Abstract
Background
Although many studies suggest that, on average, depression-specific psychotherapy and antidepressant pharmacotherapy are efficacious, we know relatively little about which patients are more likely to respond to one versus the other. We sought to determine whether measures of spectrum psychopathology are useful in deciding which patients with unipolar depression should receive pharmacotherapy vs. depression-specific psychotherapy.
Methods
318 adult outpatients with major depression were randomly assigned to escitalopram pharmacotherapy or interpersonal psychotherapy at academic medical centers at Pittsburgh, Pennsylvania and Pisa, Italy. The outcomes of primary interest were predictors and moderators time to remission on monotherapy at 12 weeks.
Results
Participants with higher scores on the need for medical reassurance factor of the PAS-SR had more rapid remission with IPT and those with lower scores on the psychomotor activation factor of the MOODS-SR experienced more rapid remission with SSRI. Nonspecific predictors of longer time to remission with monotherapy included several panic spectrum and mood spectrum factors and the social phobia spectrum total score. Higher baseline HRSD-17 and-25, and Work and Social Adjustment Scale scores also predicted longer time to remission, while being married predicted shorter time to remission.
Conclusions
This exploratory study identified several nonspecific predictors, but few moderators of psychotherapy vs. pharmacotherapy outcome. It offers useful indicators of the characteristics of patients that are generally difficult to treat, but only limited guidance as to who benefits from IPT versus SSRI pharmacotherapy.
INTRODUCTION
Although specific information about which depressed patients are best treated with medication and which with psychotherapy could provide guidance to practicing clinicians and improve outcomes, only a few studies have reported findings in this regard. In the NIMH TDCRP study, low social dysfunction predicted superior response to interpersonal psychotherapy (IPT) (Klerman, et al., 1984) (Weissmann, et al., 2000), low dysfunctional attitudes predicted better response to cognitive therapy (CT) (Beck, et al.,1979), high work dysfunction predicted superior response to imipramine and higher baseline severity predicted better response to IPT and medication (Sotsky, et al., 1991). In a subsequent report examining only the two psychotherapy conditions, Barber and Muenz (JCCP,1996) reported that among `completers,' CT was more effective than IPT for those with avoidant PD features, while the reverse was true for those with elevated levels of OC features. Married and cohabiting patients had better outcomes with CT, while single patients improved more with IPT.
In the initial report from their study comparing outcomes for CT and antidepressant medication, DeRubeis, et al., (1995) noted that the presence of generalized anxiety and the absence of chronic depression were associated with better response among those treated with antidepressant, while social phobia and absence of melancholia were associated with poorer response to CT. They did not indicate whether any of these variables predicted differential response to medication versus psychotherapy. In subsequent reports based, first, on a priori hypotheses regarding treatment moderation by previous antidepressant exposure and presence of personality disorder and then on a posteriori analyses of potential predictors and moderators, they found that previous antidepressant exposure was associated with poor response to medication, but not to CT (Leykin, et al., 2007), while presence of a personality disorder was associated with better response to medication and poorer response to CT (Fournier et al., 2008). Finally, being married, unemployed, and having a greater number of recent life events each predicted superior response to CT (Fournier et al., 2009).
Dimidjian, et al.,(2006) confirmed their hypothesis that higher initial severity would be associated with better response to pharmacotherapy than to placebo and found that it was also associated with superior response to behavioral activation than to the full CT treatment. They did not report analyses of other potential moderators.
In an earlier study, we had found that lifetime panic spectrum symptomatology predicted longer time to remission among individuals with unipolar disorder whose initial IPT treatment was augmented with SSRI in the absence of remission with IPT monotherapy (Frank, et al., 2000). In the present study, we sought to extend this work in order to explore whether panic or other mood or anxiety spectrum conditions define treatment-relevant phenotypes moderating psychotherapy vs. pharmacotherapy outcome.
The platform for this investigation was a clinical trial in which individuals presenting for treatment of non-psychotic major depression were randomly assigned to a treatment plan that began with IPT or SSRI monotherapy. Participants who had not responded by 6 weeks or remitted by 12 weeks received augmentation with the other treatment. Here we report the primary findings of this study: predictors and moderators of time to remission on monotherapy over 12 weeks of treatment.
Given the exploratory nature of this work, we did not articulate specific a priori hypotheses; however, because we had observed substantial somatic sensitivity among individuals with panic spectrum symptomatology, we anticipated that such patients would have greater difficulty achieving remission with SSRI than IPT. We also expected that higher levels of social phobia or obsessive-compulsive spectrum features would interfere with the establishment of a strong psychotherapeutic alliance and, therefore, lead to greater difficulty achieving remission with IPT than SSRI.
METHODS
Participants
Participants were outpatients in a DSM-IV-defined episode of major depression as determined by SCID (American Psychiatric Association (APA) (2000)) interview with a minimum score of 15 on the 17-item Hamilton Rating Scale for Depression (HRSD) (Hamilton, 1960). A primary diagnosis of schizophrenia, schizoaffective disorder, bipolar I or II disorder, current anorexia or bulimia, antisocial personality disorder constituted exclusions. Patients with current alcohol or substance abuse or dependence were excluded only if their drinking or substance use was unrelated to their depression. Individuals with severe, uncontrolled medical illness, those who had been unresponsive to an adequate trial of escitalopram or IPT in the current episode and women unwilling to practice an acceptable form of birth control were also excluded.
Study procedures were approved by the University of Pittsburgh IRB and the Ethics Committee of the Azienda Ospedaliero-Universitaria of Pisa. All patients signed written informed consent after receiving a complete description of the study and having an opportunity to ask questions.
Interventions
Participants were randomly assigned to a treatment plan that began with interpersonal psychotherapy (IPT) (Klerman, et al., 1984) (Weissmann, et al., 2000) or SSRI pharmacotherapy (escitalopram oxalate) monotherapy. If the initial treatment did not bring about a stable remission, the other treatment was added. The study began with a variable length acute treatment phase, defined as at least 12 weeks of treatment and stable remission (3 weeks during which mean 17-item HRSD score was ≤7), followed by a 6-month continuation treatment phase (see Figure 1). The median number of acute treatment visits in both groups was 12 (IPT-first: range=2–45; SSRI-first: range=1–34).
Figure 1.
Study Design
Participants assigned to pharmacotherapy were seen by experienced psychopharmacologists for visits lasting 20 to 30 minutes, during which psychotherapeutic interventions were specifically proscribed. Psychotherapy was provided at Pittsburgh by masters' or PhD-level psychologists or social workers who were either trained by Gerald Klerman, Myrna Weissman, Bruce Rounsaville and Eve Chevron or by one of two clinicians initially trained by that group (EF or Debra N. Frankel). Because Italian law only permits non-physicians to perform psychotherapy after completion of a four-year specialization in the specific form of psychotherapy and IPT specialization did not exist in Italy at the time of study initiation, Pisa therapists were all psychiatrists trained to research-level competence by PS. All psychotherapy sessions were tape recorded and rated for IPT specificity by blind raters using Wagner, et al., (1992) scale. IPT sessions lasted approximately 50 minutes. Participants allocated to IPT at Pittsburgh also had a physician of record who saw them briefly at baseline and study weeks 6, 12 and 20.
Participants in either condition who complained of sleep difficulties were permitted up to 2 mg of lorazepam until sleep difficulties resolved. Participants reporting agitation could receive up to 4 mg. The median dose was 1 mg (range 0.25 to 3 mg). Among participants who had at least 1 treatment visit, 31.5% of those assigned to IPT-first and 40.8% of those assigned to SSRI-first received lorazepam at some point prior to entering the continuation phase (chi square=2.7, p=.099).
Escitalopram was started at 10 mg and titrated up or down as needed, with the goal of symptom remission and/or achieving a dose of 20 mg/day. The acute phase included 3 triage points for augmentation with the other treatment at weeks 6, 12, and 20.
Measures
Lifetime mood and anxiety spectrum psychopathology was assessed using 4 self-report instruments the Mood Spectrum (MOODS-SR) (Fagiolini, et al., 1999) (Dell'Osso, et al., 2002), the Panic-Agoraphobic Spectrum (PAS-SR) (Cassano, et al., 1997) (Shear, et al., 2001), the Obsessive-Compulsive Spectrum (OBS-SR) (Dell'Osso, et al., 2002) and the Social Phobia Spectrum (SHY-SR) (Dell'Osso, et al., 2002) assessments. These instruments are based on a theoretical approach that gives clinical significance not only to typical symptoms of full blown mood disorders but also to atypical symptoms, behavioral traits and temperamental features. Furthermore, these symptoms and traits need not cluster in time in order to have clinical importance. Rather, even isolated symptoms or traits that occur over an individual's lifetime, may mark clinically important phenotypes (Cassano et al, 1997).
We examined total scores on all instruments as well as factor scores on the two factor-analyzed instruments (PAS-SR (Rucci, et al., 2009) and MOODS-SR (Cassano, et al., 2009) (Cassano, et al., 2009)). The presence of other psychiatric disorders was assessed with the SCID-I (APA Task Force for the Handbook for Psychiatric Measures, 2000) and SCID-II (APA Task Force for the Handbook for Psychiatric Measures, 2000) and we used the Work and Social Adjustment Scale (WSAS) (Mundt, et al., 2002) to assess functioning.
Treatment progress was evaluated with the Hamilton Rating Scale for Depression (HRSD-17) (Hamilton, 1960). In order to ensure consistency between sites a bilingual psychiatrist from Pisa was trained over the course of one year at Pittsburgh and was certified as the `gold standard' HRSD rater for Pisa. Inter-rater agreement at each site and between sites was recalibrated approximately every 6 months and was maintained at ICC ≥.85. Because of the nature of the interventions, neither participants nor therapists could be blinded to group assignment. Outcomes were assessed by study clinicians not involved in the patient's treatment.
Response was defined as a 50% reduction in baseline HRSD-17 score; remission was defined as a mean HRSD-17 of ≤7 over 3 consecutive weeks. Augmentation occurred at week 6 in the absence of response on 20 mg of escitalopram or at least 5 IPT sessions and at week 12 for any participant not in remission.
Outcome Measure
The primary purpose of the study was to determine for which phenotypic picture treatment should be initiated with psychotherapy vs. pharmacotherapy. Therefore, we examined time to remission on monotherapy up to the 12-week point as our primary outcome since this was the earliest point at which patients could move to the continuation phase and at which both SSRI and IPT monotherapy could be expected to have had a reasonable chance of success.
Sample Size
Power calculation for the primary study aim was based on a linear regression model with HRSD change from baseline as the dependent variable and site, treatment group, and spectrum score (dichotomized at the median) entered as independent variables and interacted with one another. Sample size and power were calculated to detect an interaction effect between the potential moderator and treatment in the linear model. With equal cell sizes, the power to detect a moderate effect size (the standardized interaction effect of about .5) at the two-tailed 5% level with more than 95% power required 290 subjects, equally divided between the sites and initial treatment groups. Power calculations were performed using Power and Sample Size Calculation, version 2.1.31 (Dupont & Plummer 1997). However, rather than analyses based on a linear regression, we report predictors and moderators of the more clinically interpretable outcome of time to remission
Statistical Methods
We used Cox regression models to analyze the effects of demographic and clinical variables on time to remission in monotherapy by 12 weeks. Data on patients who did not remit in monotherapy (including those who were discontinued for clinical reasons and those who received augmentation) were censored at 12 weeks Those who dropped out were censored at the date of drop out. All variables were centered (i.e., binary variables were coded +1/2 and −1/2, and ordinal variables centered at the mean). We used the MacArthur approach to evaluation of moderators of treatment outcome (Kraemer, et al., 2002). According to this conceptualization, non-specific predictors of treatment outcome are pre-treatment variables that predict the outcome equally in the 2 treatments. Moderators, in contrast, predict outcome differently in the 2 groups, and, thus, “specify for whom or under what conditions [a particular] treatment works…”
We identified a priori, a number of demographic and clinical variables to be tested as potential moderators of treatment outcome in individual models. Each model included treatment allocation (T), site (S), one predictor/moderator (M) and their 2-and 3-way interactions (T × S, T × M, T × S × M). When the main effect of a variable was significant, but the interactions T × M and/or T × M × S were not, the variable was considered a non-specific predictor of outcome. When the interactions were significant whether or not there was a significant main effect, the variable was considered a moderator. Site was coded as −0.5 Pittsburgh, 0.5 Pisa and treatment as −0.5 IPT, 0.5 SSRI. Analyses were carried out using SPSS 16.0.
Results
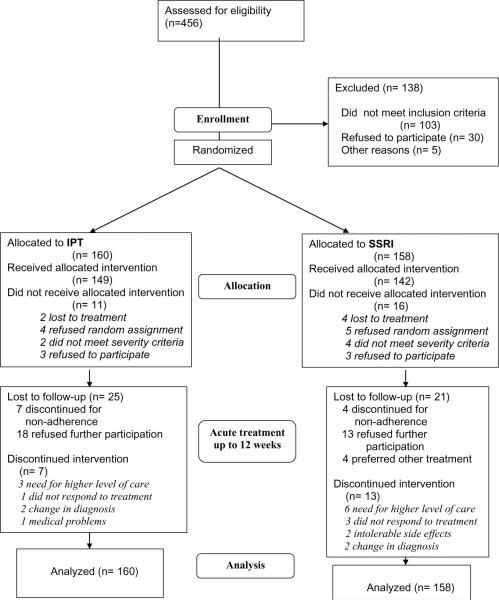
Figure 1 presents the full acute and continuation phase design and Figure 2 presents participant flow. 318 outpatients with unipolar depression were randomly allocated to treatment; 291 received the allocated intervention, 153 at Pittsburgh and 138 at Pisa. Recruitment began in April, 2003. Acute phase treatment ended in November, 2007 and continuation phase treatment in March, 2008.
Figure 2.
CONSORT Diagram
Participants had a mean age of 39.2 years and 13.8 years of education. The majority (71.7%) was female and not married (62.1%). Median duration of illness was 7.4 years and median number of lifetime episodes (including the current episode) was 2 (range=1–21). Mean HRSD-17 score at baseline was 20.0 (SD=4.10, range 15–35) and mean HRSD-25 was 24.6 (SD=5.1, range 15–43). Of the 316 patients for whom illness history was available, 97 (30.7%) were in their first episode of illness, while 219 (69.3%) had a history of recurrent depression.
At 12 weeks, the average daily dosage of escitalopram was 12.5 mg/d (SD=7.6) - . 13.3 mg/d (SD=8.1) at Pittsburgh and 11.6 mg/d (SD=7.1) at Pisa (Mann-Whitney test = 2174.5, p=.139). The mean number of IPT sessions attended in the first 12 weeks was 10.3 (SD=2.1) at Pisa and 10.3 (SD=2.7) at Pittsburgh.
Attrition and Tolerability
Of the 160 participants assigned to IPT, 11 (6.87%) did not receive the allocated intervention, 4 because they had hoped to receive pharmacotherapy. 30 (18.75%) were no longer in the trial at 12 weeks (Figure 2). Among the 19 participants who left the study after a first IPT session, 3 were discontinued because of change in diagnosis or need for a higher level of care and 13 were discontinued for non-adherence to the protocol or refusal to participate further. Of the 158 participants assigned to SSRI, 16 (10.13%) did not receive the intervention, 5 because they had hoped to receive psychotherapy. 36 (22.78%) were no longer in the trial at week 12. Among the 20 patients who left the trial after a first SSRI visit, 2 were discontinued because of intolerable side effects, 1 required a higher level of care and 17 were non-adherent to the protocol or refused to participate. The number of Patient Rated Inventory of Side Effects (PRISE) (Rush & O'Neal, 1999) items coded 2 (distressing) was 1.00 (SD=1.23) and 1.12 (SD 1.59) for patients successfully treated with IPT and SSRI monotherapy, respectively.
Remission with monotherapy
Table 1 reports the proportion of patients responding and remitting by treatment and site in the ITT sample and in those receiving the allocated intervention. There were no significant differences between the treatment conditions at Pisa (mean time to remission was 62 days with both treatments); however, time to remission was significantly longer among patients assigned to IPT vs. SSRI at Pittsburgh (80 vs. 71 days: log rank test =4.66, p<.05).
Table 1.
Response and Remission with Monotherapy at 6 and 12 Weeks by Treatment and Site:
| PISA - N (%) | ||||
|---|---|---|---|---|
| IPT | SSRI | |||
| No. of participants | ITT sample (N=74) | >=1 tx visit (N=70) | ITT sample (N=73) | >=1 tx visit (N=68) |
| Response at 6 weeks – N (%) | 61 (82.4) | 61 (87.1) | 57 (78.1) | 57 (83.8) |
| Remission at 6 weeks | 21 (28.4) | 21 (30.0) | 21 (28.8) | 21 (30.9) |
| Remission at 12 weeks | 47 (63.5) | 47 (67.1) | 44 (60.3) | 44 (64.7) |
| PITTSBURGH - N (%) | ||||
|---|---|---|---|---|
| IPT | SSRI | |||
| No. of participants | ITT sample (N=86) | >=1 tx visit (N=79) | ITT sample (N=85) | >=1 tx visit (N=74) |
| Response at 6 weeks | 37 (43.0) | 37 (46.8) | 42 (49.4) | 42 (56.8) |
| Remission at 6 weeks | 8 (9.3) | 8 (10.1) | 20 (23.5) | 20 (27.0) |
| Remission at 12 weeks | 21 (24.4) | 21 (26.6) | 30 (35.3) | 30 (40.5) |
Moderators of time to remission with monotherapy
Participants with higher lifetime PAS-SR need for medical reassurance factor scores had more rapid remission with IPT than with SSRI (HR=.59, 95%CI .39–.91). Because the hazard ratio is below unity, for each additional medical reassurance item endorsed, the likelihood of remitting with SSRI compared with IPT is decreased by 41%.
Participants with lower scores on the psychomotor activation factor from the mania/hypomania component of the lifetime Mood Spectrum Self-Report (MOODS-SR) experienced more rapid remission with SSRI than with IPT (HR=.70, 95%CI .49–1.00). Thus, for each additional psychomotor activation item endorsed the likelihood of remitting with SSRI compared to IPT is decreased by 30%.
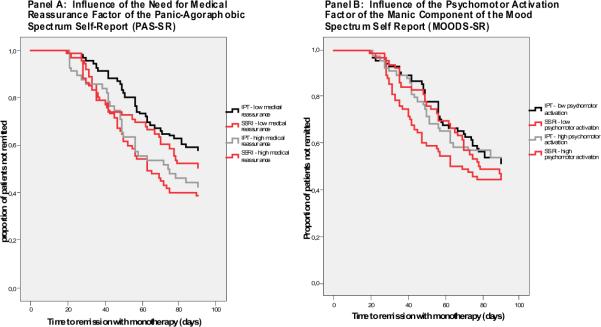
In order to better clarify the interaction between scores on these measures and treatment outcome, we plotted the survival curves for high and low scorers (using a median split) assigned to each of the two treatments in Figure 3. Panel A shows that patients with low need for medical reassurance took a median of 11 days less (64 vs. 75 days, log rank=7.3, p<0.01) to remit with SSRI and Panel B indicates that patients with low psychomotor activation remitted 9 days faster with SSRI (median 63 vs. 72 days, log rank=2.5, p=0.11).
Figure 3.
Time to Remission with Monotherapy: Influence of the Need for Medical Reassurance Factor of the Panic-Agoraphobic Spectrum Self-Report (PAS-SR) and the Psychomotor Activation Factor of the Manic Component of the Mood Spectrum Self-Report (MOODS-SR)
Two PAS-SR factors, lower lifetime separation anxiety factors scores (HR=2.33, 95%CI 1.09–5.01) and lower loss sensitivity (HR=2.29, 95%CI 1.08–4.86) were associated with longer time to remission with IPT than SSRI at Pittsburgh, but not at Pisa. No demographic or traditional clinical characteristics moderated time to remission.
Non-specific predictors of time to remission on monotherapy
As indicated in Table 2, nonspecific predictors of longer time to remission on monotherapy and, in general, of the need for combination treatment included the panic symptoms, drug/illness phobia, fear of losing control, and agoraphobia factors of the PAS-SR and the depressive mood, suicidality, psychomotor retardation, neurovegetative symptoms, and the psychotic features factors of the MOODS-SR, as well as the SHY-SR total score. For a detailed overview of the PAS-SR and MOODS-SR factors, see (Rucci, et al., 2009), (Cassano, et al., 2009), (Cassano, et al., 2009).
Table 2.
Significant Predictors and Moderators of Remission with Monotherapy by 12 Weeks of Treatments: Results from Cox Survival Regression Models
| HR (95% CI) | ||||
|---|---|---|---|---|
| Main effect | Interaction with treatment | Interaction with site | Interaction with treatment and site | |
| PAS-SR | ||||
| Panic symptoms | .76 (.63–.92) | |||
| Drug phobia | .79 (.64–.96) | |||
| Fear of losing control/depersonalization | .75 (.63–.91) | |||
| Agoraphobia | .82 (.68–1.00) | |||
| Medical reassurance | .59(.39–.91) | 2.35 (1.00–5.51) | ||
| Loss sensitivity | 2.29 (1.08–4.86) | |||
| Separation anxiety | 2.33 (1.09–5.01) | |||
| MOODS-SR (depressive component) | ||||
| Depressive mood | .74 (.62–.89) | |||
| Suicidality | .77 (.64–.93) | |||
| Psychomotor retardation | .69 (.58–.82) | |||
| Neurovegetative symptoms | .84 (.70–1.00) | |||
| Psychotic features | .82 (.68–.98) | |||
| MOODS-SR (manic/hypomania component) | ||||
| Psychomotor activation | .70 (.49–1.00) | |||
| Total SHY | .76 (.63–.91) | |||
| Baseline HDRS-17 | .74 (.61–.91) | |||
| Baseline HDRS-25 | .68 (.56–.83) | |||
| Marital status (married) | 1.69 (1.19–2.39) | |||
| WSAS | .56 (.46–.68) | |||
| Hypersomnia HDRS | .65 (.52–.81) | 2.58 (1.64−4.07) | ||
| Any anxiety disorder | .69 (.48–.99) | |||
Higher baseline scores on the HRSD-17 and HRSD-25, WSAS and a history of any anxiety disorder also predicted longer time to remission on monotherapy. Being married or living with a partner was the only variable that predicted shorter time to remission.
DISCUSSION
We sought to enhance clinicians' ability to determine which depressed outpatients should be treated initially with psychotherapy and which with pharmacotherapy, with the expectation that measures of broadly-conceptualized comorbidity represented in the Spectrum Project self-reports might lead to improved methods for making this determination (Cassano, et al., 2004). We hoped that these instruments, combined with easy-to-obtain variables such as illness history, and interview or self-report measures of depression severity, would enable clinicians to make more informed decisions about the best way to initiate treatment. This exploratory study identified several nonspecific predictors, but, contrary to our expectations, few moderators of psychotherapy vs. pharmacotherapy outcome. We did, however, find confirmation of our earlier findings regarding the relationship of panic spectrum features to time to remission and some guidance in terms of treatment selection.
Patients with lower need for medical reassurance factor scores on the Panic-Agoraphobic Spectrum self-report (PAS-SR) experienced more rapid remission with SSRI than with IPT and vice versa. Thus, this factor might be used to identify patients who are/are not as likely to benefit from drug treatment. Examining the specific items in the need for medical reassurance factor (repeated requests for diagnostic procedures, lab tests, or hospital admissions not recommended by the doctor, frequent checking of blood pressure or pulse, use of emergency services or calls to doctor for reassurance, felt need to dramatize symptoms to get reassurance or in order for others to understand your suffering, etc), one can see how such features might interfere with patients' ability to achieve remission with a potent, alerting SSRI such as escitalopram in the context of relatively brief pharmacotherapy visits. In contrast, the stance of the IPT therapist is that of a non-neutral advocate who is charged with providing reassurance that the symptoms the patient is experiencing constitute a well-known and highly treatable syndrome in medicine, and with offering specific treatment strategies – that have few, if any, somatic effects – for achieving that remission (Klerman, et al., 1984) (Weissmann, et al., 2000).
Those with lower scores on the psychomotor activation factor of the manic/hypomanic component of the MOODS-SR remitted more rapidly with SSRI than with IPT. This dimension reflects core features of mania/hypomania including flight of ideas, pressured speech, increased energy, and reduced need for sleep, activity, assertiveness, impatience, desire to reconnect with people and being noisy that, as we have reported earlier (Cassano, et al., 2009), may be experienced as isolated symptoms in patients who have never met criteria for an episode of mania or hypomania. Secondary analyses indicated that 7 o f 9 (77.8%) patients who experienced hypomania during the study had psychomotor activation scores above the median (≥5), suggesting that this factor may be useful in identifying patients at risk of switching and confirming the clinical relevance of this dimension. Indeed, Akiskal, (2007) has described clinical depressions superimposed on hyperthymic temperament (bipolar IV), referring to individuals with subthreshold hypomanic traits rather than episodes.
At Pittsburgh, but not at Pisa, PAS-SR separation anxiety factor scores also moderated outcomes and there was a trend for a similar effect for loss sensitivity factor scores, with those for whom separation issues were less salient having a clearly superior response to SSRI. For those high on these dimensions, IPT and SSRI treatment appeared to be equally successful. At least two studies suggest that separation anxiety is associated with bipolar spectrum conditions and panic disorder (Pini, et al., 2005) (Brückl, et al., 2007). Thus, the rapid remissions of those with low lifetime levels of separation distress may reflect the presence of a less-complicated, more purely unipolar disorder.
The present study replicated and extended earlier findings indicating the utility of the spectrum measurement approach in predicting generally poorer depression treatment outcome (Frank, et al., 2000). Higher scores on the panic symptoms, drug/illness phobia, fear of losing control and agoraphobia factors of the PAS-SR, the depressive mood, suicidality, psychomotor retardation and psychotic features factors of the MOODS-SR and the lifetime SHY-SR total score were all associated with longer time to remission.
These dimensions may, again, reflect the complexity of the clinical picture in patients endorsing the lifetime experience of these features, complexity that makes remission of depression more difficult to achieve with any monotherapy. In addition, higher depression severity at baseline and the presence of any lifetime anxiety disorder were associated with longer time to remission. These, too, may be considered markers of greater severity and, in general, a need for combination treatment in order to achieve remission of depression. Souery et al. (2007), identified 11 factors associated with difficult-to-treat depression, of which, comorbid panic attacks and anxiety disorders had the strongest associations. Fava,et al. (2008), proposed “anxious depression” as a discrete clinical subtype leading to poorer response to antidepressants.
Our study is among the first to focus, not only on full-blown comorbidity, but also on isolated dimensions of panic psychopathology that may permit a better characterization and management of the depressed patient. Among the PAS-SR factors, panic symptoms, agoraphobia, fear of losing control/depersonalization and drug/illness phobia factors predicted longer time to remission. The first two factors correspond to the diagnostic criteria for the diagnosis of panic disorder and should be always explored when examining a depressed patient. The other factors, including fear of losing control/depersonalization and drug/illness phobia, which may have an impact on compliance and treatment response, have been previously overlooked.
Other predictors associated with delayed response were lifetime suicidality, psychomotor retardation, neurovegetative symptoms and psychotic features. These MOODS-SR dimensions correspond to criteria or indicators of subthreshold bipolarity (Phelps & Ghaemi, 2002). While higher psychomotor activation factor scores were associated with poorer response to SSRI, they were not a predictor of difficulty in achieving remission across both treatments studied.
In the present study, evaluation by means of factor scores was limited to the panic and mood spectrum (mania and depression) assessments, as these are the only two instruments thus far subjected to factor analysis. Nevertheless, the present data give a clear idea of the range and complexity of depressive presentations, in which a number of sub-threshold psychopathological dimensions may co-occur with syndromal major depression.
By means of the methodological approach adopted in our study, we identified multiple predictors, but few moderators of treatment outcome. Thus, the study offers useful indicators of the characteristics of patients that are generally difficult to treat, but only limited guidance as to who benefits from IPT versus SSRI pharmacotherapy.
Despite the use of identical inclusion criteria and multiple efforts to minimize site differences in the study populations recruited, the US and Italian samples differed significantly in terms of gender and history of illness characteristics. Patients treated in Pittsburgh were more likely to be male, had longer histories of depression and more recurrent episodes and tended to be unmarried. Overall outcomes were better at Pisa, where patients were more likely to be married and to be experiencing their first episode of depression. While these demographic and clinical characteristics probably played some role in the differential treatment outcomes, it is more likely that unmeasured cultural factors (such as living in one's place of birth and the availability of a network of family members and friends) served to increase speed of remission at Pisa. Furthermore, as noted in our paper describing the implementation of this trial (Frank, et al., 2008), health system differences between the US and Italy led to differing incentives for participation (the availability of free treatment at Pittsburgh; the availability of IPT at Pisa) which, in turn, led to quite different participant groups. Of note, however, we observed no significant site x treatment interaction effects, suggesting that the administration of the treatments functioned in a similar manner at both sites.
In the present study of outpatients with moderately severe depression, the overall proportion of patients remitting on monotherapy at 12 weeks (44.6%) was higher than in the STAR*D (Rush, et al., 2006) efficacy/effectiveness trial. This difference is likely attributable to the comprehensiveness of the treatment protocol that involved weekly visits during the acute phase and the involvement of patients' family members through inclusion in their initial evaluation and in a psychoeducational workshop on depression and the goals of the study. The 12-week remission rates, however, are roughly comparable to those in other recent US trials conducted in academic medical centers comparing medication and psychotherapy. For example, in the DeRubeis, et al., (2005) and the Dimidjian, et al., (2006) studies, 16-week remission rates were 46% and 42% respectively for medication, and 40% for psychotherapy in both studies.
The following limitations must be considered in interpreting our results. First, the clinics in which the study was conducted were established specifically for the conduct of research, meaning that the full focus of clinicians' energy could be on the conduct of the study treatments during the time they spent in the respective clinics and that patients had access to a 24-hour on call system in case of emergencies or urgent after-hours questions about their treatment. Second, while all participants at Pisa were individuals presenting to the outpatient clinic of the hospital for treatment of depression, approximately two-thirds of study participants at Pittsburgh were recruited through public information announcements of the availability of free treatment to those willing to participate in research. Thus, different features of the study made it attractive at each site. At Pisa, the appeal was the availability of interpersonal psychotherapy for the first time in a department of psychiatry in Italy; at Pittsburgh, it was the availability of free treatment. Interestingly, however, participants at Pittsburgh were more likely to have a history of recurrent depression and had longer duration of illness, suggesting that they were not simply `symptomatic volunteers.' Third, other factors requiring more complex or time-intensive assessment that may also be related to the relative success of SSRI pharmacotherapy or interpersonal psychotherapy (e.g., personality pathology, treatment intensity – as measured by IPT specificity or escitalopram pharmacokinetics) were not discussed in this report. Future reports will examine their relationship to patient outcomes. Finally, it may be that our failure to find moderators is a function of the fact that a substantial proportion of participants in both groups were `responding' to being engaged in a comprehensive treatment protocol, thus producing sufficient noise that any moderation signal was difficult to detect.
Empirical validation of the findings of this exploratory study would require random assignment of patients who endorse high need for medical reassurance and high levels of psychomotor activation to IPT vs SSRI pharmacotherapy.
ACKNOWLEDGEMENTS
Dr. Ellen Frank has served as a consultant to Servier, has received grant/research support from The Fine Foundation, The Pittsburgh Foundation, and Forest Research Institute, and has received royalties from Guilford Press.
Dr. Cassano has served as a consultant to Aziende Chimiche Riunite Angelini Francesco ACRAF, Janssen Cilag, Abiogen Pharma, Pfizer Italy, Essex Italy, Eli Lilly Italy, GlaxoSmithKline, Boerhinger Ingelheim Italy, Sanofi Aventis, Sigma-Tau Industrie Farmaceutiche Riunite, Bristol-Meyers Squibb, Lundbeck Italy, and Innova Pharma.
Dr. Rucci has received research support from Forest Research Institute and Fondazione IDEA.
Dr. Fagiolini has been a consultant and/or a speaker for Boheringer Ingelheim, Bristol Mayer Squibb, Eli Lilly Italy, Lundbeck, Jannssen, Pfizer, Sigma Tau, and Takeida
Dr. Maggi has received honoraria for the teaching of interpersonal psychotherapy from Lundbeck, Italia.
Dr. Scocco has served as a speaker for Bristol-Myers Squibb and Eli Lilly and has received honoraria for the teaching of interpersonal psychotherapy from Lundbeck, Italia.
Drs. Grochocinski, Kupfer, Kraemer, Shear, Thompson, and, Mses. Buttenfield, Calugi, Houck, and Mr. Forgione, did not report any financial disclosures.
Dr. Victoria J. Grochocinski had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
This work was supported by National Institute of Mental Health grants MH065376 (Drs. Frank and Cassano) and MH030915 (Dr. Frank), investigator-initiated grants from Forest Research Institute (Dr. Frank) and Fondazione IDEA (Dr. Cassano).
REFERENCES
- Akiskal HS. The emergence of the bipolar spectrum: validation along clinical epidemiologic and familial-genetic lines. Psychopharmacology Bulletin. 2007;40:99–115. [PubMed] [Google Scholar]
- American Psychiatric Association Task Force for the Handbook for Psychiatric Measures: Handbook of Psychiatric Measures. 1 st Edition American Psychiatric Association; Washington DC: 2000. [Google Scholar]
- Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive Therapy of Depression Guiliford Press, New York, NY. Association; Washington, DC: 1979. [Google Scholar]
- Brückl TM, Wittchen HU, Höfler M, Pfister H, Schneider S, Lieb R. Childhood separation anxiety and the risk of subsequent psychopathology: results from a community study. Psychotherapy and Psychosomatics. 2007;76:47–56. doi: 10.1159/000096364. [DOI] [PubMed] [Google Scholar]
- Cassano GB, Benvenuti A, Miniati M, Calugi S, Mula M, Maggi L, Rucci P, Fagiolini A, Perris F, Frank E. The factor structure of lifetime depressive spectrum in patients with unipolar depression. Journal of Affective Disorders. 2009;115:87–99. doi: 10.1016/j.jad.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassano GB, Michelini S, Shear MK, Coli E, Maser JD, Frank E. The panic-agoraphobic spectrum: a descriptive approach to the assessment and treatment of subtle symptoms. American Journal of Psychiatry. 1997;154:27–38. doi: 10.1176/ajp.154.6.27. [DOI] [PubMed] [Google Scholar]
- Cassano GB, Mula M, Rucci P, Miniati M, Frank E, Kupfer DJ, Oppo A, Calugi S, Maggi L, Gibbons R, Fagiolini A. The structure of lifetime manic-hypomanic spectrum. Journal of Affective Disorders. 2009;112:59–70. doi: 10.1016/j.jad.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassano GB, Rucci P, Frank E, Fagiolini A, Dell'Osso L, Shear MK, Kupfer DJ. The mood spectrum in unipolar and bipolar disorder: arguments for a unitary approach. American Journal of Psychiatry. 2004;161:1264–1269. doi: 10.1176/appi.ajp.161.7.1264. [DOI] [PubMed] [Google Scholar]
- Dell'Osso L, Armani A, Rucci P, Frank E, Fagiolini A, Corretti G, Shear MK, Grochocinski VJ, Maser JD, Endicott J, Cassano GB. Measuring mood spectrum: comparison of interview (SCI-MOODS) and self-report (MOODS-SR) instruments. Comprehensive Psychiatry. 2002;43:69–73. doi: 10.1053/comp.2002.29852. [DOI] [PubMed] [Google Scholar]
- Dell'Osso L, Rucci P, Cassano GB, Maser JD, Endicott J, Shear MK, Sarno MK, Saettoni M, Grochocinski VJ, Frank E. Measuring social anxiety and obsessive-compulsive spectra: comparison of interviews and self-report instruments. Comprehensive Psychiatry. 2002;43:81–87. doi: 10.1053/comp.2002.30795. [DOI] [PubMed] [Google Scholar]
- DeRubeis RJ, Hollon SD, Amsterdam JD, Shelton RC, Young PR, Salomon RM, O'Reardon JP, Lovett ML, Gladis MM. Cognitive therapy vs medications in the treatment of moderate to severe depression. Archives of General Psychiatry. 2005;62:409–416. doi: 10.1001/archpsyc.62.4.409. [DOI] [PubMed] [Google Scholar]
- Dimidjian S, Dobson KS, Kohlenberg RJ, Gallop R, Markley DK, Atkins DC, Hollon SD, Schmaling KB, Addis ME, McGlinchey JB, Gollan JK, Dunner DL, Jacobson NS. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the acute treatment of adults with major depression. Journal of Consulting and Clinical Psychology. 2006;74:658–670. doi: 10.1037/0022-006X.74.4.658. [DOI] [PubMed] [Google Scholar]
- Dupont WD, Plummer WD. PS power and sample size program available for free on the Internet. Controlled Clinical Trials. 1997;18:274. [Google Scholar]
- Fagiolini A, Dell'Osso L, Pini S, Armani A, Bouanani S, Rucci P, Cassano GB, Endicott J, Maser JD, Shear MK, Grochocinski VJ, Frank E. Validity and reliability of a new instrument for assessing mood symptomatology: The Structured Clinical Interview for Mood Spectrum (SCI-MOODS) International Journal of Methods Psychiatry Research. 1999;8:71–82. [Google Scholar]
- Fava M, Rush AJ, Alpert JE, Balasubramani GK, Wisniewski SR, Carmin CN, Biggs MM, Zisook S, Leuchter A, Howland R, Warden D, Trivedi MH. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. American Journal of Psychiatry. 2008;165:342–351. doi: 10.1176/appi.ajp.2007.06111868. [DOI] [PubMed] [Google Scholar]
- Fournier JC, DeRubeis RJ, Shelton RC, Gallop R, Amsterdam JD, Hollon SD. Antidressant medications v. cognitive therapy in people with depression with or without personality disorder. The British Journal of Psychiatry. 2008;192:124–129. doi: 10.1192/bjp.bp.107.037234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier JC, DeRubeis RJ, Shelton RC, Hollon SD, Amsterdam JD, Gallop R. Prediction of response to medication and cognitive therapy in the treatment of moderate to severe depression. Journal of Consulting and Clinical Psychology. 2009;77:775–787. doi: 10.1037/a0015401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank E, Cassano GB, Rucci P, Fagiolini A, Maggi L, Kraemer HC, Kupfer DJ, Pollock B, Bies R, Nimgaonkar V, Pilkonis P, Shear MK, Thompson Grochocinski, V.J., Scocco P, Buttenfield J, Forgione RN. Addressing the challenges of a cross-national investigation: lessons from the Pittsburgh-Pisa Study of Treatment-Relevant Phenotypes of Unipolar Depression. Clinical Trials. 2008;5:253–261. doi: 10.1177/1740774508091965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank E, Shear MK, Rucci P, Cyranowski JM, Endicott J, Fagiolini A, Grochocinski VJ, Houck P, Kupfer DJ, Maser JD, Cassano GB. Influence of panic-agoraphobic spectrum symptomatology on treatment response in patients with recurrent major depression. American Journal of Psychiatry. 2000;157:1101–1107. doi: 10.1176/appi.ajp.157.7.1101. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer HC, Wilson GT, Fairburn CG, Agras WS. Mediators and moderators of treatment effects in randomized clinical trials. Archives of General Psychiatry. 2002;59:877–883. doi: 10.1001/archpsyc.59.10.877. Review. [DOI] [PubMed] [Google Scholar]
- Klerman GL, Weissmann MM, Rounsaville BJ, Chevron ES. Interpersonal Psychotherapy of Depression. Basic Books; New York, NY: 1984. [Google Scholar]
- Leykin Y, Amsterdam JD, DeRubeis RJ, Gallop R, Shelton RC, Hollon S,D. Progressive resistance to a selective serotonin reuptake inhibitor but not to cognitive therapy in the treatment of major depression. Journal of Consulting and Clinical Psychology. 2007;75:267–276. doi: 10.1037/0022-006X.75.2.267. [DOI] [PubMed] [Google Scholar]
- Mundt JC, Marks IM, Shear MK, Greist JH. The Work and Social Adjustment Scale: a simple measure of impairment in functioning. British Journal of Psychiatry. 2002;180:461–464. doi: 10.1192/bjp.180.5.461. [DOI] [PubMed] [Google Scholar]
- Phelps JR, Ghaemi SN. Improving the diagnosis of bipolar disorder: predictive value of screening tests. Journal of Affect Disorder. 2002;92:141–148. doi: 10.1016/j.jad.2006.01.029. Epub 2006 Mar 9. Review. [DOI] [PubMed] [Google Scholar]
- Pini S, Abelli M, Mauri M, Muti M, Lazzetta P, Banti S, Cassano GB. Clinical correlates and significance of separation anxiety in patients with bipolar disorder. Bipolar Disorders. 2005;7:370–376. doi: 10.1111/j.1399-5618.2005.00216.x. [DOI] [PubMed] [Google Scholar]
- Rucci P, Miniati M, Oppo A, Mula M, Calugi S, Frank E, Shear MK, Mauri M, Pini S, Cassano GB. The structure of lifetime panic-agoraphobic spectrum. Journal of Psychiatric Research. 2009;43:366–379. doi: 10.1016/j.jpsychires.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Rush AJ, O'Neal . Patient Rated Inventory of Side Effects. University of Texas Southwestern Medical Center; Dallas: 1999. unpublished rating scale. [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. American Journal of Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- Shear MK, Frank E, Rucci P, Fagiolini A, Grochocinski VJ, Houck P, Cassano GB, Kupfer DJ, Endicott J, Maser JD, Mauri M, Banti S. Panic-agoraphobic spectrum: reliability and validity of assessment instruments. Journal of Psychiatric Research. 2001;35:59–66. doi: 10.1016/s0022-3956(01)00002-4. [DOI] [PubMed] [Google Scholar]
- Sotsky SM, Glass DR, Shea T, Pilkonis PA, Collins JF, Elkin I, Watkins JT, Imber SD, Leber WR, Moyer J, Oliveri ME. Patient predictors of response to psychotherapy and pharmacotherapy: findings in the NIMH Treatment of Depression Collaborative Research Program. American Journal of Psychiatry. 1991;148:997–1008. doi: 10.1176/ajp.148.8.997. [DOI] [PubMed] [Google Scholar]
- Souery D, Oswald P, Massat I, Bailer U, Bollen J, Demyttenaere K, Kasper S, Lecrubier Y, Montgomery S, Serretti A, Zohar J, Mendlewicz J. Group for the Study of Resistant Depression. Clinical factors associated with treatment resistance in major depressive disorder: results from a European multicenter study. Journal of Clinical Psychiatry. 2007;68:1062–1070. doi: 10.4088/jcp.v68n0713. [DOI] [PubMed] [Google Scholar]
- Wagner E, Frank E, Steiner S. Discriminating maintenance treatments for recurrent depression: Development and implementation of a rating scale. Journal of Psychotherapy Practice and Research. 1992;1:280–290. [PMC free article] [PubMed] [Google Scholar]
- Weissman MM, Markowitz JC, Klerman GL. Comprehensive Guide to Interpersonal Psychotherapy. Basic Books; New York, NY: 2000. [Google Scholar]