Abstract
Human anaplastic thyroid cancer (ATC) is a lethal disease with an advanced clinical presentation and median survival of 3 months. The BRAFV600E oncoprotein is a potent transforming factor that causes human thyroid cancer cell progression in vitro and in vivo; therefore, we sought to target this oncoprotein in a late intervention model of ATC in vivo. We used the human ATC cell line 8505c, which harbors the BRAFV600E and TP53R248G mutations. Immunocompromised mice were randomized to receive the selective anti-BRAFV600E inhibitor, PLX4720, or vehicle by oral gavage 28 d after tumor implantation, 1 wk before all animals typically die due to widespread metastatic lung disease and neck compressive symptoms in this model. Mice were euthanized weekly to evaluate tumor volume and metastases. Control mice showed progressive tumor growth and lung metastases by 35 d after tumor implantation. At that time, all control mice had large tumors, were cachectic, and were euthanized due to their tumor-related weight loss. PLX4720-treated mice, however, showed a significant decrease in tumor volume and lung metastases in addition to a reversal of tumor-related weight loss. Mouse survival was extended to 49 d in PLX4720-treated animals. PLX4720 treatment inhibited cell cycle progression from 28 d to 49 d in vivo. PLX4720 induces striking tumor regression and reversal of cachexia in an in vivo model of advanced thyroid cancer that harbors the BRAFV600E mutation.
Despite tremendous advances in our understanding of the genetic and molecular events that characterize aggressive thyroid cancers, anaplastic thyroid cancer (ATC) remains a lethal disease with a median survival of less than 6 months (1, 2). ATC frequently presents as a large and rapidly expanding neck mass (3), and at the time of presentation, the tumor has often invaded surrounding structures and has metastasized to distant locations. It is for these reasons that surgical therapies consistently fail, and tracheostomy is often necessary as a palliative measure to improve patient comfort for local-regional control in the setting of a massive tumor that invades or obstructs the airway (4). These tumors tend to be resistant to standard chemotherapy, external beam radiation, and radioiodine (131I) due to loss of the sodium iodide symporter through malignant dedifferentiation (5). Because all of these treatments for ATC have marginal efficacy, new treatments are needed that use rationally designed molecularly-targeted agents and demonstrate efficacy for tumors at this late stage of presentation (6).
B-RafV600E is an oncoprotein that is implicated in the pathogenesis and progression of ATC (7–9) and is prevalent in several other human cancers, such as papillary thyroid cancer (PTC), melanoma, and colon cancer. B-RafV600E oncoprotein is a potent transforming factor that causes human thyroid cancer cell progression in vitro and in vivo (9). This mutation is present in approximately 25%–38% of ATCs (10, 11). The mutant B-RafV600E protein results from a transversion (T1799A) in exon 15 of the B-Raf gene, which causes a valine-for-glutamate substitution at residue 600 of the protein. This mutation induces a conformational change in the protein that constitutively activates the MAPK pathway (i.e. ERK1/2) (12).
PLX4720 is an ATP analog that selectively inhibits B-RafV600E by stabilizing it in an inactive conformation (13). This highly selective molecule binds with an IC50 of 13 nm, and thus its off-target binding is minimized. PLX4720 has undergone considerable in vitro and in vivo testing in animal models of melanoma and colon cancer (13–15). In addition, a recent clinical trial (16) using a related compound (PLX4032) in patients with melanoma has shown considerable efficacy and may lead to further trials with other cancers that harbor the B-RafV600E mutation, such as colorectal and thyroid cancers.
The utility of molecular targeting with PLX4720 for thyroid cancer has been tested in vitro by Nucera et al. (9) and others (17) showing strong inhibition of B-RafV600E in thyroid cancer cell lines with this mutation. We have recently demonstrated that mRNA and protein expression levels of human thrombospondin-1 (TSP-1), a key regulator of tumor invasion and extracellular matrix (ECM) remodeling, were significantly increased in B-RafV600E -positive human PTC compared with those with wild-type B-Raf or normal thyroid tissue. Additionally, we found that either TSP-1 or B-RafV600E mRNA knockdown in 8505c ATC cells by short hairpin RNA (9), or targeting B-RafV600E with PLX4720 (18), significantly inhibited proliferation, migration, and invasion of these thyroid tumor cells.
We have also recently shown that oral administration of PLX4720 within 1 wk of orthotopic ATC implantation can result in delayed tumor growth and decreased tumor volume in an early intervention in vivo model (12). Although B-RafV600E inhibition did decrease tumor growth in our previous prevention trial, it is also clinically relevant to know whether molecular targeting at a later intervention time point would also be efficacious given the uniformly late clinical presentation of patients with ATC. No study to date has tested a selective B-RafV600E inhibitor in a late-intervention orthotopic animal model of ATC, which is a model that reproduces the advanced clinical presentation in humans with this type of cancer. Therefore in this study, we began PLX4720 treatment in mice after large orthotopic ATC and widespread lung metastases were already established. Bioimaging and histology have confirmed the rapid timeline for disease progression and metastasis in this animal model of human ATC, a situation that closely resembles the rapidly fatal disease seen in humans. Our results demonstrate, for the first time, that anti-B-RafV600E therapy with orally delivered PLX4720 causes orthotopic ATC regression and reverses cachexia, even when administered just 1 wk before death using a late-intervention model of ATC in vivo.
Materials and Methods
Human ATC cell line
The human ATC 8505c cell line harboring B-RafV600E (19) was purchased from DSMZ (German collection of microorganisms and cell culture). The 8505c cells were engineered to express green fluorescent protein (GFP) as described by Nucera et al. (20). Cells were grown in RPMI 1640 with penicillin/streptomycin/amphtoericin with 10% fetal bovine serum.
Tumor implantation
The animal work was done in the animal facility at Massachusetts General Hospital (Boston, MA) in accordance with federal, local, and institutional guidelines. We used an orthotopic mouse model of ATC as previously described and validated by Nucera et al. (9. 20). Briefly, 48 female 6-wk-old severe combined immunodeficient (SCID) mice (Charles River Laboratories, Wilmington, MA) were anesthetized using ketamine and xylazine. For each mouse, the neck was shaved and prepared with betadine. The skin and sc tissues were incised with scissors, and the salivary glands were reflected superiorly. The central component of the neck was exposed, and the overlying strap muscles were bluntly dissected away from the right thyroid lobe using forceps. Once the right thyroid was exposed, 5 × 105 8505c cells resuspended in 10 μl of serum-free RPMI were injected into the thyroid using a Hamilton syringe (Fisher Scientific, Pittsburgh, PA) attached to a 27-gauge needle. After the injection, the salivary glands were repositioned, and the incision was closed using nylon sutures. Triple antibiotic ointment was applied to the wound, and the mice were placed under a warming lamp while they recovered from anesthesia. There were no surgical or anesthetic complications.
PLX4720 preparation and administration
We dissolved PLX4720 in the vehicle, dimethyl sulfoxide, initially at a concentration of 120 mg/ml (final concentration: 30 mg/kg body weight/d). It was then suspended in a 1% solution of carboxymethycellulose (Sigma Chemical Co., St. Louis, MO) daily at the time of administration. Mice were gavaged with the drug suspension using a 24-gauge blunt-tipped gavage needle.
Group stratification and randomization
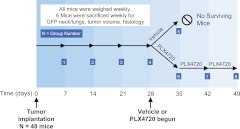
In detail, 48 mice were randomly divided into eight equal groups of six mice for the purpose of establishing a time course of tumor progression and the response to late therapeutic intervention with PLX4720 (Fig. 1). Late therapeutic intervention was defined as initiating treatment 1 wk (at 28 d) before natural (not per protocol) death (mice showed physical criteria to be killed, i.e. advanced thyroid tumors that caused distention and pressure of the trachea and esophagus), which reproducibly occurs in this model by 35 d after tumor implantation as we previously showed (20).
Fig. 1.
Experimental design. Orthotopic anaplastic tumors were implanted in 48 SCID mice. Each week, six mice were killed. Their tumors and lungs were evaluated by histology and GFP biomaging. Either vehicle or PLX4720 was begun at 28 d, and the response to therapy was evaluated weekly. “No surviving mice” reflects the fact that all mice at this time point showed physical criteria to be killed (e.g. cachexia and/or advanced thyroid tumors causing compression of the trachea and esophagus).
Groups 1–4 were assigned to no treatment and were killed after 7, 14, 21, and 28 d, respectively, to allow a detailed radiological, gross, and histological analysis of tumor progression and metastasis, knowing that these large groups serve as a timeline proxy for all the groups before the initiation of treatment.
Group 5 was a control group treated with vehicle on d 28–d 35 and then killed at 35 d given the large tumor burden and cachexia. Finally, groups 6, 7, and 8 had treatment with PLX4720 initiated at 28 d after tumor implantation at 30 mg/kg/d PLX4720 for 1, 2, and 3 wk, respectively. Each group was killed after the completion of its treatment.
Because it has been previously demonstrated that mice in this model have a large and dramatic tumor burden and cachexia at 35 d after tumor injection (9), we chose to begin treatment with PLX4720 1 wk earlier (28 d after injection) to accurately simulate the advanced disease presentation seen in humans. To characterize the growth pattern of this model, six mice were killed weekly, their tumors were measured, lungs were evaluated for metastases, and tissues were analyzed by histology. Body weight (grams) of all remaining mice was measured weekly. Tumor size was measured using an electronic caliper (Fisher Scientific, Hampton, NH), and tumor volume was calculated as (1/2) × length × width × height. Lungs were also collected and analyzed for metastasis by histology and GFP imaging as described below. Mice were killed when they had lost more than 25% of their baseline weight or had signs of tracheal compressive symptoms. As expected in this study, all control mice displayed features of advanced disease (e.g. compression of the trachea and esophagus or severe cachexia, by 35 d). We report “no surviving mice” after the 35-d time point for control mice, and this reflects the fact that these mice were intentionally killed because of these physical criteria to minimize pain and suffering for the humane treatment of the animals. These physical criteria were established a priori for both groups at the beginning of the study.
Bioimaging
To detect GFP signal in the primary tumor and lungs, we used a Multi-spectral Fluorescence Scanner (CRi Maestro 500, Woburn, MA) that was able to detect and image fluorophores between 450 and 900 nm. The tumor and lung specimens were imaged with fluorescence microscopy immediately after necropsy (wavelength absorption, 488 nm) (Leica Microsystems, Bannockburn, IL).
Histological and immunohistochemical (IHC) analysis
All tissue specimens were fixed with 10% buffered formalin phosphate and embedded in paraffin blocks. Histopathology evaluation was performed by an endocrine pathologist (P.S.) on hematoxylin and eosin-stained tissue sections of the orthotopic thyroid tumors, the surrounding perithyroidal tissues, and the lungs. These were visualized with an Olympus BX 41 microscope and the Olympus Q COLOR 5 photo camera (Olympus Corp., Lake Success, NY). Sections (4-μm thick) of formalin-fixed tissues were used for IHC procedures. After baking overnight at 37 C, deparaffinization with xylene/ethanol and rehydration were performed. IHC analysis was performed using the phospho-ERK1/2 (Cell Signaling Technology, Danvers, MA), Ki67 (Dako Envision system, DAKO Corp., Carpinteria, CA), cyclins E2 (CCNE2) (Abcam, Cambridge, MA), caspase-3 and cleaved caspase-3 (Cell Signaling Technology), Cyclin D1, Cyclin B1, p21, and p27/kip1 (Abcam) primary antibodies. The sections, treated with pressure cooker for antigen retrieval (Biocare Medical, Concord, CA), were incubated at 123 C in citrate buffer (Dako Target Retrieval Solution, S1699; DAKO Corp.), cooled and washed with PBS. Antigen retrieval was performed for 60 min at room temperature. The primary antibody was detected using a biotin-free secondary antibody (K4011) (Dako Envision system) and incubated for 30 min. All incubations were carried out in a humid chamber at room temperature. Slides were rinsed with PBS between incubations. Sections were developed using 3,3′-diaminobenzidine (Sigma Chemical Co.) as a substrate and were counterstained with Mayer's hematoxylin.
The IHC markers phospho-ERK1/2, CCNE2, p27/kip1, cascapase-3, and cleaved caspase-3 were assessed semiquantitatively using the following scoring method: 0 negative, 1 + 1–10% positive cells (low expression), 2 + 11–50% positive cells (moderate), and 3+ more than 50% positive cells (high expression).
Proliferative index and apoptosis assay in vivo
Immunostaining for nuclear Ki67 was evaluated by an endocrine pathologist (P.S.) in the PLX4720-treated and control groups. The proportion of nuclei that stained positive for Ki67 relative to background nuclei was counted for each tumor and averaged. Data are reported as mean percent for positive Ki67 staining. The apoptosis assay was performed using terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay according to manufacturer's recommendation (ApopTag peroxidase in situ detection kit) (Millipore Corp., Bedford, MA); apoptotic cells were assessed semiquantitatively using the following scoring method: 0 negative, 1 + 1–10% positive cells (low expression), 2 + 11–50% positive cells (moderate), and 3+ more than 50% positive cells (high expression).
Scoring for metastases
Metastatic dissemination in the lungs was assessed by GFP imaging at necropsy. The lungs were dissected from the mouse, washed with PBS, and visualized by GFP imaging. For each mouse, the number of metastases was counted as the number of distinct GFP signals per square centimeter (cm2) in an anterior projection of the lungs. The number of metastases found in each mouse was averaged per group.
Statistical analysis
Statistical analysis was carried out using Microsoft Excel software. Two-tailed t-tests were used to evaluate statistical differences. Data are reported as the mean value, and error bars represent the se of the mean for each group. Results with P < 0.05 were considered significant. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Results
Late intervention with PLX4720 reverses weight loss and cachexia in an in vivo model of human ATC
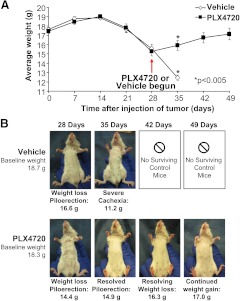
Weight measurements in control mice were used to generate a clear comparison group. Mice in all groups lost weight starting 14 d after tumor implantation. As we have previously demonstrated (20), control untreated mice in groups 1–5 (n = 6/group) consistently lost weight, developed cachexia and piloerection, and met a priori criteria to be killed (weight loss >25% from baseline or tracheal compression) by 35 d (Fig. 2A). At the 35 d time point, the control mice had lost additional weight (average weight 12.5 g ± 0.3 g) and had progression of cachexia as shown in Fig. 2B. No mice in the control group survived longer than 35 d (all six had lost >25% of their baseline weight).
Fig. 2.
PLX4720 extends survival and reverses cachexia in orthotopic mice with human ATC. A, Mouse weight over time. B, Untreated mice developed weight loss, piloerection, and cachexia by 28 d. Vehicle-treated mice progressed to severe cachexia, whereas PLX4720-treated mice had reversal of weight loss, cachexia, and piloerection over the 3-wk course of therapy. *, P < 0.05.
In the mice treated with PLX4720, however, not only was the progression of weight loss and cachexia stopped, but the process was reversed. Each treated mouse gained weight within a week of PLX4720 treatment, and there was a significant increase in weight in all treated mice compared with the control mice by 35 d after tumor implantation (15.9 g ± 0.5 g vs. 12.5 g ± 0.3 g; P < 0.005). As shown in Fig. 2A, mice in both the control and PLX4720-treated groups had a similar progression of weight loss from d 14–d 28 (from ∼19 g to 15.5 g). A typical example from each treatment group is shown in Fig. 2B. The control mouse (from group 5) had a baseline weight of 18.7 g. Its weight had declined to 16.6 g by d 28 and had progressed to severe cachexia (11.2 g) by d 35. Conversely, the mouse from group 8 began the experiment with a baseline of 18.3 g and had progressed to a weight of 14.4 g on d 28. After 3 wk of daily PLX4720 treatment, there was a progressive weight gain up to 17 g by 49 d and a clinical resolution of the cachectic phenotype.
Late intervention with PLX4720 induces tumor regression and reduces tumor volume and invasiveness in an in vivo model of human ATC
Nucera et al. (21) and Salerno et al. (17) have previously shown that PLX4720 is able to suppress B-RafV600E signaling cascade (i.e. down-regulation of ERK1/2 phosphorylation levels) in human thyroid cancer cells harboring the B-RafV600E mutation (e.g. 8505c human thyroid cancer cells). Therefore, we administered the anti-B-Raf V600E compound, PLX4720 in a late-intervention model of human ATC.
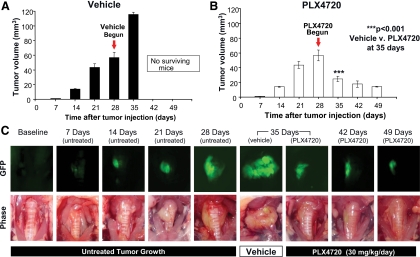
A tumor volume growth histogram demonstrates progressive growth until the tumor burden was fatal by 35 d (Fig. 3A). At 7 d after injection, small tumors measuring an average of 1 mm3 were present in the lateral aspect of the injected thyroid lobe; by 14 d after tumor implantation, there was an average tumor volume of 14 mm3 without evidence of tracheal compression or displacement. At 21 d, the tumor mass (average 44 mm3) had expanded to cause a slight compression of the trachea resulting in a leftward deviation. By 28 d, the control tumors had grown in the plane between the trachea and esophagus and extended to bilateral involvement (average 57 mm3).
Fig. 3.
PLX4720 induces tumor regression. A, Vehicle-treated mice had an exponential tumor growth up to 115 mm3 ± 14 mm3 by 35 d. No mouse in this group survived beyond 35 d. B, PLX4720-treated mice had tumor regression down to 14 mm3 ± 1 mm3 by 49 d. C, Tumor progression and regression (gross photos and GFP images). *, P < 0.05.
The mice that received vehicle from d 28–d 35 had an average tumor volume of 115 mm3 at 35 d and showed circumferential tumor involvement around the trachea with invasion into the esophagus. All of these mice were killed because of their severe tumor burden by 35 d after tumor implantation. All mice had large tumors by 28 d after implantation (average 57 mm3).
At 35 d after tumor implantation, those animals treated with 7 d of daily oral PLX4720 showed dramatically decreased tumor volume compared with controls (average 25 mm3 for PLX4720 and 115 mm3 for controls; P < 0.001). With continued treatment, tumor size continued to decrease (18 mm3 at 42 d and 14 mm3 at 49 d) as shown in Fig. 3B. Thus, 3 wk of treatment with PLX4720 reduced mean tumor volume by 75% from 57 mm3 at 28 d to 14 mm3 at 49 d. Vehicle-treated mice had large, invasive tumors that progressively grew circumferentially around the trachea as shown in Fig. 3C. Although this invasion was present after 28 d of tumor growth, the mice that were treated with 3 wk of PLX4720 had regression of invasion in addition to significantly smaller tumors (Fig. 3C).
PLX4720 induces regression of lung metastases in an in vivo model of human ATC
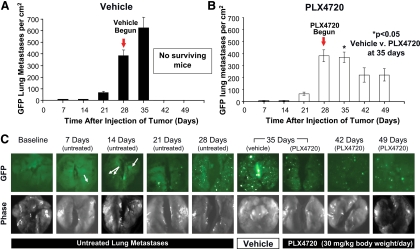
Untreated mice developed a progressive rise in the number of lung metastases as evaluated by GFP imaging at necropsy up to d 28 of the experiment (Fig. 4A). Metastatic disease to the lung was an early event, with mice showing evidence of a small number of GFP-positive tumor colonies in the lung even as early as 1 wk after tumor implantation (Fig. 4C). The six vehicle-treated mice had on average 383 GFP metastases per cm2 of lung by 28 d after tumor implantation, and the lung metastatic burden increased significantly to an average of 624 GFP metastasis/cm2 of lung (P < 0.001) by 35 d after tumor implantation, and no mouse in the vehicle-treated group survived beyond this time point (Fig. 4C). Mice treated with 1 wk of PLX4720, however, had a stabilization of the average number of lung metastases at 35 d (370 GFP metastasis/cm2), and this was significantly fewer than vehicle-treated mice at 35 d as shown in Fig. 4B (624 ± 92 in controls vs. 370 ± 43 GFP metastases/cm2 in PLX4720-treated mice; P < 0.05). Importantly, with 3 wk of PLX4720 treatment, the mice in groups 7 and 8 (42-/49-d time point) had even further significant regression in the number of lung metastases compared with the 28-d untreated mice in group 4 (down to 223 ± 51 GFP metastases/cm2; P < 0.05. The progression and regression of lung metastases are shown by GFP imaging in Fig. 4C.
Fig. 4.
PLX4720 induces regression of lung metastases. Number of metastases/cm2 as detected by GFP imaging. A, Vehicle-treated mice had exponential growth in the number of metastases, whereas PLX4720-treated mice had a significant reduction in the number of metastases by 49 d (B); *, P < 0.05. C, Anterior projections of the lungs with GFP imaging and gross photos.
PLX4720 inhibits tumor proliferation and cell cycle progression in an in vivo model of human ATC
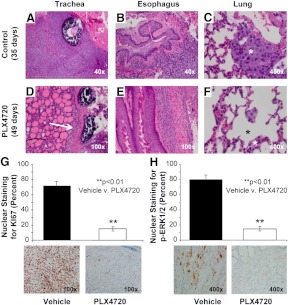
Control tumors at 35 d demonstrated extensive tracheal and esophageal invasion, and large number of lung micrometastases (Fig. 5, A–C). Mice treated with 3 wk of PLX4720, however, showed smaller tumors, less invasion, and decrease in the number of lung metastases (Fig. 5, D–F). Tumor cell proliferation rates in vivo were measured in both groups using nuclear Ki67 expression by IHC analysis (Fig. 5G). Control tumor-bearing mice treated with vehicle had an average Ki67 expression (proliferation index) of 71.7% ± 6% at 35 d after tumor implantation. Late therapeutic intervention with PLX4720 starting at 28 d after tumor implantation resulted in a significant decrease in the tumor proliferation rate (proliferation index) up to 49 d in the orthotopic tumors down to 15.0% ± 2.9% expression as shown in Fig. 5 (P < 0.01). Moreover, we found that nuclear phospho-ERK1/2 protein levels were significantly decreased in the PLX4720-treated tumors (3+) compared with controls (1+) (Fig. 5H) (P < 0.01).
Fig. 5.
Histology of orthotopic human ATC. Control tumors at 35 d demonstrated extrathyroidal extension (e.g. tracheal and esophageal invasion) (panels A and B), and lung metastases (asterisk, panel C). Mice treated with 3 wk of PLX4720, however, showed smaller and circumferential tumors (arrow, panels D and E), and decreases in lung metastases (asterisk, panel F). Ki67 immunostaining was significantly decreased with PLX4720 treatment (71.6% ± 6% in controls compared with 15% ± 2.9% in PLX4720-treated mice, P < 0.01) (panel G). Phospho-ERK1/2 immunostaining was significantly decreased with PLX4720 treatment (∼80% in controls compared with 10–20% in PLX4720-treated mice, P < 0.01) (panel H).
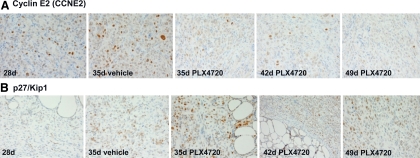
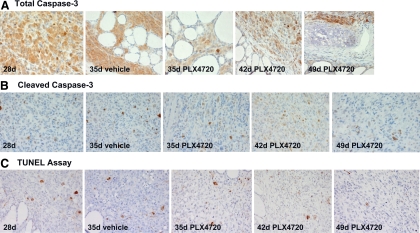
We have also analyzed protein expression of different molecules (cyclin D1, cyclin E2, cyclin B1, p27/kip1, and p21) involved in cell cycle checkpoints for the regulation of cell proliferation/DNA synthesis. Importantly, we have found that PLX4720 effectively targeted B-RafV600E in vivo and inhibited cell cycle progression in vivo by down-regulating protein levels of CCNE2 (Fig. 6A) and up-regulating cell cycle inhibitor p27/kip1(Fig. 6B). Additionally, we found that protein levels of both cleaved caspase-3 (an activated form of caspase-3) (low expression) and total caspase-3 (moderate/high expression) (Fig. 7, A and B) were not significantly different in the PLX4720-treated orthotopic ATC compared with untreated ATC; this result was also confirmed by TUNEL assay that showed a slight increase of apoptotic cells in the PLX4720-treated group at 42 d and less at 49 d compared with controls (Fig. 7C).
Fig. 6.
Immunoexpression of Cyclin E2 and p27/kip1. A, Control tumors show strong nuclear Cyclin E2 (CCNE2) protein levels (2+) at 28 d and 35 d. Orthotopic tumors treated with 3 wk of PLX4720 show a significant decrease of nuclear CCNE2 protein levels (moderate, 2+) at 35 d and 42 d, which progressively decrease up to 49 d (week, 1+). All magnifications, ×400. B, Control tumors show low nuclear p27/kip1 protein levels (2+) at 28 d and 35 d. Orthotopic tumors treated with 3 wk of PLX4720 show a significant increase of p27/kip1 protein levels (strong, 3+) at 35 d and 42 d, which progressively increase up to 49 d (strong, 3+). All magnifications, ×400.
Fig. 7.
Immunoexpression of total caspase-3 and cleaved caspase-3. A, Control tumors and PLX4720-treated tumors show strong (3+) cytoplasmic total caspase 3 protein levels (2+) at 28, 35, 42, and 49 d. All magnifications, ×400. B, Control tumors show moderate cytoplasmic cleaved caspase 3 protein levels (2+) at 28 d and 35 d. Orthotopic tumors treated with 3 wk of PLX4720 show a slight but not significant increase of cytoplasmic cleaved caspase 3 protein levels at 35 d (2+) and 42 d (3+), and moderate at 49 d (2+). C, TUNEL assay using peroxidase system. Slight increase of nuclear staining (apoptotic cells) in the PLX4720-treated orthotopic tumors at 42 d compared with vehicle (control)-treated tumors. All magnifications, ×400.
Discussion
ATC is unresponsive to all current treatment regimens. Rational drug design targeting this cancer looks at altering the level of a known mutant oncoprotein implicated in the aggressive course of this lethal disease. Molecular targets for ATC and recurrent PTC are mostly centered around the RAS/RAF/MAPK (mitogen-activated ERK1/2) kinase signaling pathway given the prominence of this pathway as an oncogenic event in PTC and ATC progression (22–25). In 2002, Davies et al. (26) identified an oncogene widespread among human cancers, mutant V600E B-Raf, or B-RafV600E. B-RafV600E is expressed in a variety of human cancers including melanoma, colorectal, glioma, sarcoma, and thyroid cancer (8, 12). Interestingly, the B-RafV600E mutation is seen exclusively in PTC and ATC but not in other histotypes, such as medullary or follicular thyroid cancers. Histopathological correlation studies in patients with PTC demonstrate an increase in the B-RafV600E mutation frequency in tumors of patients with extrathyroidal extension, thyroid capsular invasion, and lymph node metastases (27, 28). Confirmatory animal studies have also shown that thyroid specific expression of B-RafV600E in transgenic mice leads to poorly differentiated PTC (29).
Recent studies have looked for a distinct molecular explanation for how the B-RafV600E mutation leads to a more aggressive histopathological phenotype. Recently, Nucera et al. (9) have shown that B-RafV600E-positive PTCs have a distinct genetic profile with altered gene expression in critical gene sets important in tumor invasion and ECM remodeling. The presence of the B-RafV600E mutation promotes ATC invasion via TSP-1 in vitro and in vivo, a key regulator of ECM remodeling. Additionally, knockdown of B-RafV600E in vivo results in decreased tumor volume and lung metastases. Nucera et al. (9) have previously shown that oral administration PLX4720 within 1 wk (early intervention) in an orthotopic mouse model of human ATC can result in significant growth inhibition of tumor volume.
The current study demonstrates the remarkable efficacy of this compound to induce significant regression in an orthotopic mouse model of ATC even when administered at a very late therapeutic intervention stage. Although previous studies have investigated the mechanistic role of anti-B-RafV600E therapies in melanoma and colorectal cells (13, 30–32), and in thyroid cancer cells models (9, 17, 21), no previous study has investigated the effect on a late intervention model of ATC that reproduces the advanced disease seen in patients with this malignancy.
In this study, the timeframe of tumor growth and metastasis of ATC 8505c cells was characterized, and the response to a late intervention treatment was evaluated. Nucera et al. (20) have demonstrated that this orthotopic model of human ATC is characterized by the induction of intra-thyroidal tumors that grow and extend into surrounding structures, metastasize to the lungs, and result in death by 35 d after the implantation of the tumors. Interestingly, we have also found that metastasis is a very early event in this model (Fig. 4C), occurring by 7 d after tumor implantation when the primary tumor size is only 1.5 mm3 (Fig. 3C). This finding is consistent with the aggressive and lethal disease seen in humans and also highlights previous reports on the important role of the B-RafV600E mutation in the early invasion and metastasis in this human thyroid cancer model (9). Moreover, Knauf et al. (33) have shown that tumor initiation by oncogenic B-Raf renders thyroid cells susceptible to TGFβ-induced epithelial-mesenchymal transition, through a MAPK-dependent process.
Given that our previous studies have shown that anti-B-RafV600E therapy inhibits the early growth of 8505c in vivo (9), we anticipated that tumor size, mouse weight, and metastases would stabilize with PLX4720. Surprisingly, however, we found that the mice recovered 86% of weight that they lost from baseline and had clinical resolution of cachexia (piloerection, muscle atrophy, weight loss). The vehicle-treated mice progressed to severe cachexia, whereas the PLX4720-treated mice demonstrated reversal of cachexia and weight gain. The underlying cause of this weight gain is incompletely understood, but it might have resulted from the inhibition of the systemic burden of metastases, from the relief of esophageal compression by the large thyroid tumors, or a combination of these two processes. Late therapeutic intervention with PLX4720 treatment not only reversed weight loss, decreased tumor volume, tumor cell proliferation and number of lung metastasis dramatically but also extended the mice's lifespan by 40% compared with controls. All mice were killed according to a predetermined experimental plan by 49 d after tumor implantation, partially due to limited drug supply. Many of these had the equivalent tumor burden of a control mouse at 2 wk after implantation and thus may have lived several weeks longer if they had not been killed.
The tumors themselves showed a surprising degree of regression after only 1 wk of PLX4720 therapy, and this effect continued further with tumor volume decreasing down to 9 mm3 with 3 wk of PLX4720 treatment. Primary tumor regression caused by PLX4720 correlated with significant decreases in tumor cell proliferation rates (Ki67 nuclear expression), and in phospho-ERK1/2 (42 d treated ≪35 d treated, vs. 35 d untreated) protein levels downstream of B-RafV600E kinase activity. Furthermore, the number of pulmonary metastases was significantly decreased after 3 wk of PLX4720 treatment (223/cm2), even though treatment was initiated at such a late stage. Overall, our results do show that even when treating mice with initially advanced tumors for 21 d (from 28 d to 49 d) with PLX4720 we saw no increase in tumor size. Additionally, we have found that when the same 8505c human metastatic thyroid cancer cells are engineered with short hairpin RNA to stably knock-down B-RafV600E and then orthotopically injected into the thyroid of SCID mice, there was a significant delay in thyroid tumor growth and inhibition of lung metastasis at 35 d compared with controls (9). We have previously shown that PLX4720 arrests 8505c cells in G1 (32), and here we found that cyclin E2 was down-regulated in the orthotopic ATC after PLX4720 treatment. Cyclin E2 belongs to the family of E-type cyclins that operate during the G1/S phase progression in mammalian cells. It has a catalytic partner, cyclin-dependent kinase 2, and activates its associated kinase activity at similar times during cell cycle progression. Cyclin E2 controls S-phase (DNA synthesis) entry of different cell types in a tissue-specific fashion and cell cycle progression (34). Additionally, p27kip1 (but not p21), a strong inhibitor of cell proliferation, was up-regulated with PLX4720 treatment in vivo from 35 d up to 49 d. Although PLX4720 significantly inhibited cell cycle progression interfering with G1/S checkpoints (causing G1 arrest and inhibition of S-phase/DNA synthesis), overall the number of apoptotic cells assessed by cleaved caspase-3 protein expression levels and TUNEL assay were not significantly different in the PLX4720-treated orthotopic ATC compared with controls.
Although development of this drug for use in some forms of thyroid cancer is promising and should be pursued, lessons from melanoma treatment trials in patients with advanced melanoma show that in some cases human cancer cells could develop escape mechanisms to PLX4720. Further studies into molecular and stromal targets of the B-RafV600E mutation will allow rational design of serial or concurrent therapeutics (18, 35). Although resistance to therapy with this drug remains a possibility for prolonged human use, the dramatic results of our study are encouraging for the treatment of B-RafV600E-positive ATC, for which no effective therapy currently exists.
In conclusion, the anti-B-RafV600E compound PLX4720 induces striking tumor regression, reverses cachexia, and extends survival in a late-intervention orthotopic model of ATC. Our results demonstrate that even late therapeutic intervention directed at the proper molecular step in thyroid tumor progression may be effective in halting and reversing the process. Translating this therapeutic strategy to patients presenting with large ATC that harbor the B-RafV600E mutation may cause a reduction in primary tumor volume or metastatic burden, thus allowing time to proceed with additional therapies such as surgical debulking or radiation. Additionally, interesting recent in vitro data suggest potential sodium iodide symporter regulation in certain thyroid cancer cell lines by mutant B-RafV600E. If down-regulation of B-Raf with anti-B-RafV600E therapy also causes sodium iodide symporter up-regulation, then theoretically some patients may be given anti-B-RafV600E therapy both to reduce tumor size and metastatic burden and possibly to allow redifferentiation, thus making radioactive iodine administration possible in some patients for control of additional metastatic burden (36, 37). These results, in combination with other preclinical studies of PLX4720, provide justification for a human clinical trial of this drug for ATC that harbor B-RafV600E. By implementing molecularly targeted agents that inhibit this cancer's driving mutations, we may expand the armamentarium of treatments for this otherwise incurable disease.
Acknowledgments
We thank Dr. Yutaka Kawakami (Keio University, Tokyo, Japan) for providing HIV-U6 vectors. We also thank Dr. Gideon Bollag and Dr. Paul Lin of Plexxikon (Berkeley, CA) for technical assistance with PLX4720 administration.
This work was funded by National Institutes of Health Grants 1R01CA149738-01 (to S.P.) and 5T32 DK007754-10 (to R.H.). C.N. is an M.D. and recipient of Ph.D. fellowship supported from the Italian Ministry of Education, Universities and Research (MIUR) and carried out at Harvard Medical School; he was also funded through A. Gemelli Medical School (Catholic University, Rome, Italy).
Disclosure Summary: All authors declare no financial conflicts of interest.
Footnotes
- ATC
- Anaplastic thyroid cancer
- ECM
- extracellular matrix
- GFP
- green fluorescent protein
- IHC
- immunohistochemical
- PTC
- papillary thyroid cancer
- SCID
- severe combined immunodeficient
- TSP-1
- thrombospondin-1
- TUNEL
- terminal deoxynucleotidyl transferase dUTP nick end labeling.
References
- 1. Neff RL, Farrar WB, Kloos RT, Burman KD. 2008. Anaplastic thyroid cancer. Endocrinol Metab Clin North Am 37:525–538 [DOI] [PubMed] [Google Scholar]
- 2. Are C, Shaha AR. 2006. Anaplastic thyroid carcinoma: biology, pathogenesis, prognostic factors, and treatment approaches. Ann Surg Oncol 13:453–464 [DOI] [PubMed] [Google Scholar]
- 3. Broome JT, Gauger PG, Miller BS, Doherty GM. 2009. Anaplastic thyroid cancer manifesting as new-onset Horner syndrome. Endocr Pract 15:563–566 [DOI] [PubMed] [Google Scholar]
- 4. Pasieka JL. 2003. Anaplastic thyroid cancer. Curr Opin Oncol 15:78–83 [DOI] [PubMed] [Google Scholar]
- 5. Braga-Basaria M, Ringel MD. 2003. Clinical review 158: Beyond radioiodine: a review of potential new therapeutic approaches for thyroid cancer. J Clin Endocrinol Metab 88:1947–1960 [DOI] [PubMed] [Google Scholar]
- 6. Wiseman SM, Griffith OL, Deen S, Rajput A, Masoudi H, Gilks B, Goldstein L, Gown A, Jones SJ. 2007. Identification of molecular markers altered during transformation of differentiated into anaplastic thyroid carcinoma. Arch Surg 142:717–727; discussion 727–729 [DOI] [PubMed] [Google Scholar]
- 7. Nikiforov YE. 2004. Genetic alterations involved in the transition from well-differentiated to poorly differentiated and anaplastic thyroid carcinomas. Endocr Pathol 15:319–327 [DOI] [PubMed] [Google Scholar]
- 8. Xing M. 2007. BRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endocr Rev 28:742–762 [DOI] [PubMed] [Google Scholar]
- 9. Nucera C, Porrello A, Antonello ZA, Mekel M, Nehs MA, Giordano TJ, Gerald D, Benjamin LE, Priolo C, Puxeddu E, Finn S, Jarzab B, Hodin RA, Pontecorvi A, Nose V, Lawler J, Parangi S. 2010. B-Raf(V600E) and thrombospondin-1 promote thyroid cancer progression. Proc Natl Acad Sci USA 107:10649–10654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Smallridge RC, Marlow LA, Copland JA. 2009. Anaplastic thyroid cancer: molecular pathogenesis and emerging therapies. Endocr Relat Cancer 16:17–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ricarte-Filho JC, Ryder M, Chitale DA, Rivera M, Heguy A, Ladanyi M, Janakiraman M, Solit D, Knauf JA, Tuttle RM, Ghossein RA, Fagin JA. 2009. Mutational profile of advanced primary and metastatic radioactive iodine-refractory thyroid cancers reveals distinct pathogenetic roles for BRAF, PIK3CA, and AKT1. Cancer Res 69:4885–4893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nucera C, Goldfarb M, Hodin R, Parangi S. 2009. Role of B-Raf(V600E) in differentiated thyroid cancer and preclinical validation of compounds against B-Raf(V600E). Biochim Biophys Acta 1795:152–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tsai J, Lee JT, Wang W, Zhang J, Cho H, Mamo S, Bremer R, Gillette S, Kong J, Haass NK, Sproesser K, Li L, Smalley KS, Fong D, Zhu YL, Marimuthu A, Nguyen H, Lam B, Liu J, Cheung I, Rice J, Suzuki Y, Luu C, Settachatgul C, Shellooe R, Cantwell J, Kim SH, Schlessinger J, Zhang KY, West BL, Powell B, Habets G, Zhang C, Ibrahim PN, Hirth P, Artis DR, Herlyn M, Bollag G. 2008. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc Natl Acad Sci USA 105:3041–3046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Packer LM, East P, Reis-Filho JS, Marais R. 2009. Identification of direct transcriptional targets of (V600E)BRAF/MEK signalling in melanoma. Pigment Cell Melanoma Res 22:785–798 [DOI] [PubMed] [Google Scholar]
- 15. Emery CM, Vijayendran KG, Zipser MC, Sawyer AM, Niu L, Kim JJ, Hatton C, Chopra R, Oberholzer PA, Karpova MB, MacConaill LE, Zhang J, Gray NS, Sellers WR, Dummer R, Garraway LA. 2009. MEK1 mutations confer resistance to MEK and B-RAF inhibition. Proc Natl Acad Sci USA 106:20411–20416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, O'Dwyer PJ, Lee RJ, Grippo JF, Nolop K, Chapman PB. 2010. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med 363:809–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Salerno P, De Falco V, Tamburrino A, Nappi TC, Vecchio G, Schweppe RE, Bollag G, Santoro M, Salvatore G. 2010. Cytostatic activity of adenosine triphosphate-competitive kinase inhibitors in BRAF mutant thyroid carcinoma cells. J Clin Endocrinol Metab 95:450–455 [DOI] [PubMed] [Google Scholar]
- 18. Nucera C, Lawler J, Parangi S. 2011. BRAFV600E and microenvironment in thyroid cancer: a functional link to drive cancer progression. Cancer Res 71:2417–2422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Meireles AM, Preto A, Rocha AS, Rebocho AP, Máximo V, Pereira-Castro I, Moreira S, Feijão T, Botelho T, Marques R, Trovisco V, Cirnes L, Alves C, Velho S, Soares P, Sobrinho-Simões M. 2007. Molecular and genotypic characterization of human thyroid follicular cell carcinoma-derived cell lines. Thyroid 17:707–715 [DOI] [PubMed] [Google Scholar]
- 20. Nucera C, Nehs MA, Mekel M, Zhang X, Hodin R, Lawler J, Nose V, Parangi S. 2009. A novel orthotopic mouse model of human anaplastic thyroid carcinoma. Thyroid 19:1077–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nucera C, Nehs MA, Nagarkatti SS, Sadow PM, Mekel M, Fischer AH, Lin PS, Bollag GE, Lawler J, Hodin RA, Parangi S. 2011. Targeting BRAFV600E with PLX4720 displays potent antimigratory and anti-invasive activity in preclinical models of human thyroid cancer. Oncologist 16:296–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gupta-Abramson V, Troxel AB, Nellore A, Puttaswamy K, Redlinger M, Ransone K, Mandel SJ, Flaherty KT, Loevner LA, O'Dwyer PJ, Brose MS. 2008. Phase II trial of sorafenib in advanced thyroid cancer. J Clin Oncol 26:4714–4719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sherman SI, Wirth LJ, Droz JP, Hofmann M, Bastholt L, Martins RG, Licitra L, Eschenberg MJ, Sun YN, Juan T, Stepan DE, Schlumberger MJ, Motesanib Thyroid Cancer Study G 2008. Motesanib diphosphate in progressive differentiated thyroid cancer. N Engl J Med 359:31–42 [DOI] [PubMed] [Google Scholar]
- 24. Kloos RT, Ringel MD, Knopp MV, Hall NC, King M, Stevens R, Liang J, Wakely PE, Jr, Vasko VV, Saji M, Rittenberry J, Wei L, Arbogast D, Collamore M, Wright JJ, Grever M, Shah MH. 2009. Phase II trial of sorafenib in metastatic thyroid cancer. J Clin Oncol 27:1675–1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cohen EE, Rosen LS, Vokes EE, Kies MS, Forastiere AA, Worden FP, Kane MA, Sherman E, Kim S, Bycott P, Tortorici M, Shalinsky DR, Liau KF, Cohen RB. 2008. Axitinib is an active treatment for all histologic subtypes of advanced thyroid cancer: results from a phase II study. J Clin Oncol 26:4708–4713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, Floyd Y, Gray K, Hall S, Hawes R, Hughes J, Kosmidou V, Menzies A, Mould C, Parker A, Stevens C, Watt S, Hooper S, et al. 2002. Mutations of the BRAF gene in human cancer. Nature 417:949–954 [DOI] [PubMed] [Google Scholar]
- 27. Xing M, Clark D, Guan H, Ji M, Dackiw A, Carson KA, Kim M, Tufaro A, Ladenson P, Zeiger M, Tufano R. 2009. BRAF mutation testing of thyroid fine-needle aspiration biopsy specimens for preoperative risk stratification in papillary thyroid cancer. J Clin Oncol 27:2977–2982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Frasca F, Nucera C, Pellegriti G, Gangemi P, Attard M, Stella M, Loda M, Vella V, Giordano C, Trimarchi F, Mazzon E, Belfiore A, Vigneri R. 2008. BRAF(V600E) mutation and the biology of papillary thyroid cancer. Endocr Relat Cancer 15:191–205 [DOI] [PubMed] [Google Scholar]
- 29. Knauf JA, Ma X, Smith EP, Zhang L, Mitsutake N, Liao XH, Refetoff S, Nikiforov YE, Fagin JA. 2005. Targeted expression of BRAFV600E in thyroid cells of transgenic mice results in papillary thyroid cancers that undergo dedifferentiation. Cancer Res 65:4238–4245 [DOI] [PubMed] [Google Scholar]
- 30. Hatzivassiliou G, Song K, Yen I, Brandhuber BJ, Anderson DJ, Alvarado R, Ludlam MJ, Stokoe D, Gloor SL, Vigers G, Morales T, Aliagas I, Liu B, Sideris S, Hoeflich KP, Jaiswal BS, Seshagiri S, Koeppen H, Belvin M, Friedman LS, Malek S. 2010. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature 464:431–435 [DOI] [PubMed] [Google Scholar]
- 31. Heidorn SJ, Milagre C, Whittaker S, Nourry A, Niculescu-Duvas I, Dhomen N, Hussain J, Reis-Filho JS, Springer CJ, Pritchard C, Marais R. 2010. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell 140:209–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. 2010. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature 464:427–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Knauf JA, Sartor MA, Medvedovic M, Lundsmith E, Ryder M, Salzano M, Nikiforov YE, Giordano TJ, Ghossein RA, Fagin JA. 2011. Progression of BRAF-induced thyroid cancer is associated with epithelial-mesenchymal transition requiring concomitant MAP kinase and TGFβ signaling. Oncogene 30:3153–3162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Geng Y, Yu Q, Whoriskey W, Dick F, Tsai KY, Ford HL, Biswas DK, Pardee AB, Amati B, Jacks T, Richardson A, Dyson N, Sicinski P. 2001. Expression of cyclins E1 and E2 during mouse development and in neoplasia. Proc Natl Acad Sci USA 98:13138–13143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nucera C, Lawler J, Hodin R, Parangi S. 2010. The BRAFV600E mutation: what is it really orchestrating in thyroid cancer? Oncotarget 1:751–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Durante C, Puxeddu E, Ferretti E, Morisi R, Moretti S, Bruno R, Barbi F, Avenia N, Scipioni A, Verrienti A, Tosi E, Cavaliere A, Gulino A, Filetti S, Russo D. 2007. BRAF mutations in papillary thyroid carcinomas inhibit genes involved in iodine metabolism. J Clin Endocrinol Metab 92:2840–2843 [DOI] [PubMed] [Google Scholar]
- 37. Riesco-Eizaguirre G, Rodríguez I, De la Vieja A, Costamagna E, Carrasco N, Nistal M, Santisteban P. 2009. The BRAFV600E oncogene induces transforming growth factor β secretion leading to sodium iodide symporter repression and increased malignancy in thyroid cancer. Cancer Res 69:8317–8325 [DOI] [PubMed] [Google Scholar]