midline, 2.5 mm anterior to bregma, and 4.5 mm ventral from skull, with the injector aimed 6.5 mm ventral from skull. NAc shell guide cannulae were positioned ±1.0 mm lateral from midline, 2.5 mm anterior to bregma, and 5.3 mm ventral from skull, with the injector aimed 7.3 mm ventral from skull. Intraparenchymal injection sites were confirmed histologically via pontamine sky blue injections (100 nl).
Experimental procedures
Experiment 1: Fluorogold tracing and double immunohistochemistry
Rats were lightly anesthetized and received a 100-nl bilateral VTA (n = 4), unilateral NAc core (n = 6), or unilateral NAc shell (n = 4) injection of 2% Fluorogold via a high-pressure automated syringe pump (PHD Ultra; Harvard Apparatus, Holliston, MA). Rats were deeply anesthetized 3 d later and transcardially perfused with 0.1 m PBS, pH 7.4, followed with 4% formalin in 0.1 m PBS. Brains were removed and postfixed in 4% formalin for 6 h and were subsequently stored in 20% sucrose in 0.1 m PBS at 4 C for 3 d. Coronal sections (35 μm) were cut from the hindbrain using a cryostat (Leica 3050S; Leica Corp., Deerfield, IL). Brain sections were stored in 0.1 m PBS at 4 C until the start of IHC.
Double IHC for Fluorogold and PPG was conducted according to modified previous procedures (36–38). Briefly, brain sections were washed with 50% ethanol and Tris-PBS with 0.1% Triton-X. Sections were incubated on a shaker at room temperature for 1 h with a blocking solution [10% donkey serum (Jackson Immunoresearch Laboratories, West Grove, PA) in 0.1 m PBS]. Sections were subsequently incubated at room temperature with the primary antibody for PPG [GLP-1 (7–37) antiserum, Bachem Americas, Torrance, CA] at a 1:2000 concentration for 18 h, and then with a donkey antirabbit fluorescent secondary antibody (Dylight 488; Jackson ImmunoResearch Laboratories) at a 1:500 concentration for 2 h. For Fluorogold immunoreactivity, sections were again incubated at room temperature with the blocking solution followed by incubation with the antibody for Fluorogold (Fluorochrome, LLC) at a 1:2000 concentration for 18 h. Sections were then incubated with a donkey antirabbit fluorescent secondary antibody (Dylight 549; Jackson ImmunoResearch Laboratories). Using fluorescence microscopy (Nikon 80i; NIS-Elements AR 3.0) at ×10 and ×20 magnification, neurons expressing PPG and Fluorogold immunoreactivity were semiquantified by two separate experimenters blinded to treatment conditions from all coronal sections of the caudal brain stem between −14.8 mm to −14.1 mm from bregma, according to the stereotaxic atlas of Paxinos and Watson (39).
Experiment 2: effects of GLP-1R activation in the VTA on food intake
To evaluate the effects of GLP-1R activation in the VTA on sucrose and chow intake, overnight food-deprived (16 h) rats received unilateral injections of aCSF or exendin-4 (0.025 or 0.05 μg in 100 nl; n = 9–11) directed to the VTA 1 h after onset of the light cycle. Exendin-4 dose selections were based on our recent reports showing that a dose of 0.025 μg is subthreshold for intake suppression when administered intracerebroventricularly (40). Sucrose (15%) was presented immediately after injections, and intake was measured to the nearest 0.1 ml every 10 min for 1 h. After sucrose access, chow was replaced and intake was recorded to the nearest 0.1 g at 1 h, 4 h, and 23 h after its return (2 h, 5 h, and 24 h after injection), accounting for spillage. Body weight measurement was recorded 24 h after injection.
To evaluate the effects of GLP-1R activation in the VTA on high-fat (HF; 60% kcal from fat) diet (Research Diets, New Brunswick, NJ) and chow intake, nondeprived rats received unilateral injections of aCSF or exendin-4 (0.025 or 0.05 μg in 100 nl; n = 18) directed to the VTA immediately before onset of the dark cycle. Rats were given simultaneous access to both HF diet and chow, and intake and spillage measurements were made to the nearest 0.1 g at 1 h, 3 h, 6 h, and 24 h after injection. Body weight measurement was recorded 24 h after injection.
In addition to postmortem histological verification, VTA cannulae placement was functionally verified by measurement of an increase in 1 h sucrose intake after unilateral injection of DAMGO (5 nmol) as previously described (41, 42).
Experiment 3: Effects of GLP1-R activation in the NAc core and shell on food intake
To evaluate the effects of GLP-1R activation in the NAc core and shell on 15% sucrose and chow intake, overnight food-deprived (16 h) rats received counterbalanced unilateral injections of aCSF or exendin-4 (0.025 or 0.05 μg in 100 nl) directed to either the NAc core (n = 7) or shell (n = 8) 1 h after onset of the light cycle. Intake of sucrose and chow, as well as 24-h body weight change, was measured as described above.
To evaluate the effects of GLP1-R activation in the NAc on HF diet and chow intake, nondeprived rats received counterbalanced unilateral injections of aCSF or exendin-4 (0.025 or 0.05 μg in 100nl) directed to either the NAc core (n = 9) or shell (n = 9) immediately before onset of the dark cycle. Intake of HF diet and chow, as well as 24 h body weight change, was measured as described above.
Experiment 4: Effects of GLP-1R blockade in VTA, NAc core, and NAc shell on food intake
To evaluate the effects of GLP1-R blockade in the VTA (n = 12), NAc core (n = 11), and NAc shell (n = 12) on HF diet intake, nondeprived rats received injections of aCSF or exendin-9 (10 μg in 200 nl) directed to either the VTA, NAc core, or NAc shell immediately before onset of the dark cycle. HF diet intake and 24 h body weight change were measured as described above.
Experiment 5: pica effects of GLP-1R activation
Chow-maintained rats were first habituated to ad libitum access to kaolin pellets (Research Diets; K50001) for 1 wk. Probe recordings of kaolin intake conducted during the habituation phase demonstrated that baseline kaolin intake was negligible (average < 0.5 g; data not shown).
To evaluate whether GLP-1R activation in the VTA (n = 13), NAc core (n = 5), or NAc shell (n = 5) elicits a pica response, rats received aCSF or exendin-4 (0.05 μg in 100 nl) directed to either the VTA, NAc core, or NAc shell. Chow intake, kaolin intake, and body weight change were measured at 24 h. Spillages of chow and kaolin were accounted for as described above.
Data and statistical analyses
Data for each respective study were analyzed separately and expressed as mean ± sem. For all experiments, comparisons between treatment means were analyzed by one-way ANOVA and, if appropriate, post hoc Tukey test. All statistical analysis was conducted using STATISTICA software (StatSoft, Tulsa, OK). Differences of P < 0.05 were considered statistically significant.
Results
Experiment 1: PPG-expressing neurons in the NTS project directly to the VTA and the NAc
Analysis of total PPG-expressing NTS neurons in 35-μm coronal hindbrain sections between −14.8 mm and −14.1 mm from bregma, according to the atlas of Paxinos and Watson (39), showed an average of 12.1 ± 1.4 PPG neurons per coronal section. Semiquantification of double IHC for PPG neurons and Fluorogold immunoreactivity revealed that 32.4 ± 2.3%, 41.5 ± 7.0%, and 46.8 ± 6.5% of NTS PPG-expressing neurons project directly to the VTA, NAc core, and NAc shell, respectively (Fig. 1B). Additionally, semiquantification of double IHC for PPG neurons and Fluorogold immunoreactivity revealed that 30.3 ± 7.8%, 46.0 ± 2.5%, and 31.5 ± 6.5% of NTS neurons expressing Fluorogold traced from VTA, NAc core, and NAc shell, respectively, expressed immunoreactivity for PPG (Fig. 1C). Representative images of double-labeled GLP-1-producing neurons in the NTS after Fluorogold injections in the VTA, NAc core, and NAc shell are shown in Fig. 1, D, 1E, and 1F, respectively.
Fig. 1.
Colocalization of caudal NTS PPG neurons with VTA- and NAc-injected Fluorogold; green immunofluorescence represents neurons positive for PPG, red immunofluorescence represents neurons positive for Fluorogold. A, Representative low magnification (×10) image of coronal section at the level of the caudal NTS; cc, central canal. B, Semiquantification of neurons in the caudal dorsal vagal complex showed that 32.4 ± 2.3%, 41.5 ± 7.0%, and 46.8 ± 6.5% of immunofluorescing PPG neurons in the NTS project to the VTA, NAc core, and NAc shell, respectively. C, Semiquantification of neurons in the caudal dorsal vagal complex showed that 30.3 ± 7.8%, 46.0 ± 2.5%, and 31.5 ± 6.5% of caudal NTS neurons that express immunofluorescence for Fluorogold injected into the VTA, NAc core, and NAc shell, respectively, also show immunofluorescence for PPG. Representative high magnification (×20) images for colocalization of Fluorogold-expressing neurons (red) and PPG-expressing neurons (green), along with individual images for Fluorogold and PPG neurons, are shown for animals in which Fluorogold was injected in the VTA (D), the NAc core (E), and the NAc shell (F).
Experiment 2: GLP-1R activation in the VTA reduces food intake
Overnight food-deprived (16 h) rats unilaterally injected in the VTA with the GLP-1R agonist exendin-4, at doses previously determined (40) to be either subthreshold for effect in the ventricle (0.025 μg/100 nl) or just above threshold (0.05 μg/100 nl), showed a significant suppression of 15% sucrose intake at 20, 30, 40, 50, and 60 min after injection (Fig. 2A) compared with vehicle-treated rats. The magnitude of sucrose intake suppression by exendin-4 plateaued by 30 min for both doses of exendin-4. Both doses of exendin-4 significantly reduced 24 h change in body weight (Fig. 2C). Additionally, overall ANOVA revealed a trend for suppression of 24-h chow intake (Fig. 2B).
Fig. 2.
Intra-VTA injection of a GLP-1R agonist reduces intake of highly-palatable foods. Food intake and body weight measurements of rats after injection of aCSF, 0.025 μg exendin-4, or 0.05 μg exendin-4 in the VTA. A, Cumulative sucrose intake, after overnight food deprivation, at 10, 20, 30, 40, 50, and 60 min after injection. B, Modified chow intake after overnight food deprivation and 1 h of sucrose exposure at 1 h, 4 h, and 24 h after chow was returned. C, Change in body weight 24 h postinjection after overnight food deprivation and then sucrose and chow exposure. HF diet intake (D) and chow intake (E) at 1, 3, 6, and 24 h after injection when non-food-deprived rats were given choice between HF diet and chow, in addition to 24 h change in body weight (F). *, P < 0.05 from aCSF (vehicle) condition; #, P ≤ 0.057 from aCSF (vehicle) condition.
Rats who received 0.025 μg and 0.05 μg exendin-4 in the VTA given ad libitum access to both chow and HF diet (60% kcal from fat) showed significantly decreased HF diet intake at 6 h and 24 h (Fig. 2D), increased chow intake at 3 h (Fig. 2E), and reduced 24-h body weight gain (Fig. 2F) compared with intraparenchymal delivery of aCSF. The profile of food intake change after VTA GLP-1R activation when animals are given access to both chow and HF diet suggests a possible preference shift away from highly-palatable foods.
Experiment 3: GLP-1R activation in the NAc reduces food intake
Overnight food-deprived (16 h) rats unilaterally injected in the NAc core with exendin-4 (0.025 or 0.05 μg) showed a significant suppression of 15% sucrose intake at 20, 30, 40, 50, and 60 min after injection (Fig. 3A) compared with vehicle-treated rats. The magnitude of sucrose intake suppression plateaued by 40 min for both doses of exendin-4. However, exendin-4 directed to the NAc shell did not significantly alter intake of 15% sucrose at any time point (Fig. 4A). Likewise, 24 h chow intake and change in body weight were unaffected by exendin-4 delivery to either the NAc core or shell in overnight food-deprived rats (data not shown).
Fig. 3.
Intra-NAc core injection of a GLP-1R agonist reduces intake of highly-palatable foods. Food and body weight measurements in ad libitum fed rats after injection of aCSF, 0.025 μg exendin-4, or 0.05 μg exendin-4 aimed at the NAc core. A, Assessment of cumulative sucrose intake at 10, 20, 30, 40, 50, and 60 min after injection of exendin-4 aimed at the NAc core. HF diet intake (B) and chow intake (C) at 1, 3, 6, and 24 h after NAc core-aimed injection when given choice between HF diet and chow, in addition to 24 h change in body weight (D). *, P < 0.05 from aCSF (vehicle) condition; #, P ≤ 0.059 from aCSF (vehicle) condition.
Fig. 4.
Intra-NAc shell injection of a GLP-1R agonist reduces intake of highly-palatable foods. Food and body weight measurements in ad libitum fed rats after injection of aCSF, 0.025 μg exendin-4, or 0.05 μg exendin-4 aimed at the NAc shell. A, Assessment of cumulative sucrose intake at 10, 20, 30, 40, 50, and 60 min after injection of exendin-4 aimed at the NAc shell. HF diet intake (B) and chow intake (C) at 1, 3, 6, and 24 h after NAc shell-aimed injection when given choice between HF diet and chow, in addition to 24 h change in body weight (D). *, P < 0.05 from aCSF (vehicle) condition.
Unilateral injection of 0.05 μg exendin-4 directed to the NAc core of rats with ad libitum access to both chow and HF diet significantly reduced HF diet intake at 3 h, 6 h, and 24 h (Fig. 3B) and produced a significant reduction in 24-h body weight gain (Fig. 3D). NAc core-directed injection of 0.025 μg exendin-4 significantly reduced HF diet intake at 24 h (Fig. 3B). Unilateral injection of 0.05 μg exendin-4 directed to the NAc shell of rats with ad libitum access to both chow and HF diet significantly reduced HF diet intake at 6 h and 24 h (Fig. 4B). Overall ANOVA showed a significant increase in chow intake at the 3 h time point for rats that received exendin-4 directed to the NAc shell (Fig. 4C). Additionally, there was a strong trend for a reduction in 24-h body weight change (Fig. 4D); planned comparisons showed a significant suppression of body weight for 0.05 μg exendin-4 when directed to the NAc shell compared with vehicle.
Experiment 4: Exendin-9 in the VTA increases food intake
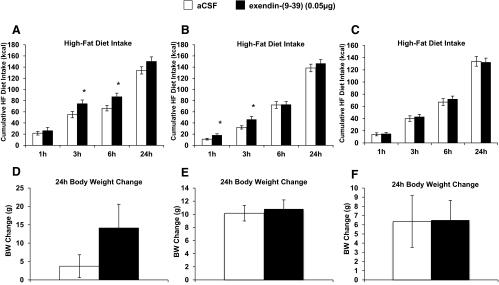
Blockade of GLP-1R in the VTA by unilateral injection of 10 μg exendin-9 directed to the VTA significantly increased HF diet intake at 3 h and 6 h compared with intake after vehicle injections (Fig. 5A). Blockade of GLP-1R in the NAc core by unilateral injection on 10 μg exendin-9 directed to the NAc core significantly increased HF diet intake at 1 h and 3 h compared with intake after vehicle injections (Fig. 5B). No significant changes in food intake occurred with GLP-1R blockade in the NAc shell (Fig. 5C); no effects on body weight occurred with GLP-1R blockade in the VTA (Fig. 5D), NAc core (Fig. 5E), or NAc shell (Fig. 5F).
Fig. 5.
Intra-VTA and NAc core injection of a GLP-1R antagonist increases intake of HF diet. HF diet and body weight measurements in ad libitum fed rats after injection of aCSF or 10 μg exendin-9 aimed at the VTA, NAc core, and NAc shell. HF diet intake (A, B, and C) at 1 h, 3 h, 6 h, and 24 h, and 24-h change in body weight (D, E, and F) after VTA-, NAc core-, or NAc shell-aimed injection, respectively. *, P < 0.05 from aCSF (vehicle) condition. BW, Body weight.
Experiment 5: Exendin-4 in the VTA, NAc core, and NAc shell does not elicit pica
Unilateral injection of 0.05 μg exendin-4 directed to the VTA, NAc core, or NAc shell of rats with ad libitum access to chow and kaolin resulted in no change in chow intake, kaolin intake, or body weight change at 24 h compared with vehicle (Fig. 6).
Fig. 6.
Intra-VTA, -NAc core, and -NAc shell injection of a GLP-1R agonist does not induce a pica response or decrease chow intake. Chow, kaolin, and body weight measurements in ad libitum fed rats after injection of aCSF or 0.05 μg exendin-4 aimed at the VTA, NAc core, and NAc shell. Chow intake (24 h) after injections aimed at the VTA (A), NAc core (B), and NAc shell (C); 24 h kaolin intake after injections aimed at the VTA (D), NAc core (E), NAc shell (F); 24 h change in body weight for rats injected in the VTA (G), NAc core (H), and NAc shell (I). BW, Body weight.
Discussion
GLP-1R signaling in the CNS reduces food intake in both humans and animal models (7, 9–12, 40); however, the brain nuclei mediating this effect are unknown. Given that the CNS control of food intake involves cross communication between classic homeostatic feeding (e.g. NTS, hypothalamus) and higher-order/hedonic nuclei (e.g. VTA, NAc) (25, 43, 44), it is possible that the intake suppression by GLP-1 involves action in homeostatic centers as well as modulation of the rewarding value of food via direct activation of the MRS. Here, we use double IHC to establish a direct connection from PPG-expressing neurons in the NTS to the VTA, NAc core, and NAc shell. Data suggest that these projections are physiologically relevant for food intake control as blockade of GLP-1R in the VTA and NAc core resulted in a significant increase in HF diet intake. Conversely, when pharmacologically activated, GLP-1R signaling in these MRS nuclei reduced food intake and body weight. The collective findings support the hypothesis that the CNS GLP-1 system controls for food intake and body weight regulation, in part, through direct action in the MRS.
There are direct projections from the NTS to the VTA and NAc (35), and in situ hybridization and immunocytochemisty confirm the presence of GLP-1R mRNA and GLP-1-immunopositive fibers, respectively, in both the VTA and NAc (17, 35). Although these data are informative about possible sites of action for GLP-1, this evidence does not directly establish monosynaptic projections from GLP-1-producing NTS neurons to the VTA or NAc, because it is possible that both of these techniques are identifying GLP-1 fibers of passage. Additionally, these aforementioned findings do not confirm that GLP-1 signaling in the VTA and NAc is relevant to food intake control. Using a similar strategy as Larsen et al., (34), we performed double IHC for Fluorogold (injected in the VTA, NAc core, or NAc shell) and PPG-expressing neurons in the caudal NTS. Semiquantitative analysis revealed that total PPG-expressing neurons in the caudal NTS were comparable to previous reports (37, 45). Further, approximately 32%, 42%, and 47% of NTS PPG-expressing neurons project directly to the VTA and the NAc, respectively. As indicated, this finding represents semiquantification, because it is possible that not all PPG axon terminals took up the Fluorogold tracer when injected into the VTA or NAc. Likewise, we cannot completely eliminate the possibility that Fluorogold was taken up by fibers of passage that were damaged during drug injection into the VTA and NAc. Nonetheless, such physical damage occurs with any method of intraparenchymal drug delivery. Moreover, it has been previously shown that Fluorogold is not taken up by undamaged fibers of passage (46), thus minimizing the likelihood of false Fluorogold tracing. The identification of direct NTS GLP-1 projections to the VTA and NAc provides a potential functional connection and mechanism by which central GLP-1 may control for food intake. Indeed, blockade of GLP-1R in the VTA and NAc core resulted in a significant increase in HF diet intake, indicating that under normal physiological circumstances, endogenous CNS GLP-1 signaling in the MRS is involved in the regulation of feeding behavior. It is interesting to note however, that although pharmacological activation of GLP-1R in the VTA and NAc produces a reduction in HF diet intake that persist for at least 24 h, the significant increase in food intake following blockade of GLP-1R by exendin-9–39 was of shorter duration. Given the limited knowledge of pharmacokinetics and degradation rates for both exendin-4 and exendin-9–39 when administered centrally, we can only speculate that these differences may be related to the drugs' pharmacodynamics.
To more extensively investigate the behavioral mechanisms by which GLP-1R activation in the VTA and the NAc reduces food intake, we administered the long-acting GLP-1R agonist exendin-4 in the VTA, the NAc core, and the NAc shell. In overnight food-deprived rats, exendin-4 delivered to the VTA dramatically reduced 1 h sucrose intake, 24 h chow intake, and 24 h body weight. In a separate paradigm in which animals were not food deprived, exendin-4 in the VTA significantly reduced HF diet intake. Similarly, when exendin-4 was directed to both the NAc core and the NAc shell, intake of HF diet was significantly reduced. Previous reports have shown that systemic (47) or forebrain intracerebroventricular (48) administration of GLP-1 reduces intake of sucrose solutions under sham feeding conditions (eliminating any postingestive consequences of the sucrose), suggesting a possible role for GLP-1R signaling in the modulation of orosensory positive feedback that drives intake of preferred foods. Therefore, it is interesting to note that exendin-4 delivered to the NAc core, but not to the shell, produced a significant reduction of sucrose intake. Thus, a speculative interpretation of these results would be that GLP-1R signaling in the NAc core plays a more important role than GLP-1 signaling in the NAc shell in control of carbohydrate intake or preference under food deprivation conditions. However, such an interpretation regarding NAc GLP-1 signaling modulating macronutrient selection or orosensory processing requires further testing. Interestingly, when animals are tested under ad libitum fed conditions, and rats were given the choice to consume standard chow or HF diet after injection of exendin-4 into the VTA, Nac core, or shell, the rats ate significantly less HF diet, but there were modest increases in chow intake. However, when animals were only maintained on chow (with kaolin access), exendin-4 injections into the VTA, NAc core, or shell were unable to significantly suppress intake of chow. Taken together, data suggest that MRS GLP-1 signaling may reduce food intake by decreasing the motivation to feed and/or the rewarding value of highly-palatable foods specifically. Both of these postulations, however, require further investigation.
Although the data clearly demonstrate that GLP-1R signaling in the MRS reduces food intake, the intracellular signaling cascades, neurotransmitters/signaling molecules and downstream neural pathways mediating this effect remain unknown. Drug addiction research has established that reward-related processing involves dopaminergic projections from the VTA to the NAc and to other forebrain structures (49, 50); similar pathways may also mediate the rewarding aspects of food intake (24, 51). Indeed, palatable food stimulates dopamine (DA) transmission in the NAc (52–54), and mice lacking tyrosine hydroxylase (enzyme involved in DA synthesis) consume less of a preferred sugar solution than control mice (55). Thus, previous research suggests that DA signaling is involved in the regulation of food intake, especially of highly-palatable foods. Given that the VTA and NAc have dense reciprocal projections (along with projections to many other nuclei involved in learning, memory, emotion, etc.), and that the dopaminergic projections from VTA to NAc are well established, it is plausible that the reduction in food intake via GLP-1R activation in the VTA and NAc involves an inhibition of DA signaling. If GLP-1R signaling in the VTA and NAc involves modulation of DA signaling, it is also unclear whether this occurs through presynaptic or postsynaptic inhibition of DA release. In addition to DA, it has been proposed that opioid, γ-aminobutyric acid, and glutamate signaling in the MRS are also involved in regulating feeding behavior (25, 41, 56–59). Thus, any of the aforementioned neurotransmitters/neuropeptides may be mediating the food intake-inhibitory effects of GLP-1 signaling in the MRS and require further investigation.
Given that the MRS is involved in the processing of not only motivational state and rewarding stimuli, but also in the mediation of aversive-like behaviors (see Ref. 60 for review), an important question is whether GLP-1R signaling in the VTA and NAc reduces intake by producing nausea/malaise. This question is underscored by clinical findings showing that 5–50% of patients prescribed long-acting GLP-1R ligands report feelings of nausea and malaise (61–63) and by the fact that GLP-1 administered centrally can produce a conditioned taste aversion/avoidance (CTA) in rats (22). In rodents that lack the anatomy and physiology necessary for vomiting, two quantitative experimental models are available for the behavioral study of nausea/malaise: 1) CTA, which is the aversion to or avoidance of flavors or foods paired with illness or change in the homeostatic state, and 2) pica, which is the consumption of nonnutritive substances (e.g. kaolin clay) exclusively in response to nausea-inducing agents (64, 65). A limitation to the CTA paradigm that has been previously described by Parker et al. (66) is that rodents learn to avoid any food or flavor that predicts a change in affective homeostatic state, a change that does not necessarily require a nausea component. To avoid this limitation, we use the pica model to demonstrate that GLP-1R activation in the VTA, NAc core, or NAc shell does not produce a pica response. The absence of kaolin ingestion supports the hypothesis that the intake-suppressive effects of palatable foods produced by GLP-1 signaling in these MRS nuclei does not occur as a result of the induction of nausea. Such a finding has strong clinical relevance for future GLP-1-based therapies aimed at targeting specific CNS GLP-1R populations for the treatment of obesity with reduced incidence of aversive events (i.e. nausea/vomiting).
Current data suggest that an endogenous central source of GLP-1 has a functional connection to the MRS, because GLP-1-producing NTS neurons project directly to the VTA and Nac, and blockade of GLP-1R in these nuclei results in a significant increase in HF diet intake. Additionally, peripheral exogenous administration of GLP-1R ligands, which reduce food intake in both humans (10, 11) and animal models (7, 67) do, in fact, enter the brain (16) and activate central GLP-1R to reduce food intake (13, 15). Thus, it is possible that peripheral administration of the FDA-approved GLP-1R agonists [e.g. exenatide (brand name Byetta) and liraglutide (brand name Victoza)] may reduce food intake, in part, through activation of MRS GLP-1R. This notion, supported by current findings, may therefore have implications for the approach to developing novel pharmacological therapeutics for the treatment of obesity. The more pronounced reduction of highly-palatable food by GLP-1R signaling in the MRS may be particularly useful in combating obesity, because one contributing factor to the development of this disease is the increased environmental availability of food with HF and sugar content (68, 69).
In summary, double IHC indicates that PPG-producing neurons in the NTS project directly to the VTA, NAc core, and NAc shell and provide a functional connection and mechanism by which central GLP-1 may reduce food intake. These connections are physiologically relevant for the normal control of feeding behavior, because blockade of GLP-1R in the VTA and NAc resulted in a significant increase in intake of HF diet. When pharmacologically activated, GLP-1R signaling in the VTA, NAc core, and NAc shell reduces overall food intake, especially intake of highly-palatable foods. These data highlight aforementioned nuclei within the mesolimbic reward system as novel sites where GLP-1R signaling controls food intake and body weight and potentially influences higher-order processing for reward and motivational behaviors.
Acknowledgments
We thank Line Stensland, Sage Rahm, and Samantha Fortin for technical assistance and Drs. Scott E. Kanoski and Harvey J. Grill (Department of Psychology, University of Pennsylvania) for continued guidance and intellectual advice.
This work was supported by developmental funds from the Translational Neuroscience Program, in the Department of Psychiatry at the University of Pennsylvania and National Institutes of Health Grant NIDDK085435 (to M.R.H.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- aCSF
- Artificial cerebrospinal fluid
- CNS
- central nervous system
- CTA
- conditioned taste aversion/avoidance
- DA
- dopamine
- GLP-1
- glucagon-like-peptide-1
- GLP-1R
- GLP-1 receptor
- IHC
- immunohistochemical
- HF
- high fat
- MRS
- mesolimbic reward system
- Nac
- nucleus accumbens
- NTS
- nucleus tractus solitarius
- PPG
- preproglucagon
- T2DM
- type 2 diabetes mellitus
- VTA
- ventral tegmental area.
References
- 1. Baggio LL, Drucker DJ. 2007. Biology of incretins: GLP-1 and GIP. Gastroenterology 132:2131–2157 [DOI] [PubMed] [Google Scholar]
- 2. Lovshin JA, Drucker DJ. 2009. Incretin-based therapies for type 2 diabetes mellitus. Nat Rev Endocrinol 5:262–269 [DOI] [PubMed] [Google Scholar]
- 3. Holst JJ. 2007. The physiology of glucagon-like peptide 1. Physiol Rev 87:1409–1439 [DOI] [PubMed] [Google Scholar]
- 4. Hayes MR, De Jonghe BC, Kanoski SE. 2010. Role of the glucagon-like-peptide-1 receptor in the control of energy balance. Physiol Behav 100:503–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Knudsen LB. 2010. Liraglutide: the therapeutic promise from animal models. Int J Clin Pract Suppl 167:4–11 [DOI] [PubMed] [Google Scholar]
- 6. Hayes MR, Skibicka KP, Grill HJ. 2008. Caudal brainstem processing is sufficient for behavioral, sympathetic, and parasympathetic responses driven by peripheral and hindbrain glucagon-like-peptide-1 receptor stimulation. Endocrinology 149:4059–4068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Scott KA, Moran TH. 2007. The GLP-1 agonist exendin-4 reduces food intake in nonhuman primates through changes in meal size. Am J Physiol Regul Integr Comp Physiol 293:R983–R987 [DOI] [PubMed] [Google Scholar]
- 8. Raun K, von Voss P, Gotfredsen CF, Golozoubova V, Rolin B, Knudsen LB. 2007. Liraglutide, a long-acting glucagon-like peptide-1 analog, reduces body weight and food intake in obese candy-fed rats, whereas a dipeptidyl peptidase-IV inhibitor, vildagliptin, does not. Diabetes 56:8–15 [DOI] [PubMed] [Google Scholar]
- 9. Raun K, von Voss P, Knudsen LB. 2007. Liraglutide, a once-daily human glucagon-like peptide-1 analog, minimizes food intake in severely obese minipigs. Obesity (Silver Spring) 15:1710–1716 [DOI] [PubMed] [Google Scholar]
- 10. Vilsboll T, Zdravkovic M, Le-Thi T, Krarup T, Schmitz O, Courrèges JP, Verhoeven R, Bugánová I, Madsbad S. 2007. Liraglutide, a long-acting human glucagon-like peptide-1 analog, given as monotherapy significantly improves glycemic control and lowers body weight without risk of hypoglycemia in patients with type 2 diabetes. Diabetes Care 30:1608–1610 [DOI] [PubMed] [Google Scholar]
- 11. Blonde L, Klein EJ, Han J, Zhang B, Mac SM, Poon TH, Taylor KL, Trautmann ME, Kim DD, Kendall DM. 2006. Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes Obes Metab 8:436–447 [DOI] [PubMed] [Google Scholar]
- 12. Turton MD, O'Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, Wilding JP, Smith DM, Ghatei MA, Herbert J, Bloom SR. 1996. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 379:69–72 [DOI] [PubMed] [Google Scholar]
- 13. Rüttimann EB, Arnold M, Hillebrand JJ, Geary N, Langhans W. 2009. Intrameal hepatic portal and intraperitoneal infusions of glucagon-like peptide-1 reduce spontaneous meal size in the rat via different mechanisms. Endocrinology 150:1174–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barrera JG, Jones KR, Herman JP, D'Alessio DA, Woods SC, Seeley RJ. 2011. Hyperphagia and increased fat accumulation in two models of chronic CNS glucagon-like peptide-1 loss of function. J Neurosci 31:3904–3913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kanoski SE, Fortin SM, Arnold M, Grill HJ, Hayes MR. 2011. Peripheral and central GLP-1 receptor populations mediate the anorectic effects of peripherally administered GLP-1 receptor agonists, liraglutide and exendin-4. Endocrinology 152:3103–3112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Göke R, Larsen PJ, Mikkelsen JD, Sheikh SP. 1995. Distribution of GLP-1 binding sites in the rat brain: evidence that exendin-4 is a ligand of brain GLP-1 binding sites. Eur J Neurosci 7:2294–2300 [DOI] [PubMed] [Google Scholar]
- 17. Merchenthaler I, Lane M, Shughrue P. 1999. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol 403:261–280 [DOI] [PubMed] [Google Scholar]
- 18. Hayes MR, Leichner TM, Zhao S, Lee GS, Chowansky A, Zimmer D, De Jonghe BC, Kanoski SE, Grill HJ, Bence KK. 2011. Intracellular Signals mediating the food intake-suppressive effects of hindbrain glucagon-like peptide-1 receptor activation. Cell Metab 13:320–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vrang N, Hansen M, Larsen PJ, Tang-Christensen M. 2007. Characterization of brainstem preproglucagon projections to the paraventricular and dorsomedial hypothalamic nuclei. Brain Res 1149:118–126 [DOI] [PubMed] [Google Scholar]
- 20. Hayes MR, Bradley L, Grill HJ. 2009. Endogenous hindbrain glucagon-like peptide-1 receptor activation contributes to the control of food intake by mediating gastric satiation signaling. Endocrinology 150:2654–2659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McMahon LR, Wellman PJ. 1998. PVN infusion of GLP-1-(7–36) amide suppresses feeding but does not induce aversion or alter locomotion in rats. Am J Physiol 274:R23–R29 [DOI] [PubMed] [Google Scholar]
- 22. Kinzig KP, D'Alessio DA, Seeley RJ. 2002. The diverse roles of specific GLP-1 receptors in the control of food intake and the response to visceral illness. J Neurosci 22:10470–10476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dossat AM, Lilly N, Kay K, Williams DL. 2011. Glucagon-like Peptide 1 receptors in nucleus accumbens affect food intake. J Neurosci 31:14453–14457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kenny PJ. 2011. Reward mechanisms in obesity: new insights and future directions. Neuron 69:664–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Narayanan NS, Guarnieri DJ, DiLeone RJ. 2010. Metabolic hormones, dopamine circuits, and feeding. Front Neuroendocrinol 31:104–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hajnal A, Norgren R, Kovacs P. 2009. Parabrachial coding of sapid sucrose: relevance to reward and obesity. Ann NY Acad Sci 1170:347–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nathan PJ, Bullmore ET. 2009. From taste hedonics to motivational drive: central mu-opioid receptors and binge-eating behaviour. Int J Neuropsychopharmacol 12:995–1008 [DOI] [PubMed] [Google Scholar]
- 28. Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, Maratos-Flier E, Flier JS. 2006. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron 51:811–822 [DOI] [PubMed] [Google Scholar]
- 29. Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, Thurmon JJ, Marinelli M, DiLeone RJ. 2006. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron 51:801–810 [DOI] [PubMed] [Google Scholar]
- 30. Dickson SL, Egecioglu E, Landgren S, Skibicka KP, Engel JA, Jerlhag E. 2011. The role of the central ghrelin system in reward from food and chemical drugs. Mol Cell Endocrinol 340:80–87 [DOI] [PubMed] [Google Scholar]
- 31. Abizaid A. 2009. Ghrelin and dopamine: new insights on the peripheral regulation of appetite. J Neuroendocrinol 21:787–793 [DOI] [PubMed] [Google Scholar]
- 32. Vaccarino FJ, Koob GF. 1984. Microinjections of nanogram amounts of sulfated cholecystokinin octapeptide into the rat nucleus accumbens attenuates brain stimulation reward. Neurosci Lett 52:61–66 [DOI] [PubMed] [Google Scholar]
- 33. Babcock AM, Livosky M, Avery DD. 1985. Cholecystokinin and bombesin suppress operant responding for food reward. Pharmacol Biochem Behav 22:893–895 [DOI] [PubMed] [Google Scholar]
- 34. Larsen PJ, Tang-Christensen M, Holst JJ, Orskov C. 1997. Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience 77:257–270 [DOI] [PubMed] [Google Scholar]
- 35. Rinaman L. 2010. Ascending projections from the caudal visceral nucleus of the solitary tract to brain regions involved in food intake and energy expenditure. Brain Res 1350:18–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huo L, Maeng L, Bjørbaek C, Grill HJ. 2007. Leptin and the control of food intake: neurons in the nucleus of the solitary tract are activated by both gastric distension and leptin. Endocrinology 148:2189–2197 [DOI] [PubMed] [Google Scholar]
- 37. Vrang N, Phifer CB, Corkern MM, Berthoud HR. 2003. Gastric distension induces c-Fos in medullary GLP-1/2-containing neurons. Am J Physiol Regul Integr Comp Physiol 285:R470–R478 [DOI] [PubMed] [Google Scholar]
- 38. De Jonghe BC, Horn CC. 2008. Chemotherapy-induced pica and anorexia are reduced by common hepatic branch vagotomy in the rat. Am J Physiol Regul Integr Comp Physiol 294:R756–R765 [DOI] [PubMed] [Google Scholar]
- 39. Paxinos G, Watson C. 2005. The rat brain in stereotaxic coordinates. 5th ed San Diego: Academic Press [Google Scholar]
- 40. Hayes MR, Leichner TM, Zhao S, Lee GS, Chowansky A, Zimmer D, De Jonghe BC, Kanoski SE, Grill HJ, Bence KK. 2011. Intracellular signals mediating the food intake suppressive effects of hindbrain glucagon-like-peptide-1 receptor activation. Cell Metab 13:320–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. MacDonald AF, Billington CJ, Levine AS. 2003. Effects of the opioid antagonist naltrexone on feeding induced by DAMGO in the ventral tegmental area and in the nucleus accumbens shell region in the rat. Am J Physiol Regul Integr Comp Physiol 285:R999–R1004 [DOI] [PubMed] [Google Scholar]
- 42. Kim EM, Quinn JG, Spanswick D, O'Hare E. 2009. Feeding association between the nucleus of the solitary tract and the ventral tegmental area. Appetite 53:457–460 [DOI] [PubMed] [Google Scholar]
- 43. Grill HJ, Skibicka KP, Hayes MR. 2007. Imaging obesity: fMRI, food reward, and feeding. Cell Metab 6:423–425 [DOI] [PubMed] [Google Scholar]
- 44. Berthoud HR, Morrison C. 2008. The brain, appetite, and obesity. Annu Rev Psychol 59:55–92 [DOI] [PubMed] [Google Scholar]
- 45. Rinaman L. 1999. Interoceptive stress activates glucagon-like peptide-1 neurons that project to the hypothalamus. Am J Physiol Regul Integr Comp Physiol 277:R582–R590 [DOI] [PubMed] [Google Scholar]
- 46. Schmued LC, Fallon JH. 1986. Fluoro-Gold: a new fluorescent retrograde axonal tracer with numerous unique properties. Brain Res 377:147–154 [DOI] [PubMed] [Google Scholar]
- 47. Chelikani PK, Haver AC, Reidelberger RD. 2005. Intravenous infusion of glucagon-like peptide-1 potently inhibits food intake, sham feeding, and gastric emptying in rats. Am J Physiol Regul Integr Comp Physiol 288:R1695–R1706 [DOI] [PubMed] [Google Scholar]
- 48. Asarian L, Corp ES, Hrupka B, Geary N. 1998. Intracerebroventricular glucagon-like peptide-1 (7–36) amide inhibits sham feeding in rats without eliciting satiety. Physiol Behav 64:367–372 [DOI] [PubMed] [Google Scholar]
- 49. Wise RA. 1996. Neurobiology of addiction. Curr Opin Neurobiol 6:243–251 [DOI] [PubMed] [Google Scholar]
- 50. Schmidt HD, Famous KR, Pierce RC. 2009. The limbic circuitry underlying cocaine seeking encompasses the PPTg/LDT. Eur J Neurosci 30:1358–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wise RA. 2006. Role of brain dopamine in food reward and reinforcement. Philos Trans R Soc Lond B Biol Sci 361:1149–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Roitman MF, Stuber GD, Phillips PE, Wightman RM, Carelli RM. 2004. Dopamine operates as a subsecond modulator of food seeking. J Neurosci 24:1265–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Roitman MF, Wheeler RA, Wightman RM, Carelli RM. 2008. Real-time chemical responses in the nucleus accumbens differentiate rewarding and aversive stimuli. Nat Neurosci 11:1376–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Norgren R, Hajnal A, Mungarndee SS. 2006. Gustatory reward and the nucleus accumbens. Physiol Behav 89:531–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cannon CM, Palmiter RD. 2003. Reward without dopamine. J Neurosci 23:10827–10831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Miner P, Borkuhova Y, Shimonova L, Khaimov A, Bodnar RJ. 2010. GABA-A and GABA-B receptors mediate feeding elicited by the GABA-B agonist baclofen in the ventral tegmental area and nucleus accumbens shell in rats: reciprocal and regional interactions. Brain Res 1355:86–96 [DOI] [PubMed] [Google Scholar]
- 57. Khaimova E, Kandov Y, Israel Y, Cataldo G, Hadjimarkou MM, Bodnar RJ. 2004. Opioid receptor subtype antagonists differentially alter GABA agonist-induced feeding elicited from either the nucleus accumbens shell or ventral tegmental area regions in rats. Brain Res 1026:284–294 [DOI] [PubMed] [Google Scholar]
- 58. MacDonald AF, Billington CJ, Levine AS. 2004. Alterations in food intake by opioid and dopamine signaling pathways between the ventral tegmental area and the shell of the nucleus accumbens. Brain Res 1018:78–85 [DOI] [PubMed] [Google Scholar]
- 59. Badiani A, Leone P, Noel MB, Stewart J. 1995. Ventral tegmental area opioid mechanisms and modulation of ingestive behavior. Brain Res 670:264–276 [DOI] [PubMed] [Google Scholar]
- 60. Carlezon WA, Jr, Thomas MJ. 2009. Biological substrates of reward and aversion: a nucleus accumbens activity hypothesis. Neuropharmacology 56(Suppl 1):122–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Buse JB, Rosenstock J, Sesti G, Schmidt WE, Montanya E, Brett JH, Zychma M, Blonde L. 2009. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet 374:39–47 [DOI] [PubMed] [Google Scholar]
- 62. Astrup A, Rössner S, Van Gaal L, Rissanen A, Niskanen L, Al Hakim M, Madsen J, Rasmussen MF, Lean ME. 2009. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet 374:1606–1616 [DOI] [PubMed] [Google Scholar]
- 63. Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD. 2004. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care 27:2628–2635 [DOI] [PubMed] [Google Scholar]
- 64. Andrews PL, Horn CC. 2006. Signals for nausea and emesis: implications for models of upper gastrointestinal diseases. Auton Neurosci 125:100–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Garcia J, Kimeldorf DJ, Koelling RA. 1955. Conditioned aversion to saccharin resulting from exposure to γ radiation. Science 122:157–158 [PubMed] [Google Scholar]
- 66. Parker LA, Rana SA, Limebeer CL. 2008. Conditioned nausea in rats: assessment by conditioned disgust reactions, rather than conditioned taste avoidance. Can J Exp Psychol 62:198–209 [DOI] [PubMed] [Google Scholar]
- 67. Hayes MR, Kanoski SE, Alhadeff AL, Grill HJ. 2011. Comparative effects of the long-acting GLP-1 receptor ligands, liraglutide and exendin-4, on food intake and body weight suppression in rats. Obesity (Silver Spring) 19:1342–1349 [DOI] [PubMed] [Google Scholar]
- 68. Drewnowski A. 2004. Obesity and the food environment: dietary energy density and diet costs. Am J Prev Med 27:154–162 [DOI] [PubMed] [Google Scholar]
- 69. Drewnowski A, Rolls BJ. 2005. How to modify the food environment. J Nutr 135:898–899 [DOI] [PubMed] [Google Scholar]