Abstract
There is growing appreciation that estrogen signaling pathways can be modulated by naturally occurring environmental compounds such as phytoestrogens and the more recently discovered xenoestrogens. Many researchers studying the effects of estrogens on brain function or behavior in animal models choose to use phytoestrogen-free food for this reason. Corncob bedding is commonly used in animal facilities across the United States and has been shown to inhibit estrogen-dependent reproductive behavior in rats. The mechanism for this effect was unclear, because the components of corncob bedding mediating this effect did not bind estrogen receptors. Here, we show in the California mouse (Peromyscus californicus) that estrogens decrease aggression when cardboard-based bedding is used but that this effect is absent when corncob bedding is used. California mice housed on corncob bedding also had fewer estrogen receptor-α-positive cells in the bed nucleus of the stria terminalis and ventromedial hypothalamus compared with mice housed on cardboard-based bedding. In addition, corncob bedding suppressed the expression of phosphorylated ERK in these brain regions as well as in the medial amygdala and medial preoptic area. Previous reports of the effects of corncob bedding on reproductive behavior are not widely appreciated. Our observations on the effects of corncob bedding on behavior and brain function should draw attention to the importance that cage bedding can exert on neuroendocrine research.
Estrogens have powerful effects on behavior in a variety of contexts. These effects are mediated by the classical estrogen receptors (ER) such as ERα as well as membrane receptors such as G protein-coupled receptor 30 (1, 2). Recent evidence shows that the effects of estrogen signaling on behavior are dependent on the environment. For example, several forms of endocrine disruptor compounds have been identified that can directly or indirectly alter estrogen signaling (3). The phytoestrogen isoflavone, which is present in many animal feeds, inhibits ER expression in the brain and inhibits female sexual behavior (4). An unappreciated source of estrogenic compounds that may influence laboratory animal research is cage bedding. A series of papers demonstrated that corncob bedding, which is used in many animal facilities in the United States, inhibited sexual behavior in both female (5) and male (6) rats. These effects were linked to specific components of corncob bedding: linoleic acid-derived tetrahydrofuran (THF)-diols (7). Interestingly, THF-diols do not bind to ERα, so it has been unclear how THF-diols affect behavior.
Here, we examine the effects of corncob bedding on estrogen-dependent aggressive behavior in the California mouse (Peromyscus californicus). Previous studies in Peromyscus demonstrated that male-male aggression is increased under winter-like short-day photoperiods and that this effect is facilitated by rapid effects of estradiol (8, 9). We examined the levels of THF-diols in plasma as well as ERα immunoreactivity in the bed nucleus of the stria terminalis (BNST), ventromedial hypothalamus (VMH), medial preoptic area (MPOA), and medial amygdala (MEA). These brain regions form part of a circuit that regulates male social behaviors, including aggression (10). We also examined expression of phospho-ERK (pERK) because ERK function has been linked to aggressive behaviors (11–13). At present, we have not yet discovered the specific mechanisms mediating the estrogenic properties of corncob bedding. However, our data and those of others (5–7) indicate that the type of cage bedding used has the potential to influence the results of virtually any study examining the effects of estrogens on physiology or behavior.
Materials and Methods
Adult male P. californicus mice bred in our laboratory colony and purchased from the Peromyscus Stock Center (University of South Carolina, Columbia, SC) were housed in clear polypropylene cages provided with either 500 ml Carefresh (Absorption Corp., Fernadale, WA; no. 868744) or 300 ml corncob bedding (1/8 in.; Andersons, Maume, OH; no. 88) and cotton nestlets. Harlan Teklad 2016 food (phytoestrogen free, Hayward, CA) and water was provided ad libitum in glass bottles with rubber stoppers. The beddings were not irradiated, and cages were changed once per week. All animals were maintained in accordance with the recommendations of the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Experiments were approved by the Animal Use and Care Advisory Committee at the University of California, Davis. Linoleic acid-derived THF-diols were prepared as previously described (14).
Behavioral experiments
Experimental male mice ages 3–6 months old were housed under a short-day photoperiod (8 h light, 16 h dark) for at least 6 wk, after which the mice were abdominally castrated and randomly assigned to receive osmotic mini-pumps (implanted sc) filled with fadrozole (0.25 mg/kg · day; Sigma Chemical Co., St. Louis, MO) or saline. No other hormone replacement was administered. Each mouse recovered while single housed for 10 d and was then tested in a resident-intruder aggression test for 7 min. Intruders were group housed and similar in age, unrelated to the residents and housed under long-day photoperiod (16 h light, 8 h dark). Behavioral tests were conducted under dim red light. Immediately after the test, each mouse was anesthetized with isoflurane and rapidly decapitated. Brains were fixed in 5% acrolein in 0.1 m PBS overnight at 4 C, transferred to 20% sucrose in PBS for 48 h, and then frozen. Trunk blood was centrifuged and plasma frozen at −40 C for analysis.
Immunohistochemistry
Brains were sectioned at 40 μm and stored in cryopotectant at −20 C. Immunohistochemistry methods for ERα (9) and pERK (11) in California mice have been previously described. Briefly, brain sections were washed in PBS, incubated in 1% sodium borohydride, and blocked in 10% normal goat serum and 0.3% hydrogen peroxide in PBS. Sections were then incubated in either ERα antibody for 48 h (C1355; Millipore, Billerica, MA; 1:100,000 dilution) or overnight pERK antibody (no. 4370; Cell Signaling Technology, Danvers, MA; 1:250 dilution), dissolved in 2% normal goat serum and 0.5% Triton X-100 in PBS. Sections were then washed with PBS and incubated in biotinylated goat antirabbit antibody (Vector Laboratories, Burlingame, CA; 1:500), then incubated in avidin-biotin complex (ABC Elite Kit; Vector); and finally developed in nickel-enhanced diaminobenzidine (Vector).
Image analysis
Images of the MEA, BNST, MPOA, and VMH were taken under bright-field conditions using a Zeiss Axioimager equipped with an Axiocam MRC camera. Cellular quantification was performed with Image J (National Institutes of Health, Bethesda, MD) where boxes were used to count the number of positive cells within each brain area (dorsal BNST, 0.27 × 0.33 mm; ventral BNST, 0.29 × 0.23 mm; MEA, 0.25 × 0.22 mm; VMH, 0.27 × 0.27 mm; MPOA, 0.24 × 0.40 mm).
THF-diol measurements
For measurements of linoleic-derived THF-diol isomers in blood samples, 200 μl plasma were loaded onto a Waters Oasis-HLB cartridge (1 ml, 30 mg; Waters Corp., Milford, MA) equilibrated with 94.9:5:0.1 vol/vol water/methanol/acetic acid. The cartridge was washed with 1.5 ml 94.9:5:0.1 vol/vol water/methanol/acetic acid. After drying, the solid phase extraction cartridges were further dried with low vacuum about 20 min. Solid phase extraction cartridge was eluted with 0.5 ml methanol followed by 2 ml ethyl acetate into tubes containing 6 μl 30% glycerol in methanol as a trap solution. The solvents were removed under vacuum, and the residues were reconstituted in 50 μl methanol containing 200 nm internal standard [12-(3-cyclohexylureido)dodecanoic acid].
Liquid chromatography tandem mass spectrometry analysis were performed on an Agilent 1200 liquid chromatography system (Agilent, Santa Clara, CA) equipped with a ABI 4000 TRAP tandem mass spectrometer (Applied Biosystems, Foster City, CA). After injection of 25 μl extract, the analytes were separated by a binary gradient of 0.1% acetic acid in water as solvent A and 800:150:1 methanol/acetonitrile/acetic acid as solvent B on a reverse-phase column (Zorbax Eclipse Plus C18, 2.1 × 150 mm, 1.8-μm, 600 bar, Santa Clara, CA). The analytes were separated by a binary gradient of 25 mm ammonium acetate containing 0.1% acetic acid 95%/5% acetonitrile/water (vol/vol). Elution was performed with a gradient of 70–95% of solvent B from time 0–4 min, followed by 95% of solvent B for an additional minute, with a flow rate of 300 μl/min. Elution times of 1.74 and 2.11 min were obtained for trans-THF diols and cis-THF diols, respectively.
The mass spectrometer was operated in negative ion mode with a capillary voltage of −4500 V, using 25 psi curtain gas, 40 psi nebulizer gas, and 71 psi drying gas at a temperature of 500 C. The dwell time was set to 50 msec and collision energy to −34 V. Analyst software (version 1.5.1; Applied Biosystems) was used for data analysis. In selected reaction-monitoring mode, the mass to charge ratio 329/201 transition was used to monitor both trans-THF diols and cis-THF diols, whereas the 339/214 transition was used for the internal standard. The method was calibrated using a series of standard solutions. The limit of detection (signal-to-noise ratio = 3) was 0.1 nm for both trans-THF diols and cis-THF diols. The method provided a broad linear range of detection from 0.3–1000 nm (r2 ≥ 0.99), suggesting a limit of quantification around 0.1 nm. To validate the method, plasma samples were spiked with 10 and 100 nm of the analytes and processed like samples. The present method shows recovery rate of 85–119% of the spiked analytes. Dried corncob bedding was extracted with methanol, and THF-diols levels were then measured.
Fecundity analysis and corncob ingestion
Colony records were used to estimate the effects of corncob bedding on reproductive success. We recorded the number of pups weaned per month over a 3-month period from established male-female breeder pairs (n = 19). These breeders were switched to corncob bedding, and we recorded the number of pups weaned per month over a 3-month period starting 2 months after the switch.
Statistical analyses
All behavioral, brain, and THF-diol data were analyzed with two-way ANOVA testing for effects of bedding, fadrozole, and the interaction. To improve normality of datasets, attack latency data were log transformed, whereas pERK cell counts were square root transformed (15). Fecundity data were averaged across months for each bedding type and analyzed using paired t tests.
Results
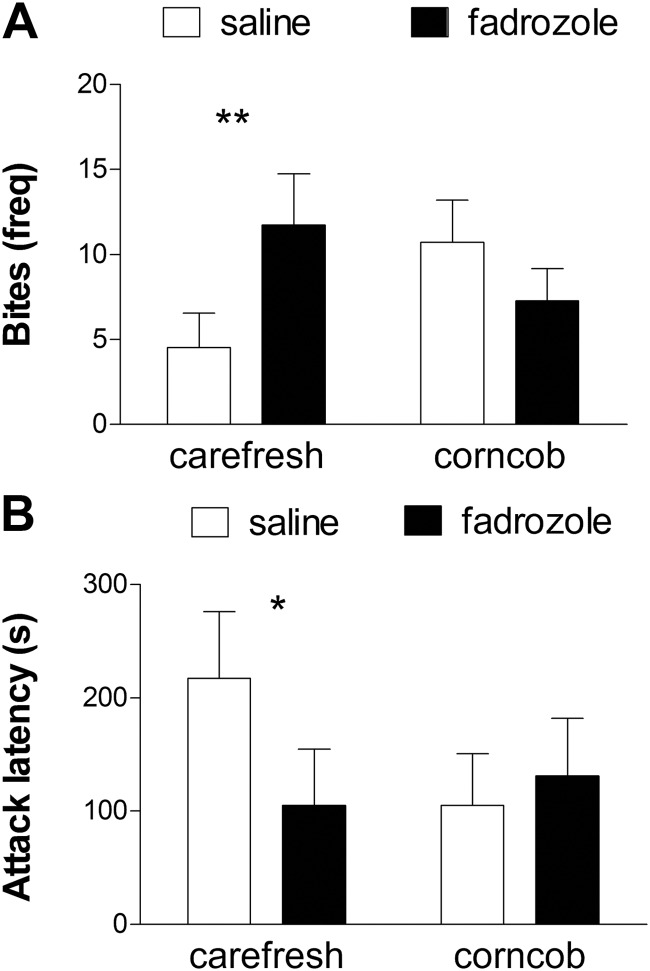
The effect of fadrozole on aggressive behavior depended on which bedding was used [Fig. 1A, bedding × fadrozole, F(1,45 = 4.88; P = 0.03]. On Carefresh bedding, fadrozole increased the number of times mice attacked intruders (bites, planned comparison, P < 0.01). On corncob bedding, mice treated with fadrozole attacked intruders less frequently than mice treated with saline, but this difference was not significant. The latency to first attack showed a similar pattern [Fig. 1B, bedding × fadrozole, F(1,45) = 2.52; P = 0.12]. Planned comparisons indicated that fadrozole decreased attack latency in mice on Carefresh bedding (planned comparison, P < 0.05) but had no effect in mice on corncob bedding.
Fig. 1.
The effects of estrogens on aggression depend on cage bedding. Aromatase inhibition with fadrozole increased the number of bites (A) and decreased attack latency (B) on Carefresh bedding but not corncob bedding. *, P < 0.05; **, P < 0.01, planned comparison for effect of fadrozole; n = 10–14 per group.
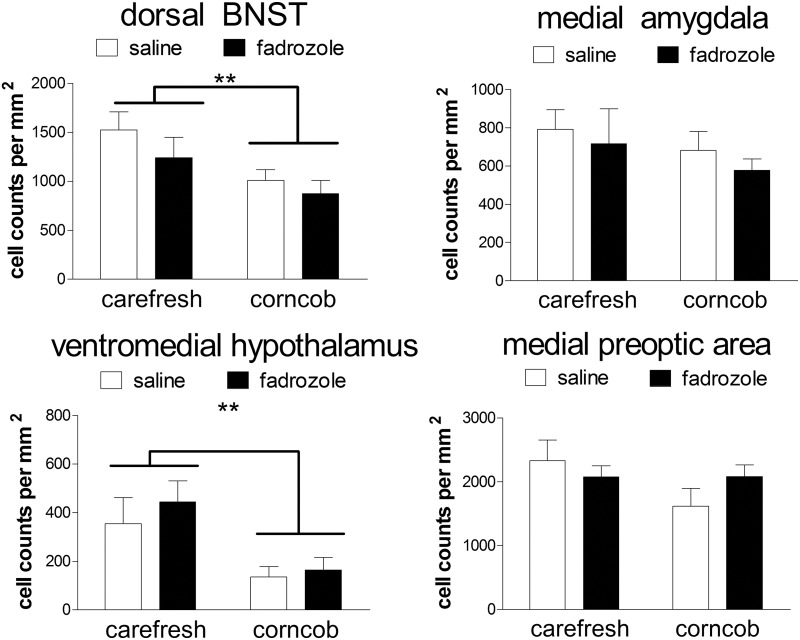
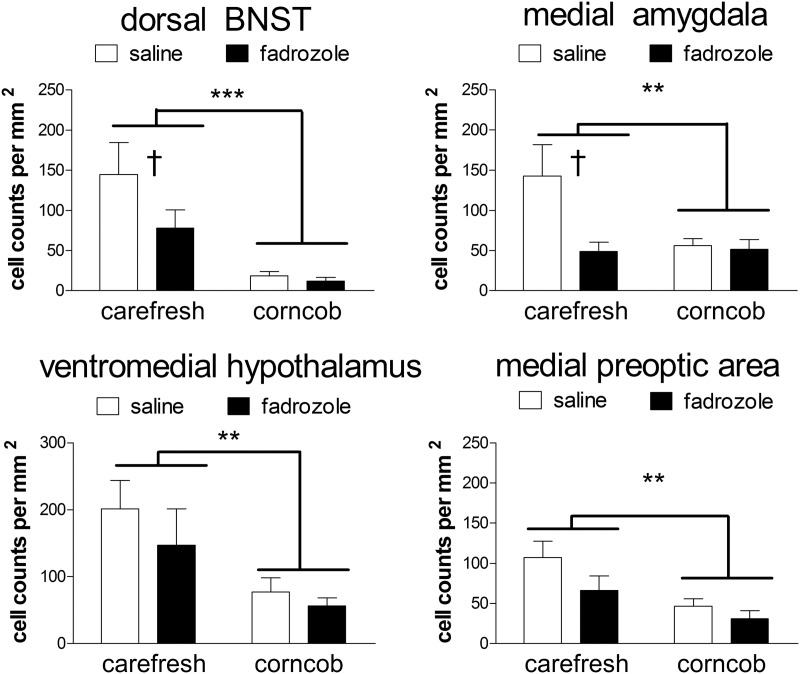
Corncob bedding also had important effects in the brain. Corncob bedding suppressed the number of ERα-positive cells in the dorsal BNST [Fig. 2, F(1,33) = 6.87; P = 0.01] and VMH [Fig. 2, F(1,33) = 11.5; P = 0.02]. There were no differences in ERα-positive cells in the ventral BNST, MPOA, or MEA (Fig. 2, all P > 0.14). Measurements of pERK immunostaining demonstrated that corncob bedding suppressed the number of pERK cells in every brain region examined (Fig. 3, all main effects of bedding P < 0.05). In the ventral and dorsal BNST and MEA, fadrozole also reduced the number of pERK-positive cells for mice housed on Carefresh bedding (planned comparisons, P < 0.05) but not mice housed on corncob bedding.
Fig. 2.
Corncob bedding suppresses the number of ER-α-immunoreactive cells in the BNST and VMH but not the MEA or MPOA. **, P < 0.01, main effect of bedding; n = 7–11 per group.
Fig. 3.
Corncob bedding suppresses the number of pERK-immunoreactive cells. Fadrozole reduces the number of pERK-immunoreactive cells in the BNST and VMH but not the MEA or MPOA. **, P < 0.01; ***, P < 0.001, main effect of bedding; †, P < 0.01, planned comparison for effect of fadrozole; n = 7–11 per group.
Surprisingly, we found evidence that the levels of total THF-diols in plasma were influenced by both corncob bedding and fadrozole [Table 1, interaction, F(1,33) = 3.2; P = 0.08]. Corncob bedding increased total THF-diol levels for mice treated with saline (planned comparison, P < 0.01) but not fadrozole. Differences in THF-diol levels were driven primarily by differences in trans-THF-diols [interaction, F(1,33) = 4.3; P = 0.04] vs. cis-THF-diols [interaction, F(1,33) = 2.4; P = 0.14]. These data were consistent with measurements of corncob extract, which contained approximately 5-fold more trans-THF-diols (22.0 ± 4.0 nmol/g) compared with cis-THF-diols (4.2 ± 0.6). There were no significant differences in the number of pups born (mean ± se; Carefresh 2.6 ± 0.1, corncob 2.4 ± 0.2; paired t18 = 0.7; P > 0.4) or weaned (Carefresh 2.4 ± 0.1, corncob 2.4 ± 0.2; paired t18 = 0.3; P > 0.7) after switching breeders to corncob bedding.
Table 1.
Effects of bedding and fadrozole on THF-diol levels
| Carefresh |
Corncob |
|||||
|---|---|---|---|---|---|---|
| Trans-THF (nm) | Cis-THF (nm) | Total (nm) | Trans-THF (nm) | Cis-THF (nm) | Total (nm) | |
| Saline | 26 ± 7a | 32 ± 10 | 59 ± 18a | 84 ± 28 | 110 ± 41 | 194 ± 69 |
| Fadrozole | 50 ± 15 | 47 ± 15 | 95 ± 29 | 34 ± 8 | 47 ± 15 | 82 ± 24 |
P < 0.01, planned comparison with corncob.
Discussion
Our results show that the effects of corncob bedding on behavior can be even more dramatic than previously observed with reproductive behavior. Estrogens increased aggressive behavior on cardboard-based bedding, whereas this effect was essentially reversed on corncob bedding. We also demonstrated that corncob bedding reduced the number ERα-positive cells in both the BNST and VMH and that corncob bedding suppressed pERK expression across the hypothalamus and extended amygdala. The widespread suppression of pERK by corncob bedding has important implications for many areas of neuroscience. For example, ERK signaling is known to regulate a diverse range of behaviors including learning (16), addiction (17), and social behavior (18). Our results suggest that these mechanisms would be impacted by the use of corncob bedding.
We demonstrated that corncob bedding significantly increased plasma levels of THF-diols in mice treated with saline. This difference was driven primarily by increases in trans-THF-diols, and we observed that concentrations of trans-THF-diols were 5-fold higher than cis-THF-diols in corncob bedding. The most likely route of administration of THF-diols is orally, because the authors and animal care staff observed mice ingesting corncob bedding (75 ± 13 mg over a 30-min period). It has been hypothesized that dietary polyunsaturated fats (such as linoleate contained in corn products) may be beneficial for reducing high-density lipoproteins (19). Linoleate can be metabolized into THF-diols (20), which our data suggest could influence brain function. Although linoleate-derived THF-diols are known to suppress estrogen-dependent reproductive behavior, they do not bind to ERα. We showed that corncob bedding reduces ERα immunoreactivity in the BNST and VMH, which regulate both male (21) and female (22) reproductive behavior. Thus, one potential mechanism through which corncob bedding may alter estrogen-sensitive behavior is by decreasing ERα expression in specific brain regions. Intriguingly, corncob bedding did not significantly increase plasma THF-diol levels in fadrozole-treated mice. One possible explanation is that fadrozole may affect the clearance of fatty acids from the blood, which would influence levels of highly polar THF-diols.
Studies of neuroendocrine mechanisms of behavior and brain function are frequently conducted in laboratories, because this allows for more control over experimental conditions. However, scientists need to be creative in thinking about the factors that could be influencing observed results. Recent reports have demonstrated that factors such as nearby construction (23) or the age at which mice are shipped (24) can have very strong effects on neuroendocrine function. The potential effects of phytoestrogens in soy-based animal feeds on behavior and brain function have received more attention (25), and many vendors now emphasize this in their literature. However, citation analyses indicate that the behavioral effects of corncob bedding are much less appreciated, despite its widespread use. An informal survey of attending veterinarians at six University of California laboratory animal facilities indicated that roughly half use corncob as the primary bedding. Although it is possible that there could be differences in the effects of corncob from different suppliers, previous work reported that the estrogenic properties of corn were detected in other commercially available products such as corn tortillas (5). Our results suggest that journals should be more diligent in requiring investigators to report the type of bedding used in experiments. The potential impact of bedding needs to be considered by researchers studying the effects of estrogens on brain function and behavior.
Acknowledgments
We thank Kelsey Hogeman, Sarah Laredo, Michael Pride, Veronica Orr, and Andrea Silva for technical assistance and Victor Lucas and Cindy Clayton for helpful discussions.
This work supported by NIEHS R01 ES002710 and the Superfund Program ES004669 to B.D.H. and a Hellman Fellowship and NIH MH085069-02 to B.C.T.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BNST
- bed nucleus of the stria terminalis
- ER
- estrogen receptor
- MEA
- medial amygdala
- MPOA
- medial preoptic area
- pERK
- phospho-ERK
- THF
- tetrahydrofuran
- VMH
- ventromedial hypothalamus.
References
- 1. Vasudevan N, Pfaff DW. 2007. Membrane-initiated actions of estrogens in neuroendocrinology: emerging principles. Endocr Rev 28:1–19 [DOI] [PubMed] [Google Scholar]
- 2. Rissman EF. 2008. Roles of oestrogen receptors α and β in behavioural neuroendocrinology: beyond yin/yang. J Neuroendocrinol 20:873–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lóránd T, Vigh E, Garai J. 2010. Hormonal action of plant derived and anthropogenic non-steroidal estrogenic compounds: phytoestrogens and xenoestrogens. Curr Med Chem 17:3542–3574 [DOI] [PubMed] [Google Scholar]
- 4. Patisaul HB, Dindo M, Whitten PL, Young LJ. 2001. Soy isoflavone supplements antagonize reproductive behavior and estrogen receptor α- and β-dependent gene expression in the brain. Endocrinology 142:2946–2952 [DOI] [PubMed] [Google Scholar]
- 5. Markaverich B, Mani S, Alejandro MA, Mitchell A, Markaverich D, Brown T, Velez-Trippe C, Murchison C, O'Malley B, Faith R. 2002. A novel endocrine-disrupting agent in corn with mitogenic activity in human breast and prostatic cancer cells. Environ Health Perspect 110:169–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mani SK, Reyna AM, Alejandro MA, Crowley J, Markaverich BM. 2005. Disruption of male sexual behavior in rats by tetrahydrofurandiols (THF-diols). Steroids 70:750–754 [DOI] [PubMed] [Google Scholar]
- 7. Markaverich BM, Alejandro MA, Markaverich D, Zitzow L, Casajuna N, Camarao N, Hill J, Bhirdo K, Faith R, Turk J, Crowley JR. 2002. Identification of an endocrine disrupting agent from corn with mitogenic activity. Biochem Biophys Res Commun 291:692–700 [DOI] [PubMed] [Google Scholar]
- 8. Trainor BC, Lin S, Finy MS, Rowland MR, Nelson RJ. 2007. Photoperiod reverses the effects of estrogens on male aggression via genomic and non-genomic pathways. Proc Natl Acad Sci USA 104:9840–9845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Trainor BC, Finy MS, Nelson RJ. 2008. Rapid effects of estradiol on male aggression depend on photoperiod in reproductively non-responsive mice. Horm Behav 53:192–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goodson JL. 2005. The vertebrate social behavior network: evolutionary themes and variations. Horm Behav 48:11–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Trainor BC, Crean KK, Fry WH, Sweeney C. 2010. Activation of extracellular signal-regulated kinases in social behavior circuits during resident-intruder aggression tests. Neuroscience 165:325–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Silva AL, Fry WH, Sweeney C, Trainor BC. 2010. Effects of photoperiod and experience on aggressive behavior in female California mice. Behav Brain Res 208:528–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Satoh Y, Endo S, Nakata T, Kobayashi Y, Yamada K, Ikeda T, Takeuchi A, Hiramoto T, Watanabe Y, Kazama T. 2011. ERK2 Contributes to the Control of Social Behaviors in Mice. J Neurosci 31:11953–11967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nourooz-Zadeh J, Uematsu T, Borhan B, Kurth MJ, Hammock BD. 1992. Characterization of the cytosolic epoxide hydrolase-catalyzed hydration products from 9,10:12,13-diepoxy stearic esters. Arch Biochem Biophys 294:675–685 [DOI] [PubMed] [Google Scholar]
- 15. Zar JH. 1996. Biostatistical analysis. 3rd ed Upper Saddle River, NJ: Prentice Hall [Google Scholar]
- 16. Samuels IS, Saitta SC, Landreth GE. 2009. MAP'ing CNS development and cognition: an ERKsome process. Neuron 61:160–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lu L, Koya E, Zhai H, Hope BT, Shaham Y. 2006. Role of ERK in cocaine addiction. Trends Neurosci 29:695–703 [DOI] [PubMed] [Google Scholar]
- 18. Iñiguez SD, Vialou V, Warren BL, Cao JL, Alcantara LF, Davis LC, Manojlovic Z, Neve RL, Russo SJ, Han MH, Nestler EJ, Bolaños-Guzmán CA. 2010. Extracellular signal-regulated kinase-2 within the ventral tegmental area regulates responses to stress. J Neurosci 30:7652–7663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grundy SM, Denke MA. 1990. Dietary influences on serum lipids and lipoproteins. J Lipid Res 31:1149–1172 [PubMed] [Google Scholar]
- 20. Moghaddam M, Motoba K, Borhan B, Pinot F, Hammock BD. 1996. Novel metabolic pathways for linoleic acid and arachidonic acid metabolism. Biochim Biophys Acta 1290:327–339 [DOI] [PubMed] [Google Scholar]
- 21. Hull EM, Dominguez JM. 2006. Getting his act together: Roles of glutamate, nitric oxide, and dopamine in the medial preoptic area. Brain Res 1126:66–75 [DOI] [PubMed] [Google Scholar]
- 22. Meisel RL, Dohanich GP, McEwen BS, Pfaff DW. 1987. Antagonism of sexual-behavior in female rats by ventromedial hypothalamic implants of antiestrogen. Neuroendocrinology 45:201–207 [DOI] [PubMed] [Google Scholar]
- 23. Raff H, Bruder ED, Cullinan WE, Ziegler DR, Cohen EP. 2011. Effect of animal facility construction on basal hypothalamic-pituitary-adrenal and renin-aldosterone activity in the rat. Endocrinology 152:1218–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Laroche J, Gasbarro L, Herman JP, Blaustein JD. 2009. Reduced behavioral response to gonadal hormones in mice shipped during the peripubertal/adolescent period. Endocrinology 150:2351–2358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brown NM, Setchell KDR. 2001. Animal models impacted by phytoestrogens in commercial chow: implications for pathways influenced by hormones. Lab Invest 81:735–747 [DOI] [PubMed] [Google Scholar]