Abstract
Thyroid hormone (T3) plays an important role in regulating multiple cellular and metabolic processes, including cell proliferation, cell death, and energy metabolism, in vertebrates. Dysregulation of T3 signaling results in developmental abnormalities, metabolic defects, and even cancer. We used T3-dependent Xenopus metamorphosis as a model to study how T3 regulates transcription during vertebrate development. T3 exerts its metamorphic effects through T3 receptors (TR). TR recruits, in a T3-dependent manner, cofactor complexes that can carry out chromatin remodeling/histone modifications. Whether and how histone modifications change upon gene regulation by TR during vertebrate development is largely unknown. Here we analyzed histone modifications at T3 target genes during intestinal metamorphosis, a process that involves essentially total apoptotic degeneration of the simple larval epithelium and de novo development of the adult epithelial stem cells, followed by their proliferation and differentiation into the complex adult epithelium. We demonstrated for the first time in vivo during vertebrate development that TR induces the removal of core histones at the promoter region and the recruitment of RNA polymerase. Furthermore, a number of histone activation and repression marks have been defined based on correlations with mRNA levels in cell cultures. Most but not all correlate with gene expression induced by liganded TR during development, suggesting that tissue and developmental context influences the roles of histone modifications in gene regulation. Our findings provide important mechanistic insights on how chromatin remodeling affects developmental gene regulation in vivo.
Thyroid hormone (T3) plays a critical role in adult organ function and development in mammals (1–5). In humans, the most important period of T3 action responsible for cretinism appears to be the so-called postembryonic period, a few months before and several months after birth, which is critical for growth and maturation of many organs, including the brain (3–5). Unfortunately, the difficulty in manipulating uterus-enclosed mammalian embryos has severely limited molecular and functional studies of T3 action during this critical late embryonic developmental period. Metamorphosis in amphibians serves as an excellent model to study T3 function during postembryonic development in vertebrate due to its total dependence on T3 (3–5). All changes during metamorphosis in amphibians such as Xenopus laevis and X. tropicalis, two highly related species, are initiated and controlled by T3 through gene regulation by T3 receptor (TR) (4, 6, 7). During this period, the endogenous T3 peaks at the climax of metamorphosis to induce the metamorphic changes and organ maturation, similar to the high levels of T3 present in human fetal plasma during the postembryonic period of extensive organ development and maturation (3, 4).
Molecular and genetic studies have demonstrated that the metamorphic effects of T3 are mediated by TR (6, 8–12). TR forms heterodimers with 9-cis retinoic acid receptors (RXR), and these dimers bind to T3 response element (TRE) in TR target genes (1, 2, 13, 14). In the absence of T3, TR/RXR functions as a repressor, whereas in the presence of T3, TR/RXR functions as an activator. In both transcriptional activation and repression, different cofactor complexes are recruited by TR to TRE to affect transcription (2, 15–23). Using the metamorphosis model system, we and others have provided strong in vivo evidence to support the importance of histone modifying cofactor complexes in mediating gene regulation by liganded or unliganded TR and in regulating Xenopus development (8, 24–30). On the other hand, relatively little is known on whether the recruitment of the histone modifying cofactor complexes by TR is associated with changes in histone modifications and chromatin remodeling at target genes during development in vivo.
Eukaryotic DNA is wrapped around nucleosomes, composed of an octamer of four core histones (H2A, H2B, H3, and H4), which form the primary units of the chromatin. The histones, particularly their N-terminal tails, are subject to a large number of posttranslational modifications, including acetylation, methylation, phosphorylation, and ubiquitylation (31). These histone modifications seem to have a substantial influence on chromatin structure and gene function. Transcription initiation by RNA polymerase II (Pol II) involves the coordinated action of the general transcription machinery with chromatin modifying and remodeling enzymes (32). In particular, di- and trimethylation of H3 lysine 9 (H3K9me2, H3K9me3) and trimethylation of H3K27 (H3K27me3) can elicit the formation of repressive heterochromatin through the recruitment of heterochromatin protein 1 (33) and polycomb group proteins, respectively (34–36). In contrast, histone modifications that are associated with active transcription, such as acetylation of histone 3 and histone 4 (H3 and H4) or di- or trimethylation of H3K4 (H3K4me2, H3K4me3), are commonly referred to as euchromatin modifications (37, 38). Most modifications are distributed in distinct patterns within the upstream region, the core promoter, the 5′ end of the open reading frame and the 3′ end of the open reading frame (38, 39). Although extensive studies in cell cultures have provided strong evidence to suggest that histone modifications function in a combinatorial fashion to regulate the diverse activities associated with chromatin, whether these patterns of histone modifications are associated with gene regulation by nuclear receptors during vertebrate development remains to be investigated.
Here we use the intestinal metamorphosis as a model to investigate the possible involvement of histone modification in gene regulation by TR during vertebrate development. The vertebrate intestine is one of the best-studied organs in which self-renewal is an integral part of the physiological function of the organs (40, 41). The establishment of this self-renewal system in all vertebrates takes place during the postembryonic period when plasma T3 concentrations are high. For example, in mammals such as mouse, the intestine changes from an embryonic form to a more complex adult form during this T3-dependent period (around birth) as the crypt-villus axis is established for the self-renewal of the adult epithelium. In frogs such as X. laevis, this transition takes place during metamorphosis (4). The tadpole intestine consists of predominantly a monolayer of larval epithelial cells (41). During metamorphosis, the larval epithelial cells undergo apoptosis and concurrently, adult epithelial stem/progenitor cells develop de novo from the larval epithelium through yet unknown mechanism and then rapidly proliferate (24, 42–46). Toward the end of metamorphosis, the adult epithelial cells differentiate to establish a trough-crest axis of epithelial fold, resembling the crypt-villus axis in adult mammalian intestine. This remodeling is entirely dependent on T3, making it an excellent model to investigate in vivo whether histone modifications play a role in gene regulation by T3.
Our goal is to eventually determine at the genome wide level the changes in histone modifications at TR target genes in the intestine. Because X. laevis is pseudotetraploid and its genome sequence has not been determined, it is impossible to carry out genome-wide analyses. On the other hand, X. tropicalis is diploid and its genome has been sequenced. To investigate the feasibility of using X. tropicalis metamorphosis as a model to study global regulation mechanisms by TR during intestinal remodeling, we have analyzed, by using chromatin immunoprecipitation (ChIP) assay, the changes in histone acetylation and methylation at two known TR target genes, the TRβ and T3- induced basic leucine zipper transcription factor (TH/bZIP) genes, in the intestine during the metamorphosis of X. tropicalis. Our results show for the first time that T3 induces histone removal at TR-activated genes during vertebrate development. In addition, our findings suggest that changes in histone modifications during developmental gene regulation may be different from their correlations with mRNA levels in culture cells, arguing for the importance of carrying out in vivo studies to understand gene regulation during development.
Materials and Methods
Experimental animals
Wild-type tadpoles of X. tropicalis were obtained from Nasco (Fort Atkinson, MI), and developmental stages were determined according to Nieuwkoop and Faber (47). Stage 54 tadpoles were treated for 2 d at 22 C with 10 nm T3, close to the peak levels of T3 at the climax of metamorphosis in X. laevis (48), although the levels of the hormone in the treated tadpole tissues might be higher (49). Animal studies were done as approved by Eunice Kennedy Shriver National Institute of Child Health and Human Development Animal Use and Care Committee.
Quantitative RT-PCR (qRT-PCR)
Total RNA was isolated from the intestine of tadpoles at premetamorphic stage 54, early metamorphic climax (stage 58), metamorphic climax (stages 60–62), and the end of metamorphosis (stage 66). The cDNA was prepared from 2.5 μg of total RNA using the Applied Biosystems high-capacity cDNA archive kit (Foster City, CA) according to the manufacturer's instructions in a total volume of 50 μl. qRT-PCR based on SYBR Green detection was carried out to quantify gene expression levels on an ABI 7000 (Applied Biosystems) and elongation factor-1α (EF1α) was used as the normalization control as described previously (50). The primers used for SYBR Green PCR were the following: forward, 5′-TGACTGTCAGGACTCTAGCAGCTGA-3′ and reverse, 5′-TGGGTTTTGTTCCTGGTGCTTGACG-3′ for TRβ, forward, 5′-CATTCAGGCATCTGCCCTT-3′ and reverse, 5′-CATAAGCCTCCAGTGTGGGA-3′ for TH/bZIP, forward, 5′-AGCACACGACAACCTGAAGA-3′ and reverse, 5′-GGAACCATGAGCCATTGAGT-3′ for intestinal fatty acid binding protein (IFABP), forward, 5′-CTATCCCCGCCAAACATCT-3′ and reverse, 5′-CCATCTCAGCAGCTTCCTTC-3′ for EF1α, and the rest were as published (50).
ChIP assay
A ChIP assay on tadpole intestine was done as described previously (51) with anti-TR (new PB), which recognized both TRα and TRβ (7, 52), and commercial antibodies, which included H2B (07-371), H3 (07-690), H3K4me3 (07-473), H3K9me3 (07-442), H3K27me3 (07-449), H3ac (06-599), H4ac (06-866), and asymmetric dimethylation of H3 arginine 17 (H3R17me2a) (07-214) from Millipore (formerly Upstate, Lake Placid, NY), and H3K79me3 (ab2621) and RNA polymerase II (ab5095) from Abcam (Cambridge, MA) (Table 1). As a negative control, a polyclonal antibody against Id14, an extracellular protein (53), was also used. Each sample had six to eight tadpoles. All ChIP experiments were done at least twice with similar results.
Table 1.
Antibodies used for ChIP assays
| Antibody specificity | Source | Catalog no. | Immunogen | Biological function | Commercial antibody name |
|---|---|---|---|---|---|
| TR | Full length Xenopus laevis TRβ | TR | |||
| ID14 | ETKCRCNMDGDVE | Extracellular protein expressed by intestinal epithelial cells | |||
| RNA Pol II | Abcam | ab5095 | Amino acid 1600–1700 (CTD repeat YSPTSPS, phosphorylated at S2) of Saccharomyces cerevisiae RNA Pol II | RNA Pol II | RNA Pol II CTD repeat YSPTSPS (phospho S2) antibody-ChIP grade (ab5095) |
| H2B | Millipore | 07-371 | Amino acids 118–126 (CG-AVTKYTSSK) of human histone H2B | Core histone | Antihistone H2B |
| H3 | Millipore | 07-690 | C terminus of human histone H3 | Core histone | Antihistone H3, CT, pan |
| H3K4me3 | Millipore | 07-473 | Residues surrounding and including trimethlyated Lys 4 of histone H3 | Transcriptional activation | Antitrimethyl-histone H3 (Lys4) |
| H3K79me3 | Abcam | ab2621 | Synthetic peptide conjugated to KLH derived from within residues 50 to the C terminus of human histone H3, trimethylated at K79 | Transcriptional activation | Histone H3 (trimethyl K79) antibody-ChIP grade (ab2621) |
| H3R17me2a | Millipore | 07-214 | Amino acid 11–21 (TGGKAPMe2RKQLA-C) of human histone H3 | Transcriptional activation | Antidimethyl-histone H3 (Arg17) |
| H3K9me3 | Millipore | 07-442 | … AR[me3K]ST … in which me3K corresponds to trimethyl-lysine at residue 9 of human histone H3 | Transcriptional repression | Antitrimethyl-histone H3 (Lys9) |
| H3K27me3 | Millipore | 07-449 | … AR (me3K)SAP … in which me3K corresponds to trimethyl-lysine at residue 27 of human histone H3 | Transcriptional repression | Antitrimethyl-histone H3 (Lys27) |
| AcH3 | Millipore | 06-599 | Amino acids 1–20 of tetrahymena histone H3 [ARTKQTAR(K*)STGG(K*)APRKQLC] where K* is acetylated (K9 and 14) | Transcriptional activation | Antiacetyl-histone H3 |
| AcH4 | Millipore | 06-866 | Amino acids 2–19 of tetrahymena histone H4 [AGG(K*)GG(K*)GMG(K*)VGA(K*)RHS-C] where K* is acetylated (K5, 8, 12, and 16) | Transcriptional activation | Antiacetyl-histone H4 |
For immunoprecipitation, the DNA concentration of the chromatin was diluted to 10 ng/μl with ChIP dilution buffer (Millipore). After precleaning with salmon sperm DNA/protein A-agarose beads, input samples were taken, and 500 μl of each chromatin sample was immunoprecipitated with the indicated antibodies and salmon sperm DNA/protein A-agarose beads. The mixtures were incubated overnight at 4 C followed by spinning down the beads. The beads were washed with ChIP buffer I, ChIP buffer II, ChIP buffer III, and Tris/EDTA (Millipore). After the last wash, 200 μl of elution buffer was added to the samples as well as the input controls and incubated at 65 C overnight, and the immunoprecipitated DNA was purified. The DNA was then analyzed by quantitative PCR on an ABI 7000 with the gene-specific Taqman primers/probes for X. laevis TRβ promoter and TH/bZIP promoter (51) because the primer/probe sequences are conserved in X. tropicalis (7). For the detection of exon 5 of X. tropicalis TRβ, forward, primer 5′-CCCCGAAAGTGAAACTCTAACTCT-3′, reverse primer, 5′-CCACACCGAGTCCTCCATTTT-3′ and FAM-labeled Taqman probe, CTGCCATCTCACCATTC, were used. For the detection of X. tropicalis IFABP (or Fabp2) promoter, forward, primer 5′-CCCTACATTGGTTGAGCCAGTTTT-3′, reverse primer, 5′-TCAAAGGCCATGGTGATTGGT-3′ and FAM-labeled Taqman probe, CCTAGCCAACATCTCC, were used.
The ChIP signals were expressed as the percentage of the total input DNA before immunoprecipitations. Due to nucleosome removal by liganded TR at the TRE regions, the ChIP signals for modified histones were thus expressed relative to the total histones associated with the DNA regions of interest. Thus, the ChIP signals for modified histones were normalized with the ChIP signals of histone H3 in the same tissue samples for the corresponding promoter/exon regions.
Results
Intestinal expression profiles of two known TR-target genes during natural and T3-induced metamorphosis of X. tropicalis tadpoles
We first determined whether the X. tropicalis TRβ and TH/bZIP genes are regulated in the intestine during X. tropicalis metamorphosis similarly as in X. laevis. As a control, we also analyzed the expression of IFABP, a gene expressed in differentiated intestinal epithelium and down-regulated indirectly by T3 during X. laevis metamorphosis. As shown in Fig. 1, the mRNA levels of TRβ and TH/bZIP were found to be very low at stage 54 but up-regulated during metamorphosis, reached a peak at climax, and then down-regulated by the end of metamorphosis. On the other hand, IFABP mRNA was present at high levels in the larval and adult epithelium before and after metamorphosis, respectively, but was repressed at the climax of metamorphosis when remodeling took place (Fig. 1A). Likewise, when premetamorphic tadpoles at stage 54 were treated with 10 nm T3 for 2 d, the expression of both TRβ and TH/bZIP were drastically induced, whereas IFABP expression was reduced (Fig. 1B). These temporal patterns are similar to those observed during natural and T3-induced intestinal remodeling in X. laevis (54–56). In fact, bioinformatics analysis has shown that both the X. tropicalis TRβ and TH/bZIP genes contained TRE at similar locations as in the corresponding X. laevis genes (Fig. 1C) (7), suggesting that they are directly regulated by TR just like in X. laevis.
Fig. 1.
Developmental expression profiles and T3 regulation of TRβ, TH/bZIP, and IFABP in the intestine during natural and T3-induced metamorphosis of X. tropicalis. A and B, The expression of two known direct TR target genes TRβ and TH/bZIP, and an indirectly repressed gene IFABP were analyzed during natural (A) and T3-induced (B) metamorphosis by qRT-PCR. EF1α was also analyzed as the control gene (nonregulated) and used to normalize the expression of the other genes. Note that the levels of TRβ and TH/bZIP mRNA were gradually up-regulated during natural development and reached a peak at stage 60 and 62, respectively (A). The expression of mRNA was then reduced by stage 66, the end of metamorphosis (A). On the other hand, the level of IFABP mRNA was down-regulated at stage 62, at the climax of metamorphosis. After 2 d of T3 treatment of premetamorphic tadpoles at stage 54, the expression of TRβ and TH/bZIP mRNA were up-regulated, whereas that of IFABP was down-regulated (B), just like during natural metamorphosis. Error bars indicate sem. C, Schematics of the promoters of X. tropicalis TRβ and TH/bZIP genes showing the locations of the TRE relative to the transcription start site (+1). Note that the positions were based on those for the corresponding X. laevis genes (52, 68) because the exact start sites of the X. tropicalis genes have not been mapped but the TRE regions are conserved (7). The arrows indicate the relative locations of the PCR primers used for the analysis of the ChIP DNA.
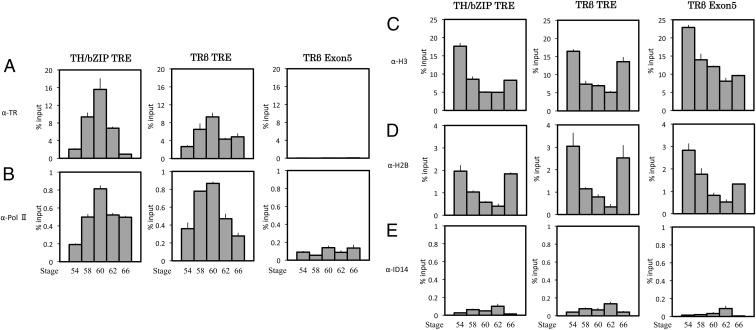
TR binds to target genes and recruits RNA polymerase II during T3-induced intestinal remodeling
To investigate how TR regulates target genes in X. tropicalis intestine, we analyzed the binding of TR to TRE in tadpole intestine in vivo. Premetamorphic tadpoles at stage 54 were treated with 10 nm T3 for 2 d, and the intestine was isolated for ChIP assay with an anti-TR antibody. As shown in Fig. 2, TR was bound to the TRE regions of both TRβ and TH/bZIP genes and T3 treatment led to increased binding. In contrast, no TR was found in the exon 5 of the TRβ gene or in the promoter region of the IFABP gene (Fig. 2). In addition, for each batch of chromatin samples, we also included a negative control for nonspecific antibody binding by carrying out a ChIP assay with an antibody against an extracellular protein encoded by X. laevis gene ID14 (53), which yielded low background signals for all genomic loci analyzed (Fig. 2). These results confirmed the specificity of the TR binding.
Fig. 2.
TR binding to target genes leads to the recruitment of RNA Pol II and loss of core histones during T3-induced metamorphosis. Tadpoles at stage 54 were treated with or without T3 for 2 d, and the intestine was isolated for ChIP assay with the anti-TR (A), anti-Pol II (B), anti-H3 (C), anti-H2B (D), or anti-Id14 (extracellular protein, as a negative control) (E) antibody. The immunoprecipitated DNA was analyzed by qPCR for the presence of the TRE regions of the TRβ and TH/bZIP promoters. A region of exon 5 of the TRβ gene was analyzed as a negative control. The promoter region of the IFABP gene was also analyzed as a non-TR direct target gene control. Note that TR bound to both genes in the absence of T3 in premetamorphic tadpoles. In the presence of T3, TR binding to the TRE was increased, accompanied by the recruitment Pol II and reduction in histones at the TRE regions of both TH/bZIP and TRβ promoters. There was no TR binding to exon 5 in the presence or absence of T3. However, increased Pol II was observed in the exon region due to increased transcription in the presence of T3. Only background signals were observed with the anti-ID14 antibody. Error bars indicate sem (n = 3). Student's t test was carried out between pairs of control and T3-treated groups. The one and two stars indicate pairs of samples with significant differences (P < 0.05 and P < 0.01, respectively).
To determine whether T3 activation of the TR-target genes was associated with the recruitment of RNA polymerase II, a ChIP assay was performed on the same intestinal samples above with an anti-RNA polymerase II antibody. The results showed increased RNA polymerase II recruitment at the promoter regions of both genes and exon 5 of TRβ gene upon T3 treatment (Fig. 2). In contrast, RNA polymerase recruitment at the IFABP gene was reduced as the gene was down-regulated, although indirectly by TR. Thus, liganded TR recruits RNA polymerase to the promoter regions of T3-induced genes.
Nucleosome removal accompanies gene activation by liganded TR
We have shown previously in the frog oocyte transcription system, liganded TR induced chromatin remodeling on a reporter plasmid assembled into chromatin in vivo, leading to the loss of a significant fraction of the nucleosomes on the plasmid (57, 58). To investigate whether this occurs on endogenous TR target genes during vertebrate development, we carried out ChIP assays with antibodies against total H2B and H3 of the core histones on the intestine isolated from premetamorphic tadpoles. As shown in Fig. 2, upon T3 treatment of premetamorphic tadpoles, the amount of histones H2B and H3 were drastically reduced at the TRE regions of both the TRβ and TH/bZIP genes. On the other hand, little changes were observed at exon 5 of the TRβ gene or at the promoter region of the IFABP gene, indicating specific nucleosome removal associated with gene activation by liganded TR during intestinal remodeling.
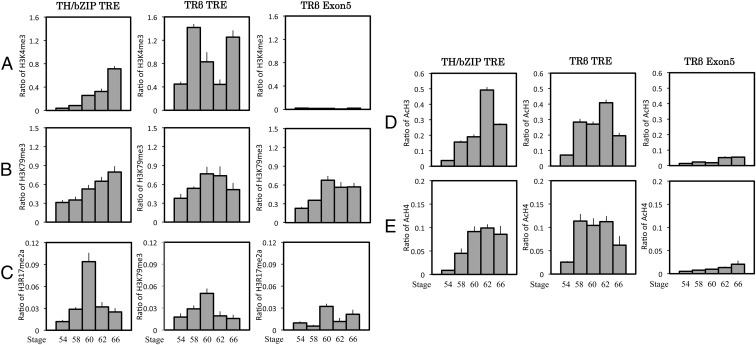
The levels of activation histone marks increase at TR target genes during T3-induced metamorphosis
Cell culture studies have identified a number of histone modifications (histone marks) that are associated with high level of gene expression or gene silencing (34, 35, 37, 38, 59–63). To determine whether these modifications play a role in gene regulation by TR during development, we carried out ChIP assays with antibodies again different histone modifications. We analyzed five activation marks, H3K4me3 (trimethylated K4), H3K79me3 (trimethylated K79), H3R17me2a (asymmetrically dimethylated R17), acetylated H3 (AcH3), and acetylated H4 (AcH4), on intestine isolated from premetamorphic tadpoles treated with or without 10 nm T3 for 2 d. The ChIP signals for the modified histones were normalized against the level of total histone H3 associated with each DNA region analyzed. The results showed that at the TRE regions of both T3-activated genes, the levels of all these activation marks were increased upon T3 treatment (Fig. 3). Few changes were observed at the IFABP promoter, which was indirectly down-regulated by T3 (Fig. 3). Interestingly, the exon 5 of the TRβ gene had very little histone acetylation or H3K4me3 in both the presence or absence of T3, although the levels of H3R17me2a and H3K79me3 increased upon gene activation by T3 (Fig. 3). Thus, the histone marks associated with high levels of gene expression in mammalian cells are used by TR for transcriptional activation of target genes during intestinal metamorphosis.
Fig. 3.
Histone modifications associated with gene activation are increased at TR-target genes during T3-induced metamorphosis. Premetamorphic tadpoles at stage 54 were treated with T3 for 2 d. The intestine was isolated and subjected to a ChIP assay with anti-H3K4me3 (A), anti-H3K79me3 (B), or anti-H3R17me2a (C), anti-AcH3 (D), or anti-AcH4 antibody (E). ChIP signals were normalized with the ChIP signals of histone H3 in Fig. 2 for the corresponding promoter/exon regions. Error bars indicate sem (n = 3). A Student's t test was carried out between pairs of control and T3-treated groups. The one and two stars indicate pairs of samples with significant differences (P < 0.05 and P < 0.01, respectively).
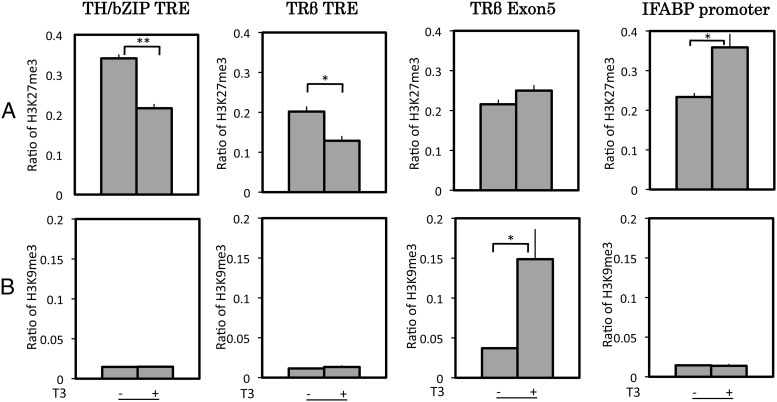
Distinct changes of two repression histone marks at TR target genes during T3-induced metamorphosis
To determine whether repression marks found in mammalian cells are also similarly associated with TR-dependent gene regulation during intestinal metamorphosis, we analyzed two such marks: H3K27me3 (trimethylated K27) and H3K9me3 (trimethylated K9). Consistent with the findings in mammalian cells, H3K27me3 level was reduced at the TRE regions of both TR-target genes after T3 treatment of premetamorphic tadpoles, whereas it changed little in the downstream transcribed region of the TRβ gene and increased at the down-regulated gene IFABP (Fig. 4). On the other hand, the ChIP signals for H3K9me3 were very low at the TRE regions of both TR-target genes or the IFABP promoter in the presence or absence of T3 (Fig. 4). Interestingly, it actually increased in the transcribed exon 5 of the TRβ gene when the gene was activated upon T3 treatment. Thus, of the two repression histone marks found in mammalian cells, only one, H3K27me3, was correlated with gene regulation by TR during intestinal remodeling.
Fig. 4.
Of the two histone marks associated with gene silencing, H3K9me3 and H3K27me3, only H3K27me3 was reduced upon gene activation by T3 during metamorphosis. Premetamorphic tadpoles at stage 54 were treated with T3 for 2 d. The intestine was isolated and subjected to a ChIP assay with anti-H3K27me3 (A) or anti-H3K9me3 antibody (B). ChIP signals were normalized with the ChIP signals of histone H3 in Fig. 2 for the corresponding promoter/exon regions. Note that surprisingly, the amount of H3K9me3 was increased in the downstream transcribed region of the TRβ gene (exon 5) upon gene activation by T3. Error bars indicate sem (n = 3). A Student's t test was carried out between pairs of control and T3-treated groups. The one and two stars indicate pairs of samples with significant differences (P < 0.05 and P < 0.01, respectively).
Chromatin remodeling and RNA polymerase recruitment at TR target genes in the intestine during natural metamorphosis
The T3 treatment of premetamorphic tadpoles induces accelerated and more synchronized changes among different tadpoles than natural metamorphosis, making it ideal to study how TR regulates gene expression in vivo. To determine whether the findings during T3-induced metamorphosis mimic those during natural development, we next carried out analyses on intestines isolated from tadpoles during natural metamorphosis. As shown in Fig. 5, TR was bound to the TRE of both target genes at stage 54 (compare with the negative control at exon 5 or with the ChIP data with anti-ID14 antibody), and this binding was increased by stage 58 when the endogenous T3 level was high (resembling 2 d of T3 treatment). The TR binding remained high at the climax and was reduced, especially for the TH/bZIP gene, by the end of metamorphosis. Also similar to that observed during T3 treatment, RNA polymerase II recruitment was drastically increased by stage 58 (Fig. 5). Its recruitment was reduced somewhat toward the end of metamorphosis. Interestingly, for unknown reasons, RNA polymerase association with TRβ exon 5 was low and changed little during natural metamorphosis, unlike that during T3 treatment.
Fig. 5.
TR binding to target genes increases during natural metamorphosis and is accompanied by increased recruitment of RNA Pol II and reduced core histones at the TRE. The intestine was isolated from tadpoles at premetamorphosis (stage 54), early metamorphic climax (stage 58), metamorphic climax (stage 60–62), and the end of metamorphosis (stage 66) and subjected to ChIP assay with the anti-TR (A), anti-Pol II (B), anti-H3 (C), anti-H2B (D), or anti-Id14 (extracellular protein, as a negative control) (E) antibody. The ChIP DNA was analyzed as in Fig. 2. Note that TR binding to the TRE was higher during metamorphosis (stages 58–62), accompanied by increased Pol II and reduced amounts of core histones at TRE of both TR-target promoters at stages 58–62. Only background signals were observed with the anti-ID14 antibody. Error bars indicate sem.
Analysis of cores histone H2B and H3 at the TR-target genes again showed that they were removed when T3 became available by stage 58 (Fig. 5). Interestingly, this removal persisted during metamorphosis, and their association with the TRE regions increased only by the end of metamorphosis as the genes were down-regulated. In addition, histone H2B and H3 at TRβ exon 5 was also lower during metamorphosis, suggesting that active transcription also affects downstream chromatin structure.
More importantly, ChIP assays showed that the histone modification patterns at the TR-target genes during natural metamorphosis were the same as those during T3 treatment. That is, all activation histone marks were increased during metamorphosis at the TRE regions of both genes (Fig. 6). Of the two repression marks, H3K27me3 was reduced at the TRE regions, but little signal for H3K9me3 was detected at the TRE regions (Fig. 7). Again, like during T3 treatment, the repression mark H3K9me3 was actually increased at TRβ exon 5 during natural metamorphosis when the gene was activated, indicating that H3K9me3 is not a repression mark for gene regulation by TR during intestinal metamorphosis.
Fig. 6.
Histone modifications associated with gene activation are increased at TR-target genes during natural metamorphosis. The ChIP assay was performed as in Fig. 5 on intestines isolated from tadpoles at the indicated stages with anti-H3K4me3 (A), anti-H3K79me3 (B), anti-H3R17me2a (C), anti-AcH3 (D), or anti-AcH4 antibody (E). ChIP signals were normalized with the ChIP signals of histone H3 in Fig. 5 for the corresponding promoter/exon regions. Note that in general, these activation histone marks were increased at the two promoters during metamorphosis. Error bars indicate sem.
Fig. 7.
A and B, Of the two histone marks associated with gene silencing, H3K9me3 and H3K27me3, only K3K27me3 was reduced upon gene activation during natural metamorphosis. The ChIP assay was performed as in Fig. 5 on intestines isolated from tadpoles at the indicated stages during metamorphosis with anti-H3K27me3 (A) or anti-H3K9me3 antibody (B). ChIP signals were normalized with the ChIP signals of histone H3 in Fig. 5 for the corresponding promoter/exon regions. Note that surprisingly but in agreement with T3-induced metamorphosis, the amount of H3K9me3 was increased in the downstream transcribed region of TRβ gene (exon 5) upon gene activation during natural metamorphosis (stages 58–62). Error bars indicate sem. C, A model for nucleosome removal and histone modifications during gene activation by liganded TR. In the absence of T3, TR recruits corepressor complexes containing histone deacetylases, which results in a repressed chromatin state (dashed arrow) with many repression histone marks such as H3K27me3 (triangles). The presence of T3 induces a conformational change in TR, leading to the recruitment of coactivator complexes containing chromatin remodeling and histone modification enzymes. This results in nucleosomal removal at the promoter region, a reduction in repression histone marks, an increase in activation histone marks such as H3K4me3, H3K7me3, R17me2a, AcH3, and AcH4 (circles), and gene activation (thick arrow).
Discussion
Genome-wide ChIP and gene expression analyses have identified a number of histone marks that are associated with active or repressed genes (34, 35, 37, 38, 59–63). It is thus important to determine whether such activation and repression histone marks correlate with gene regulation in vivo, especially during development. Amphibian metamorphosis offers a unique opportunity for such studies in vertebrates because it can be easily manipulated by controlling the availability of T3, the causative agent of metamorphosis. Earlier studies by us and others have shown that TR has dual functions during Xenopus development (6, 8–12, 25–30). It first functions as repressors of T3-inducible genes in premetamorphic tadpoles by recruiting corepressor complexes containing histone deacetylases to ensure proper growth of the tadpoles before metamorphosis. As endogenous T3 becomes available later, liganded TR releases corepressor complexes and recruits coactivator complexes that contain chromatin-remodeling proteins, histone acetyltransferases, and methyltransferases to activate gene expression, thus leading to the larval to adult transformation. Here we have analyzed whether the recruitment of these complexes alter chromatin structure and histone modifications at two TR target genes by focusing on the early phase of intestinal metamorphosis, i.e. premetamorphic, stage 54 tadpoles with up to 2 d of T3 treatment or natural metamorphosis from stage 54 to stage 58. This is a period during which the larval epithelial apoptosis is being induced and adult epithelial stem cells develop. Our studies have demonstrated here for the first time in any vertebrate, gene activation by liganded TR during development involves nucleosome removal at the promoter, accompanied by the recruitment of RNA polymerase II. We have further shown that liganded TR increases all five studied histone marks that are associated with high levels of gene expression in cell cultures. On the other hand, the two histone repression marks analyzed here showed opposite changes upon gene activation by liganded TR, suggesting that correlations of histone marks with mRNA levels may not be indicative of their roles in transcriptional regulation during development.
Nucleosome removal by liganded TR
Early studies on gene regulation by TR in the context of chromatin in the frog oocyte transcription system revealed that gene activation by liganded TR leads to chromatin disruption (57, 58, 64). However, it has been unclear whether chromatin disruption occurs on endogenous TR-target genes. Our results here show that during X. tropicalis metamorphosis, the activation of target genes by TR in the animal intestine involves the removals of nucleosomes around the TR binding sites. This is likely the manifestation of the chromatin disruption observed in the frog oocyte transcription system. There the chromatin disruption was determined based on supercoiling assays on the reporter plasmids injected into oocyte in the presence and/or absence of TR and/or T3. It has been shown that the binding of TR to a single location, independent of the number of TRE present there, leads to the loss of three negative superhelical turns on the plasmid minichromosome, suggesting a loss of up to three nucleosomes (57). Although the mechanism of this nucleosome loss in the oocyte or on the endogenous target genes during intestinal metamorphosis remains to be determined, it has been shown that liganded TR can recruit chromatin remodeling complexes containing Brg1 and BAF57 to the TRE of the reporter plasmid assembled into chromatin in the frog oocyte (65, 66). Furthermore, both Brg1 and BAF57 are expressed during intestinal metamorphosis in X. laevis (66). Thus, it is likely that in the presence of T3, TR bound to the TRE of both TRβ and TH/bZIP genes in X. tropicalis recruits chromatin remodeling complexes such as those containing Brg1 and BAF57 to remove the nucleosomes to facilitate gene activation. Regardless, our findings suggest that it is important to take into account such nucleosome removal when analyzing histone modifications and that the levels of histone modifications caused by liganded TR as reported in previous studies by various groups were possibly underestimated.
Liganded TR induces alterations in histone marks to affect gene expression
Histone modifications have long been recognized as an important factor in regulation gene expression. Many histone modifications have been correlated with high levels of gene expression or repressed gene transcription, thus termed activation or repression histone marks, respectively. Much less is known whether such modifications are related to gene expression changes during development and/or induced by various signals such as hormones, especially during vertebrate development. Here we analyzed five activation histone marks and found that all the marks were increased upon gene activation by TR in the presence of T3 during both natural and T3-induced metamorphosis. These results suggest that these histone marks are likely involved in the activation of gene transcription and that their roles are conserved in different vertebrate species.
The two repression histone marks that we analyzed, however, showed distinct patterns. H3K27me3 was reduced at the TRE regions of both TR target genes when they are up-regulated in the presence of T3. This agrees with their known association with repressed genes in mammalian cells and other species. On the other hand, H3K9me3 signals at the both promoters in the intestine were low in premetamorphic tadpoles and did not change in response to T3 during both natural and T3-induced metamorphosis. At the exon 5 of the TRβ gene, the H3K9me3 level actually increased because the gene was activated by T3 during both natural and T3-induced metamorphosis. This suggests that although globally, high levels of H3K9me3 at different genes seem to correlate with low or repressed levels of gene expression in mammalian cell cultures, it is not involved in the repression of TR target genes in the absence of T3. Instead, it appears to be associated with ligand-induced transcription of TR target genes in the downstream transcribed region. Like most histone modifications, whether and how H3K9me3 participates actively in regulating gene transcription remains to be determined. Nonetheless, our findings suggest that global correlations of particular histone modifications with mRNA levels may be distinct from the changes in the levels of these modifications upon transcriptional regulation of specific genes under in vivo conditions.
A recent study on the same two TR target genes in the tail fin and brain after T3 treatment of premetamorphic tadpoles also revealed changes in histone modifications (67). The authors observed gene- and tissue-specific patterns of histone acetylation and methylation, including two histone marks that were also analyzed here: H3K4m3 (activation mark) and H3K27me3 (repression mark). Their findings appear to be different from what we observed here in the animal intestine. Although it is possible that the histone modification patterns may be tissue dependent, another explanation may be related to the fact that these authors did not normalize the histone modification levels against the total histones present at the TRE regions. We have observed greater than 50% reduction in total histones at the TRE regions upon gene activation by T3 during both natural and T3-induced metamorphosis. If similar levels of nucleosome removal take place at the TRE of the TRβ and TH/bZIP genes in the tail fin and brain after T3 treatment, then the H3K4m3 levels after normalizing against total histones at the TRE regions of both genes would be higher after T3 treatment in both the brain and tail fin. These results would be similar to what we observed in the intestine during both natural and T3 induced metamorphosis. Thus, it would be very interesting in the future to determine whether nucleosome removal indeed takes place after T3 treatment in the brain and tail and more importantly during natural metamorphosis.
In summary, by using intestinal metamorphosis as a model, we have provided the first in vivo evidence during vertebrate development that gene activation by liganded TR involves nucleosome removal at the target genes. We have further shown that histone marks that are associated with high level gene expressions in cell cultures are increased by liganded TR, suggesting that they participate in gene activation. On the other hand, one of the two analyzed repression histone marks had no significant presence at TR target sites both in the presence or absence of T3 and was actually increased in the transcribed coding region upon gene activation by TR. Thus, the involvement and function of individual histone marks are likely gene and/or tissue dependent in the context of vertebrate development. Functional assays in the future would be required to determine whether such histone marks are important for transcriptional regulation by TR in vivo and for the T3-induced larval epithelial apoptosis and the development of the adult epithelial stem cells in the metamorphosing intestine. Additionally, it would be interesting to use this model system to investigate at genome-wide level how histone modifications change at different target genes by TR in response to T3 during vertebrate development.
Acknowledgments
This work was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AcH3
- Acetylated H3
- AcH4
- acetylated H4
- ChIP
- chromatin immunoprecipitation
- EF1α
- elongation factor-1α
- H3
- histone 3
- H3K9me2
- dimethylation of H3 lysine 9
- H3K9me3
- trimethylation of H3 lysine 9
- H3K27me3
- trimethylation of H3K27
- H3R17me2a
- asymmetric dimethylation of H3 arginine 17
- H4
- histone 4
- IFABP
- intestinal fatty acid binding protein
- Pol II
- polymerase II
- qRT-PCR
- quantitative RT-PCR
- RXR
- 9-cis retinoic acid receptor
- T3
- thyroid hormone
- TH/bZIP
- T3-induced basic leucine zipper transcription factor
- TR
- T3 receptor
- TRE
- T3 response element.
References
- 1. Lazar MA. 1993. Thyroid hormone receptors: multiple forms, multiple possibilities. Endocr Rev 14:184–193 [DOI] [PubMed] [Google Scholar]
- 2. Yen PM. 2001. Physiological and molecular basis of thyroid hormone action. Physiol Rev 81:1097–1142 [DOI] [PubMed] [Google Scholar]
- 3. Tata JR. 1993. Gene expression during metamorphosis: an ideal model for post-embryonic development. Bioessays 15:239–248 [DOI] [PubMed] [Google Scholar]
- 4. Shi Y-B. 1999. Amphibian metamorphosis: from morphology to molecular biology. New York: John Wiley, Sons, Inc [Google Scholar]
- 5. Hetzel BS. 1989. The story of iodine deficiency: an international challenge in nutrition. Oxford, UK: Oxford University Press [Google Scholar]
- 6. Buchholz DR, Paul BD, Fu L, Shi YB. 2006. Molecular and developmental analyses of thyroid hormone receptor function in Xenopus laevis, the African clawed frog. Gen Comp Endocrinol 145:1–19 [DOI] [PubMed] [Google Scholar]
- 7. Wang X, Matsuda H, Shi YB. 2008. Developmental regulation and function of thyroid hormone receptors and 9-cis retinoic acid receptors during Xenopus tropicalis metamorphosis. Endocrinology 149:5610–5618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shi YB. 2009. Dual functions of thyroid hormone receptors in vertebrate development: the roles of histone-modifying cofactor complexes. Thyroid 19:987–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nakajima K, Yaoita Y. 2003. Dual mechanisms governing muscle cell death in tadpole tail during amphibian metamorphosis. Dev Dyn 227:246–255 [DOI] [PubMed] [Google Scholar]
- 10. Schreiber AM, Das B, Huang H, Marsh-Armstrong N, Brown DD. 2001. Diverse developmental programs of Xenopus laevis metamorphosis are inhibited by a dominant negative thyroid hormone receptor. Proc Natl Acad Sci USA 98:10739–10744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Buchholz DR, Tomita A, Fu L, Paul BD, Shi YB. 2004. Transgenic analysis reveals that thyroid hormone receptor is sufficient to mediate the thyroid hormone signal in frog metamorphosis. Mol Cell Biol 24:9026–9037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Buchholz DR, Hsia SC, Fu L, Shi YB. 2003. A dominant negative thyroid hormone receptor blocks amphibian metamorphosis by retaining corepressors at target genes. Mol Cell Biol 23:6750–6758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P. 1995. The nuclear receptor superfamily: the second decade. Cell 83:835–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tsai MJ, O'Malley BW. 1994. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Ann Rev Biochem 63:451–486 [DOI] [PubMed] [Google Scholar]
- 15. Ito M, Roeder RG. 2001. The TRAP/SMCC/Mediator complex and thyroid hormone receptor function. Trends Endocrinol Metab 12:127–134 [DOI] [PubMed] [Google Scholar]
- 16. Rachez C, Freedman LP. 2000. Mechanisms of gene regulation by vitamin D(3) receptor: a network of coactivator interactions. Gene 246:9–21 [DOI] [PubMed] [Google Scholar]
- 17. Zhang J, Lazar MA. 2000. The mechanism of action of thyroid hormones. Annu Rev Physiol 62:439–466 [DOI] [PubMed] [Google Scholar]
- 18. Burke LJ, Baniahmad A. 2000. Co-repressors 2000. FASEB J 14:1876–1888 [DOI] [PubMed] [Google Scholar]
- 19. Jepsen K, Rosenfeld MG. 2002. Biological roles and mechanistic actions of co-repressor complexes. J Cell Sci 115:689–698 [DOI] [PubMed] [Google Scholar]
- 20. Jones PL, Shi Y-B. 2003. N-CoR-HDAC corepressor complexes: roles in transcriptional regulation by nuclear hormone receptors. In: Workman JL. ed. Current topics in microbiology and immunology: protein complexes that modify chromatin. Berlin: Springer-Verlag; 237–268 [DOI] [PubMed] [Google Scholar]
- 21. Rachez C, Freedman LP. 2001. Mediator complexes and transcription. Curr Opin Cell Biol 13:274–280 [DOI] [PubMed] [Google Scholar]
- 22. Hu X, Lazar MA. 2000. Transcriptional repression by nuclear hormone receptors. Trends Endocrinol Metab 11:6–10 [DOI] [PubMed] [Google Scholar]
- 23. Perissi V, Jepsen K, Glass CK, Rosenfeld MG. 2010. Deconstructing repression: evolving models of co-repressor action. Nat Rev Genet 11:109–123 [DOI] [PubMed] [Google Scholar]
- 24. Matsuda H, Shi YB. 2010. An essential and evolutionarily conserved role of protein arginine methyltransferase 1 for adult intestinal stem cells during postembryonic development. Stem Cells 28:2073–2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Matsuda H, Paul BD, Choi CY, Hasebe T, Shi YB. 2009. Novel functions of protein arginine methyltransferase 1 in thyroid hormone receptor-mediated transcription and in the regulation of metamorphic rate in Xenopus laevis. Mol Cell Biol 29:745–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sato Y, Buchholz DR, Paul BD, Shi YB. 2007. A role of unliganded thyroid hormone receptor in postembryonic development in Xenopus laevis. Mech Dev 124:476–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Paul BD, Shi YB. 2003. Distinct expression profiles of transcriptional coactivators for thyroid hormone receptors during Xenopus laevis metamorphosis. Cell Res 13:459–464 [DOI] [PubMed] [Google Scholar]
- 28. Paul BD, Buchholz DR, Fu L, Shi YB. 2005. Tissue- and gene-specific recruitment of steroid receptor coactivator-3 by thyroid hormone receptor during development. J Biol Chem 280:27165–27172 [DOI] [PubMed] [Google Scholar]
- 29. Havis E, Sachs LM, Demeneix BA. 2003. Metamorphic T3-response genes have specific co-regulator requirements. EMBO Rep 4:883–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Paul BD, Fu L, Buchholz DR, Shi YB. 2005. Coactivator recruitment is essential for liganded thyroid hormone receptor to initiate amphibian metamorphosis. Mol Cell Biol 25:5712–5724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kouzarides T. 2007. Chromatin modifications and their function. Cell 128:693–705 [DOI] [PubMed] [Google Scholar]
- 32. Roeder RG. 2005. Transcriptional regulation and the role of diverse coactivators in animal cells. FEBS Lett 579:909–915 [DOI] [PubMed] [Google Scholar]
- 33. Lachner M, O'Carroll D, Rea S, Mechtler K, Jenuwein T. 2001. Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature 410:116–120 [DOI] [PubMed] [Google Scholar]
- 34. Cao R, Wang L, Wang H, Xia L, Erdjument-Bromage H, Tempst P, Jones RS, Zhang Y. 2002. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science 298:1039–1043 [DOI] [PubMed] [Google Scholar]
- 35. Cao R, Zhang Y. 2004. The functions of E(Z)/EZH2-mediated methylation of lysine 27 in histone H3. Curr Opin Genet Dev 14:155–164 [DOI] [PubMed] [Google Scholar]
- 36. Pasini D, Bracken AP, Jensen MR, Lazzerini Denchi E, Helin K. 2004. Suz12 is essential for mouse development and for EZH2 histone methyltransferase activity. EMBO J 23:4061–4071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li B, Carey M, Workman JL. 2007. The role of chromatin during transcription. Cell 128:707–719 [DOI] [PubMed] [Google Scholar]
- 38. Wang Z, Schones DE, Zhao K. 2009. Characterization of human epigenomes. Curr Opin Genet Dev 19:127–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shilatifard A. 2006. Chromatin modifications by methylation and ubiquitination: implications in the regulation of gene expression. Annu Rev Biochem 75:243–269 [DOI] [PubMed] [Google Scholar]
- 40. van der Flier LG, Clevers H. 2009. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol 71:241–260 [DOI] [PubMed] [Google Scholar]
- 41. Shi YB, Ishizuya-Oka A. 1996. Biphasic intestinal development in amphibians: embryogensis and remodeling during metamorphosis. Curr Top Dev Biol 32:205–235 [DOI] [PubMed] [Google Scholar]
- 42. Ishizuya-Oka A, Hasebe T, Buchholz DR, Kajita M, Fu L, Shi YB. 2009. Origin of the adult intestinal stem cells induced by thyroid hormone in Xenopus laevis. FASEB J 23:2568–2575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schreiber AM, Cai L, Brown DD. 2005. Remodeling of the intestine during metamorphosis of Xenopus laevis. Proc Natl Acad Sci USA 102:3720–3725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hasebe T, Buchholz DR, Shi YB, Ishizuya-Oka A. 2011. Epithelial-connective tissue interactions induced by thyroid hormone receptor are essential for adult stem cell development in the Xenopus laevis intestine. Stem Cells 29:154–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sun G, Hasebe T, Fujimoto K, Lu R, Fu L, Matsuda H, Kajita M, Ishizuya-Oka A, Shi YB. 2010. Spatio-temporal expression profile of stem cell-associated gene LGR5 in the intestine during thyroid hormone-dependent metamorphosis in Xenopus laevis. PLoS One 5:e13605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shi YB, Hasebe T, Fu L, Fujimoto K, Ishizuya-Oka A. 2011. The development of the adult intestinal stem cells: insights from studies on thyroid hormone-dependent amphibian metamorphosis. Cell Biosci 1:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nieuwkoop PD, Faber J. 1965. Normal table of Xenopus laevis. Amsterdam: North Holland Publishing [Google Scholar]
- 48. Leloup J, Buscaglia M. 1977. La triiodothyronine: hormone de la métamorphose des amphibiens. C R Acad Sci 284:2261–2263 [Google Scholar]
- 49. Krain LP, Denver RJ. 2004. Developmental expression and hormonal regulation of glucocorticoid and thyroid hormone receptors during metamorphosis in Xenopus laevis. J Endocrinol 181:91–104 [DOI] [PubMed] [Google Scholar]
- 50. Das B, Heimeier RA, Buchholz DR, Shi YB. 2009. Identification of direct thyroid hormone response genes reveals the earliest gene regulation programs during frog metamorphosis. J Biol Chem 284:34167–34178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Buchholz DR, Paul BD, Shi YB. 2005. Gene-specific changes in promoter occupancy by thyroid hormone receptor during frog metamorphosis. Implications for developmental gene regulation. J Biol Chem 280:41222–41228 [DOI] [PubMed] [Google Scholar]
- 52. Wong J, Shi YB. 1995. Coordinated regulation of and transcriptional activation by Xenopus thyroid hormone and retinoid X receptors. J Biol Chem 270:18479–18483 [DOI] [PubMed] [Google Scholar]
- 53. Buchholz DR, Ishizuya-Oka A, Shi YB. 2004. Spatial and temporal expression pattern of a novel gene in the frog Xenopus laevis: correlations with adult intestinal epithelial differentiation during metamorphosis. Gene Expr Patterns 4:321–328 [DOI] [PubMed] [Google Scholar]
- 54. Shi YB, Ishizuya-Oka A. 1997. Autoactivation of Xenopus thyroid hormone receptor beta genes correlates with larval epithelial apoptosis and adult cell proliferation. J Biomed Sci 4:9–18 [DOI] [PubMed] [Google Scholar]
- 55. Ishizuya-Oka A, Ueda S, Shi YB. 1997. Temporal and spatial regulation of a putative transcriptional repressor implicates it as playing a role in thyroid hormone-dependent organ transformation. Dev Genet 20:329–337 [DOI] [PubMed] [Google Scholar]
- 56. Shi YB, Hayes WP. 1994. Thyroid hormone-dependent regulation of the intestinal fatty acid-binding protein gene during amphibian metamorphosis. Dev Biol 161:48–58 [DOI] [PubMed] [Google Scholar]
- 57. Wong J, Shi YB, Wolffe AP. 1997. Determinants of chromatin disruption and transcriptional regulation instigated by the thyroid hormone receptor: hormone-regulated chromatin disruption is not sufficient for transcriptinal activation. EMBO J 16:3158–3171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wong J, Shi YB, Wolffe AP. 1995. A role for nucleosome assembly in both silencing and activation of the Xenopus TR βA gene by the thyroid hormone receptor. Genes Dev 9:2696–2711 [DOI] [PubMed] [Google Scholar]
- 59. Wang Z, Zang C, Rosenfeld JA, Schones DE, Barski A, Cuddapah S, Cui K, Roh TY, Peng W, Zhang MQ, Zhao K. 2008. Combinatorial patterns of histone acetylations and methylations in the human genome. Nat Genet 40:897–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Roh TY, Cuddapah S, Cui K, Zhao K. 2006. The genomic landscape of histone modifications in human T cells. Proc Natl Acad Sci USA 103:15782–15787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K. 2007. High-resolution profiling of histone methylations in the human genome. Cell 129:823–837 [DOI] [PubMed] [Google Scholar]
- 62. Maunakea AK, Chepelev I, Zhao K. 2010. Epigenome mapping in normal and disease states. Circ Res 107:327–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Barth TK, Imhof A. 2010. Fast signals and slow marks: the dynamics of histone modifications. Trends Biochem Sci 35:618–626 [DOI] [PubMed] [Google Scholar]
- 64. Hsia SC, Shi YB. 2002. Chromatin disruption and histone acetylation in regulation of the human immunodeficiency virus type 1 long terminal repeat by thyroid hormone receptor. Mol Cell Biol 22:4043–4052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Huang ZQ, Li J, Sachs LM, Cole PA, Wong J. 2003. A role for cofactor—cofactor and cofactor—histone interactions in targeting p300, SWI/SNF and mediator for transcription. EMBO J 22:2146–2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Heimeier RA, Hsia VS, Shi YB. 2008. Participation of BAF57 and BRG1-containing chromatin remodeling complexes in thyroid hormone-dependent gene activation during vertebrate development. Mol Endocrinol 22:1065–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bilesimo P, Jolivet P, Alfama G, Buisine N, Le Mevel S, Havis E, Demeneix BA, Sachs LM. 2011. Specific histone lysine 4 methylation patterns define TR-binding capacity and differentiate direct T3 responses. Mol Endocrinol 25:225–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Furlow JD, Brown DD. 1999. In vitro and in vivo analysis of the regulation of a transcription factor gene by thyroid hormone during Xenopus laevis metamorphosis. Mol Endocrinol 13:2076–2089 [DOI] [PubMed] [Google Scholar]