Abstract
Kisspeptin, encoded by the Kiss1 gene, stimulates GnRH secretion and is therefore critical for sex steroid secretion at puberty and in adulthood. However, kisspeptin's role in regulating sex steroid secretion earlier in development is unexplored. In rodents, testosterone (T) levels are higher in prenatal and newborn males than females. We determined whether kisspeptin-Kiss1r and GnRH signaling plays a role in sexually dimorphic perinatal T secretion in mice. Our results demonstrate that 1) T levels in newborn males are elevated at 4 h but not 20 h after birth, but hypothalamic Kiss1 and neurokinin B (NKB) levels in males are not different between these time points (and both are lower than in females); 2) serum T levels in newborn Kiss1r knockout (KO) males are higher than in newborn females and similar to wild-type (WT) males; 3) perinatal hypothalamic progesterone receptor (Pgr) expression, which is dependent on circulating levels of gonadally produced T, is significantly higher in prenatal and newborn Kiss1r KO and WT males than similarly aged females; 4) multiple measures of testicular growth and function are not different between developing Kiss1r KO and WT mice until after postnatal d 5; and 5) GnRH neurons of newborn males do not exhibit high c-fos coexpression, and newborn hypogonadal (hpg) male mice (lacking GnRH) secrete elevated T, similar to newborn WT males. We conclude that, unlike in puberty and adulthood, elevated T secretion in prenatal and neonatal mice is independent of both kisspeptin and GnRH signaling, and the necessity of kisspeptin-Kiss1r signaling for testicular function is first apparent after d 5.
The neuropeptide kisspeptin and its receptor, Kiss1r, have emerged as essential upstream regulators of the reproductive axis during puberty and adulthood (1–4). At these ages, kisspeptin's primary action is to stimulate GnRH secretion via binding of the Kiss1r in GnRH neurons (3–6). This is evidenced by kisspeptin's ability to robustly stimulate GnRH-dependent gonadotropin secretion, increase electrical firing of GnRH neurons, induce c-fos in GnRH cells, and evoke GnRH secretion (7–12). Given kisspeptin's potent activation of GnRH neurons, it is not surprising that secretion of gonadal sex steroids in adulthood is also influenced by kisspeptin signaling. Indeed, kisspeptin treatment substantially increases testosterone (T) secretion in numerous mammals, including rodents, primates, and humans (13–16), and adult Kiss1 and Kiss1r knockout (KO) mice lacking kisspeptin-Kiss1r signaling have virtually undetectable circulating T levels (1, 17).
In rodents, the kisspeptin protein and its gene, Kiss1, are expressed in two hypothalamic regions: the continuum comprising the anteroventral periventricular and rostral periventricular nuclei (AVPV/PeN) and the arcuate nucleus (ARC) (7, 18–20), the latter of which coexpresses neurokinin B (NKB), another key modulator of GnRH release (21–24). In peripubertal and adult rodents, sex steroids stimulate Kiss1 levels in the AVPV/PeN and inhibit Kiss1 in the ARC, suggesting that Kiss1 neurons in the AVPV/PeN and ARC mediate positive and negative feedback effects of gonadal sex steroids, respectively (6, 18, 25–27). Although less is known about kisspeptin signaling before puberty, some studies have reported that Kiss1 mRNA and kisspeptin protein are expressed in the rodent ARC as early as the day of birth [postnatal day (PND)1], whereas Kiss1 expression in the AVPV/PeN does not emerge until the second postnatal week (28–30). The functional significance of this differential developmental ontogeny of ARC and AVPV/PeN Kiss1 expression is unknown.
In mammals, sex steroid levels are typically low in juveniles, rise during puberty, and are elevated in adulthood. However, T secretion is also temporarily elevated at particular stages of perinatal development. Specifically, in many species, gonadal T secretion occurs in a sexually dimorphic pattern on PND1, being elevated in newborn males relative to newborn females (31). In rodents, this elevated T secretion in newborn males, sometimes referred to as the PND1 T surge, is transient, typically lasting less than a day, and in some species, such as mice, only a few hours (31). This sexually dimorphic PND1 T surge has been detected in numerous rodent and nonrodent species (31–34) and is believed to contribute to masculinization of many sexually dimorphic phenotypes (35, 36). In addition to the male-biased PND1 T surge, T is also secreted in a sexually dimorphic manner in prenatal rodents, with circulating T levels being elevated several days before birth in male but not female fetuses (37, 38). This elevated T secretion in late-prenatal males lasts for several days but is apparently independent from elevated PND1 T secretion, because there is a sharp decrease in fetal T levels shortly before birth which then rapidly increase again just after parturition (37).
The regulatory mechanisms underlying the timing and sexually dimorphic nature of prenatal and PND1 T secretion are poorly understood. Because kisspeptin-Kiss1r signaling is essential for pubertal and adult T secretion via kisspeptin's activation of GnRH secretion, we asked whether kisspeptin signaling also plays a role in regulating T secretion during perinatal development. Supporting this possibility, Kiss1 and kisspeptin are expressed in the hypothalamus on PND1 and prenatally (28, 29, 39, 40), and GnRH neurons have fully migrated and express Kiss1r by embryonic day (E)17 (41, 42), indicating the possibility of a potentially functional kisspeptin-GnRH circuit in early development. Moreover, at least one clinical case reported the presence of a micropenis is a newborn patient with a KISS1R mutation, also suggesting that kisspeptin could act perinatally (43, 44). We therefore assessed the contribution of both kisspeptin and GnRH signaling in governing sexually dimorphic T secretion in prenatal and postnatal mice.
Materials and Methods
Animals
Mice were housed on a 12-h light, 12-h dark cycle (lights off at 1800 h) with food and water available ad libitum. Adult C57BL/6 breeder mice were purchased from Harlan Laboratories (Indianapolis, IN). Adult Kiss1r heterozygous mice, originally created by Omeros, Inc. (Seattle, WA), were a gift from Robert Steiner (University of Washington, Seattle, WA) and were used to generate Kiss1r KO mice (10, 45). Adult Gnrh1hpg heterozygous mice (on a C57BL/6 background; a gift from Pamela Mellon, University of California, San Diego) were used to generate hypogonadal (hpg) mutant (i.e. GnRH deficient) mice (46). Genotypes and sexes of Kiss1r and hpg mice were determined by PCR of tail DNA. All experiments were conducted in accordance with National Institutes of Health Animal Care and Use Guidelines and with approval of the Animal Care and Use Committee of University of California, San Diego.
Timed breeding paradigms for PND1 pup and E18 fetus collection
The PND1 T surge in mice only occurs within the first 4–5 h of life (31). To collect blood and tissue from PND1 pups within this narrow temporal window, we used a timed-breeding paradigm: breeder pairs were set up for three nights, and breeder males were removed after the third night; bred females remained single housed for the remainder of gestation. Beginning 16 d after the breeder males were removed, pup checks were performed every 4 h for the next 72 h to determine exactly when litters were born (i.e. litter checks performed at 1600, 2000, 0000, 0400, 0800, and 1200 h for three consecutive days). Pup checks during lights off periods were performed under red-light illumination. Newborn pups were either collected immediately (0–4 h group, “4 h”) or 16 h later (16–20 h group, “20 h”). If a dam was observed actively delivering a litter, she was left undisturbed for 1 h before the pups were collected. This pregnancy check paradigm ensured that all newborn animals were collected between 0 and 4 h old or 16 and 20 h old. All PND1 pups were killed by rapid decapitation, and blood and tissue collected.
To accurately kill fetuses specifically on E18, one-night timed breedings were used. Female and male breeders were housed together for one night and the male removed the next morning (designated E1). On E18 (∼2 d before parturition normally occurs), females were killed via rapid decapitation, their uterine horns rapidly removed onto ice, and the fetuses quickly dissected out.
Blood collection and hormone assays
Retro-orbital blood (adult mice) or trunk blood (PND1 mice) was collected at killing in BD Microtainer SST tubes. Serum was isolated and stored at −20 C. Serum samples were assayed for hormones by the University of Virginia's Ligand Assay Core (Charlottesville, VA). Given the low serum volumes obtained from individual PND1 mice, serum samples within each group were pooled into several samples for subsequent T RIA (three to seven pups pooled per sample; six to eight pooled samples per group). Because the LH/FSH multiplex ELISA uses a smaller serum sample, LH and FSH levels were measured from individual serum samples.
Brain collection and in situ hybridization (ISH)
Collected brains were immediately frozen on dry ice and stored at −80 C. Five series of 20-μm brain sections (experiments 1 and 5) or four series of 25-μm brain sections (experiment 3) were cut on a cryostat, thaw mounted onto Superfrost-plus slides, and stored at −80 C until processing via ISH.
Single-label ISH for Kiss1, NKB, or progesterone receptor (Pgr) mRNA was performed as previously described (7, 20, 25, 28, 47), using previously validated mouse Kiss1 (7) and NKB riboprobes (23). Pgr was cloned using primers designed and validated by the Allen Brain Atlas (http://mouse.brain-map.org/) to produce a cRNA probe that binds bases 901-1810 of the mouse Pgr mRNA (GenBank accession no. NM_008829). For single-label ISH assays, slide-mounted sections encompassing the entire PND1 hypothalamus were fixed in 4% paraformaldehyde, pretreated with acetic anhydride, rinsed in 2× sodium citrate, sodium chloride (SSC), delipidated in chloroform, dehydrated in ethanols, and air dried. Radiolabeled (33P) antisense riboprobe (0.05 pmol/ml) was combined with 1/20 volume yeast tRNA (Roche Biochemicals, Indianapolis, IN), heat denatured, added to hybridization buffer, and applied to each slide (100 μl/slide). Slides were cover slipped and placed in a humidity chamber at 55 C for 18 h. After hybridization, slides were washed in 4× SSC and then placed into ribonuclease (RNase) [37 mg/ml RNase A in 0.15 m sodium chloride, 10 mm Tris, and 1 mm EDTA (pH 8.0)] for 30 min at 37 C, then in RNase buffer without RNase at 37 C. After washing in 2× SSC at room temperature, slides were washed in 0.1× SSC at 62 C for 1 h, dehydrated in ethanols, and air dried. Slides were then dipped in Kodak NTB emulsion (Kodak, New York, NY), air dried, and stored at 4 C for 6–8 d (depending on the assay). Slides were then developed and cover slipped as previously described (28).
For double-label ISH, slide-mounted brain sections were treated similarly to single-label ISH with the following modifications. Digoxigenin (DIG)-labeled antisense mouse Gnrh cRNA was synthesized with DIG labeling mix (Roche Biochemicals). Radio-labeled (33P) antisense c-fos (0.05 pmol/ml) and DIG-labeled Gnrh (1:400) riboprobes, previously described (48), were combined with tRNA, denatured, and dissolved together in hybridization buffer. The probe mix was then applied to slides (100 μl/slide) and hybridized at 55 C for 18 h. After the 62 C washes on d 2, slides were incubated in 2× SSC with 0.05% Triton X-100 containing 2% normal sheep serum for 1 h at room temperature. The slides were then washed in buffer 1 and incubated overnight at room temperature with anti-DIG antibody conjugated to alkaline phosphatase [diluted 1:250 in buffer 1 containing 1% normal sheep serum and 0.3% Triton X-100 (Roche Biochemicals)]. The next day, slides were washed with buffer 1 and incubated with Vector Red alkaline phosphatase substrate (Vector Laboratories, Burlingame, CA) for 2 h at room temperature. The slides were then air dried, dipped in emulsion, stored at 4 C, and developed 9 d later.
Quantification and analysis of ISH data
ISH slides were analyzed with an automated image processing system and custom silver grain-counting software (Don Clifton, University of Washington) by a person blind to the treatment group (49). For single-label experiments, the number of silver grain clusters representing cells was counted, as was the number of silver grains over each cell (a semiquantitative index of mRNA content per cell) (23, 50–52). Cells were considered Kiss1, NKB, or Pgr positive when the number of silver grains in a cluster exceeded background by 3-fold. For double-label assays, DIG-containing cells (GnRH cells) were identified under fluorescence microscopy and the grain-counting software quantified the silver grains (representing c-fos mRNA) overlying each cell. Signal-to-background ratios for individual cells were calculated, and a cell was considered double labeled if it had a ratio greater than four.
Experiment 1. Analysis of Kiss1 and NKB levels in the brains of mice during the postnatal T surge
Kisspeptin-Kiss1r signaling is essential for T secretion in adulthood. This experiment addressed whether the elevated T secretion observed in newborn males is driven by enhanced kisspeptin signaling in the brain. We hypothesized that, as in adulthood, hypothalamic Kiss1 expression would correlate with T levels, such that newborn males would have higher Kiss1 expression, along with higher T levels, than newborn females. C57BL/6 breeder pairs were set up for timed pregnancy. On the day of birth, blood and brains of newborn male and female pups were collected when the mice were either 0–4 h old (during the presumptive T surge in males) or 16–20 h old (after the T surge was over). Blood serum from males and females at each time point were pooled (three to seven pups of each sex per sample) and T levels measured by RIA (six to eight pooled samples per group) to confirm elevated T at 0–4 h but not 16–20 h after birth. Brains from each group were examined for Kiss1 levels in the hypothalamus using single-label ISH for Kiss1 mRNA (7–12 animals per group). Because NKB has recently been implicated in stimulating the reproductive axis via its activation of Kiss1 neurons (21, 53, 54), an alternate set of brain sections from the same newborn animals was assayed with ISH for NKB levels in the ARC.
Experiment 2. Assessment of a PND1 T surge in Kiss1r KO mice
Functional kisspeptin-Kiss1r signaling is required for elevated T secretion in pubertal and adult animals. Here, we tested the necessity of kisspeptin-Kiss1r signaling for PND1 T secretion, using Kiss1r KO mice, which lack kisspeptin signaling. Timed breeding of Kiss1r heterozygous breeders was used to generate PND1 Kiss1r KO and wild-type (WT) offspring. Blood serum was collected from newborn Kiss1r KO and WT pups of both sexes when they were 0–4 h old (when the T surge normally occurs). Serum T levels were measured in pooled samples by RIA (three to five pups pooled per sample; six to eight pooled samples per group).
Experiment 3. Evaluation of prenatal T levels in Kiss1r KO mice via analysis of hypothalamic Pgr levels
In late fetal life, as on the day of birth, male rodents secrete higher levels of T than females (37). Here, we determined whether functional Kiss1r signaling is required for elevated T secretion during late fetal life. Kiss1r heterozygous breeders were set up for timed matings. Kiss1r KO and WT fetuses of both sexes were killed on E18 and their brains collected. Brains were assayed using single-label ISH for hypothalamic Pgr levels, a well-established biomarker for circulating T levels in perinatal rodents: prenatal males have higher gonadal T secretion, which, after its aromatization to estradiol (E2) in the brain, induces higher expression of hypothalamic Pgr than in prenatal females (who lack elevated levels of T and, hence, are not exposed to high prenatal E2 in the brain) (55–58). For comparison, a set of brain sections from PND1 Kiss1r KO and WT mice was also examined, because hypothalamic Pgr expression is also higher in newborn males than females, again reflecting higher T levels in the former sex (five to eight animals per group).
Experiment 4. Developmental profile of kisspeptin's involvement in gonadal activation
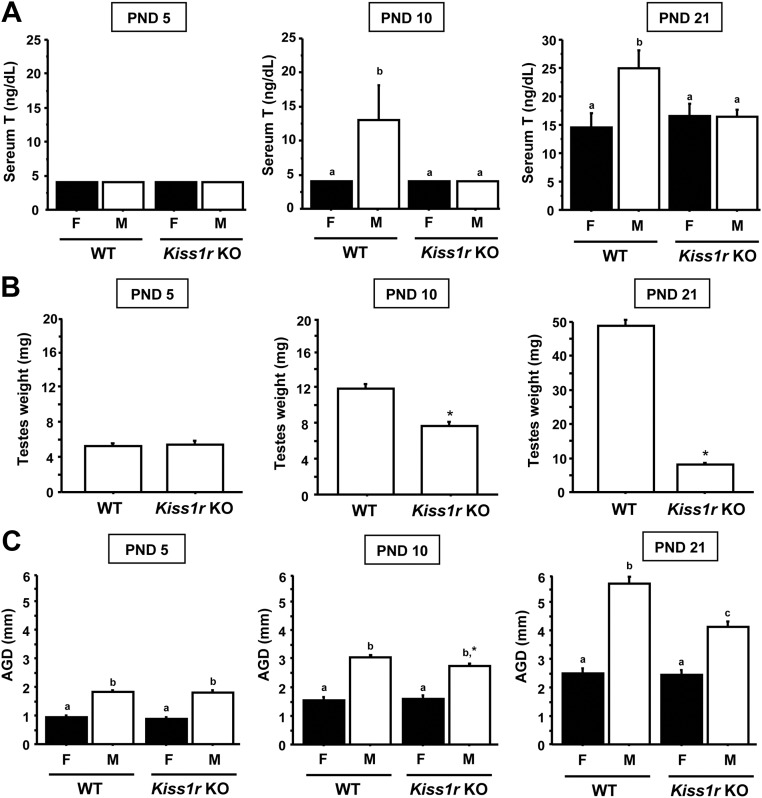
Experiments 2 and 3 determined that Kiss1r KO males were capable of secreting elevated T on the day of birth and also during late fetal life but not in adulthood. Here, we determined when in postnatal development kisspeptin-Kiss1r signaling becomes required for T secretion and testicular growth. Male and female Kiss1r KO and WT littermates were killed on PND5, PND10, and PND21. Paired testis weight in males and anogenital distance (AGD) (an external marker of androgen action) in both sexes were measured (n = 6–12 per group). Blood was also collected and the serum pooled (for PND5 and PND10) and assayed for T levels.
Experiment 5. Assessment of the involvement of GnRH signaling in PND1 T secretion
Experiments 2 and 3 demonstrated that kisspeptin-Kiss1r signaling is not necessary for either prenatal or postnatal T secretion. Here, we asked whether GnRH signaling promotes elevated T secretion in newborn males. In experiment 5A, we determined whether GnRH neurons of PND1 males coexpress higher levels of c-fos, a marker of neuronal activation, than PND1 females during the time of the PND1 T surge. We tested this possibility using double-label ISH for Gnrh and c-fos coexpression in an alternate set of brain sections from PND1 C57BL/6 mice from experiment 1. Newborn animals from both the 4 h (elevated T in males) and 20 h (T not elevated in males) groups were examined (five to seven animals per group). Next, in experiment 5B, we determined whether elevated T secretion in PND1 males requires GnRH. Blood serum was collected from newborn hpg mutant (hpg) and WT pups of both sexes when they were 0–4 h old (during the time of any T surge). Serum T levels in each group were measured in pooled samples by RIA (three to four pups pooled per sample; four to eight pooled samples per group).
Experiment 6. Serum gonadotropins during the PND1 T surge
Experiments 2 and 5 determined that elevated T secretion in newborn males is independent of both kisspeptin and GnRH signaling. We therefore asked whether gonadotropin secretion in newborn mice is similarly independent of kisspeptin and/or GnRH control. Separate cohorts of newborn male and female Kiss1r KO and hpg pups (and WT littermates) were killed between 0 and 4 h after birth, and their blood was collected. Blood serum from individual animals was assayed for both LH and FSH using a multiplex ELISA (7–10 animals per group).
Statistical analysis
All data are expressed as the mean ± sem for each group. In all experiments, differences were analyzed by Student's t test or two-way ANOVA, followed by post hoc comparisons via Fisher's (protected) Least Significant Difference test. For all comparisons, statistical significance was set at P < 0.05. All analyses were performed in StatView 5.0.1 (SAS Institute, Cary, NC).
Results
Experiment 1. Determination of Kiss1 and NKB levels in the hypothalamus during the PND1 T surge
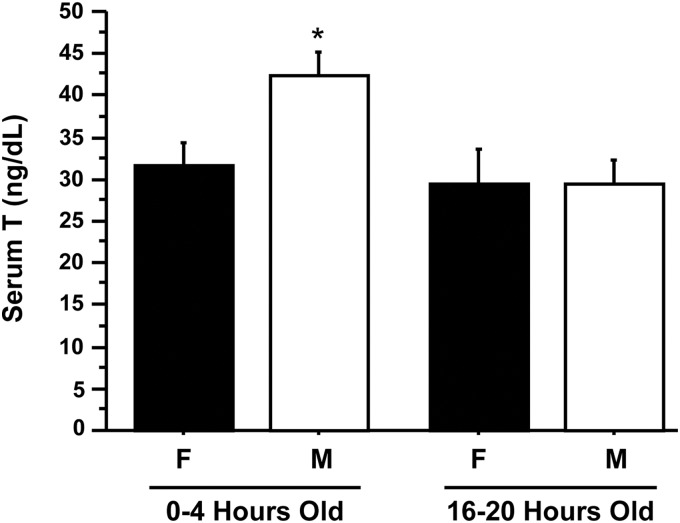
Blood serum was analyzed for circulating T levels from newborn male and female C57BL/6 mice that were either 0–4 h old (4 h) or 16–20 h old (20 h); 4-h males had significantly greater serum T levels than 4-h females, indicating a sexually dimorphic pattern of T secretion at this time (P < 0.05) (Fig. 1). Serum T levels of 4-h males were also significantly higher than T levels of both 20-h males and 20-h females (P < 0.05) (Fig. 1), indicating that elevated T secretion in males decreases by 16–20 h after birth.
Fig. 1.
Serum T in PND1 C57BL/6 males (M) and females (F). Serum was isolated from blood collected from C57BL/6 pups killed between 0 and 4 h old and 16 and 20 h old, pooled within groups, and assayed for T. Males had significantly higher T levels than females at 0–4 h old (P < 0.05). There was no significant difference in T between males and females at 16–20 h old.
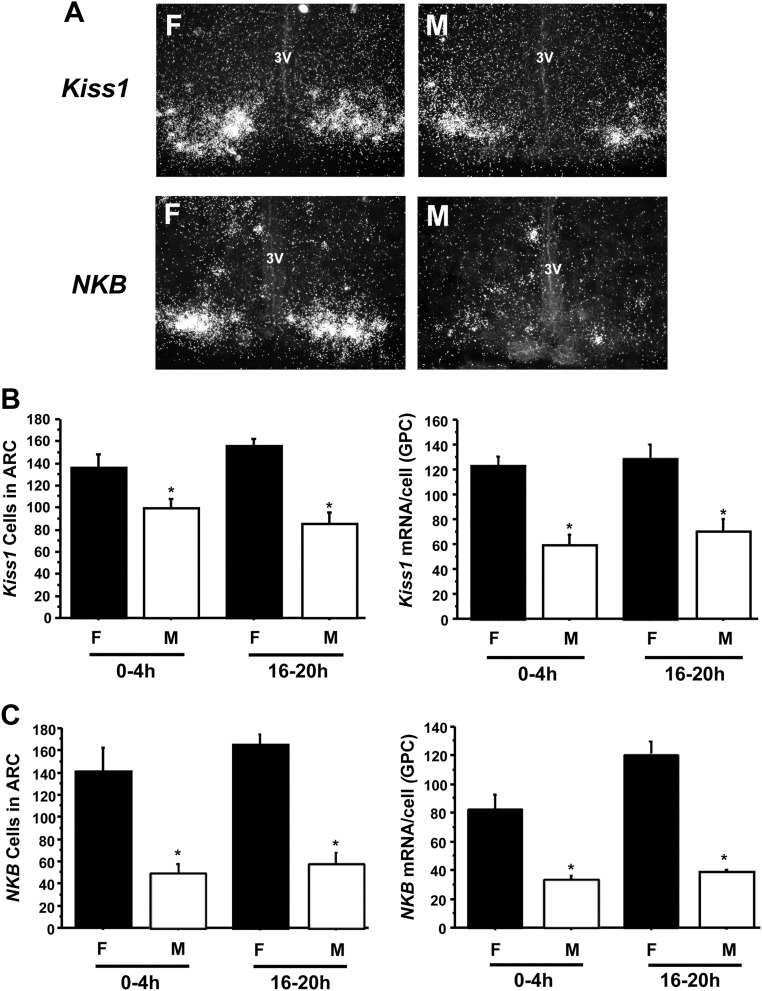
Hypothalamic Kiss1 expression was examined to determine whether activation of the Kiss1 system might contribute to elevated T secretion in 4-h males. In all PND1 mice, regardless of sex, Kiss1 mRNA was detected only in the ARC (and not the AVPV/PeN), consistent with our previous findings (28). Despite higher T levels in 4-h males than 20-h males (Fig. 1), ARC Kiss1 expression was not significantly different between these two groups: 4- and 20-h males had similar numbers of ARC Kiss1 cells and similar Kiss1 mRNA per cell (Fig. 2B). Thus, Kiss1 levels in newborn males did not correlate with T levels. Interestingly, the number of ARC Kiss1 cells and the relative amount of Kiss1 mRNA/cell were both significantly higher in PND1 females than PND1 males at each time point (P < 0.05) (Fig. 2B). Similar to Kiss1 expression, there were no significant differences in NKB expression in the ARC between 4- and 20-h males, both in terms of NKB cell number and relative NKB mRNA/cell (Fig. 2C). Additionally, like Kiss1, NKB levels were significantly higher in PND1 females than PND1 males at both time points (P < 0.05) (Fig. 2C).
Fig. 2.
Gene expression of GnRH-stimulating neuropeptides in the ARC on PND1. A, Representative photomicrographs for Kiss1 and NKB mRNA expression in females (F) and males (M) that were 0–4 h old. The mean number of Kiss1 (B) and NKB (C) neurons in the arcuate, as well as the relative level of Kiss1 (B) and NKB (C) mRNA/cell (determined by grains per cell), were quantified for males and females that were ages 0–4 h old and 16–20 h old. Males had significantly fewer Kiss1 and NKB cells and lower mRNA per cell than females at either time point (P < 0.05). There were no significant differences in the number of neurons or mRNA/cell between 0 and 4 h and 16 and 20 h. 3V, Third ventricle; GPC, grains per cell.
Experiment 2. Assessment of a PND1 T surge in Kiss1r KO mice
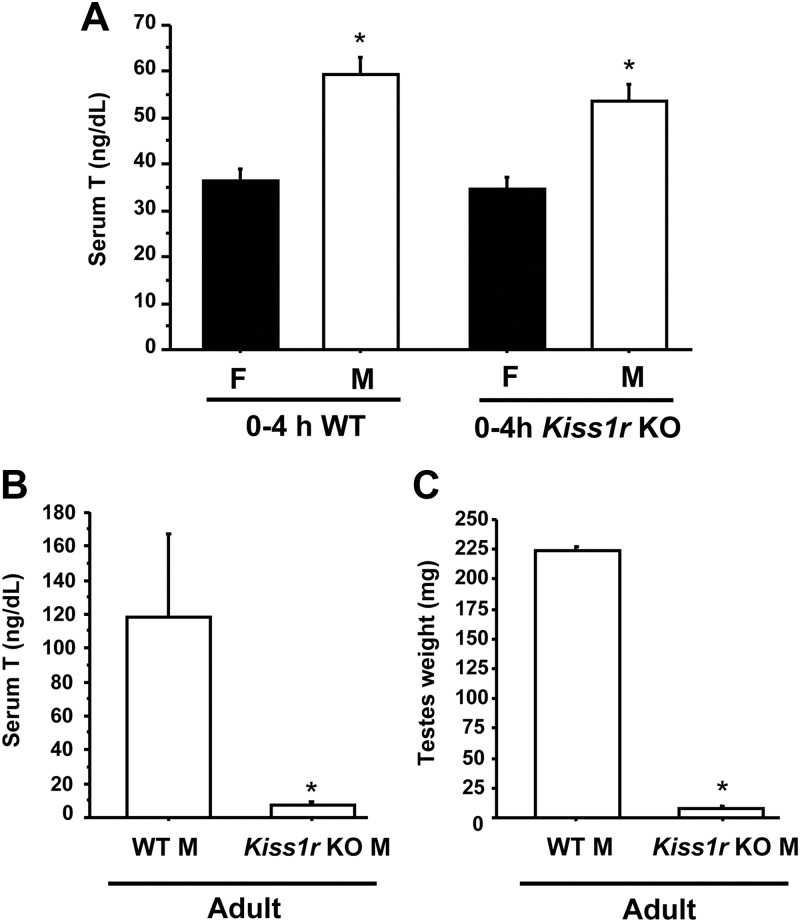
This experiment used Kiss1r KO mice to determine whether kisspeptin-Kiss1r signaling is necessary for elevated T secretion in newborn 4-h males. Interestingly, newborn Kiss1r KO mice had elevated T levels between 0–4 h of life, similar to those of WT males (P < 0.05) (Fig. 3A). This elevated T in 4-h Kiss1r KO males was significantly higher than that of 4-h WT and Kiss1r KO females, indicating that sexually dimorphic PND1 T secretion persists in the absence of kisspeptin signaling. In contrast to newborn mice, adult Kiss1r KO male mice had virtually undetectable serum T (P < 0.05) (Fig. 3B), highly reduced paired testis weight (P < 0.05) (Fig. 3C), and significantly shortened AGD (P < 0.05) (data not shown) compared with adult WT male littermates.
Fig. 3.
Serum T in Kiss1r KO males and females. A. Blood serum was isolated from newborn male (M) and female (F) mice less than 4 h old and assayed for T. Both WT and Kiss1r KO males had significantly higher T levels than females of the same genotype (P < 0.05). There was no significant difference in T levels between 4-h WT and KO males. B, In contrast to neonatal mice, serum T was significantly reduced in adult Kiss1r KO males compared with adult WT male littermates (P < 0.05). C, Testis weight was also significantly lower in adult Kiss1r KO males than adult WT littermates (P < 0.05).
Experiment 3. Evaluation of prenatal T levels in Kiss1r KO mice via analysis of hypothalamic Pgr levels
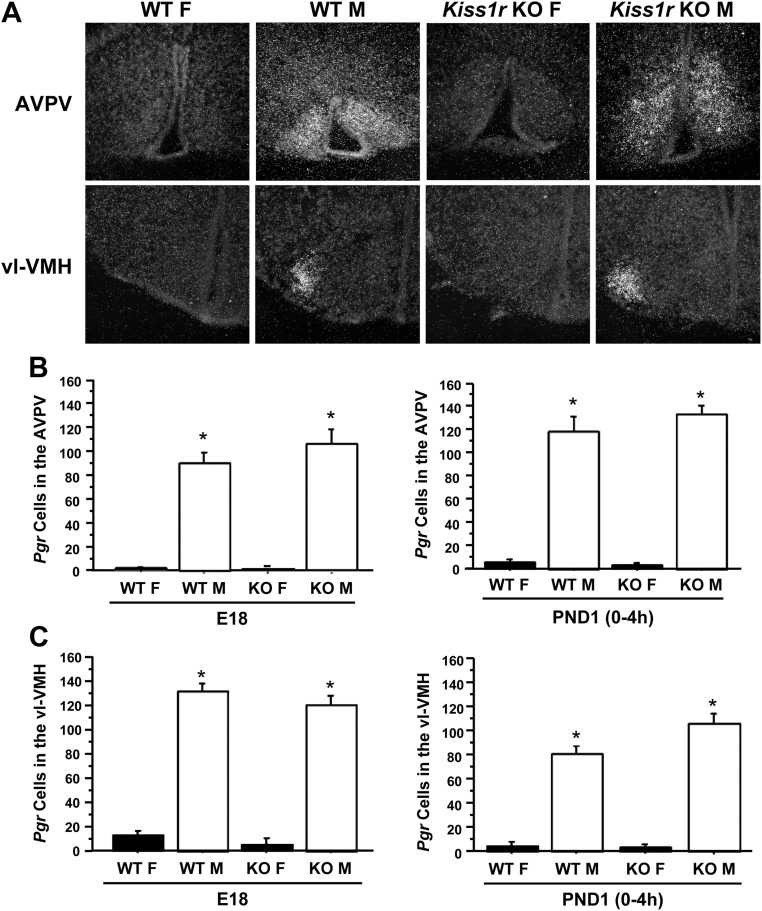
In perinatal rodents, elevated E2 in the brain, aromatized from circulating T of gonadal origin, induces Pgr expression in select hypothalamic regions (56–58). Hypothalamic Pgr expression is therefore greater in prenatal and PND1 males than females, reflecting higher gonadal T secretion in males at these ages (56, 57). We detected perinatal Pgr expression in two hypothalamic nuclei: the AVPV and the ventrolateral portion of the ventromedial nucleus (vl-VMH), consistent with previous reports of Pgr-immunoreactivity in perinatal rodents (55, 56). Reflecting the normal sex difference in perinatal T levels, Pgr expression was significantly higher in both hypothalamic regions of WT males than WT females, both at E18 and PND1 (P < 0.05 for each region) (Fig. 4). Similarly, Pgr expression in both brain regions was significantly higher in Kiss1r KO males than Kiss1r KO and WT females, both at E18 and PND1 (P < 0.05) (Fig. 4). Thus, kisspeptin-Kiss1r signaling is not necessary for elevated perinatal Pgr levels, which are reflective of circulating T levels.
Fig. 4.
Pgr mRNA expression in the hypothalamus of fetal and neonatal Kiss1r KO and WT mice. Hypothalamic Pgr levels are an established indicator of fetal and neonatal androgen milieu and therefore can serve as a biomarker to recent T exposure. A, Representative photomicrographs of Pgr expression in WT and Kiss1r KO males (M) and females (F) in the AVPV and vl-VMH on E18. B and C, The mean number of Pgr neurons in the AVPV and vl-VMH on E18 and PND1 was significantly higher in males than females, regardless of age or genotype (P > 0.05).
Experiment 4. Developmental onset of kisspeptin requirement for gonadal activation
Experiments 2 and 3 indicated that kisspeptin-Kiss1r signaling is not essential for prenatal or neonatal T secretion but is necessary for adulthood T secretion. Here, we determined when in postnatal development kisspeptin signaling becomes necessary for normal gonadal function. Kiss1r KO male mice killed at either PND10 or PND21 had serum T levels, AGD, and paired testis weights that were significantly lower than those of their WT male littermates (P < 0.05) (Fig. 5). In contrast, younger Kiss1r KO males killed on PND5 had circulating T levels, AGD, and testis weights that were not significantly different from WT males. At all three ages, the AGD of Kiss1r KO males was higher than that of WT and Kiss1r KO females (P < 0.05) (Fig. 5).
Fig. 5.
Serum T, testis weight, and AGD were measured in WT and Kiss1r KO mice of different postnatal ages. Serum T (A), testis weight (B), and AGD (C) were not significantly different between genotypes on PND5 but were lower in KO Kiss1r males than WT males on PND 10 and PND 21 (P < 0.05). *, Significantly different from WT males. Bars labeled with different letters designate significantly different groups (P < 0.05).
Experiment 5. Evaluation of the role of GnRH signaling in the PND1 T surge
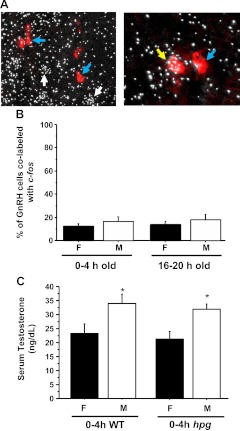
Having determined that kisspeptin-Kiss1r signaling is not required for elevated T secretion on PND1, we next tested whether GnRH is involved in elevated neonatal T secretion. Experiment 5A determined whether GnRH neurons of newborn males exhibit enhanced c-fos coexpression during the time of elevated T secretion, similar to elevated GnRH-c-fos coexpression during the preovulatory LH surge in adult females (48, 59–61). However, we found that only a small minority of GnRH cells coexpress c-fos on PND1: just 14–18% of GnRH neurons of 4- and 20-h PND1 males coexpressed c-fos, despite the presence of higher T levels in the former group (Fig. 6B). There were no significant differences in GnRH-c-fos coexpression between sexes or time points (Fig. 6B).
Fig. 6.
A, Representative low power (left) and higher power (right) photomicrographs of GnRH (red DIG staining) and c-fos (silver grains) mRNA expression in PND1 mice. Blue arrows designate example GnRH neurons lacking c-fos expression; white arrows designate single-label c-fos cells that are not GnRH neurons; yellow arrow designates one of the few GnRH cells coexpressing c-fos. B, Quantification of the percent of total GnRH neurons double labeled with c-fos on PND1. There were no significant differences between sexes or between time points. C, Serum T levels in hpg males (M) and females (F) on the day of birth. Blood serum collected from WT and hpg mice that were killed between 0 and 4 h old was assayed for T. Both WT and hpg males had significantly higher T levels than females from the same genotype (P < 0.05). There was no significant difference in T levels between 4-h WT and hpg males.
Next, using hpg mice, experiment 5B tested whether GnRH signaling is required for elevated T secretion in PND1 males. Interestingly, we detected elevated T levels in both 4-h WT and 4-h hpg males, with no significant differences between the two groups. These elevated T levels of 4-h hpg and WT males were significantly higher than those of 4-h females (P < 0.05) (Fig. 6C).
Experiment 6. LH and FSH levels in PND1 mice lacking GnRH or kisspeptin signaling
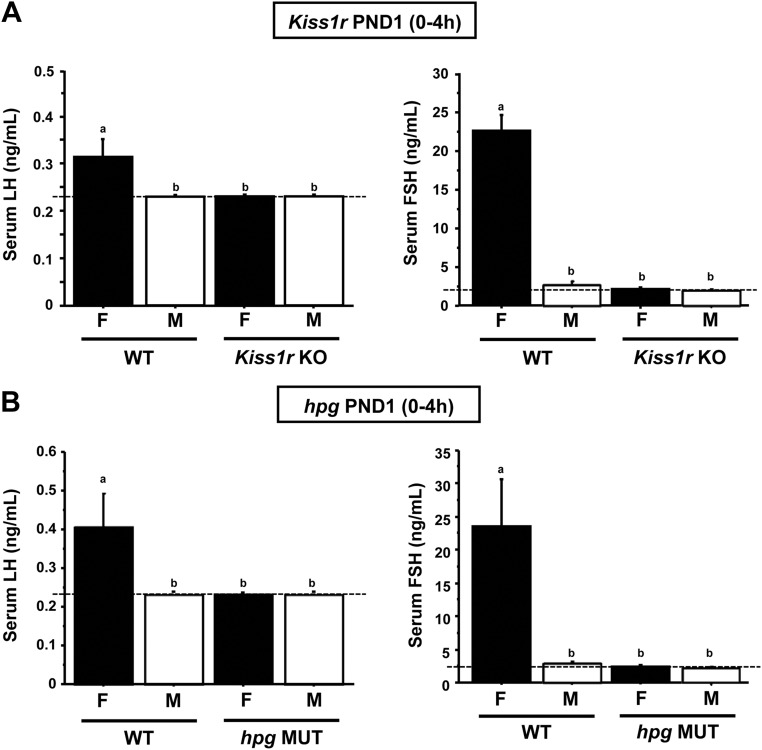
Here, we assessed gonadotropin levels in PND1 males and females lacking functional GnRH or kisspeptin signaling. We first measured serum LH and FSH of 4-h Kiss1r KO and WT newborn mice. In 4-h WT females, serum LH and FSH were low but readily detectable, whereas LH and FSH were undetectable in both 4-h WT males and 4-h Kiss1r KO males. Similarly, neither gonadotropin was detectable in 4 h Kiss1r KO females, despite being detectable in WT females (P < 0.05) (Fig. 7A). Similar results were obtained in PND1 hpg mutant mice: although LH and FSH were both detectable in 4-h WT females, neither gonadotropin was detectable in 4-h hpg females or in 4-h males of either genotype (P < 0.05) (Fig. 7B).
Fig. 7.
Serum gonadotropin concentrations from PND1 WT, Kiss1r KO (A), and hpg mutant (B) mice. Individual serum samples were collected from newborn mice between 0 and 4 h after birth and assayed for LH and FSH using an LH/FSH multiplex ELISA. LH and FSH were readily detectable in newborn WT females (F) but undetectable in newborn WT males (M). LH and FSH were undetectable in Kiss1r KO and hpg mice, regardless of sex. Bars labeled with different letters designate significantly different groups (P < 0.05). The dashed line designates the lower limit of detection for the assays.
Discussion
Kisspeptin and its receptor, Kiss1r, are critical for controlling the reproductive axis in mammalian species, including humans. In the absence of kisspeptin signaling in adulthood, such as cases with Kiss1r mutations, reproductive hormone levels, including T, are virtually undetectable (1, 2, 17). Thus, kisspeptin-Kiss1r signaling, presumably acting to stimulate GnRH neurons, is necessary for normal T secretion in adulthood. Here, we examined the possible role of kisspeptin-Kiss1r signaling in promoting T secretion during the perinatal period, when T levels are higher in males than females in numerous species (31, 62). Our findings clearly indicate that, despite its requisite role in stimulating T secretion in adulthood, kisspeptin-Kiss1r signaling is not necessary for T secretion before birth or on PND1, at least in mice (Fig. 8). Our data also demonstrate that GnRH secretion is similarly not needed for elevated T secretion on PND1. Our findings therefore support the model that sexually dimorphic T secretion on both E18 and PND1 is independent of hypothalamic kisspeptin and GnRH signaling. However, in contrast to their dispensability for perinatal T secretion, Kiss1r and GnRH signaling were both found to be required for gonadotropin secretion in PND1 females.
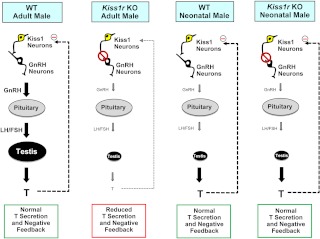
Fig. 8.
Schematic summary of differences between the adult and neonatal reproductive axis in WT and Kiss1r KO males. In adulthood, kisspeptin is a required upstream stimulant of GnRH release and subsequent secretion of gonadotropins and T. When kisspeptin signaling is disrupted in adults by a Kiss1r mutation, GnRH, gonadotropin, and T secretion decreases considerably, and there is no longer sex steroid-mediated negative feedback on the brain. In contrast to adulthood, kisspeptin-Kiss1r signaling is not required for T production on PND1. Kiss1r KO male mice are able to secrete similar elevated T as WT males, despite presumed decreased in GnRH signaling and gonadotropin production. Moreover, the presence of elevated T in PND1 Kiss1r KO males suggests that T-mediated negative feedback to the brain is still present and not impaired in newborns, unlike in older Kiss1r KO animals.
Because kisspeptin-Kiss1r signaling is necessary for elevated T secretion during puberty and adulthood, we predicted that T secretion on the day of birth would also be dependent on functional Kiss1r. Surprisingly, we found that neonatal Kiss1r KO males, which lack kisspeptin-Kiss1r signaling, have no detectable impairment in T secretion on PND1: newborn Kiss1r KO males displayed elevated T levels that were no different from WT males but were significantly higher than females. This finding was paralleled by our measures of T-dependent hypothalamic Pgr expression on PND1 as well as in late fetal life: both E18 and PND1 Kiss1r KO males had high AVPV and vl-VMN Pgr expression that was no different from WT males and which was significantly greater than that of WT and KO females. Thus, contrary to the assumption that kisspeptin signaling is required throughout life for activation of the reproductive axis, our findings clearly demonstrate that kisspeptin-Kiss1r signaling is not required for elevated gonadal hormone secretion in male rodents before and on the day of birth.
In pubertal and adult animals, higher Kiss1 levels in the ARC correlate with higher activation of the reproductive axis (i.e. elevated reproductive hormone secretion) (3, 4, 6). However, we found that Kiss1 and NKB levels in the ARC of newborn C57BL/6 mice were not correlated with serum T levels. Specifically, higher T levels in 4-h males than 20-h males did not equate with higher Kiss1 or NKB expression in the former group. These findings support the notion that enhanced kisspeptin (and NKB) signaling is not a principal component of elevated PND1 T secretion in newborn males, supporting our finding of a T surge in PND1 Kiss1r KO mice. Another unexpected finding was that both Kiss1 and NKB expression were lower in males than females on PND1. This sex difference in neonatal ARC Kiss1 and NKB levels differs from adult rodents, in which ARC Kiss1 and NKB cell number is not markedly different between sexes (20, 23, 28) but is similar to recent findings showing that Kiss1 levels are sexually dimorphic (favoring females) during the first postnatal week (30, 40). The mechanistic basis (and functional significance) for the PND1 Kiss1 sex difference in the ARC is unclear but may reflect, in part, sex differences in the neonatal sex steroid milieu: higher gonadal T secretion in newborn males may provide more negative feedback inhibition of Kiss1 and NKB expression in the ARC, as in adulthood (18, 20, 23, 25). If so, the lower Kiss1 and NKB levels in PND1 males than females may simply reflect higher T-mediated negative feedback in males. However, additional factors may also be involved, because the Kiss1 and NKB sex differences were also evident at 20 h after birth, when male T levels were no longer higher than those of females. Thus, lower Kiss1/NKB levels in neonatal males than females may be due, in part, to sex steroid-independent mechanisms. This possibility is supported by recent findings in mice that indicate that nongonadal factors suppress ARC Kiss1/NKB expression in juvenile males to a greater extent than in juvenile females (23).
Our findings suggest that kisspeptin-Kiss1r signaling is not required for prenatal or neonatal testicular endocrine function. We therefore determined when in postnatal development Kiss1r KO males first show defects in gonadal growth and T secretion. Unlike on PND1, on both PND10 and PND21, there were clear defects in reproductive development of Kiss1r KO males, exemplified by reduced testis weight, decreased T levels, and slightly lower AGD (an androgen-dependent measure). In contrast, PND5 Kiss1r KO males displayed no significant differences in AGD, testes weight, or serum T levels compared with WT males. Thus, kisspeptin necessity for testicular endocrine function and growth in mice first becomes apparent sometime between PND5 and PND10. Whether or not kisspeptin is actually required before PND5 for other unstudied testicular processes remains to be tested. Notably, the postnatal age period when kisspeptin appears to become necessary for testicular growth and physiology coincides with developmental changes in rodent Leydig cells during the second postnatal week (38), but whether these events are related remains unexplored.
Having excluded Kiss1r as a necessary player in the PND1 T surge, we questioned whether GnRH is required for elevated PND1 T secretion. To our surprise, newborn hpg males had elevated T levels comparable with their newborn WT brothers. Additionally, unlike during the preovulatory LH surge in adult females when most GnRH neurons coexpress c-fos (48, 61), the vast majority of GnRH neurons (∼85%) did not coexpress c-fos during the PND1 T surge. Together, these data demonstrate that GnRH, like kisspeptin, is not required for elevated T secretion in newborn rodents and is unlikely to be a major contributor to neonatal T secretion. This agrees with previous data demonstrating that testicular T content is similar in neonatal hpg and WT mice (63), although that report did not discriminate when mice were killed on PND1 (most neonates appear to have been collected after the T surge ended). Because our newborn hpg pups were killed specifically within the 4-h window when T is elevated on PND1, we can definitively conclude that sexually dimorphic neonatal T secretion is preserved in the absence of GnRH signaling, at least in mice.
The mechanism(s) underlying the neonatal induction of T remains ill-defined. Interestingly, in mice, ACTH can stimulate T production in neonatal but not adult testes (64, 65). However, genetic deletion of proopiomelanocortin, from which ACTH is derived, does not diminish neonatal T levels (66), suggesting that ACTH is sufficient but not necessary for neonatal T secretion. Additional evidence in rats suggests that neonatal elevations in circulating T levels may be regulated, in part, by alterations in the rate of T metabolism in the liver (34), although this has not been examined in mice yet, nor would it account for elevations in intratesticular T levels in newborn males. Lastly, although the adrenal makes androgens as well, it is not the source of the perinatal T surge, because castrated newborn rats fail to exhibit elevated T levels, suggesting a gonadal origin of T (67). Whether or not the lower T levels detected in our newborn females is of adrenal origin is unknown, as is whether these lower T levels fail to achieve a threshold level needed to induce hypothalamic PR expression, as occurs in males.
Although perinatal T secretion appears to be independent of kisspeptin and GnRH signaling, gonadotropin secretion is not, at least in females. Unlike their WT littermates, female PND1 Kiss1r and hpg mice had undetectable serum LH and FSH levels, providing evidence that neonatal gonadotropin secretion is dependent on kisspeptin and GnRH signaling. Although evidence for postnatal LH dependence on GnRH has been demonstrated before (63), we are the first to show that kisspeptin signaling is required as early as PND1 for both LH and FSH production, at least in females. Unfortunately, we cannot conclude whether gonadotropin secretion in PND1 males is also regulated by kisspeptin or GnRH signaling, because gonadotropins were already completely undetectable in newborn WT males (likely reflecting a combination of high T-mediated negative feedback and additional gonadal hormone-independent suppression).
In summary, our data demonstrate that, unlike in adulthood, neither kisspeptin nor GnRH signaling is required for elevated T secretion in fetal and neonatal males and that kisspeptin-Kiss1r signaling's involvement in testicular growth and endocrine function becomes apparent sometime around the second postnatal week. Conversely, gonadotropin secretion on the first postnatal day is dependent on kisspeptin and GnRH, at least in females. Thus, gonadal T secretion on PND1 is independent of both gonadotropin secretion and upstream hypothalamic factors (GnRH, kisspeptin), but the pituitary's dependence on upstream hypothalamic GnRH and kisspeptin signaling is already established at birth.
Acknowledgments
We thank Sangeeta Dhamija, Joshua Kim, and Daniel Clark for their support with the various mouse colonies.
This research was supported by National Institutes of Health (NIH) Grants R01 HD065856 and R00 HD056157. M.C.P. received support from The Endocrine Society's 2010 Summer Research Fellowship. Additional support was provided by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/NIH (Specialized Cooperative Centers Program in Reproduction and Infertility Research) Grants U54-HD012303 (University of California, San Diego) and U54 HD-28934 (University of Virginia Ligand Assay and Analysis Core).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AGD
- Anogenital distance
- ARC
- arcuate nucleus
- AVPV/PeN
- anteroventral periventricular and rostral periventricular nuclei
- DIG
- digoxigenin
- E
- embryonic day
- E2
- estradiol
- hpg
- hypogonadal
- ISH
- in situ hybridization
- KO
- knockout
- NKB
- neurokinin B
- Pgr
- progesterone receptor
- PND
- postnatal day
- RNase
- ribonuclease
- SSC
- sodium citrate, sodium chloride
- T
- testosterone
- vl-VMH
- ventrolateral portion of the ventromedial nucleus
- WT
- wild type.
References
- 1. Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. 2003. The GPR54 gene as a regulator of puberty. N Engl J Med 349:1614–1627 [DOI] [PubMed] [Google Scholar]
- 2. de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. 2003. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA 100:10972–10976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kauffman AS. 2010. Coming of age in the kisspeptin era: sex differences, development, and puberty. Mol Cell Endocrinol 324:51–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kauffman AS. 2010. Gonadal and nongonadal regulation of sex differences in hypothalamic Kiss1 neurones. J Neuroendocrinol 22:682–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, Caraty A, Aparicio SA. 2005. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA 102:1761–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oakley AE, Clifton DK, Steiner RA. 2009. Kisspeptin signaling in the brain. Endocr Rev 30:713–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. 2004. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 145:4073–4077 [DOI] [PubMed] [Google Scholar]
- 8. Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. 2004. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology 80:264–272 [DOI] [PubMed] [Google Scholar]
- 9. Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. 2005. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci 25:11349–11356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kauffman AS, Park JH, McPhie-Lalmansingh AA, Gottsch ML, Bodo C, Hohmann JG, Pavlova MN, Rohde AD, Clifton DK, Steiner RA, Rissman EF. 2007. The kisspeptin receptor GPR54 is required for sexual differentiation of the brain and behavior. J Neurosci 27:8826–8835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu X, Lee K, Herbison AE. 2008. Kisspeptin excites gonadotropin-releasing hormone neurons through a phospholipase C/calcium-dependent pathway regulating multiple ion channels. Endocrinology 149:4605–4614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang C, Roepke TA, Kelly MJ, Rønnekleiv OK. 2008. Kisspeptin depolarizes gonadotropin-releasing hormone neurons through activation of TRPC-like cationic channels. J Neurosci 28:4423–4434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dhillo WS, Chaudhri OB, Patterson M, Thompson EL, Murphy KG, Badman MK, McGowan BM, Amber V, Patel S, Ghatei MA, Bloom SR. 2005. Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. J Clin Endocrinol Metab 90:6609–6615 [DOI] [PubMed] [Google Scholar]
- 14. Castellano JM, Navarro VM, Fernández-Fernández R, Roa J, Vigo E, Pineda R, Dieguez C, Aguilar E, Pinilla L, Tena-Sempere M. 2006. Expression of hypothalamic KiSS-1 system and rescue of defective gonadotropic responses by kisspeptin in streptozotocin-induced diabetic male rats. Diabetes 55:2602–2610 [DOI] [PubMed] [Google Scholar]
- 15. Patterson M, Murphy KG, Thompson EL, Patel S, Ghatei MA, Bloom SR. 2006. Administration of kisspeptin-54 into discrete regions of the hypothalamus potently increases plasma luteinising hormone and testosterone in male adult rats. J Neuroendocrinol 18:349–354 [DOI] [PubMed] [Google Scholar]
- 16. Ramaswamy S, Seminara SB, Pohl CR, DiPietro MJ, Crowley WF, Jr, Plant TM. 2007. Effect of continuous intravenous administration of human metastin 45–54 on the neuroendocrine activity of the hypothalamic-pituitary-testicular axis in the adult male rhesus monkey (Macaca mulatta). Endocrinology 148:3364–3370 [DOI] [PubMed] [Google Scholar]
- 17. d'Anglemont de Tassigny X, Fagg LA, Dixon JP, Day K, Leitch HG, Hendrick AG, Zahn D, Franceschini I, Caraty A, Carlton MB, Aparicio SA, Colledge WH. 2007. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci USA 104:10714–10719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. 2005. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 146:3686–3692 [DOI] [PubMed] [Google Scholar]
- 19. Smith JT, Clay CM, Caraty A, Clarke IJ. 2007. KiSS-1 messenger ribonucleic acid expression in the hypothalamus of the ewe is regulated by sex steroids and season. Endocrinology 148:1150–1157 [DOI] [PubMed] [Google Scholar]
- 20. Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, Hoffman GE, Steiner RA, Tena-Sempere M. 2007. Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology 148:1774–1783 [DOI] [PubMed] [Google Scholar]
- 21. Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. 2009. Regulation of GnRH secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci 29:11859–11866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Navarro VM, Castellano JM, McConkey SM, Pineda R, Ruiz-Pino F, Pinilla L, Clifton DK, Tena-Sempere M, Steiner RA. 2011. Interactions between kisspeptin and neurokinin B in the control of GnRH secretion in the female rat. Am J Physiol Endocrinol Metab 300:E202–E210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kauffman AS, Navarro VM, Kim J, Clifton D, Steiner RA. 2009. Sex differences in the regulation of Kiss1/NKB neurons in juvenile mice: implications for the timing of puberty. Am J Physiol Endocrinol Metab 297:E1212–E1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Ozbek MN, Imamoglu S, Akalin NS, Yuksel B, O'Rahilly S, Semple RK. 2009. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat Genet 41:354–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA. 2005. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology 146:2976–2984 [DOI] [PubMed] [Google Scholar]
- 26. Herbison AE. 2008. Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: the case for the rostral periventricular area of the third ventricle (RP3V). Brain Res Rev 57:277–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Khan AR, Kauffman AS. 8 May 2011. The role of kisspeptin and RFRP-3 neurons in the circadian-timed preovulatory luteinizing hormone surge. J Neuroendocrinol 10.1111/j.1365-2826.2011.02162.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Semaan SJ, Murray EK, Poling MC, Dhamija S, Forger NG, Kauffman AS. 2010. BAX-dependent and BAX-independent regulation of Kiss1 neuron development in mice. Endocrinology 151:5807–5817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Desroziers E, Mikkelsen JD, Bentsen AH, Keller M, Caraty A, Duittoz AH, Franceschini I. 2010. Kisspeptins in the developing and adult rat brain. Annual Meeting of the Society for Neuroscience, San Diego, 2010 [Google Scholar]
- 30. Takumi K, Iijima N, Ozawa H. 2011. Developmental changes in the expression of kisspeptin mRNA in rat hypothalamus. J Mol Neurosci 43:138–145 [DOI] [PubMed] [Google Scholar]
- 31. Corbier P, Edwards DA, Roffi J. 1992. The neonatal testosterone surge: a comparative study. Arch Int Physiol Biochim Biophys 100:127–131 [DOI] [PubMed] [Google Scholar]
- 32. Pang SF, Tang F. 1984. Sex differences in the serum concentrations of testosterone in mice and hamsters during their critical periods of neural sexual differentiation. J Endocrinol 100:7–11 [DOI] [PubMed] [Google Scholar]
- 33. Motelica-Heino I, Castanier M, Corbier P, Edwards DA, Roffi J. 1988. Testosterone levels in plasma and testes of neonatal mice. J Steroid Biochem 31:283–286 [DOI] [PubMed] [Google Scholar]
- 34. Baum MJ, Brand T, Ooms M, Vreeburg JT, Slob AK. 1988. Immediate postnatal rise in whole body androgen content in male rats: correlation with increased testicular content and reduced body clearance of testosterone. Biol Reprod 38:980–986 [DOI] [PubMed] [Google Scholar]
- 35. Simerly RB. 2002. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci 25:507–536 [DOI] [PubMed] [Google Scholar]
- 36. Kauffman AS, Bojkowska K, Rissman EF. 2010. Critical periods of susceptibility to short-term energy challenge during pregnancy: impact on fertility and offspring development. Physiol Behav 99:100–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Weisz J, Ward IL. 1980. Plasma testosterone and progesterone titers of pregnant rats, their male and female fetuses, and neonatal offspring. Endocrinology 106:306–316 [DOI] [PubMed] [Google Scholar]
- 38. Scott HM, Mason JI, Sharpe RM. 2009. Steroidogenesis in the fetal testis and its susceptibility to disruption by exogenous compounds. Endocr Rev 30:883–925 [DOI] [PubMed] [Google Scholar]
- 39. Fiorini Z, Jasoni CL. 2010. A novel developmental role for kisspeptin in the growth of gonadotrophin-releasing hormone neurites to the median eminence in the mouse. J Neuroendocrinol 22:1113–1125 [DOI] [PubMed] [Google Scholar]
- 40. Cao J, Patisaul HB. 2011. Sexually dimorphic expression of hypothalamic estrogen receptors α and β and kiss1 in neonatal male and female rats. J Comp Neurol 519:2954–2977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wray S, Nieburgs A, Elkabes S. 1989. Spatiotemporal cell expression of luteinizing hormone-releasing hormone in the prenatal mouse: evidence for an embryonic origin in the olfactory placode. Brain Res Dev Brain Res 46:309–318 [DOI] [PubMed] [Google Scholar]
- 42. Tobet S. 2011. Organization effects of estrogen across the lifespan. 93rd Annual Meeting of The Endocrine Society, Boston, 2011 (Abstract S75–3) [Google Scholar]
- 43. Teles MG, Trarbach EB, Noel SD, Guerra-Junior G, Jorge A, Beneduzzi D, Bianco SD, Mukherjee A, Baptista MT, Costa EM, De Castro M, Mendonça BB, Kaiser UB, Latronico AC. 2010. A novel homozygous splice acceptor site mutation of KISS1R in two siblings with normosmic isolated hypogonadotropic hypogonadism. Eur J Endocrinol 163:29–34 [DOI] [PubMed] [Google Scholar]
- 44. Silveira LG, Tusset C, Latronico AC. 2010. Impact of mutations in kisspeptin and neurokinin B signaling pathways on human reproduction. Brain Res 1364:72–80 [DOI] [PubMed] [Google Scholar]
- 45. Dungan HM, Gottsch ML, Zeng H, Gragerov A, Bergmann JE, Vassilatis DK, Clifton DK, Steiner RA. 2007. The role of kisspeptin-GPR54 signaling in the tonic regulation and surge release of gonadotropin-releasing hormone/luteinizing hormone. J Neurosci 27:12088–12095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cattanach BM, Iddon CA, Charlton HM, Chiappa SA, Fink G. 1977. Gonadotrophin-releasing hormone deficiency in a mutant mouse with hypogonadism. Nature 269:338–340 [DOI] [PubMed] [Google Scholar]
- 47. Robertson JL, Clifton DK, de la Iglesia HO, Steiner RA, Kauffman AS. 2009. Circadian regulation of Kiss1 neurons: implications for timing the preovulatory gonadotropin-releasing hormone/luteinizing hormone surge. Endocrinology 150:3664–3671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Finn PD, Steiner RA, Clifton DK. 1998. Temporal patterns of gonadotropin-releasing hormone (GnRH), c-fos, and galanin gene expression in GnRH neurons relative to the luteinizing hormone surge in the rat. J Neurosci 18:713–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chowen JA, Clifton DK. 1991. Semiquantitative analysis of cellular somatostatin mRNA levels by in situ hybridization histochemistry. Method Neurosci 5:137–158 [Google Scholar]
- 50. Smith JT. 2009. Sex steroid control of hypothalamic Kiss1 expression in sheep and rodents: comparative aspects. Peptides 30:94–102 [DOI] [PubMed] [Google Scholar]
- 51. Plant TM, Ramaswamy S. 2009. Kisspeptin and the regulation of the hypothalamic-pituitary-gonadal axis in the rhesus monkey (Macaca mulatta). Peptides 30:67–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Krishnan S, Intlekofer KA, Aggison LK, Petersen SL. 2009. Central role of TRAF-interacting protein in a new model of brain sexual differentiation. Proc Natl Acad Sci USA 106:16692–16697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ramaswamy S, Seminara SB, Ali B, Ciofi P, Amin NA, Plant TM. 2010. Neurokinin B stimulates GnRH release in the male monkey (Macaca mulatta) and is colocalized with kisspeptin in the arcuate nucleus. Endocrinology 151:4494–4503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Navarro VM, Castellano JM, McConkey SM, Pineda R, Ruiz-Pino F, Pinilla L, Clifton DK, Tena-Sempere M, Steiner RA. 2011. Interactions between kisspeptin and neurokinin B in the control of GnRH secretion in the female rat. Am J Physiol Endocrinol Metab 300:E202–E210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wagner CK, Nakayama AY, De Vries GJ. 1998. Potential role of maternal progesterone in the sexual differentiation of the brain. Endocrinology 139:3658–3661 [DOI] [PubMed] [Google Scholar]
- 56. Wagner CK, Pfau JL, De Vries GJ, Merchenthaler IJ. 2001. Sex differences in progesterone receptor immunoreactivity in neonatal mouse brain depend on estrogen receptor α expression. J Neurobiol 47:176–182 [DOI] [PubMed] [Google Scholar]
- 57. Quadros PS, Goldstein AY, De Vries GJ, Wagner CK. 2002. Regulation of sex differences in progesterone receptor expression in the medial preoptic nucleus of postnatal rats. J Neuroendocrinol 14:761–767 [DOI] [PubMed] [Google Scholar]
- 58. Quadros PS, Pfau JL, Goldstein AY, De Vries GJ, Wagner CK. 2002. Sex differences in progesterone receptor expression: a potential mechanism for estradiol-mediated sexual differentiation. Endocrinology 143:3727–3739 [DOI] [PubMed] [Google Scholar]
- 59. Wu TJ, Segal AZ, Miller GM, Gibson MJ, Silverman AJ. 1992. FOS expression in gonadotropin-releasing hormone neurons: enhancement by steroid treatment and mating. Endocrinology 131:2045–2050 [DOI] [PubMed] [Google Scholar]
- 60. Rajendren G. 2001. Subsets of gonadotropin-releasing hormone (GnRH) neurons are activated during a steroid-induced luteinizing hormone surge and mating in mice: a combined retrograde tracing double immunohistochemical study. Brain Res 918:74–79 [DOI] [PubMed] [Google Scholar]
- 61. Clarkson J, d'Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE. 2008. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci 28:8691–8697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. O'Shaughnessy PJ, Fowler PA. 2011. Endocrinology of the mammalian fetal testis. Reproduction 141:37–46 [DOI] [PubMed] [Google Scholar]
- 63. O'Shaughnessy PJ, Baker P, Sohnius U, Haavisto AM, Charlton HM, Huhtaniemi I. 1998. Fetal development of Leydig cell activity in the mouse is independent of pituitary gonadotroph function. Endocrinology 139:1141–1146 [DOI] [PubMed] [Google Scholar]
- 64. O'Shaughnessy PJ, Fleming LM, Jackson G, Hochgeschwender U, Reed P, Baker PJ. 2003. Adrenocorticotropic hormone directly stimulates testosterone production by the fetal and neonatal mouse testis. Endocrinology 144:3279–3284 [DOI] [PubMed] [Google Scholar]
- 65. Johnston H, King PJ, O'Shaughnessy PJ. 2007. Effects of ACTH and expression of the melanocortin-2 receptor in the neonatal mouse testis. Reproduction 133:1181–1187 [DOI] [PubMed] [Google Scholar]
- 66. O'Shaughnessy PJ, Morris ID, Huhtaniemi I, Baker PJ, Abel MH. 2009. Role of androgen and gonadotrophins in the development and function of the Sertoli cells and Leydig cells: data from mutant and genetically modified mice. Mol Cell Endocrinol 306:2–8 [DOI] [PubMed] [Google Scholar]
- 67. Rhoda J, Corbier P, Roffi J. 1983. Hypothalamic testosterone increase in the male rat at birth. Int J Devl Neuroscience 1:187–190 [DOI] [PubMed] [Google Scholar]