Abstract
Adipose tissue secretes a variety of bioactive signaling molecules, termed adipokines, which regulate numerous biological functions including appetite, energy balance, glucose homeostasis, and inflammation. Chemerin is a novel adipokine that regulates adipocyte differentiation and metabolism by binding to and activating the G protein-coupled receptor, chemokine like receptor-1 (CMKLR1). In the present study, we investigated the impact of CMKLR1 deficiency on adipose development, glucose homeostasis, and inflammation in vivo. Herein we report that regardless of diet (low or high fat), CMKLR1−/− mice had lower food consumption, total body mass, and percent body fat compared with wild-type controls. CMKLR1−/− mice also exhibited decreased hepatic and white adipose tissue TNFα and IL-6 mRNA levels coincident with decreased hepatic dendritic cell infiltration, decreased adipose CD3+ T cells, and increased adipose natural killer cells. CMKLR1−/− mice were glucose intolerant compared with wild-type mice, and this was associated with decreased glucose stimulated insulin secretion as well as decreased skeletal muscle and white adipose tissue glucose uptake. Collectively these data provide compelling evidence that CMKLR1 influences adipose tissue development, inflammation, and glucose homeostasis and may contribute to the metabolic derangement characteristic of obesity and obesity-related diseases.
Obesity is characterized by an excess accumulation of white adipose tissue (WAT) and is associated with a variety of metabolic derangements that increase the risk for developing a number of diseases including hypertension, cardiovascular disease, and type 2 diabetes (1, 2). Despite the clear link between obesity and the development of these prevalent diseases, the mechanisms responsible for this relationship are not fully understood. In addition to serving an important role in energy storage, adipose tissue secretes a number of bioactive signaling molecules known as adipokines (3, 4). Through endocrine actions in various tissues of the body, adipokines affect many physiological processes, including appetite and energy balance, insulin sensitivity, and glucose uptake (4, 5). The synthesis and secretion of many adipokines is dynamic and modifiable, with levels of proinflammatory/prodiabetic adipokines commonly increasing with obesity and levels of antiinflammatory/antidiabetic adipokines decreasing with obesity (6). This imbalance in adipokine secretion is proposed to contribute to metabolic syndrome (7, 8).
Chemerin is a recently discovered secreted protein with a role in adaptive and innate immunity (9–12). The first recognized function of chemerin, acting through the G protein-coupled receptor chemokine-like receptor 1 (CMKLR1), was to promote chemotaxis of dendritic cells and macrophages (9, 11). Subsequently, chemerin and CMKLR1 were shown to be highly expressed in WAT (13, 14). Chemerin expression and secretion increases dramatically with adipogenesis, and loss of chemerin or CMKLR1 in preadipocytes severely impairs adipocyte differentiation in vitro (13, 15, 16). Chemerin may also contribute to adipose tissue inflammation commonly found with obesity by serving as a chemoattractant for various types of immune cells (17). Serum chemerin levels are elevated in obese humans and rodents (14, 18–23), suggesting that chemerin also contributes to the dysregulation of glucose metabolism that often occurs with obesity. However, in vitro studies have provided conflicting results. Chemerin is reported to both augment (24) and reduce (25) insulin-stimulated glucose uptake in 3T3-L1 adipocytes as well as decrease insulin-stimulated glucose uptake in primary human skeletal muscle cells (26). In mice, acute chemerin treatment exacerbated glucose intolerance in obese/diabetic, but not normoglycemic models, by decreasing serum insulin levels as well as reducing adipose tissue and liver glucose uptake (18). Similarly, chronic overexpression of chemerin in mice worsened glucose intolerance in skeletal muscle (27). Thus, the relationship between chemerin, obesity, and energy homeostasis remains unclear. In the present study, we examined the impact of genetic ablation of the chemerin receptor CMKLR1 on adiposity, inflammation, and glucose metabolism in a mouse model of obesity. We report for the first time that loss of chemerin/CMKLR1 in vivo profoundly reduces adipose tissue accumulation, modifies white adipose immune cell infiltration, and is associated with undesirable changes in insulin secretion and tissue glucose uptake.
Materials and Methods
Animals
All protocols and procedures were approved by the Dalhousie University Committee on Laboratory Animals and are in accordance with the Canadian Council on Animal Care guidelines. Whole-body CMKLR1 knockout mice were originally generated by Deltagen and fully backcrossed into the C57BL/6 background (28, 29). RT-PCR was used to confirm the absence of a CMKLR1 transcript (Supplemental Fig. 1). Mice were placed on a low-fat (LF; 10 kcal% fat; D12450B; Research Diets, New Brunswick, NJ) or high-fat (HF; 60 kcal% fat; D12492) diet beginning at 6 wk of age.
Dual-energy x-ray absorptiometry
Mice were anesthetized using isoflurane and whole-body measurements of prostrate mice, excluding the head, were made by dual-energy x-ray absorptiometry (DEXA; Lunar PIXImus2, GE Medical Systems, Milwaukee, WI). The DEXA instrument was calibrated before each use and one person performed all scans.
Blood chemistry
Serum levels of insulin, leptin, adiponectin, chemerin, IL-6, and TNFα were determined using ELISA, as per the manufacturer's instructions (R&D Systems, Minneapolis, MN). Blood glucose levels were measured using a glucometer (Freestyle Freedom; Abbott Laboratories, Abbott Park, IL).
Quantitative real-time PCR
RNA isolation and quantitative real-time PCR was performed as described previously (18). Primer sequences are shown in Supplemental Table 1.
Flow cytometry
Mice were perfused with PBS, and liver and WAT were collected. Tissues were minced using scissors to a homogeneous consistency in 2 ml HEPES buffer and incubated with 1000 U collagenase IV (liver) and 2500 U of collagenase I (WAT), respectively, for 120 min at 37 C in a shaking incubator at 200 rpm. The resulting suspension was passed through a 75-μm mesh filter to remove undigested tissue. The liver filtrate was centrifuged (30 × g, 3 min), and the supernatant was transferred and centrifuged (300 × g, 10 min). The resulting pellet was resuspended in 35% percoll and centrifuged (800 × g, 20 min). The remaining red blood cells were lysed by incubating with red blood cell lysis buffer (140 mm NH4Cl; 20 mm Tris-Cl, pH 7.2) for 3 min, and the resulting cell suspension was centrifuged (800 × g, 5 min). The pellet was resuspended in fluorescence-activated cell sorting (FACS) buffer (2.0% fetal bovine serum and 20 mm sodium azide in PBS). The WAT filtrate was centrifuged (50 × g, 5 min) and the supernatant was transferred and centrifuged (200 × g, 10 min). The resulting pellet was resuspended in red blood cell lysis buffer and incubated for 3 min at room temperature, and the resulting cell suspension was centrifuged (200 × g, 5 min). The pellet was resuspended in FACS buffer. The cells were analyzed by flow cytometry for the expression of Ly-6G, F4/80, and CD11c to identify neutrophils as Ly-6G+ and F4/80−, macrophages as Ly-6G− and F4/80+, and dendritic cells as F4/80−, CD11c+, and CD3, CD19, and NK1.1 to identify B cells as CD3−, CD19+, CD3+ T cells as CD3+, NK1.1−, natural killer T cells as CD3+, NK1.1+, and natural killer cells as CD3−, NK1.1+. Cells were collected on a FACSAria system (BD Biosciences, San Jose, CA) and were analyzed with FCS Express version 3.0 software (DeNovo Software, Los Angeles, CA).
Insulin sensitivity, glucose tolerance, and glucose uptake
Insulin sensitivity and glucose tolerance tests were performed as previously described (18). Glucose tolerance tests were performed on LF and HF wild-type and CMKLR1−/− at wk 6. To measure tissue glucose uptake, mice were weighed before the test and after an 18-h overnight fast were injected intraperitoneally with filter sterilized d-glucose (BDH Inc., Toronto, Canada) at 2 mg/g and 10 μCi of 2-[1,2-3H(N)]-deoxy-d-glucose (2-DOG) (PerkinElmer, Waltham, MA). Blood samples were collected from the saphenous vein at 0, 15, 30, 45, and 60 min after the injection, and glucose concentrations were measured using a glucometer (Freestyle Freedom; Abbott Laboratories). At 60 min, mice were euthanized and liver, skeletal muscle, and WAT were snap frozen in liquid nitrogen. To determine glucose-specific activity, plasma samples from 0, 15, 30, 45, and 60 min were deproteinized using perchloric acid and neutralized with KHCO3. Radioactivity was measured using a scintillation counter, and glucose-specific activity was calculated by determining the area under the curve of sample radioactivity divided by the glucose concentration for the duration of the experiment. To determine tissue accumulation of 2-DOG, 100–500 mg of tissue was homogenized in distilled water, and the homogenate was transferred to perchloric acid. The sample was centrifuged to remove precipitated protein, and the supernatant was neutralized with KHCO3. The precipitate was removed by centrifugation, and the radioactivity in the supernatant was measured in a scintillation counter. To calculate 2-DOG uptake, tissue radioactivity was divided by mass of tissue homogenized and the glucose-specific activity.
Statistics
All data are expressed as mean ± sem. All comparisons were performed using an unpaired t test or a one- or two-way ANOVA unless otherwise stated. Bonferroni's test was used for post hoc analysis of the significant ANOVA.
Results
CMKLR1 deficiency is associated with reduced food consumption, body mass, and adiposity
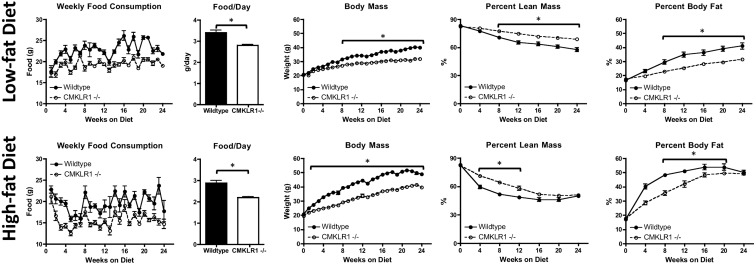
To determine the effect of CMKLR1 loss on food consumption and body weight, wild-type and CMKLR1−/− mice were fed a LF (10 kcal% fat) or HF (60 kcal% fat) diet for 24 wk. Regardless of diet, the food consumption of CMKLR1−/− mice was lower at all measured time points and was approximately 25% less than that of wild-type mice when expressed as daily food consumption (Fig. 1). Although the hypothalamic mRNA levels of the leptin receptor and agouti-related peptide were increased after 24 wk of the HF diet, there was no effect of CMKLR1 loss on either transcript (Supplemental Fig. 2). Hypothalamic neuropeptide Y mRNA levels were similar for LF- and HF-fed mice, regardless of genotype (Supplemental Fig. 2). CMKLR1−/− mice exhibited significantly lower body weights compared with wild-type mice beginning at wk 8 (LF) or wk 3 (HF), and these differences persisted for the remainder of the study (Fig. 1). DEXA analysis revealed a significantly higher percent lean mass for LF-fed CMKLR1−/− mice from wk 8 through wk 24 and for HF-fed mice from wk 4 through wk 12 (Fig. 1). However, the difference in total body mass was largely a consequence of differing fat mass as LF-fed CMKLR1−/− mice exhibited significantly lower percent body fat from wk 8 through wk 24, whereas HF-fed CMKLR1−/− mice had significantly lower percent body fat from wk 4 through wk 16 (Fig. 1).
Fig. 1.
CMKLR1−/− mice have reduced food consumption and a lean phenotype compared with wild-type mice. Weekly and daily food consumption and total body mass of wild-type and CMKLR1−/− mice fed a LF or HF diet for 24 wk are shown. Percent fat and lean mass were measured at wk 0, 4, 8, 12, 16, 20, and 24 using DEXA analysis. Values are expressed as means ± sem. *, P < 0.05, comparing wild-type with CMKLR1−/− mice within the diet (n = 6–10 mice/group).
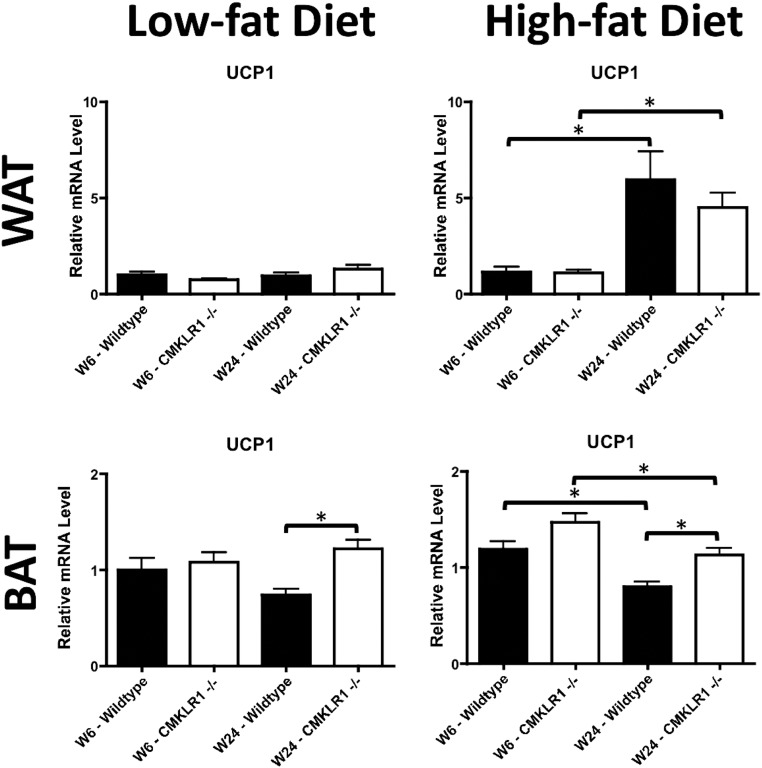
Activity levels and indirect calorimetry were also used as a measure of energy expenditure. Similar activity levels were observed for all experimental groups (Supplemental Fig. 3). However, two-way ANOVA analysis indicated that HF-fed CMKLR1−/− mice had significantly lower oxygen consumption (P = 0.0029), carbon dioxide production (P = 0.03), and energy expenditure (P = 0.0055) as well as a significantly higher respiratory quotient (P < 0.0001) compared with wild-type mice (Supplemental Fig. 3). However, post hoc analysis did not reveal any individual time points as being significantly different. No significant effect of genotype was found in the LF-fed groups using the same statistical analyses, (Supplemental Fig. 3). As a further indicator of energy expenditure, uncoupling protein 1 (UCP1) mRNA levels were measured in WAT and brown adipose tissue (BAT). Although WAT UCP1 mRNA levels were elevated after 24 wk (vs. 6 wk) of the HF diet, there were no significant differences between genotypes (Fig. 2). In BAT, UCP1 mRNA levels were significantly higher for CMKLR1−/− vs. wild-type mice at wk 24 on the LF diet (Fig. 2). Moreover, although BAT UCP1 mRNA levels were decreased with long-term (wk 24 vs. wk 6) feeding of the HF diet to both genotypes, CMKLR1−/− exhibited significantly higher levels of this transcript at both time points.
Fig. 2.
BAT UCP1 mRNA levels are higher in CMKLR1−/− mice. WAT and BAT UCP1 mRNA levels were measured by quantitative real-time PCR at wk 6 and 24 in wild-type and CMKLR1−/− mice fed a LF or HF diet. Values are expressed as means ± sem. *, P < 0.05, comparing wild-type with CMKLR1−/− mice within the diet (n = 6–10 mice/group).
CMKLR1−/− mice have lower blood glucose and serum insulin, leptin, and adiponectin
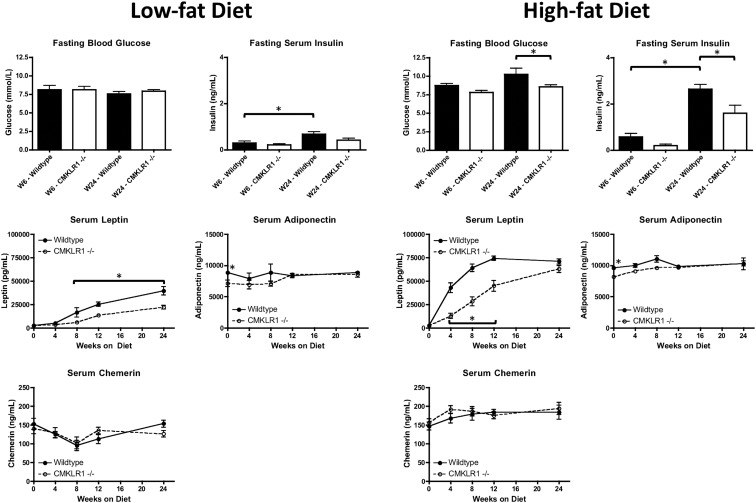
Fasting blood glucose levels were similar for CMKLR1−/− and wild-type mice when fed a LF diet for 6 or 24 wk (Fig. 3). However, when fed a HF diet for 24 wk, fasting blood glucose levels were significantly reduced for CMKLR1−/− vs. wild-type mice (Fig. 3). Moreover, when fed a LF diet, wild-type mice at wk 24 had significantly higher serum insulin levels than wild-type mice at wk 6 (Fig. 3). HF-fed CMKLR1−/− mice at wk 24 had significantly lower serum insulin levels than wild-type mice at wk 24, and HF-fed wild-type mice at wk 24 had significantly higher serum insulin levels than wild-type mice at wk 6 (Fig. 3).
Fig. 3.
CMKLR1 deficiency reduces blood glucose and serum insulin, leptin, and adiponectin levels. Fasting blood glucose and serum insulin levels were measured using a glucometer and ELISA, respectively, at wk 6 and 24 in wild-type and CMKLR1−/− mice fed a LF or HF diet. Serum leptin, adiponectin, and chemerin levels were quantified by ELISA at wk 0, 4, 8, 12, and 24 in wild-type and CMKLR1−/− mice fed a LF or HF diet. Values are expressed as means ± sem. *, P < 0.05, comparing wild-type with CMKLR1−/− mice within the diet (n = 6–10 mice/group).
Serum levels of several adipokines including leptin and adiponectin correlate with adiposity and this is believed to underlie in part some of the undesirable metabolic changes that often coincide with obesity. Serum leptin levels were significantly lower in LF-fed CMKLR1−/− mice from wk 8 through wk 24 and HF-fed CMKLR1−/− mice from wk 4 through wk 12 (Fig. 3). Serum levels of the insulin-sensitizing adipokine adiponectin were significantly lower in CMKLR1−/− mice at wk 0, regardless of diet (Fig. 3). Serum chemerin levels, although generally higher in HF-fed mice, were similar between wild-type and CMKRL1−/− mice, regardless of diet (Fig. 3).
CMKLR1−/− mice have altered proinflammatory cytokine levels and leukocyte distributions in liver and WAT
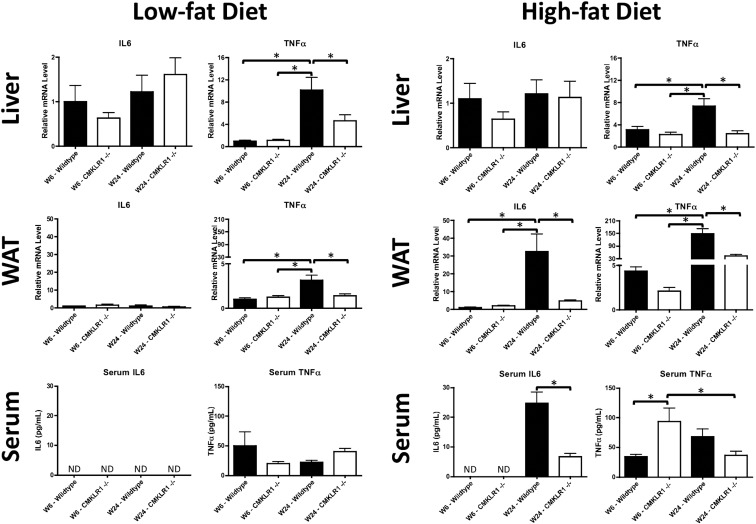
Obesity is commonly associated with hepatic steatosis due to impairment of lipid storage and metabolism in WAT. Wild-type and CMKLR1−/− mice fed a LF diet for 6 wk displayed a similar liver histology; however, at wk 24, CMKLR1−/− mice had visibly less liver steatosis than wild-type mice (Supplemental Figure 4). By comparison, no differences were observed between wild-type and CMKLR1−/− mice fed a HF diet for 6 or 24 wk. Quantitation of the lipid deposits demonstrated that the area fraction in LF-fed CMKLR1−/− mice was significantly lower than wild-type mice at wk 24 (Supplemental Fig. 4). In addition, the area fraction in LF fed wild-type mice was significantly higher at wk 24 than wk 6 (Supplemental Fig. 4). No significant differences were observed in HF-fed mice (Supplemental Fig. 4). Although liver steatosis and obesity are commonly associated with inflammation, hepatic IL-6 mRNA levels were similar between CMKLR1−/− and wild-type mice, regardless of age or diet (Fig. 4). However, within both the LF- and HF-fed groups, wild-type mice at wk 24 had hepatic TNFα mRNA levels significantly greater than CMKLR1−/− mice at wk 24 and wild-type mice at wk 6 (Fig. 4). For WAT, no significant differences of IL-6 mRNA levels were observed within the LF-fed groups (Fig. 4). However, within the HF group, IL-6 mRNA levels were significantly higher for wild-type vs. CMKLR1−/− mice at wk 24 and wild-type mice at wk 6 (Fig. 4). Similar to liver tissue, within both the LF and HF groups, wild-type mice at wk 24 had WAT TNFα mRNA levels that were significantly greater than CMKLR1−/− mice at wk 24 and wild-type mice at wk 6 (Fig. 4). Serum IL-6 levels were undetectable in LF-fed mice at wk 6 or 24 as well as HF-fed mice at wk 6, regardless of genotype (Fig. 4). After 24 wk exposure to the HF diet, CMKLR1−/− mice had significantly lower serum IL-6 levels than wild-type mice (Fig. 4). No significant differences in serum TNFα levels were found within the LF-fed group (Fig. 4). However, within the HF-fed group, CMKLR1−/− mice at wk 6 had significantly higher serum TNFα levels compared with wild-type mice at wk 6 and CMKLR1−/− mice at wk 24 (Fig. 4).
Fig. 4.
IL-6 and TNFα levels are altered in CMKLR1−/− mice. Liver and WAT IL-6 and TNFα mRNA levels were measured by quantitative real-time PCR and serum IL6 and TNFα levels quantified using ELISA at wk 6 and 24 in wild-type and CMKLR1−/− mice fed a LF or HF diet. Values are expressed as mean ± sem. *, P < 0.05, comparing wild-type with CMKLR1−/− mice within the diet (n = 6–10 mice/group).
Given that chemerin is a chemoattractant for subsets of dendritic cells, macrophages, and natural killer (NK) cells, immune cell infiltration in the liver and WAT was assessed using flow cytometry. In the liver, the percentage of dendritic cells was significantly lower in CMKLR1−/− mice compared with wild-type mice within the LF fed group (24 wk; Table 1). In contrast, no significant differences were observed for the hepatic immune cell population of HF-fed mice. In WAT, both LF- and HF-fed CMKLR1−/− mice had a significantly lower percentage of CD3+ T cells and a significantly greater percentage of NK cells compared with wild-type mice (Table 1).
Table 1.
CMKLR1 loss alters immune cell infiltration in liver and white adipose tissue
| Cell type | Low-fat diet |
High-fat diet |
||||
|---|---|---|---|---|---|---|
| Wild type (%) | CMKLR1−/− (%) | P | Wild type (%) | CMKLR1−/− (%) | P | |
| Liver | ||||||
| Neutrophils | 0.34 ± 0.09 | 0.14 ± 0.03 | 0.14 | 1.74 ± 0.90 | 0.06 ± 0.02 | 0.13 |
| Macrophages | 1.76 ± 0.91 | 3.84 ± 1.04 | 0.20 | 6.42 ± 2.83 | 5.59 ± 0.90 | 0.77 |
| Dendritic cells | 3.13 ± 0.19 | 1.70 ± 0.41 | 0.03 | 2.99 ± 0.62 | 2.43 ± 0.45 | 0.48 |
| B cells | 15.95 ± 7.70 | 37.44 ± 10.60 | 0.19 | 14.25 ± 7.37 | 32.69 ± 4.92 | 0.07 |
| CD3+ T cells | 50.52 ± 8.59 | 29.55 ± 6.03 | 0.09 | 34.19 ± 7.94 | 32.90 ± 4.18 | 0.88 |
| NK T cells | 1.75 ± 0.33 | 1.81 ± 0.30 | 0.89 | 2.69 ± 0.73 | 2.43 ± 0.37 | 0.75 |
| NK cells | 7.46 ± 0.49 | 8.12 ± 1.39 | 0.67 | 7.18 ± 1.13 | 7.17 ± 0.67 | 1.00 |
| WAT | ||||||
| Neutrophils | — | — | — | — | — | — |
| Macrophages | 8.21 ± 1.61 | 7.34 ± 0.99 | 0.65 | 10.23 ± 4.99 | 13.15 ± 3.43 | 0.63 |
| Dendritic cells | 1.58 ± 0.50 | 2.33 ± 0.97 | 0.52 | 1.91 ± 0.29 | 2.98 ± 1.59 | 0.58 |
| B cells | 2.71 ± 1.24 | 6.61 ± 3.73 | 0.36 | 12.05 ± 1.54 | 7.79 ± 1.47 | 0.12 |
| CD3+ T cells | 54.62 ± 6.87 | 30.98 ± 6.59 | 0.05 | 59.38 ± 6.14 | 40.68 ± 3.51 | 0.04 |
| NK T cells | 1.32 ± 0.55 | 1.80 ± 0.64 | 0.59 | 2.03 ± 0.17 | 4.33 ± 0.79 | 0.07 |
| NK cells | 6.61 ± 1.73 | 15.08 ± 1.37 | 0.01 | 5.33 ± 0.50 | 14.52 ± 2.04 | 0.02 |
Flow cytometry analysis of liver and WAT from wild-type and CMKLR1−/− mice fed a LF or HF diet for 24 wk. Values are expressed as mean ± sem.
P < 0.05, comparing wild-type with CMKLR1−/− mice within the diet (n = 3–5).
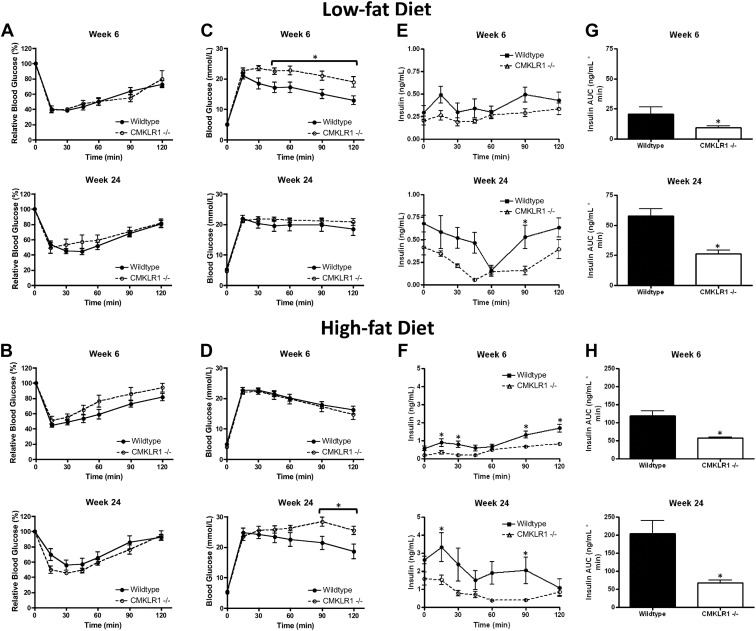
Reduced glucose tolerance and insulin secretion in CMKLR1−/− mice
To investigate the impact of CMKLR1 gene deletion on glucose homeostasis, insulin sensitivity and glucose tolerance tests (GTT) were performed with wild-type and CMKLR1−/− mice fed a LF or HF diet. A single bolus of insulin produced a similar decline in blood glucose levels for both wild-type and CMKLR1−/− mice at 6- and 24-wk time points, regardless of diet, indicating that CMKLR1 deletion did not impact insulin sensitivity (Fig. 5, A and B). However, CMKLR1−/− mice were significantly more glucose intolerant than wild-type mice at wk 6 when fed a LF diet (Fig. 5C) and at wk 24 when fed a HF diet (Fig. 5D). In LF-fed mice, a significant difference in serum insulin levels during the GTT was found between wild-type and CMKLR1−/− mice only at the 90-min sample point for animals maintained on the diet for 24 wk (Fig. 5E). However, area under the curve (AUC) analysis of these data indicated that the total exposure to insulin was significantly lower in CMKLR1−/− mice compared with wild-type mice after 6 or 24 wk on the LF diet. In HF fed mice, wk 6 and wk 24 GTT serum insulin levels were significantly lower in CMKLR1−/− mice at the 15-, 30-, 90-, and 120-min and the 15- and 90-min sample points, respectively (Fig. 5F). Consistent with the LF group, the insulin AUC was significantly lower in HF-fed CMKLR1−/− mice compared with wild-type mice after 6 and 24 wk on the HF diet.
Fig. 5.
CMKLR1 loss is associated with glucose intolerance and decreased glucose-stimulated insulin secretion. Insulin sensitivity tests (A and B), GTT (C and D), serum insulin levels during GTT (E and F), and AUC analysis of serum insulin levels (G and H) from LF- or HF-fed wild-type and CMKLR1−/− mice at wk 6 and 24. Values are expressed as mean ± sem. *, P < 0.05, comparing wild-type with CMKLR1−/− mice within the diet (n = 5–10 mice/group).
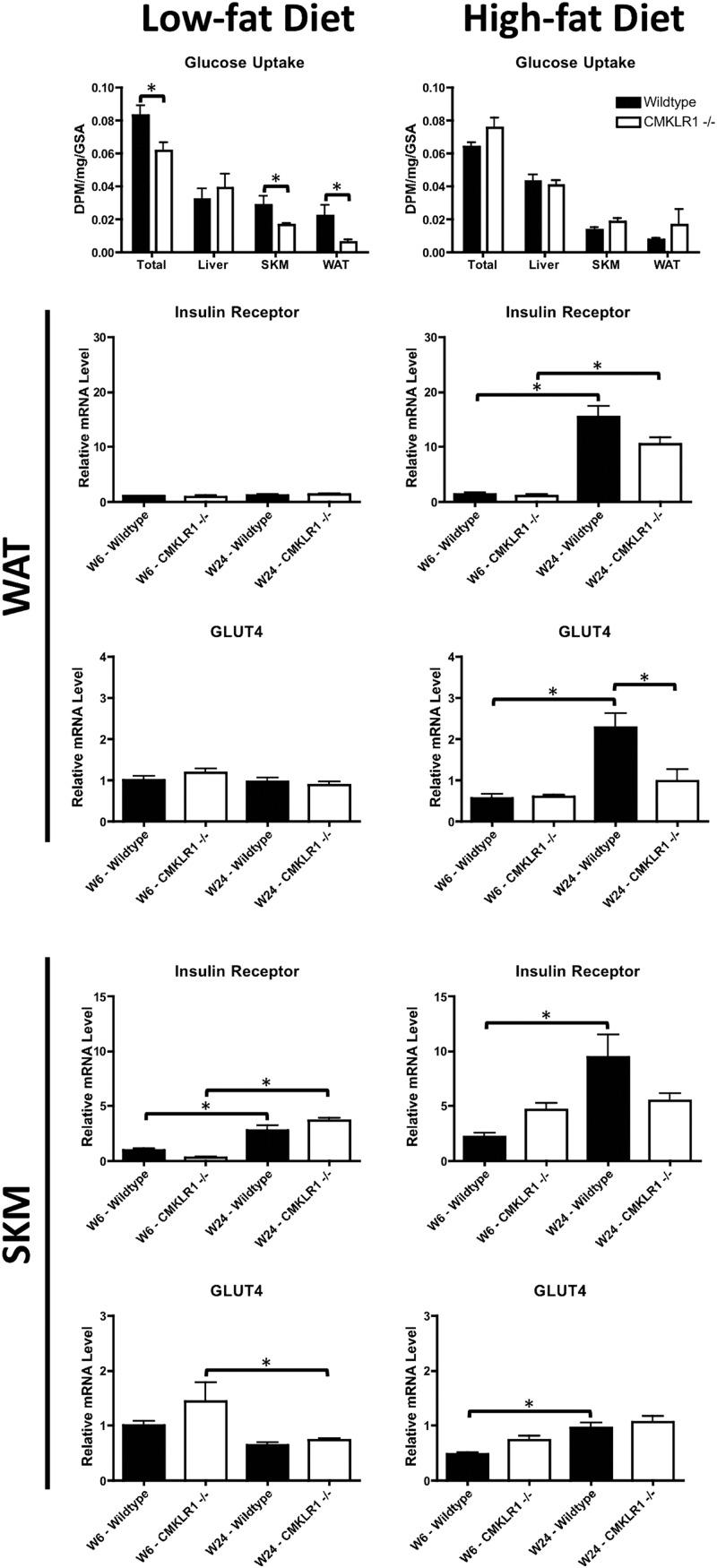
To determine whether the reduced glucose tolerance in CMKLR1−/− mice reflected a decrease in tissue glucose uptake, we performed in vivo tissue glucose uptake experiments. Consistent with GTT results, CMKLR1 null mice fed the LF diet for 6 wk had significantly lower total tissue, skeletal muscle, and WAT glucose uptake compared with wild-type controls (Fig. 6). Also, consistent with the GTT results, although there was a general trend toward reduced tissue glucose uptake in HF vs. LF mice overall, there was no significant difference between the genotypes after 6 wk on the HF diet (Fig. 6). The mRNA levels of the insulin receptor and glucose transporter 4 (GLUT4) were measured in WAT and skeletal muscle (SKM) to determine whether differences in glucose uptake were due to differences in expression of these genes. In LF-fed mice, no significant differences in WAT insulin receptor or GLUT4 mRNA levels were observed with respect to genotype or time on diet (Fig. 6). However, WAT insulin receptor mRNA levels were significantly elevated with increasing time (wk 6 vs. wk 24) on the HF diet for both genotypes. Although WAT GLUT4 mRNA levels were similarly elevated in wild-type mice with increased time on the HF diet, a similar effect was not apparent for CMKLR1−/− mice. In SKM, insulin receptor mRNA levels were significantly elevated with increasing time on the LF diet for both genotypes (Fig. 6). In contrast, CMKLR1−/−, but not wild-type mice, exhibited decreased GLUT4 mRNA levels with increasing time on the LF diet. Moreover, although wild-type mice exhibited a significant time-dependent increase of insulin receptor and GLUT4 mRNA levels with the HF diet, a similar effect was not seen for CMKLR−/− mice.
Fig. 6.
CMKLR1 loss decreases in vivo tissue glucose uptake and alters WAT and SKM insulin receptor and GLUT4 mRNA levels. Total, liver, SKM, and WAT glucose uptake were measured in wild-type and CMKLR1−/− mice. Mice were injected ip with glucose and 2-[1,2-3H(N)]deoxy-d-glucose. Blood samples were collected over a 1-h period. WAT and SKM insulin receptor and GLUT4 mRNA levels were measured by quantitative real-time PCR at wk 6 and 24 in wild-type and CMKLR1−/− mice fed a LF or HF diet. Values are expressed as mean ± sem. *, P < 0.05, comparing wild-type with CMKLR1−/− mice within the diet (n = 5–10 mice/group).
Discussion
Herein we report for the first time that genetic ablation of CMKLR1, a receptor for the adipokine chemerin, is associated with reduced food consumption, weight gain, and adiposity in mice. Consistent with a leaner phenotype, CMKLR1−/− mice have lower fasting blood glucose and serum insulin levels. CMKLR1 loss also protected against hepatic steatosis and resulted in a decrease in the mRNA levels of the proinflammatory cytokines TNFα and IL-6 in WAT. However, our novel findings have also revealed a complex phenotype whereby the apparently beneficial effects of CMKLR1 deficiency in vivo are balanced by potentially deleterious changes in glucose tolerance associated with decreased glucose-stimulated insulin secretion and tissue glucose uptake.
Previous studies have shown that loss of CMKLR1 expression/function impairs adipogenesis in cell culture models (13, 15). Consistent with these findings, CMKLR1 loss in vivo resulted in a lower total body mass, primarily due to significantly lower fat mass. CMKLR1−/− mice had markedly lower food consumption compared with wild-type controls. This unanticipated finding suggests that the loss of CMKLR1 affects other regulators of appetite or that chemerin is an orexigenic adipokine acting through CMKLR1. Although the mechanism(s) remain unclear, our data provide empirical evidence that changes in hypothalamic leptin receptor, agouti-related peptide, or neuropeptide Y expression are not involved. Activity levels were similar in wild-type and CMKLR1−/− mice, indicating that energy expenditure through locomotor activity was not a factor contributing to the decreased body mass. Although the indirect calorimetry indicated a subtle alteration of metabolic phenotype for HF-fed CMKLR1−/− mice, interpretation of these data are confounded by the differences in food consumption because the reduced energy expenditure could simply reflect a reduction in energy intake. UCP1 generates heat by uncoupling oxidative phosphorylation and mice lacking this protein are more susceptible to diet-induced obesity (30). Therefore, the increased UCP1 mRNA levels found in CMKLR1−/− BAT could contribute to the differences in body weight between wild-type and CMKLR1−/− mice. However, at present we can only conclude that the reduced body weight and adiposity associated with CMKLR1 loss primarily derives from differences in feeding and possibly to some extent from reduced adipogenesis. Further studies are clearly required to elucidate the specific role of chemerin/CMKLR1 signaling in appetite regulation.
Obesity-associated imbalances in adipokine secretion are believed to contribute to the risk for development of various deleterious metabolic changes characteristic of metabolic syndrome (7, 8). We found significantly lower serum leptin levels in both LF- and HF-fed CMKLR1−/− mice, a finding consistent with the well-established positive correlation of leptin levels with fat mass (31). However, leptin is also an adipocyte-derived anorexigenic factor (32) and despite having lower serum leptin levels throughout the study, food consumption was consistently lower in CMKLR1−/− mice. This reiterates the need for further studies to examine the role of CMKLR1 in appetite regulation and any mechanistic interactions between chemerin and leptin signaling. Although the serum levels of adiponectin were significantly lower in CMKLR1−/− mice at baseline, the lack of persistent difference throughout the remainder of the study indicates that alterations in the secretion of this insulin-sensitizing adipokine did not contribute substantially to the metabolic phenotype of the knockout mice.
In addition to the growing body of evidence linking chemerin/CMKLR1 signaling to adiposity and energy metabolism, this signaling pathway also has physiological relevance to immune function. CMKLR1 is expressed by a number of immune cells, including plasmacytoid dendritic cells, myeloid dendritic cells, macrophages, and NK cells (11, 33). After activation, these cells synthesize and secrete a number of proinflammatory cytokines, including IL-6 and TNFα. Consistent with the reduced hepatic steatosis, TNFα mRNA levels were significantly lower in the livers of CMKLR1−/− mice. It is well established that obesity is associated with chronic low-grade inflammation (34). Interestingly, WAT IL-6 and TNFα mRNA levels were significantly lower in CMKLR1−/− mice. Consistent with WAT mRNA, serum IL-6 levels were significantly lower in CMKLR1−/− mice; however, TNFα levels were not reflective of the mRNA levels of liver or WAT. Serum TNFα concentrations were similar in LF-fed mice but were significantly higher in wk 6 HF-fed CMKLR1−/− mice, suggesting that tissues other than liver and WAT are responsible for the observed increase in TNFα levels. Flow cytometry of liver leukocytes from CMKLR1−/− mice fed a LF diet for 24 wk showed a decrease in dendritic cell infiltration compared with wild-type mice, an effect not observed in HF-fed counterparts. Liver dendritic cells can contribute to hepatic inflammation by activating NK cells and T cells (35). Further analysis of WAT from LF- and HF-fed mice showed significantly less CD3+ T cells in CMKLR1−/− compared with wild-type and a significant increase in NK cells in CMKLR1−/− mice. WAT infiltration by NK cells is elevated in mice lacking mature T or B cells when fed a HF diet (36). Given the reduction in WAT CD3+ T cells in CMKLR1 KO mice, the increase in NK cells may reflect a similar finding. Taken together, our data provide novel evidence implicating CMKLR1 in the recruitment of various immune cells to liver and WAT.
A growing body of human clinical data indicates that serum chemerin levels are elevated with obesity and correlate with many facets of metabolic syndrome, including insulin resistance and type 2 diabetes (14, 19, 20). In the present study, we have shown that CMKLR1 loss results in decreased glucose-stimulated insulin secretion and decreased insulin-stimulated glucose uptake in skeletal muscle and WAT. Analysis of WAT and skeletal muscle insulin receptor or GLUT4 expression revealed no substantial effect of CMKLR1 gene deletion at 6 wk, regardless of diet. Thus, changes in these genes did not contribute to the differences in glucose uptake or glucose tolerance observed at that time point. We cannot rule out that changes in the expression and/or activity of these proteins contributed to the reduction of glucose tolerance exhibited for CMKLR1−/− mice after 24 wk on the HF diet. However, given that differences in insulin sensitivity were not observed, this appears unlikely. Interestingly, although clear differences in glucose tolerance were observed wild-type and CMKLR1−/− mice fed the LF diet for 6 wk, these differences were not apparent after 24 wk. It appears that as the mice age (wk 24 of study = 30 wk of age), a degree of glucose intolerance begins to develop in the wild-type mice that may mask the impact of CMKLR1−/− loss. In essence, we interpret these findings to indicate that CMKLR1−/− mice exhibit an early onset of this glucose tolerance that develops later in LF-fed wild-type mice. Thus, the lack of difference for glucose tolerance in LF-fed mice at 24 wk does not reflect an improvement of the metabolic phenotype of the CMKLR1−/− mice but rather a worsening of the wild-type mice with time.
Previously, we have reported that recombinant chemerin administration exacerbates glucose intolerance in obese/diabetic mice (18), and others have reported independently that chronic overexpression of chemerin causes glucose intolerance by inducing skeletal muscle insulin resistance in mice (27). Reconciliation of the present results with these previous findings is difficult, given fundamental differences in the experimental models and procedures. For example, recombinant chemerin administration exacerbated glucose tolerance in severely obese/diabetic mice (ob/ob and db/db) that lacked leptin function (18). In contrast, chemerin was without effect in lean, normoglycemic mice. In addition to lacking leptin function, ob/ob and db/db mice exhibited profoundly reduced expression of G protein-coupled receptor-1, another receptor for chemerin (37) that is highly expressed in the skeletal muscle and WAT of lean mice (18). Although the biological function of this receptor in mammals is not well characterized, the closest homolog in yeast has been implicated in nutrient metabolism (38). Thus, the confounds of fundamental differences in leptin signaling and the likelihood that both CMKLR1 and G protein-coupled receptor-1 mediate the biological effects chemerin in models other than the CMKLR1−/− mouse render direct comparisons impossible. Nonetheless, our data provide important new information and together with these other studies will aid in the further elucidation of chemerin/CMKLR1 function at the physiological level.
The reduction in glucose evoked insulin secretion reported in the present study suggests a direct or indirect impact of chemerin/CMKLR1 signaling on pancreatic insulin release. Indeed, CMKLR1 activation is reported to stimulate ERK phosphorylation and calcium influx (9, 13), both of which are known to promote the exocytosis of insulin-containing vesicles (39–41). However, experiments with murine MIN6 pancreatic β-cells (42) failed to demonstrate an effect of CMKLR1 knockdown or exogenous chemerin application on glucose evoked insulin release (data not shown). Thus, although mechanism(s) underlying the influence of chemerin/CMKLR1 signaling on glucose stimulated insulin release remains unknown, at present an indirect impact appears more likely. Interestingly, despite being glucose intolerant relative to wild-type mice, CMKLR1−/− mice remain lean compared with wild-type mice, exhibit lower fasting blood glucose and serum insulin levels and generally exhibit lower circulating concentrations and tissue expression levels of proinflammatory cytokines. Similarly, individuals exist who are not obese on the basis of height and weight but are predisposed to glucose intolerance and type 2 diabetes. These individuals are classified as metabolically obese normal weight (43). Therefore, the lean phenotype in conjunction with glucose intolerance suggests that CMKLR1−/− mice may display a similar metabolically obese normal-weight phenotype.
In summary, we provide evidence for a complex phenotype associated with CMKLR1 loss that is characterized by lower food consumption and reduced body mass and adiposity, coincident with worsened glucose tolerance as well as decreased glucose stimulated insulin secretion and tissue glucose uptake. Moreover, our data also demonstrate that CMKLR1 influences the expression of proinflammatory cytokines and composition of leukocytes present in the liver and WAT. Thus, characterization of the function of CMKLR1 in satiety, weight gain, and glucose tolerance has the potential to lead to novel therapeutic approaches for the treatment of obesity and type 2 diabetes.
Supplementary Material
Acknowledgments
We are grateful to Dr. Jean Marshall (Dalhousie University) for her invaluable assistance with the flow cytometry analysis.
This work was supported by funding from the Canadian Institutes of Health Research, Nova Scotia Health Research Foundation, and the National Institutes of Health. B.A.Z. is supported by National Institutes of Health Grant AI-079320. L.A.Z. was supported by National Institutes of Health Predoctoral Fellowship AI-073198. E.C.B. is supported by grants from the National Institutes of Health and a Merit Award from the Department of Veterans Affairs.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AUC
- Area under the curve
- BAT
- brown adipose tissue
- CMKLR1
- chemokine-like receptor 1
- DEXA
- dual-energy x-ray absorptiometry
- 2-DOG
- 2-[1,2-3H(N)]-deoxy-d-glucose
- FACS
- fluorescence-activated cell sorting
- GLUT4
- glucose transporter 4
- GTT
- glucose tolerance test
- HF
- high fat
- LF
- low fat
- NK
- natural killer
- SKM
- skeletal muscle
- UCP1
- uncoupling protein 1
- WAT
- white adipose tissue.
References
- 1. Hossain P, Kawar B, El Nahas M. 2007. Obesity and diabetes in the developing world—a growing challenge. N Engl J Med 356:213–215 [DOI] [PubMed] [Google Scholar]
- 2. Muoio DM, Newgard CB. 2006. Obesity-related derangements in metabolic regulation. Annu Rev Biochem 75:367–401 [DOI] [PubMed] [Google Scholar]
- 3. Frühbeck G, Gómez-Ambrosi J, Muruzábal FJ, Burrell MA. 2001. The adipocyte: a model for integration of endocrine and metabolic signaling in energy metabolism regulation. Am J Physiol Endocrinol Metab 280:E827–E847 [DOI] [PubMed] [Google Scholar]
- 4. Goralski KB, Sinal CJ. 2007. Type 2 diabetes and cardiovascular disease: getting to the fat of the matter. Can J Physiol Pharmacol 85:113–132 [DOI] [PubMed] [Google Scholar]
- 5. Havel PJ. 2004. Update on adipocyte hormones: regulation of energy balance and carbohydrate/lipid metabolism. Diabetes 53(Suppl 1):S143–S151 [DOI] [PubMed] [Google Scholar]
- 6. Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW. 2004. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology 145:2273–2282 [DOI] [PubMed] [Google Scholar]
- 7. Gade W, Schmit J, Collins M, Gade J. 2010. Beyond obesity: the diagnosis and pathophysiology of metabolic syndrome. Clin Lab Sci 23:51–61; quiz 62–65 [PubMed] [Google Scholar]
- 8. Maury E, Brichard SM. 2010. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol Cell Endocrinol 314:1–16 [DOI] [PubMed] [Google Scholar]
- 9. Wittamer V, Franssen JD, Vulcano M, Mirjolet JF, Le Poul E, Migeotte I, Brézillon S, Tyldesley R, Blanpain C, Detheux M, Mantovani A, Sozzani S, Vassart G, Parmentier M, Communi D. 2003. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J Exp Med 198:977–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wittamer V, Bondue B, Guillabert A, Vassart G, Parmentier M, Communi D. 2005. Neutrophil-mediated maturation of chemerin: a link between innate and adaptive immunity. J Immunol 175:487–493 [DOI] [PubMed] [Google Scholar]
- 11. Zabel BA, Silverio AM, Butcher EC. 2005. Chemokine-like receptor 1 expression and chemerin-directed chemotaxis distinguish plasmacytoid from myeloid dendritic cells in human blood. J Immunol 174:244–251 [DOI] [PubMed] [Google Scholar]
- 12. Vermi W, Riboldi E, Wittamer V, Gentili F, Luini W, Marrelli S, Vecchi A, Franssen JD, Communi D, Massardi L, Sironi M, Mantovani A, Parmentier M, Facchetti F, Sozzani S. 2005. Role of ChemR23 in directing the migration of myeloid and plasmacytoid dendritic cells to lymphoid organs and inflamed skin. J Exp Med 201:509–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goralski KB, McCarthy TC, Hanniman EA, Zabel BA, Butcher EC, Parlee SD, Muruganandan S, Sinal CJ. 2007. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J Biol Chem 282:28175–28188 [DOI] [PubMed] [Google Scholar]
- 14. Bozaoglu K, Bolton K, McMillan J, Zimmet P, Jowett J, Collier G, Walder K, Segal D. 2007. Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology 148:4687–4694 [DOI] [PubMed] [Google Scholar]
- 15. Muruganandan S, Roman AA, Sinal CJ. 2010. Role of chemerin/CMKLR1 signaling in adipogenesis and osteoblastogenesis of bone marrow stem cells. J Bone Miner Res 25:222–234 [DOI] [PubMed] [Google Scholar]
- 16. Muruganandan S, Parlee SD, Rourke JL, Ernst MC, Goralski KB, Sinal CJ. 2011. Chemerin, a novel peroxisome proliferator-activated receptor γ (PPARγ) target gene that promotes mesenchymal stem cell adipogenesis. J Biol Chem 286:23982–23995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ernst MC, Sinal CJ. 2010. Chemerin: at the crossroads of inflammation and obesity. Trends Endocrinol Metab 21:660–667 [DOI] [PubMed] [Google Scholar]
- 18. Ernst MC, Issa M, Goralski KB, Sinal CJ. 2010. Chemerin exacerbates glucose intolerance in mouse models of obesity and diabetes. Endocrinology 151:1998–2007 [DOI] [PubMed] [Google Scholar]
- 19. Weigert J, Neumeier M, Wanninger J, Filarsky M, Bauer S, Wiest R, Farkas S, Scherer MN, Schäffler A, Aslanidis C, Schölmerich J, Buechler C. 2010. Systemic chemerin is related to inflammation rather than obesity in type 2 diabetes. Clin Endocrinol 72:342–348 [DOI] [PubMed] [Google Scholar]
- 20. Lehrke M, Becker A, Greif M, Stark R, Laubender RP, von Ziegler F, Lebherz C, Tittus J, Reiser M, Becker C, Göke B, Leber AW, Parhofer KG, Broedl UC. 2009. Chemerin is associated with markers of inflammation and components of the metabolic syndrome but does not predict coronary atherosclerosis. Eur J Endocrinol 161:339–344 [DOI] [PubMed] [Google Scholar]
- 21. Yang M, Yang G, Dong J, Liu Y, Zong H, Liu H, Boden G, Li L. 2010. Elevated plasma levels of chemerin in newly diagnosed type 2 diabetes mellitus with hypertension. J Invest Med 58:883–886 [DOI] [PubMed] [Google Scholar]
- 22. Pfau D, Stepan H, Kratzsch J, Verlohren M, Verlohren HJ, Drynda K, Lössner U, Blüher M, Stumvoll M, Fasshauer M. 2010. Circulating levels of the adipokine chemerin in gestational diabetes mellitus. Horm Res Paediatr 74:56–61 [DOI] [PubMed] [Google Scholar]
- 23. Sell H, Divoux A, Poitou C, Basdevant A, Bouillot JL, Bedossa P, Tordjman J, Eckel J, Clément K. 2010. Chemerin correlates with markers for fatty liver in morbidly obese patients and strongly decreases after weight loss induced by bariatric surgery. J Clin Endocrinol Metab 95:2892–2896 [DOI] [PubMed] [Google Scholar]
- 24. Takahashi M, Takahashi Y, Takahashi K, Zolotaryov FN, Hong KS, Kitazawa R, Iida K, Okimura Y, Kaji H, Kitazawa S, Kasuga M, Chihara K. 2008. Chemerin enhances insulin signaling and potentiates insulin-stimulated glucose uptake in 3T3-L1 adipocytes. FEBS Lett 582:573–578 [DOI] [PubMed] [Google Scholar]
- 25. Roh SG, Song SH, Choi KC, Katoh K, Wittamer V, Parmentier M, Sasaki S. 2007. Chemerin—a new adipokine that modulates adipogenesis via its own receptor. Biochem Biophys Res Commun 362:1013–1018 [DOI] [PubMed] [Google Scholar]
- 26. Sell H, Laurencikiene J, Taube A, Eckardt K, Cramer A, Horrighs A, Arner P, Eckel J. 2009. Chemerin is a novel adipocyte-derived factor inducing insulin resistance in primary human skeletal muscle cells. Diabetes 58:2731–2740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Becker M, Rabe K, Lebherz C, Zugwurst J, Göke B, Parhofer KG, Lehrke M, Broedl UC. 2010. Expression of human chemerin induces insulin resistance in the skeletal muscle but does not affect weight, lipid levels, and atherosclerosis in LDL receptor knockout mice on high-fat diet. Diabetes 59:2898–2903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Graham KL, Zabel BA, Loghavi S, Zuniga LA, Ho PP, Sobel RA, Butcher EC. 2009. Chemokine-like receptor-1 expression by central nervous system-infiltrating leukocytes and involvement in a model of autoimmune demyelinating disease. J Immunol 183:6717–6723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Luangsay S, Wittamer V, Bondue B, De Henau O, Rouger L, Brait M, Franssen JD, de Nadai P, Huaux F, Parmentier M. 2009. Mouse ChemR23 is expressed in dendritic cell subsets and macrophages, and mediates an anti-inflammatory activity of chemerin in a lung disease model. J Immunol 183:6489–6499 [DOI] [PubMed] [Google Scholar]
- 30. Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. 2009. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab 9:203–209 [DOI] [PubMed] [Google Scholar]
- 31. Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, et al. 1996. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med 334:292–295 [DOI] [PubMed] [Google Scholar]
- 32. Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. 1995. Weight-reducing effects of the plasma protein encoded by the obese gene. Science 269:543–546 [DOI] [PubMed] [Google Scholar]
- 33. Zabel BA, Ohyama T, Zuniga L, Kim JY, Johnston B, Allen SJ, Guido DG, Handel TM, Butcher EC. 2006. Chemokine-like receptor 1 expression by macrophages in vivo: regulation by TGF-β and TLR ligands. Exp Hematol 34:1106–1114 [DOI] [PubMed] [Google Scholar]
- 34. Cottam DR, Mattar SG, Barinas-Mitchell E, Eid G, Kuller L, Kelley DE, Schauer PR. 2004. The chronic inflammatory hypothesis for the morbidity associated with morbid obesity: implications and effects of weight loss. Obes Surg 14:589–600 [DOI] [PubMed] [Google Scholar]
- 35. Connolly MK, Bedrosian AS, Mallen-St Clair J, Mitchell AP, Ibrahim J, Stroud A, Pachter HL, Bar-Sagi D, Frey AB, Miller G. 2009. In liver fibrosis, dendritic cells govern hepatic inflammation in mice via TNF-α. J Clin Invest 119:3213–3225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Duffaut C, Galitzky J, Lafontan M, Bouloumi é A. 2009. Unexpected trafficking of immune cells within the adipose tissue during the onset of obesity. Biochem Biophys Res Commun 384:482–485 [DOI] [PubMed] [Google Scholar]
- 37. Barnea G, Strapps W, Herrada G, Berman Y, Ong J, Kloss B, Axel R, Lee KJ. 2008. The genetic design of signaling cascades to record receptor activation. Proc Natl Acad Sci USA 105:64–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Van Dijck P. 2009. Nutrient sensing G protein-coupled receptors: interesting targets for antifungals? Med Mycol 47:671–680 [DOI] [PubMed] [Google Scholar]
- 39. Maechler P, Carobbio S, Rubi B. 2006. In β-cells, mitochondria integrate and generate metabolic signals controlling insulin secretion. Int J Biochem Cell Biol 38:696–709 [DOI] [PubMed] [Google Scholar]
- 40. Fridlyand LE, Tamarina N, Philipson LH. 2003. Modeling of Ca2+ flux in pancreatic β-cells: role of the plasma membrane and intracellular stores. Am J Physiol Endocrinol Metab 285:E138–E154 [DOI] [PubMed] [Google Scholar]
- 41. Lin YF, Chai Y. 2008. Functional modulation of the ATP-sensitive potassium channel by extracellular signal-regulated kinase-mediated phosphorylation. Neuroscience 152:371–380 [DOI] [PubMed] [Google Scholar]
- 42. Ishihara H, Asano T, Tsukuda K, Katagiri H, Inukai K, Anai M, Kikuchi M, Yazaki Y, Miyazaki JI, Oka Y. 1993. Pancreatic β cell line MIN6 exhibits characteristics of glucose metabolism and glucose-stimulated insulin secretion similar to those of normal islets. Diabetologia 36:1139–1145 [DOI] [PubMed] [Google Scholar]
- 43. Ruderman N, Chisholm D, Pi-Sunyer X, Schneider S. 1998. The metabolically obese, normal-weight individual revisited. Diabetes 47:699–713 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.