Abstract
The master circadian clock located within the hypothalamic suprachiasmatic nucleus (SCN) is necessary for the circadian rhythm of glucocorticoid (GC) release. The pathways by which the SCN sustains rhythmic GC release remain unclear. We studied the circadian regulation of cortisol release in the behaviorally split golden hamster, in which the single bout of circadian locomotor activity splits into two bouts approximately12 h apart after exposing the animals to constant light conditions. We show that unsplit control hamsters present a single peak of cortisol release that is concomitant with a single peak of ACTH release. In contrast, split hamsters show two peaks of cortisol release that are approximately12 h appart and are appropriately phased to each locomotor activity bout but surprisingly do not rely on rhythmic release of ACTH. Our results are consistent with a model in which the circadian pacemaker within the SCN regulates the circadian release of GC via input to the hypothalamo-pituitary-adrenal axis and via a second regulatory pathway, which likely involves sympathetic innervation of the adrenal and can operate even in the absence of ACTH circadian rhythmic release. Furthermore, we show that although the overall 24-h cortisol output in split hamsters is lower than in unsplit controls, split hamsters release constant low levels of ACTH. This result suggests that the timing, rather than the absolute amount, of cortisol release is more critical for the induction of negative feedback effects that regulate the hypothalamo-pituitary-adrenal axis.
Virtually all organisms have circadian clocks (or pacemakers) functioning as endogenous timekeeping mechanisms that drive daily biological rhythms. Under natural conditions, circadian (∼24 h) pacemakers entrain to 24-h exogenous cycles (such as the light-dark cycle), harmonizing daily physiological and behavioral processes with the external environment. In mammals, the master circadian clock within the hypothalamic suprachiasmatic nucleus (SCN) coordinates the precise timing of daily biological rhythms through neural and humoral outputs to other brain regions and extra-SCN circadian clocks (1).
One critical process regulated by the SCN is the circadian release of glucocorticoids (GC) from the adrenal cortex (2). Circadian GC release exhibits a peak near the onset of locomotor activity, preparing the organism for the increased energetic demands of wake relative to sleep.
When golden hamsters (Mesocricetus auratus) are housed under constant light conditions (LL), the circadian locomotor activity pattern of approximately 60% of the animals will split into two bouts of locomotor activity approximately 12 h apart. Each of these peaks presumably represents the independent outputs of the asymmetrically active bilaterally paired left and right SCN (3). This model has been used to delineate pathways underlying the circadian regulation of the preovulatory LH surge (4, 5). To our knowledge, however, no studies have used the split hamster model to elucidate regulatory pathways by which the circadian system controls GC release.
The first potential regulatory branch controlling circadian GC release is via the hypothalamic-pituitary-adrenal (HPA) axis. In this branch, SCN efferents directly (6) and indirectly (7) regulate CRH-containing cells in the paraventricular nucleus. These neurosecretory cells subsequently release CRH, which induces the release of ACTH from the anterior pituitary gland, which in turn, triggers the release of GC from the adrenal cortex.
Recent evidence suggests there is potentially another regulatory branch governing circadian GC release (8). In the mouse, light induces the release of corticosterone without a corresponding rise of plasma ACTH. This light-induced corticosterone release is dependent on an intact SCN and innervation of the adrenal by the thoracic splanchnic nerve (9). In line with these results, tract tracing studies in the rat demonstrate a putative multisynaptic neural pathway between the SCN and the adrenal cortex (10).
Finally, SCN coordination of the phase of a circadian clock within the adrenal gland is also implicated in circadian GC release. Adrenal gland-specific circadian clock knockdown mice exhibit dampened circadian oscillations of corticosterone (11). Interestingly, circadian clocks of the left and right adrenal glands in split hamsters oscillate in antiphase, mirroring the activity of the SCN and suggesting the phase of the adrenal circadian clock is regulated through neural pathways instead of systemically released humoral factors such as ACTH (12).
The current study exploited the split hamster to test the hypothesis that the circadian release of GC is the result of the integration of SCN-adrenal communication via the following: 1) a multisynaptic neural pathway and 2) the HPA axis.
Materials and Methods
Animals and monitoring of locomotor activity
Male golden hamsters were purchased from Charles River Laboratories (Wilmington, MA) and used in accordance with regulations established by the University of Washington Institutional Animal Care and Use Committee. Animals were 30 d old upon their arrival and individually housed under LL (∼250 lux). Animals were provided with food and water ad libitum, which was restocked randomly, and wheel-running locomotor activity was continuously monitored via ClockLab software (Actimetrics, Wilmette, IL). After 2 months under LL, approximately 67% of the hamsters exhibited stable behavioral splitting of locomotor activity as determined by visual inspection of actograms. The remaining unsplit hamsters were used as control animals.
Surgeries
Animals were anesthetized and implanted with jugular catheters. Briefly, the right external jugular vein was exposed and SILASTIC brand (Dow Corning Corp., Midland, MI) tubing (inner diameter × outer diameter: 0.020 × 0.037) was inserted into the vessel until the end reached the right atrium. The tubing was stitched to the vein with two nylon sutures to hold its position. An exit site for the cannula was created in the scapular region of the animal by sc tunneling with a 16-gauge needle. The catheter was then filled with heparinized saline (20 U/ml) and sealed with the tip of a 22-gauge needle that had been filled with SILASTIC glue (Dow Corning). Animals were allowed to recover from the surgery for 3–5 d before blood collection.
Blood collections
Blood was serially collected from unstressed animals to generate complete 24-h hormone profiles of plasma cortisol and ACTH. In the group of hamsters used for cortisol measurements (n = 8 control; n = 6 split), 25 μl of blood were collected every hour for a total of 23 h. In the group of animals used for ACTH measurements (n = 6 control; n = 8 split), 250 μl of blood was collected every 3 h for 21 h. After each blood draw, the volume was replaced 1:1 (25 and 250 μl for cortisol and ACTH collections, respectively) with warm (37 C) saline. Potential cortisol and ACTH release in response to the stress of the sampling procedure was controlled in several ways. First, the start of sampling for each individual hamster varied relative to the individual's onset of wheel-running activity; thus, peaks in hormone levels that correlated with activity onsets were not due to the initiation of the blood collections. Second, animals underwent mock collection procedures at least three times per day for 1–2 d before collection to acclimate the animals to frequent human handling. Third, all samples were collected within 3 min of removing the animals from their home cages. In split animals, the asymmetry between the length of each of the two activity bouts (if any) was not taken into account for the bleeding schedule because this asymmetry bears no relationship to which side of the SCN (left or right) is responsible for each bout (3). Blood was collected in cold EDTA-treated syringes for ACTH assays and heparin-treated syringes for cortisol assays. After blood collection, plasma was isolated by centrifuging at 4 C and 2000 × g for 15 min and stored at −80 C until conducting hormone assays.
Determining plasma cortisol and ACTH concentrations
Although hamsters are dual secretors of both corticosterone and cortisol, the release of cortisol shows a higher amplitude circadian rhythm (13, 14), and cortisol is clearly a more potent regulator of energy homeostasis in this species (15). Plasma cortisol concentrations were determined in triplicate with a cortisol express enzyme immunoassay kit (Cayman Chemical, Ann Arbor, MI) in accordance with the manufacture's protocol. This assay has minimal cross reactivity (0.14%) with corticosterone. Samples were run at 1:50 dilution. Inter- and intraassay coefficients of variability were 11.57 and 11.86%, respectively. Plasma ACTH concentrations were determined by RIA according to previously established methodology (16). Antibody directed against rat ACTH (Rb7) was acquired as a generous gift from Dr. Bill Engeland (University of Minnesota, Minneapolis, MN). Briefly, plasma and a serial dilution of rat ACTH 1-39 standards (Bachem, Torrance, CA) were exposed to ACTH antibody (1:120,000 antibody in phosphate buffer with EDTA, Triton X-100, aprotinin, BSA, and blue food coloring, which does not interfere with assay and helps with keeping track of the pipetting sequence) overnight at 4 C. Then 125I-labeled ACTH 1-39 (DiaSorin, Stillwater, MN) was added to a final concentration of 25 cpm/μl per reaction and allowed to incubate overnight at 4 C. On the third day of the assay, goat antirabbit IgG secondary antibody and normal rabbit serum (Peninsula Laboratories) were added to the reaction and incubated for 4 h at 4 C, after which separation buffer (phosphate buffer with EDTA and 99.9% protease free BSA) was added to the reaction and centrifuged at 4 C for 25 min at 2500 rpm. The supernatant was then aspirated and the remaining pellets were counted in duplicate on a γ-counter (Wizard 1470 automatic γ-counter; PerkinElmer, Waltham, MA) for 10 min each. Serially diluted ACTH standards were used to generate a four-parameter logistic standard curve via AssayZap software (Biosoft, Cambridge, UK) from which unknown concentrations were calculated. Inter- and intraassay coefficients of variability were 6.28 and 5.17%, respectively. Areas under the curve for complete cortisol and ACTH 24-h outputs were relatively quantified in Photoshop (Adobe, San Jose, CA) by using the magic wand tool, which returns a pixel number for each area.
Statistics
Plasma hormone concentrations were log transformed to obtain homogeneity of variance as determined by Fligner and Bartlett's tests, and statistical differences were then determined by ANOVA. For hormone profiles, the effect of time was determined by one-way ANOVA. Interaction of treatment (control or split) and time were determined by two-way ANOVA. Repeated-measure ANOVA was not used because a subset of hamsters lacked a complete 24-h profile (the minimum number of samples for any single time point was 4; see figure legends for details). Specific differences were determined by planned comparisons between split and controls for each individual time point.
Results
Circadian release of cortisol exhibits two peaks in behaviorally split hamsters
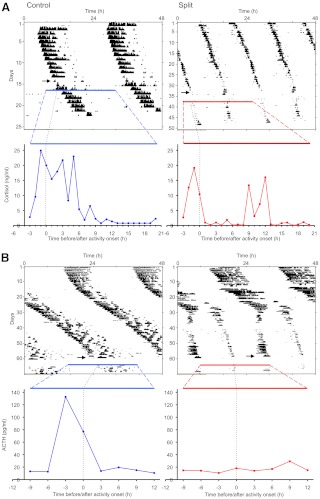
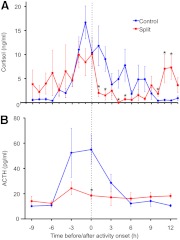
After serially bleeding behaviorally split and unsplit hamsters housed in LL, plasma cortisol profiles were determined for each individual (Fig. 1A). Over a 24-h period, split hamsters exhibited two phases of elevated plasma cortisol, each preceding one of the two projected onsets of wheel-running activity. In contrast, control unsplit hamsters exhibited a single peak in plasma cortisol before the onset of wheel-running activity. Figure 2A shows mean plasma cortisol for all split and unsplit hamsters. There was an effect of sample time on plasma cortisol levels in both groups as assessed by one-way ANOVA for control [F(23) = 5.39, P < 0.001; n = 8 and 6–8 samples per time point] and split [F(23) = 3.36, P < 0.001; n = 6 and 4–6 samples per time point] hamsters. Additionally, two-way ANOVA revealed an interaction of control/split state with sample time [F(1, 23) = 3.13, P < 0.001] and a marginally nonsignificant control/split group effect [F(1) = 3.07, P = 0.081]. Post hoc analysis showed split hamsters had a lower cortisol peak before one of the two bouts of locomotor activity, compared with the single cortisol peak observed in control animals. Additionally, split hamsters exhibited a second significant elevation of plasma cortisol 10–12 h after one of the locomotor activity onsets, which was also just before the onset of the other locomotor activity bout. Furthermore, the area under the curve for hamsters with a complete 24-h profile demonstrates a significant reduction of the overall 24 h cortisol output in split hamsters relative to controls [t(5.95) = 2.47, P < 0.05].
Fig. 1.
Representative double-plotted actograms of wheel-running activity and hormone profiles. Control (blue) and split (red) hamsters were surgically implanted with jugular catheters (arrows), and blood was collected for 24 h (colored horizontal lines on actograms). A, Plasma cortisol of representative control and split hamsters relative to wheel-running activity onset (dotted line). B, Plasma ACTH of representative control and split hamsters relative to wheel-running activity onset (dotted line). Dashed diagonal colored bands expand the 24-h scale on the day of bleeding for better visualization.
Fig. 2.
Mean plasma cortisol and ACTH 24-h profiles from control and split hamsters. A, Twenty-four-hour plasma cortisol of control (blue; n = 6–8) and split (red; n = 4–6) hamsters. B, Twenty-four-hour plasma ACTH of control (blue; n = 4–6) and split (red; n = 6–8) hamsters. Onset of wheel-running activity is indicated by dotted line. Data are shown as mean ± sem. *, P < 0.05 vs. control.
Plasma ACTH is not concomitantly elevated with cortisol in split hamsters
To test potential mechanisms regulating the dual peaks of cortisol in split hamsters, we repeated the above experiment but collected blood every 3 h for a total duration of 24 h to create plasma ACTH profiles from split (n = 8 and 6–8 samples per time point) and unsplit control (n = 6 animals and 4–6 samples per time point) hamsters (Figs. 1B and 2B). The sampling frequency was reduced to eight time points because a larger volume of plasma (100 μl) was required to assay ACTH. However, the 3-h sampling frequency is well within the minimum Nyquist frequency (one third of period) to detect an approximately 12 h oscillation (17). As illustrated in Fig. 1B, unsplit control hamsters displayed a single peak in plasma ACTH, occurring before the onset of locomotor activity, as seen for plasma cortisol in the first experiment. In contrast, split hamsters exhibited no significant elevation in plasma ACTH at any time (Fig. 1B). One-way ANOVA revealed an effect of time on plasma ACTH in control [F(7) = 4.61, P < 0.001] but not in split [F(7) = 1.15, P = 0.346] hamsters (Fig. 2B). Two-way ANOVA, with group (split/unsplit) and time as factors, revealed a significant interaction [F(1, 7) = 2.47, P = 0.02] but no significant effect of group [F(1) = 1.53, P = 0.21]. Post hoc analysis revealed control hamsters had significantly higher ACTH levels at the onset of locomotor activity. Although the two-way ANOVA yielded no effect of group, analysis of area under the curve showed there was a decrease in the overall 24-h ACTH output of split relative to control hamsters [t(8.18) = 2.2611, P = 0.05].
Discussion
The circadian release of GC was characterized as an SCN-dependent rhythm 4 decades ago (2), yet the pathways by which the master circadian clock regulates circadian GC release remain unknown. We show LL-housed hamsters that display a single approximately 24-h locomotor activity rhythm also display a single peak of cortisol release that is concomitant with ACTH release. Conversely, LL-housed hamsters that behaviorally split show two corresponding peaks of cortisol approximately 12 h apart, which are independent of circadian rhythmic ACTH release. These results suggest that the circadian release of cortisol is the result of the dual contribution of ACTH and non-ACTH regulatory pathways.
Two master clocks for physiology and behavior
Splitting in the hamster is the result of antiphase oscillations of the left and right SCN (3). Here we demonstrate that these antiphase circadian pacemakers can sustain two rhythms of cortisol release that are 180° out of phase. This finding is consistent with previous work in which behaviorally split female hamsters display LH surges approximately 12 h apart (4). Collectively, these data suggest circadian endocrine rhythms in general might show a dual oscillatory pattern in behaviorally split hamsters. Furthermore, core body temperature rhythms in the split hamster also show an approximately 12-h oscillation (18). To our knowledge, no circadian outputs studied in behaviorally split hamsters have escaped the approximately 12-h modulation by the left and right SCN, indicating the neural and peripheral targets that produce these outputs remain responsive to ∼12-h signals emerging from the master circadian pacemaker. Together, these results provide strong evidence, from a neurologically, genetically, and pharmacologically intact animal model, for the unequivocal preeminence of the SCN in the regulation of rhythmic, physiological and behavioral processes and for an unparalleled localization of function in the brain. Importantly, work by Kalsbeek et al. (19, 20) indicates the SCN may send signals that stimulate or inhibit GC release, depending on the time of the day. Thus, the two antiphase-oscillating SCN in the split hamster could respectively and simultaneously send inhibitory and stimulatory signals to the adrenal glands. This idea is supported by the fact that within each SCN of the split hamster two subsets of neurons also oscillate in antiphase (21, 22).
Although the split master circadian clocks can time a multiplicity of rhythms to an approximately 12-h period, the pathways by which they do so depend on the specific circadian rhythm examined. Locomotor activity rhythms rely on diffusible factors released by the SCN (23). In contrast, the circadian regulation of cortisol, corticosterone, LH, and melatonin release relies on synaptic connections of SCN efferent fibers on specific neural targets (14). The regulation of the LH surge in split female hamsters is associated with the activation of the left- or right-sided GnRH network by ipsilateral SCN efferents, likely involving direct and indirect synaptic connections with GnRH neurons (5, 24–26). In the split female hamster, these connections presumably lead to the approximately 12-h activation of the hypothalamo-pituitary-gonadal axis. In contrast, the dual cortisol peaks we describe cannot be explained by the approximately 12-h activation of the HPA axis. Split hamsters show no significant rise in plasma ACTH corresponding with the two elevations in cortisol. In the mouse, light-induced corticosterone release has been shown to occur without a concomitant increase in ACTH and is abolished by SCN lesions as well as by removing sympathetic input to the adrenal via splanchnic denervation (9).
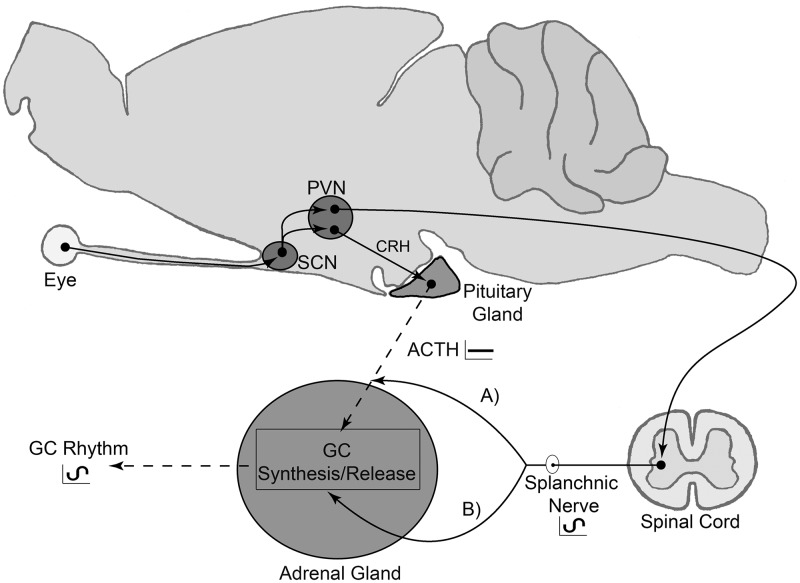
Our results in the split hamster suggest this neural pathway from the SCN to the adrenal could contribute to the circadian release of cortisol without the intervention of rhythmic ACTH input. Such a neural pathway could drive circadian cortisol release by direct stimulation of adrenal cortical cells or by modulating adrenal sensitivity to ACTH (27) (Fig. 3). Recent evidence indicates the adrenal peripheral clock locally regulates the amplitude of circadian GC release (11), and it is conceivable that SCN signals relayed through the splanchnic nerve may set the phase of the adrenal clock, which in turn may sustain a rhythm in GC synthesis and release. In striking support of this model, circadian expression of Period1 (Per1), a genetic component of the molecular circadian clock, in the left and right adrenal cortex, and particularly the adrenal medulla, of the split hamster oscillate in antiphase, mirroring the expression of Per1 in the SCN (12). Although it is not clear what the role of medullary Per1 expression could be, it may also modulate glucocorticoid synthesis and release by the cortex. In summary, circadian GC release is likely the result of combined SCN input to the adrenal through the HPA axis and the sympathetic nervous system (Fig. 3). This latter pathway could directly influence GC synthesis and release and/or impose a rhythm in sensitivity to ACTH. Although evidence for a role of sympathetic innervation on the regulation of GC release has been previously described (9, 27, 28), our study is the first to suggest its involvement in circadian GC release in the absence of rhythmic ACTH release. The dual regulation of circadian GC release by the HPA axis and sympathetic innervation could represent a mechanism that assures proper phase and amplitude of circadian GC release. Furthermore, each pathway's contribution could change under different conditions. For instance, sympathetic innervation could be more prominent in split than unsplit hamsters and therefore sufficient to sustain a circadian rhythm of GC release in the absence of an ACTH rhythm.
Fig. 3.
Circadian regulation of GC release. Light input from the retina entrains SCN master circadian clock, which in turn can time GC release through input to preautonomic or CRH-containing neurons in the paraventricular nucleus (PVN) and two distinct regulatory branches: one regulatory pathway dependent on ACTH release and the other through an autonomic multisynaptic pathway. Autonomic multisynaptic input to the adrenal either modulates adrenal sensitivity to ACTH (A) or GC synthesis/release directly (B).
Cortisol negative feedback is likely altered in split hamsters
Our study demonstrates behaviorally split hamsters show two peaks of cortisol release; however, their overall 24-h cortisol output is reduced compared with unsplit control animals. Despite the observed overall reduction in plasma cortisol, we hypothesize the bimodal pattern of cortisol release leads to increased negative feedback, reducing the levels of ACTH and the general drive of the HPA axis. Because we observed a diminished total 24-h cortisol and ACTH output in split hamsters compared with unsplit animals, we hypothesize that the timing of release, not the absolute amount of cortisol per se, may represent a more critical signal to increase negative feedback. Alternatively, the antiphase oscillation of the left and right SCN in the split hamster could lead to changes in pulsatile release of ACTH that are undetected by our sampling frequency (29); a recent study has shown that exogenously administered ACTH induces corticosterone release only if administered at a pulsatile but not at a constant rate (30). Finally, we did not measure corticosterone in our study. Although the circadian regulation of both cortisol and corticosterone is similar in golden hamsters, the rhythm of cortisol secretion has higher amplitude in intact animals (13, 14). On the other hand, chronic stress in the hamster has been shown to increase cortisol but not corticosterone (31). We do not think this differential stress-induced regulation of both GC is relevant to our results because unsplit hamsters in our study show similar peak levels of cortisol as hamsters housed under 14-h light, 10-h dark cycles (13); future studies should determine whether corticosterone is regulated similarly to cortisol in the split hamster and whether negative feedback is indeed increased by the bimodal secretion of GC.
Our results in a neurologically and genetically intact animal support a model in which the circadian release of GCs is the result of the dual contribution of ACTH and non-ACTH regulatory branches emerging from the master circadian pacemaker located in the SCN. Furthermore, our data suggest the negative feedback systems regulating GC release may be more sensitive to the timing of glucocorticoid release than to the absolute levels of circulating GCs.
Acknowledgments
We thank Dr. David Perkel, Dr. Robert Steiner, and Dr. Eliot Brenowitz for thoughtful discussions.
This work was supported by National Institutes of Health Research Grants R01 MH075016, and R03 HD061853. T.R.L. was supported in part by Public Health Service Grant NRSA 2T32 GM007270 from the National Institute of General Medical Sciences, and J.M.L. was supported by the Washington Research Foundation Fellowship for Advanced Undergraduates.
Current address for C.W.: Department of Biology, Seattle University, P.O. Box 222000, Seattle, Washington 98122.
Current address for J.M.L.: The White House, Executive Office of the President, Office of Science and Technology Policy, 725 17th Street, NW, Washington, DC 20502.
The views expressed in this article do not necessarily represent the views of the Office of Science and Technology Policy or the United States Government. This article is a result of the authors' independent research; this research was not funded by the Office of Science and Technology Policy.
Disclosure Summary: T.R.L., C.W., D.T., J.M.L., and H.O.d.l.I. have nothing to declare.
For editorial see page 546
- GC
- Glucocorticoid
- HPA
- hypothalamic-pituitary-adrenal
- LL
- constant light conditions
- SCN
- suprachiasmatic nucleus.
References
- 1. Hastings MH, Reddy AB, Maywood ES. 2003. A clockwork web: circadian timing in brain and periphery, in health and disease. Nat Rev Neurosci 4:649–661 [DOI] [PubMed] [Google Scholar]
- 2. Moore RY, Eichler VB. 1972. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res 42:201–206 [DOI] [PubMed] [Google Scholar]
- 3. de la Iglesia HO, Meyer J, Carpino A, Jr, Schwartz WJ. 2000. Antiphase oscillation of the left and right suprachiasmatic nuclei. Science 290:799–801 [DOI] [PubMed] [Google Scholar]
- 4. Swann JM, Turek FW. 1985. Multiple circadian oscillators regulate the timing of behavioral and endocrine rhythms in female golden hamsters. Science 228:898–900 [DOI] [PubMed] [Google Scholar]
- 5. de la Iglesia HO, Meyer J, Schwartz WJ. 2003. Lateralization of circadian pacemaker output: activation of left- and right-sided luteinizing hormone-releasing hormone neurons involves a neural rather than a humoral pathway. J Neurosci 23:7412–7414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vrang N, Larsen PJ, Mikkelsen JD. 1995. Direct projection from the suprachiasmatic nucleus to hypophysiotrophic corticotropin-releasing factor immunoreactive cells in the paraventricular nucleus of the hypothalamus demonstrated by means of Phaseolus vulgaris-leucoagglutinin tract tracing. Brain Res 684:61–69 [DOI] [PubMed] [Google Scholar]
- 7. Buijs RM, Markman M, Nunes-Cardoso B, Hou YX, Shinn S. 1993. Projections of the suprachiasmatic nucleus to stress-related areas in the rat hypothalamus: a light and electron microscopic study. J Comp Neurol 335:42–54 [DOI] [PubMed] [Google Scholar]
- 8. Bornstein SR, Engeland WC, Ehrhart-Bornstein M, Herman JP. 2008. Dissociation of ACTH and glucocorticoids. Trends Endocrinol Metab 19:175–180 [DOI] [PubMed] [Google Scholar]
- 9. Ishida A, Mutoh T, Ueyama T, Bando H, Masubuchi S, Nakahara D, Tsujimoto G, Okamura H. 2005. Light activates the adrenal gland: timing of gene expression and glucocorticoid release. Cell Metab 2:297–307 [DOI] [PubMed] [Google Scholar]
- 10. Buijs RM, Wortel J, Van Heerikhuize JJ, Feenstra MG, Ter Horst GJ, Romijn HJ, Kalsbeek A. 1999. Anatomical and functional demonstration of a multisynaptic suprachiasmatic nucleus adrenal (cortex) pathway. Eur J Neurosci 11:1535–1544 [DOI] [PubMed] [Google Scholar]
- 11. Son GH, Chung S, Choe HK, Kim HD, Baik SM, Lee H, Lee HW, Choi S, Sun W, Kim H, Cho S, Lee KH, Kim K. 2008. Adrenal peripheral clock controls the autonomous circadian rhythm of glucocorticoid by causing rhythmic steroid production. Proc Natl Acad Sci USA 105:20970–20975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mahoney CE, Brewer D, Costello MK, Brewer JM, Bittman EL. 2010. Lateralization of the central circadian pacemaker output: a test of neural control of peripheral oscillator phase. Am J Physiol Regul Integr Comp Physiol 299:R751–R761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Albers HE, Yogev L, Todd RB, Goldman BD. 1985. Adrenal corticoids in hamsters: role in circadian timing. Am J Physiol 248:R434–R438 [DOI] [PubMed] [Google Scholar]
- 14. Meyer-Bernstein EL, Jetton AE, Matsumoto SI, Markuns JF, Lehman MN, Bittman EL. 1999. Effects of suprachiasmatic transplants on circadian rhythms of neuroendocrine function in golden hamsters. Endocrinology 140:207–218 [DOI] [PubMed] [Google Scholar]
- 15. Solomon MB, Sakai RR, Woods SC, Foster MT. 2011. Differential effects of glucocorticoids on energy homeostasis in Syrian hamsters. Am J Physiol Endocrinol Metab 301:E307–E316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jasper MS, Engeland WC. 1991. Synchronous ultradian rhythms in adrenocortical secretion detected by microdialysis in awake rats. Am J Physiol 261:R1257–R1268 [DOI] [PubMed] [Google Scholar]
- 17. Marmarelis PZ, Marmarelis VZ. 1978. Analysis of physiological systems: the white-noise approach. New York: Plenum Press [Google Scholar]
- 18. Pickard GE, Kahn R, Silver R. 1984. Splitting of the circadian rhythm of body temperature in the golden hamster. Physiol Behav 32:763–766 [DOI] [PubMed] [Google Scholar]
- 19. Kalsbeek A, van Heerikhuize JJ, Wortel J, Buijs RM. 1996. A diurnal rhythm of stimulatory input to the hypothalamo-pituitary-adrenal system as revealed by timed intrahypothalamic administration of the vasopressin V1 antagonist. J Neurosci 16:5555–5565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kalsbeek A, van der Vliet J, Buijs RM. 1996. Decrease of endogenous vasopressin release necessary for expression of the circadian rise in plasma corticosterone: a reverse microdialysis study. J Neuroendocrinol 8:299–307 [DOI] [PubMed] [Google Scholar]
- 21. Yan L, Foley NC, Bobula JM, Kriegsfeld LJ, Silver R. 2005. Two antiphase oscillations occur in each suprachiasmatic nucleus of behaviorally split hamsters. J Neurosci 25:9017–9026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tavakoli-Nezhad M, Schwartz WJ. 2005. c-Fos expression in the brains of behaviorally “split” hamsters in constant light: calling attention to a dorsolateral region of the suprachiasmatic nucleus and the medial division of the lateral habenula. J Biol Rhythms 20:419–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Silver R, LeSauter J, Tresco PA, Lehman MN. 1996. A diffusible coupling signal from the transplanted suprachiasmatic nucleus controlling circadian locomotor rhythms. Nature 382:810–813 [DOI] [PubMed] [Google Scholar]
- 24. de la Iglesia HO, Blaustein JD, Bittman EL. 1995. The suprachiasmatic area in the female hamster projects to neurons containing estrogen receptors and GnRH. Neuroreport 6:1715–1722 [DOI] [PubMed] [Google Scholar]
- 25. Van der Beek EM, Horvath TL, Wiegant VM, Van den Hurk R, Buijs RM. 1997. Evidence for a direct neuronal pathway from the suprachiasmatic nucleus to the gonadotropin-releasing hormone system: combined tracing and light and electron microscopic immunocytochemical studies. J Comp Neurol 384:569–579 [DOI] [PubMed] [Google Scholar]
- 26. Vida B, Deli L, Hrabovszky E, Kalamatianos T, Caraty A, Coen CW, Liposits Z, Kall ó I. 2010. Evidence for suprachiasmatic vasopressin neurones innervating kisspeptin neurones in the rostral periventricular area of the mouse brain: regulation by oestrogen. J Neuroendocrinol 22:1032–1039 [DOI] [PubMed] [Google Scholar]
- 27. Ulrich-Lai YM, Arnhold MM, Engeland WC. 2006. Adrenal splanchnic innervation contributes to the diurnal rhythm of plasma corticosterone in rats by modulating adrenal sensitivity to ACTH. Am J Physiol Regul Integr Comp Physiol 290:R1128–R1135 [DOI] [PubMed] [Google Scholar]
- 28. Jasper MS, Engeland WC. 1994. Splanchnic neural activity modulates ultradian and circadian rhythms in adrenocortical secretion in awake rats. Neuroendocrinology 59:97–109 [DOI] [PubMed] [Google Scholar]
- 29. Gudmundsson A, Carnes M. 1997. Pulsatile adrenocorticotropic hormone: an overview. Biol Psychiatry 41:342–365 [DOI] [PubMed] [Google Scholar]
- 30. Spiga F, Waite EJ, Liu Y, Kershaw YM, Aguilera G, Lightman SL. 2011. ACTH-dependent ultradian rhythm of corticosterone secretion. Endocrinology 152:1448–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ottenweller JE, Tapp WN, Burke JM, Natelson BH. 1985. Plasma cortisol and corticosterone concentrations in the golden hamster, (Mesocricetus auratus). Life Sci 37:1551–1558 [DOI] [PubMed] [Google Scholar]