Abstract
The circadian clock located in the mammalian suprachiasmatic nucleus (SCN) exhibits substantial heterogeneity in both its neurochemical and functional organization, with retinal input and oscillatory timekeeping functions segregated to different regions within the nucleus. Although it is clear that photic information must be relayed from directly retinorecipient cells to the population of oscillator cells within the nucleus, the intra-SCN signal (or signals) underlying such communication has yet to be identified. Gastrin-releasing peptide (GRP), which is found within calbindin-containing retinorecipient cells and causes photic-like phase shifts when applied directly to the SCN, is a candidate molecule. Here we examine the effect of GRP on both molecular and behavioral properties of the hamster circadian system. Within 30 min a third ventricle injection of GRP produces an increase in the number of cells expressing the phosphorylated form of extracellular signal-regulated kinases 1/2 (p-ERK1/2), localized in a discrete group of SCN cells that form a cap dorsal to calbindin cells and lateral to vasopressin cells. At 1 h after the peak of p-ERK expression these cap cells express c-fos, Period1, and Period2. Pharmacological blockade of ERK phosphorylation attenuates phase shifts to GRP. These data indicate that GRP is an output signal of retinorecipient SCN cells and activates a small cluster of SCN neurons. This novel cell group likely serves as a relay or integration point for communicating photic phase-resetting information to the rhythmic cells of the SCN. These findings represent a first step in deconstructing the SCN network constituting the brain clock.
Keywords: p44/p42 MAPK, hamster, gate, cap, core, shell, U0126
Introduction
Daily oscillations in physiology and behavior are generated endogenously and synchronized to environmental light/dark cycles by the mammalian circadian clock located in the suprachiasmatic nucleus (SCN). Although many of the cells within the SCN contain an intracellular molecular clock driven by a transcription-translation feedback loop (Reppert and Weaver, 2001), this is not true of all SCN cells (Hamada et al., 2001; Karatsoreos et al., 2004). Instead, this nucleus is heterogeneous in both structure and function (Moore, 1996; Antle et al., 2003; de la Iglesia et al., 2004; Antle and Silver, 2005).
In hamsters a group of calbindin-D28K-containing (CalB-containing) cells in the caudal SCN are retinorecipient (Bryant et al., 2000) and respond to photic input by expressing c-fos (Silver et al., 1996). After a light pulse these cells also express the Period genes haPer1 and haPer2, negative elements of the transcription-translation loops that form the molecular clock (Hamada et al., 2001). Cells in this region do not express detectable rhythms in haPer1 and haPer2 (Hamada et al., 2001). Furthermore, the CalB-containing cells lack daily rhythms in electrical activity (Jobst and Allen, 2002). The SCN region delineated by vasopressin-containing (VP-containing) cells rhythmically expresses haPer1 and haPer2, suggesting that this is a locus of cellular oscillators of the circadian clock. A similar organization has been noted in the mouse (Karatsoreos et al., 2004).
We have proposed a mathematical model that explains how neurons that receive and respond to light input can serve as gate cells that maintain phase coherence among a population of oscillator cells that do not themselves receive direct retinal input (Antle et al., 2003). This model is based on the physiology and anatomy of the SCN, in that the properties of the gates are based on hamster CalB cells and the properties of the oscillators are based on rhythmic cells in the VP region of the SCN.
Given this functional organization, it is important to identify the output signal from the light-responsive CalB cells to the rest of the SCN to gain an understanding of the neurochemical cascade producing photic entrainment. Gastrin-releasing peptide (GRP) is a promising candidate for such a signal. GRP is colocalized with CalB (LeSauter et al., 2002). c-fos expression is observed in GRP neurons after a light pulse in hamsters (Munch et al., 2002) and rats (Earnest et al., 1993; Romijn et al., 1996). GRP applied to the SCN resets the circadian clock in a manner similar to that of light both in vivo (Piggins et al., 1995) and in vitro (McArthur et al., 2000). GRP fibers leave the CalB region and course toward the dorsal SCN (LeSauter et al., 2002). GRP receptor-deficient mice demonstrate impaired phase shifts and gene expression responses to light pulses (Aida et al., 2002). Together, these data demonstrate that GRP has all of the properties required to signal photic-resetting information from the retinorecipient CalB region to the oscillators in the VP region of the SCN.
The current study was designed to determine sites of action of GRP. The results demonstrate that GRP activates extracellular signal-regulated kinase 1/2 (ERK1/2) phosphorylation and c-fos, haPer1, and haPer2 expression in a group of cells within the SCN that form a “cap” lying dorsal to the CalB cells and lateral to the VP neuronal population. Furthermore, the results show that behavioral phase shifts resulting from GRP application are dependent on the activation of a mitogen-activated protein kinase (MAPK) pathway involving the phosphorylation of ERK1/2. Together, these findings identify a novel SCN compartment that responds to GRP stimulation and is part of the neurochemical cascade associated with the synchronization of endogenous circadian oscillators to environmental time.
Materials and Methods
Animals and housing. Male hamsters (Lak:LVG; n = 65) were obtained at 6 weeks of age from Charles River Laboratories (Kingston, NY). Food and water were provided ad libitum. The animal colony room was kept on a 12 h light/dark cycle with a light intensity of 600 lux. The room was equipped with a white noise generator (91 dB sound pressure level) to mask environmental noise. For animals housed in constant darkness, a dim red light (<1 lux; Delta 1, Dallas, TX) and night-vision goggles (Alista, Richmond Hill, Ontario, Canada) allowed for husbandry and manipulations.
Before manipulations or before they were killed, the animals were housed for at least 1 week in cages equipped with running wheels (diameter, 16 cm). Locomotor activity was monitored continuously via a computer-based data acquisition system (Dataquest; Data Sciences, St. Paul, MN) that summed wheel revolution counts into 10 min bins. Data were downloaded and transferred to a Macintosh computer where they were visualized and analyzed with Circadia (Behavioral Cybernetics, Cambridge, MA).
All handling of animals was done in accordance with the Institutional Animal Care and Use Committee guidelines of Columbia University.
Experiment 1: examining GRP-induced phase shifts and gene expression in the circadian clock. Intra-SCN injections of GRP are known to produce phase shifts (Piggins et al., 1995). Because we wanted to examine GRP-induced changes in gene expression and because substantial nonspecific haPer1 and c-fos expression is associated with the presence of an intra-SCN cannula (data not shown), it was necessary to use third ventricle injections for the current experiments. The first experiment was designed to confirm that GRP phase-shifts the circadian clock when injected into the third ventricle. Because of the greater distance and potential for the injected chemical to be diluted in CSF, a larger volume (5 μl) was injected than has been used in tissue injections (i.e., 0.5 μl) (Piggins et al., 1995). Free-running animals received an injection of GRP (n = 11) or saline (n = 6) at circadian time 13 (CT13), which is 1 h after activity onset (by convention, activity onset in nocturnal animals is defined as CT12). Animals were left undisturbed for 10 d. Phase shifts were quantified.
After the behavioral phase shift experiment, some of the same animals were given GRP (n = 6) or saline (n = 6) at CT13. At CT14.5, they were anesthetized deeply and perfused. Their brains were processed for haPer1 and haPer2 mRNA in situ hybridization (ISH) and c-fos/CalB/VP immunocytochemistry (ICC).
So that we could compare GRP-induced expression of haPer1 and c-fos with the well described light-induced expression patterns of these genes (Hamada et al., 2001), additional animals received a light pulse (30 min, 2100 lux) beginning at CT13 (n = 2) or were left undisturbed (n = 1). These animals were anesthetized deeply and perfused at CT14.5.
Experiment 2: examining the time course of GRP-induced ERK1/2 phosphorylation. Animals were allowed to free run in constant darkness for 7-10 d, after which they received a CT13 injection of either GRP (n = 18) or saline (n = 13). They were anesthetized deeply and perfused 15, 30, 45, 60, 75, or 90 min (saline, two to three animals per time point; GRP, three animals per time point) after the injection started. Additionally, two untreated and uninjected control animals were perfused at CT13. All brains were processed for triple-labeled ICC for the phosphorylated form of ERK (p-ERK)/CalB/VP.
Experiment 3: examining whether the phosphorylation of ERK1/2 is necessary for GRP-induced phase shifts. Animals (n = 11) were allowed to free run in constant darkness for 10 d, after which they were given an injection of either the MAP kinase kinase (MEK) inhibitor 1,4-diamino-2,3-dicyano-1,4-bis(o-aminophenylmercapto) butadiene (U0126) or its vehicle control (50% DMSO/water) at CT12.5, followed by a GRP injection at CT13. After 10 d, the animals received the opposite CT12.5 treatment, either U0126 or its vehicle, before the CT13 GRP injection. An additional set of control animals (n = 5; previously treated with GRP alone from experiment 1) received only 50% DMSO at CT12.5.
Surgery. Animals were allowed at least 1 week acclimatization to the laboratory before undergoing surgery. They first were anesthetized deeply and placed in a stereotaxic frame. They then were implanted with a 9 mm, 22 gauge guide cannula (Plastics One, Roanoke, VA) aimed at either the third ventricle anterior to the SCN (coordinates 1.36 mm anterior to bregma, at midline, and either 7.3 or 7.0 mm below the skull surface with the incisor bar set at 2 mm below interaural level). The cannula was fixed in place with three stainless-steel screws and cranioplastic cement. Dummy cannulas and injection cannulas were cut to extend 1 mm beyond the end of the guide cannula. Animals were allowed to recover for 1 week before being placed in an activity-monitoring cage in constant darkness.
Behavior. Activity onsets for the 7 d before a manipulation were determined objectively as the first 10 min bin each day to exceed 100 counts without another such bin occurring in the preceding 240 min. For behavioral manipulations, the activity onsets also were determined for 3-10 d after the manipulation. Line fits were performed with Circadia, using linear regression. The difference in intercepts of these regression lines on the day after the manipulation was taken as the phase shift.
Injections. Substances injected included the following: GRP (100 pmol/μl sterile saline; Sigma, St. Louis, MO), the MEK1/2 inhibitor U0126 (5 nmol/μl 50% DMSO; Sigma), and their respective vehicle controls. Injection cannulas were connected to a 10 μl Hamilton syringe with polyethylene 20 tubing filled with distilled water. A small air bubble was introduced between the water and drug to prevent the two from mixing. Using night-vision goggles, we gently restrained animals by hand and injected them with 5 μl over 5 min. After administration of the drug, the injection cannula was left in place for an additional 3 min to allow the drug to diffuse.
Perfusion and tissue preparation. In the dark, the animals were given an overdose of pentobarbital. Once unconscious, their heads were wrapped in aluminum foil to prevent the eyes from being exposed to light. They then were perfused intracardially with PBS, followed by 4% paraformaldehyde. Brains were postfixed overnight and then cryoprotected in 20% sucrose for 24 h. With the use of a cryostat, we collected sections 35 μm thick (c-fos ICC and haPer1 and haPer2 ISH) or 50 μm thick (p-ERK ICC) through the anterior hypothalamus.
Immunocytochemistry. c-fos ICC (rabbit anti-c-fos, 1:20,000; Santa Cruz Biotechnology, Santa Cruz, CA), haPer1, and haPer2 ISH were performed on adjacent sections. p-ERK ICC (rabbit anti-phospho-42/44 MAPK, 1:400; Cell Signaling Technology, Beverly, MA) was performed on alternate sections. For c-fos and p-ERK ICC, the tissue also was immunolabeled with antibodies for CalB (monoclonal mouse anti-CalB, 1:20,000; Sigma) and VP (guinea pig anti-VP, 1:10,000; Peninsula Laboratories, San Carlos, CA).
Free-floating ICC for c-fos, CalB, and VP started with a 1 h blocking incubation in 2% normal donkey serum (NDS) in phosphate buffer with 0.1% Triton X-100 (0.1% PBT), followed by a 48 h incubation in the primary antibodies (in 0.3% PBT plus NDS) at 4°C. The tissue was rinsed three times in 0.1% PBT and then incubated with the following secondary antibodies: cyanine 2 (CY2) donkey anti-rabbit, CY3 donkey antiguinea pig, and CY5 donkey anti-mouse (each at 1:200; Jackson ImmunoResearch, West Grove, PA). Then the tissue was rinsed three times in phosphate buffer, mounted on gelatin-coated slides, dehydrated with alcohol rinses, cleared with xylene, air dried, and coverslipped with Krystalon.
Free-floating ICC for p-ERK was performed with the use of an amplification protocol. After the blocking step in 2% normal goat serum (NGS), the tissue was incubated in the p-ERK primary antibody (in 0.3% PBT plus NGS) for 48 h at 4°C. The tissue was rinsed six times in 0.1% PBT and then incubated for 1 h in a biotinylated goat anti-rabbit secondary antibody (Vector Laboratories, Burlingame, CA). After three rinses in 0.1% PBT, the tissue was incubated for 1 h in 0.2% avidinbiotin complex (Vectastain Elite ABC; Vector Laboratories). After three more 0.1% PBT rinses, the tissue was incubated for 30 min in biotinylated tyramide with 0.1% hydrogen peroxide in 0.1 m PBS. After three rinses in PBS, the tissue was incubated for 1 h in CY2-conjugated egg-white avidin (1:200; Jackson ImmunoResearch) in 0.1% PBT. After this, the tissue was rinsed five times in 0.1% PBT and then immunoreacted for CalB and VP as described above. In all cases the tissue was protected from light exposure once a fluorescent secondary was applied. Unless otherwise noted, all incubations were performed at room temperature on a shaker tray.
In situ hybridization. For haPer1 and haPer2 ISH, all instruments and trays were cleaned with RNase Zap (Ambion, Austin, TX), and all solutions were prepared with RNase-free water. Free-floating tissue sections were processed according to the digoxigenin (DiG) protocol of Yan and Silver (2002). First, tissue was treated with proteinase K (1 mg/ml; 0.1 m Tris buffer, pH 8.0, 50 mm EDTA for 10 min) at 37°C. This reaction was stopped by adding 2 ml of 4% paraformaldehyde. After rinses in SSC (in mm: 300 NaCl and 30 sodium citrate), the tissue was treated with 0.25% acetic anhydride in 0.1 m triethanolamine for 10 min. Then the sections were incubated in 1.5 ml of hybridization buffer (50% formamide, 60 mm sodium citrate, 600 mm NaCl, 10% dextran sulfate, 1% N-laurylsarcosine, 25 mg/ml tRNA, 1× Denhardt's solution, and 0.25 mg/ml salmonsperm DNA) containing the DiG-labeled haPer1 or haPer2 antisense cRNA probes (0.1 μg/ml) (for details, see Hamada et al., 2001) for 16 h at 60°C. After two 30 min high-stringency posthybridization washes (50% formamide/SSC at 60°C), the sections were treated with RNase A. After more high-stringency washes, the tissue then was processed for immunodetection with a nucleic acid detection kit (Roche, Indianapolis, IN). The sections were incubated in 1.0% of blocking reagent in buffer 1 (in mm: 100 Tris-HCl buffer, 150 NaCl, pH 7.5) for 1 h at room temperature. Sections then were incubated at 4°C in alkaline phosphatase-conjugated DiG antibodies diluted 1:5000 in buffer 1 for 3 d. On the following day, the sections were washed in buffer 1 two times (15 min each) and incubated in buffer 3 (in mm: 100 Tris-HCl buffer, pH 9.5, containing 100 NaCl and 50 MgCl2) for 5 min. Then they were incubated in a solution containing nitroblue tetrazolium salt (0.34 mg/ml) and 5-bromo-4-chloro-3-indolyl phosphate toluidinium salt (0.18 mg/ml; Roche) for 16 h. The colorimetric reaction was stopped by immersing the sections in buffer 4 (in mm:10 Tris-HCl containing 1 EDTA, pH 8.0). Then the tissue was mounted on gelatin-coated slides, briefly dehydrated in an alcohol series, cleared with Citrasolve, briefly air-dried, and coverslipped with Permount.
Image analysis. Photographs of immunofluorescently labeled tissue were taken on a Nikon (Tokyo, Japan) Eclipse E800 microscope equipped with the following filters: GFP, Texas Red, and CY5, each of which passes the signal for CY2, CY3, and CY5, respectively, while filtering out the signal from the other two fluorophores. Bright-field photographs of DiG-labeled tissue were taken on an Olympus (Tokyo, Japan) BH2 microscope. Each microscope was fit with a cooled CCD digital camera with SPOT software (Diagnostic Instruments, Sterling Heights, MI). Images were loaded into Photoshop (Adobe Systems, San Jose, CA) for cell counting. Templates of the CalB and VP phenotypic regions were delineated with smooth lines on triple-labeled images. These templates then were used as best estimates of regional boundaries for adjacent sections from the same animal processed for in situ hybridization. Cells were counted bilaterally in the section containing the CalB subregion (ICC) or in the section adjacent to the section containing the CalB subregion (ISH).
Statistical analysis. Data were analyzed with paired or independent sample t tests or ANOVAs as appropriate. Significant differences detected by ANOVAs were analyzed additionally with Tukey's honestly significant difference (HSD) post hoc tests. All means are reported ± SEM.
Results
Experiment 1: GRP phase shifts and induced gene expression in the circadian clock
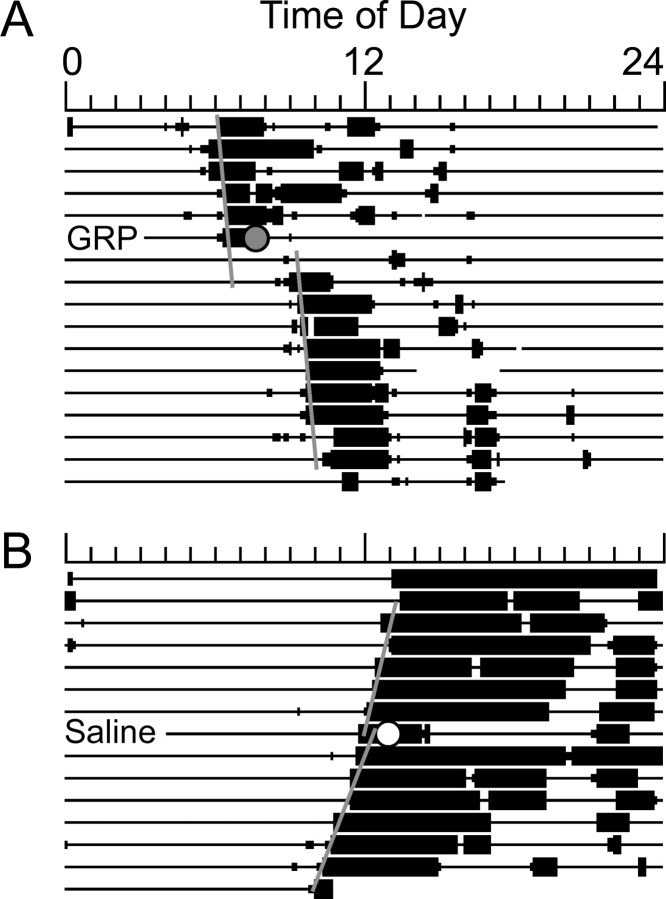
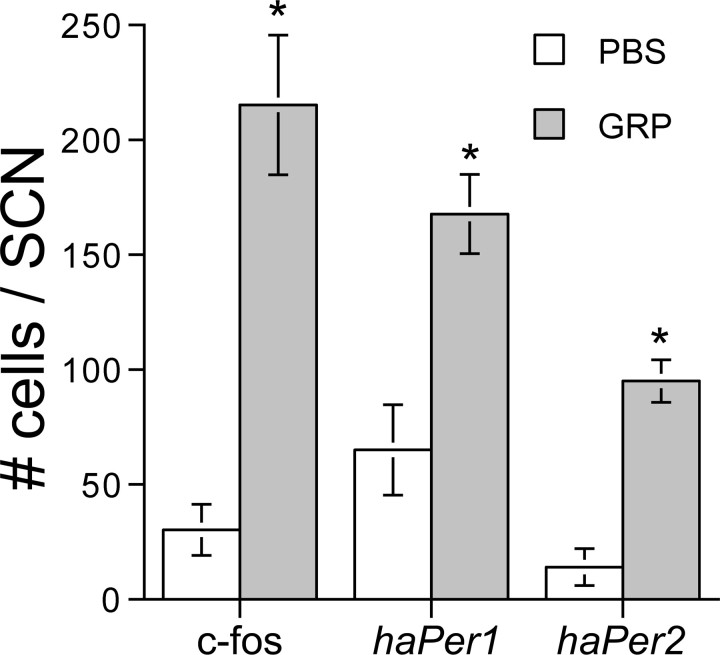
Injections of GRP produced significant phase delays of behavioral circadian rhythms compared with vehicle controls (1.53 ± 0.31 vs 0.15 ± 0.14 h delays; t(9) = 4.29; p < 0.01) (Fig. 1). GRP injections in the six animals with more ventrally placed cannulas [dorsoventral (DV), 7.3 mm below skull surface] produced phase shifts that were significantly greater from vehicle control (0.89 ± 0.17 h; t(10) = 3.14; p < 0.01) but significantly smaller (one-tailed t(9) = 2.00; p < 0.05) than shifts observed in animals with more dorsal cannula placements (DV, 7.0 mm below skull surface). GRP in these animals induced significantly more c-fos (t(10) = 5.88; p < 0.001), haPer1 (t(10) = 3.93; p < 0.01), and haPer2 (t(6) = 6.61; p < 0.001) expression than did saline treatment. GRP induced significantly more c-fos in cells in the cap region than in the CalB region (cap, 167 ± 23; CalB, 48 ± 8; t(10) = 4.84; p < 0.001). Representative photomicrographs are presented in Figure 2, and mean cell counts are presented in Figure 3. In extra-SCN areas, c-fos induction in response to GRP was not noted, although this was not analyzed quantitatively. There was no relationship between the amount of c-fos expression observed and the size of the GRP-induced phase shift in hamsters in which both responses were examined (n = 6; Pearson's r = 0.16; p > 0.05), although gene expression was not examined in animals with more dorsally located cannulas that had exhibited large phase shifts (i.e., >70 min).
Figure 1.
Sample actograms from animals housed in constant darkness depicting typical phase shifts that follow an anterior third ventricle injection of either 5 μl of 100 pmol/μl GRP (A) or 5 μl of saline (B) 1 h after activity onset (i.e., CT13).
Figure 2.
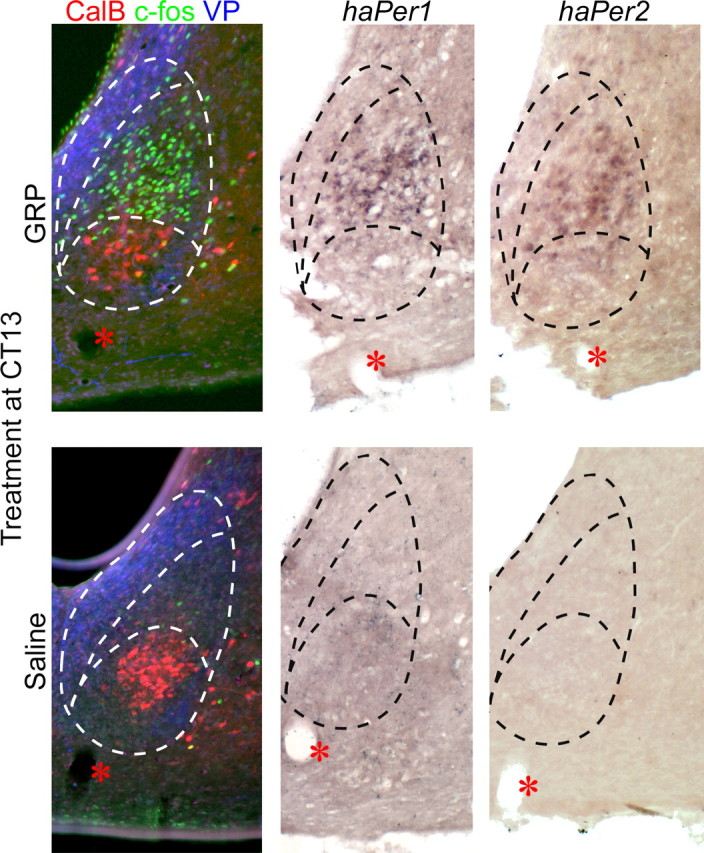
Sample SCN photomicrographs from animals given an injection of either GRP (5 μl of 100 pmol/μl; top row) or saline (5 μl; bottom row) to the anterior third ventricle 1 h after activity onset (i.e., CT13) and perfused 90 min later (i.e., CT14.5). Each row represents three adjacent sections from the same animal processed for triple-labeled ICC (red, CalB; green, c-fos; blue, VP), haPer1 DiG ISH, and haPer2 DiG ISH, respectively. Dashed black and white lines represent the borders of the SCN and of the CalB and VP immunoreactivity defined in the first column. Given that the templates were defined in an adjacent section, the dashed black lines on the in situ hybridization photomicrographs represent a best estimate of regional boundaries in that section. The red asterisk indicates the location of a blood vessel found in all three adjacent sections.
Figure 3.
Bar graph representing the mean ± SEM of cell counts expressing c-fos, haPer1, or haPer2 after an injection of either GRP (5 μl of 100 pmol/μl) or saline (5 μl) to the anterior third ventricle 1 h after activity onset (i.e., CT13) and perfused 90 min later (i.e., CT14.5). The asterisk indicates a significant difference in the number of cells compared with the saline control (independent t tests; p < 0.01).
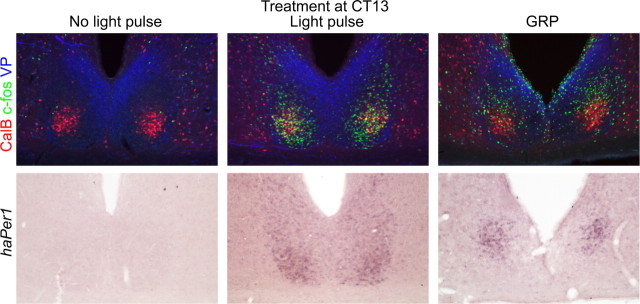
c-fos, haPer1, and haPer2 expression after GRP administration was restricted almost exclusively to a region within the SCN that was dorsal to the CalB-immunoreactive and lateral to the VP-immunoreactive subregions, as determined from triple-labeled (c-fos) or adjacent (haPer1 and haPer2) sections, although a small number of cells expressing c-fos, haPer1, or haPer2 were seen within the dorsal portion of the CalB region template in most animals. Based on their appearance resting on top of the CalB region, this population of GRP-induced cells has been termed “cap cells.” This pattern of activation is in contrast to light pulse-induced expression. After light exposure, the c-fos expression was found mostly in the CalB region, although some c-fos-immunoreactive cells also were located in the dorsolateral region where expression was observed after GRP (Fig. 4). haPer1 expression patterns were similar, based on adjacent sections immunolabeled for CalB and VP (Fig. 4).
Figure 4.
Sample SCN photomicrographs from an untreated animal (first column), an animal exposed to a 30 min, 2100 lux light pulse at CT13 (second column), and an animal given an injection of GRP (5 μl of 100 pmol/μl) at CT13. All animals were perfused at CT14.5. Each column represents adjacent sections from the same animal processed either for triple-labeled immunocytochemistry (top row; red, CalB; green, c-fos; blue, VP) or for haPer1 DiG ISH (bottom row). Little c-fos or haPer1 expression is observed in the control animal. After a light pulse, the expression of both c-fos and haPer1 is observed in the CalB subregion as well as in the dorsolateral SCN. Most CalB cells in these light-treated animals appeared to contain c-fos. After a GRP injection, the expression of both c-fos and haPer1 is observed in the SCN, primarily in cells dorsal to the CalB region and lateral to the VP region. Little GRP-induced c-fos expression is observed in the CalB region.
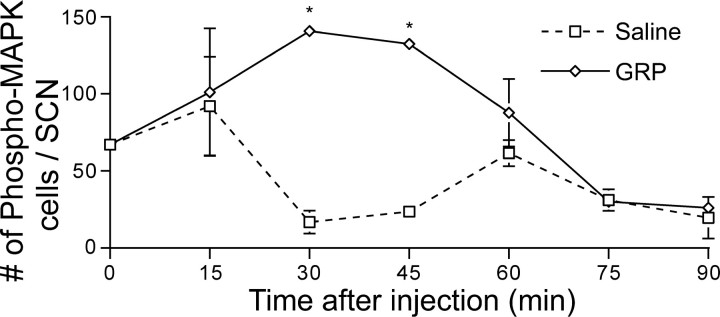
Experiment 2: GRP rapidly induces transient phosphorylation of ERK1/2 in the SCN
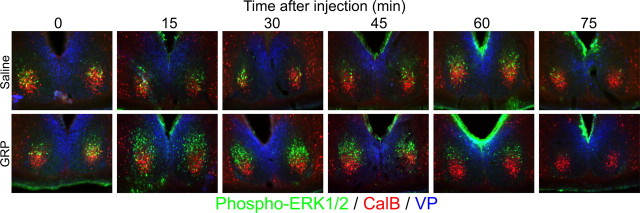
GRP induced a significant transient increase in the number of cells containing the phosphorylated form of MAPK p42/p44 (treatment × time interaction; F(5,19) = 5.25; p < 0.01) (Figs. 5, 6). The number of p-ERK-containing cells was greatest at 30 and 45 min after GRP administration. These cells were restricted to a subregion of the SCN dorsal to the CalB region and lateral to the VP region. The number of cells containing phosphorylated MAPK p42/p44 fell from 60 min after injection and was significantly below untreated baseline levels (sampled at CT13) after 75 and 90 min (t(3) = 5.856 and p < 0.05; t(3) = 6.127 and p < 0.01, respectively).
Figure 5.
Photomicrographs triple-labeled for CalB (red), p-ERK (green), and VP (blue) depicting the spatial and temporal expression pattern of p-ERK in the SCN after injections of either saline (5 μl; top row) or GRP (5 μl of 100 pmol/μl; bottom row). Animals were given an injection to the anterior third ventricle at CT13 and were perfused 0, 15, 30, 45, 60, or 75 min later.
Figure 6.
Quantification of p-ERK expression (mean ± SEM number of cells) in the SCN after injections of either saline (5 μl) or GRP (5 μl of 100 pmol/μl). Animals were given an injection to the anterior third ventricle at CT13 and were perfused 0, 15, 30, 45, 60, 75, or 90 min later. The asterisk indicates a significant difference in the number of cells compared with the saline-treated control (Tukey's HSD; p < 0.05).
Experiment 3: phosphorylation of ERK1/2 is necessary for GRP-induced phase shifts
Pretreatment with the MEK inhibitor U0126 at CT12.5 significantly attenuated phase shifts to GRP injections at CT13 when compared with animals pretreated with vehicle (DMSO at CT12.5) before the GRP injection (paired t test; t(10) = 3.892; p < 0.01) (Fig. 7). The phase delays were larger in the DMSO plus GRP (t(14) = 4.648; p < 0.001) and DMSO plus U0126 plus GRP (t(14) = 2.887; p < 0.05) groups than were the phase shifts observed in the DMSO control group.
Figure 7.
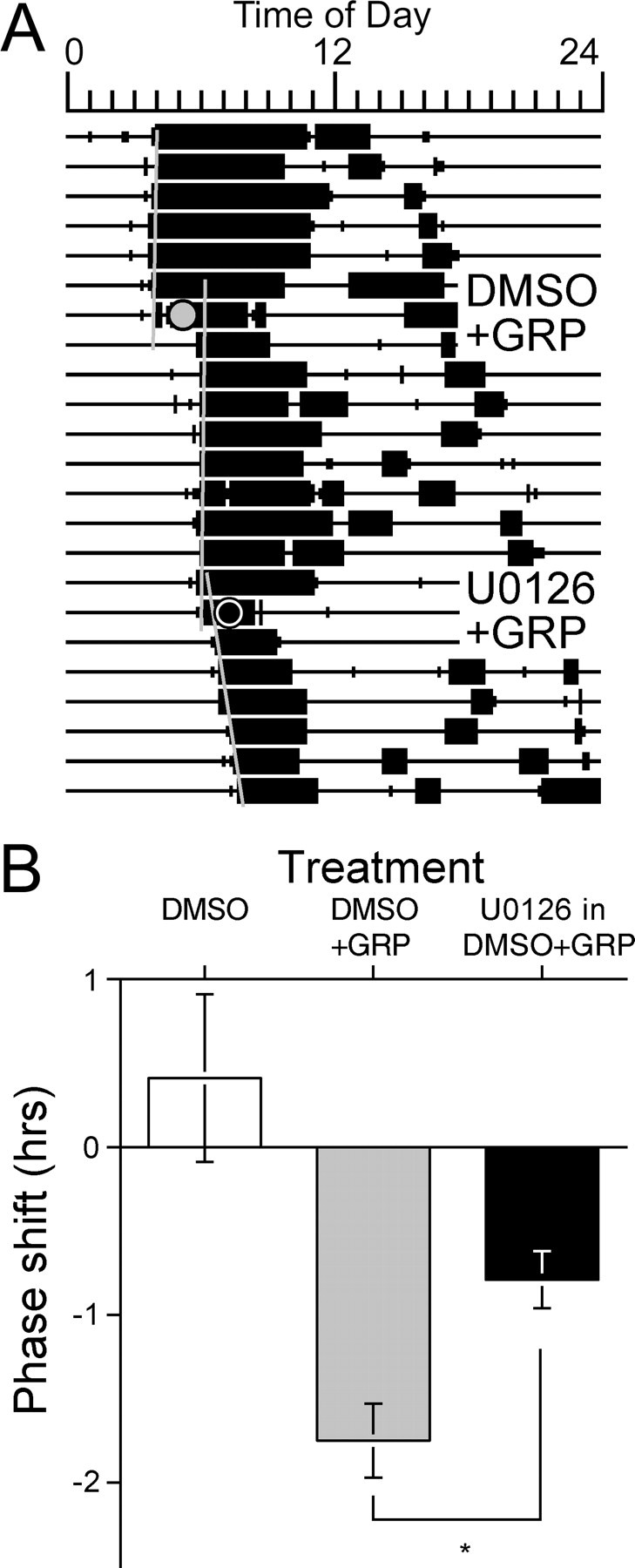
A, Sample actogram from an animal receiving a CT13 third ventricle injection of GRP (5 μl of 100 pmol/μl) after CT12.5 pretreatment with either DMSO vehicle control (5 μl of 50% DMSO) or the MEK inhibitor U0126 (25 nmol in 5 μl of 50% DMSO). B, Bar graph representing the mean ± SEM phase shifts resulting from the following treatment: DMSO alone at CT12.5; DMSO at CT12.5 followed by GRP at CT13; and U0126 in DMSO at CT12.5 followed by GRP at CT13. The asterisk indicates a significant attenuation of the phase shift compared with the DMSO plus GRP control treatment (paired t test; p < 0.01).
Discussion
The SCN is a heterogeneous structure composed of a nonrhythmic retinorecipient region and a rhythmic, non-retinorecipient region (Hamada et al., 2001; Antle et al., 2003). For light to reset the phase of the clock, photic-resting signals must be relayed from the directly retinorecipient cells to the rhythmic cells in the rest of the nucleus. We hypothesized that GRP could serve as such a signal to the rhythmic region based on two lines of evidence. First, ∼50% of CalB cells contain GRP, and ∼50% of GRP cells contain CalB (LeSauter et al., 2002). Second, when applied to the SCN in vivo or in vitro, GRP produces photic-like phase shifts (Piggins et al., 1995; McArthur et al., 2000). Although our results are consistent with GRP being an output signal of the retinorecipient region, we were surprised to find that GRP does not act throughout the nucleus. Instead, GRP induces the expression of haPer1, haPer2, and c-fos in a small population of cells in the SCN, suggesting that the retinorecipient CalB/GRP region communicates photic information directly to these cells. These cells form a cap lying dorsal to the retinorecipient and light-responsive CalB region and lateral to VP cell population that contains rhythmic clock cells (Hamada et al., 2001). Presumably, the phase-setting photic information then is relayed onward to cells located in the rhythmic VP region. The observation that GRP injections transiently activate the MAPK pathway and that impairment of the MAPK pathway attenuates GRP-induced phase shifts implicates this pathway as an important downstream mediator for GRP. Together, these findings delineate the network properties of the SCN downstream of the retinorecipient CalB region and identify important intracellular events underlying GRP-induced phase shifts.
Functional responses in cells with an anatomical location similar to the cap cells have been identified with other experimental designs. Although light pulses induce c-fos expression primarily in the region delineated by calbindin cells in the ventrolateral SCN, some scattered expression is found in the dorsolateral SCN (Rusak et al., 1990; Silver et al., 1996). Pretreatment with glutamate antagonists prevents light-induced c-fos expression in the ventrolateral SCN, but not in the dorsolateral SCN (Abe et al., 1991, 1992). Stimulation of the intergeniculate leaflet (IGL) induces c-fos expression in dorsolateral SCN cells (Abe and Rusak, 1994). During the night, the cells dorsal to the CalB region contain p-ERK (Lee et al., 2003). This nocturnal expression of p-ERK requires input from the eye, because enucleation eliminates this rhythm but does not affect daytime expression of p-ERK in VP cells. Together, these results suggest that these cap cells form a separate cluster of input cells in the SCN, distinct from those located in the CalB region. They receive photic information from three separate sources: directly from the retina (Lee et al., 2003), indirectly via the IGL (Abe and Rusak, 1994), and indirectly from GRP cells in the CalB region (this study). Given these various photic inputs, the cap cells likely serve an integrating function. In support, GRP receptor-deficient mice exhibit smaller phase shifts to light pulses than do wild-type mice, but only for bright 300 lux light pulses; phase shifts to dim 30 lux light pulses do not differ between GRP receptor-deficient and wild-type mice (Aida et al., 2002). Small responses to dim light may be mediated primarily by innervation from the retina and IGL, whereas responses to brighter light may require the involvement of a GRP signal from the CalB region as well.
Animals pretreated with the NMDA antagonist MK-801 exhibit attenuated behavioral phase shifts after exposure to a light pulse (Colwell et al., 1990). Despite this, animals treated with a wide range of glutamate antagonists before a light pulse still exhibit light-induced c-fos expression in the dorsolateral SCN (Abe et al., 1991, 1992), the region of cap cells. These results suggest that activation of the cap cells (indexed by c-fos induction) may not be sufficient for phase shifts. It is not known whether NMDA antagonists attenuate GRP-induced phase shifts, and it is possible that, although these drugs do not block c-fos induction in cap cells, they may block their outputs.
Several findings are consistent with GRP being an important signal from cells in the retinorecipient SCN. GRP frequently is colocalized with CalB (LeSauter et al., 2002). Also, GRP cells are retinorecipient in rats (Tanaka et al., 1997) and mice (Karatsoreos et al., 2004). Cells containing GRP express c-fos and Per1 after a light pulse (Earnest et al., 1993; Romijn et al., 1996, 1998; Dardente et al., 2002). When applied in an acute brain slice preparation, GRP increases the firing rate of ∼50% of SCN neurons (Piggins and Rusak, 1993; Tang and Pan, 1993; Piggins et al., 1994; Pinnock et al., 1994). GRP phase-shifts the circadian clock in vivo (Piggins et al., 1995) and in vitro (McArthur et al., 2000). If GRP is the output signal of the retinorecipient cells, then it should activate only events downstream of these retinorecipient cells. Comparison of the c-fos expression patterns after a light pulse with those observed after a GRP injection confirms this hypothesis. Although light pulses activate cells in both the CalB region (Silver et al., 1996; Hamada et al., 2001) and the dorsal SCN (Rusak et al., 1990), GRP administration induces gene expression only in the cap cells. Similarly, GRP activated ERK only in the cap cells. Although not examined specifically in previous studies (Butcher et al., 2002, 2003; Coogan and Piggins, 2003; Dziema et al., 2003), it appears that light activates ERK in both the retinorecipient ventrolateral SCN and the cap region. Given that GRP-immunoreactive fibers (LeSauter et al., 2002), GRP-induced gene expression, and ERK activation occur in a common region of the SCN, it is likely that GRP is acting on these cap cells, although the current experiments cannot distinguish direct from indirect routes of action. Mapping of GRP receptor distribution and regional analysis of cellular responses to in vitro GRP application will help to determine the precise site of action for GRP.
The present results are consistent with GRP acting as an intra-SCN signal originating from retinorecipient cells in the SCN. It should be noted that differences in the amount and precise localization of gene expression in the dorsal SCN after GRP injection versus a light pulse are to be expected. This could result from global pharmacological activation consequent to an acute increase in GRP levels in the extracellular space, whereas light pulse-induced physiological activation results from transmitter release at specific synaptic terminals. Furthermore, terminals of the retinohypothalamic tract terminate not only on GRP cells but also on other peptidergic SCN cells (Antle and Silver, 2005). Finally, it is unlikely that GRP is the only chemical messenger released by these retinorecipient cells, and the physiological expression patterns observed after a light pulse likely result from an interaction of GRP with other transmitter substances, such as GABA, glutamate, vasoactive intestinal polypeptide (VIP), and substance P (Antle and Silver, 2005).
The phenotypic heterogeneity of the SCN has long been appreciated (van den Pol and Tsujimoto, 1985; Moore, 1996). Originally, the SCN was subdivided into a ventrolateral “core,” defined by cells and fibers that are VIP-immunoreactive, and a dorsomedial “shell,” defined by VP immunoreactivity. These phenotypic divisions have been recapitulated only partially by functional heterogeneity. Whereas VIP itself has been implicated in regulation of rhythmicity (Reed et al., 2001; Dardente et al., 2002; Harmar et al., 2002; Colwell et al., 2003; Cutler et al., 2003; Kawamoto et al., 2003; Piggins and Cutler, 2003; Hughes et al., 2004; Meyer-Spasche and Piggins, 2004) and whereas VIP-containing cell bodies define a subcompartment of the SCN core, recent findings suggest that photic responses are associated primarily with a distinct subset of SCN core cells that contain CalB in hamsters (Silver et al., 1996; Hamada et al., 2001, 2003, 2004). Rats also have a group of VIP cells in the ventral SCN, but only the ventrolateral VIP cells, which are light-responsive, contain GRP (Kawamoto et al., 2003), suggesting again that GRP-containing cells underlie initial responses to photic stimulation. Although the phenotype and projection patterns of the cap cells are not yet known, the present results demonstrate the existence of a third SCN core cell population in addition to the VIP and the CalB/GRP populations.
In conclusion, the present experiments identify GRP as a candidate output signal from the retinorecipient CalB cells. Furthermore, the results have revealed a novel subregion of the SCN that responds to GRP application with activation of the p44/p42 MAPK pathway and with c-fos, haPer1, and haPer2 expression. This region lies within the SCN core and is downstream of the CalB region in the cascade of events that follow exposure to phase-shifting light pulses. Together, the present findings further elucidate the mechanisms by which light information is received and interpreted by the SCN and subsequently is transmitted to pacemaker cells to synchronize their activity with the environmental light/dark cycle. Likewise, these findings underscore the importance of investigations into the functional segregation of the SCN as well as the use of this tractable system for understanding neural networks within the CNS.
Footnotes
This research was supported by National Institutes of Health Grant NS37919 (R.S.) and by a fellowship from the Canadian Institutes of Health Research (M.C.A.). We thank Dr. Joseph LeSauter for insightful comments on a previous draft of this manuscript and Jasmine Sasanian for technical assistance.
Correspondence should be addressed to Michael C. Antle, Department of Psychology, University of Calgary, 2500 University Drive Northwest, Calgary, Alberta, Canada T2N 1N4. E-mail: antlem@ucalgary.ca.
L. J. Kriegsfeld's present address: Psychology Department, University of California Berkeley, 3210 Tolman Hall, Box 1650, Berkeley, CA 94720-1650.
Copyright © 2005 Society for Neuroscience 0270-6474/05/252447-08$15.00/0
References
- Abe H, Rusak B (1994) Physiological mechanisms regulating photic induction of Fos-like protein in hamster suprachiasmatic nucleus. Neurosci Biobehav Rev 18: 531-536. [DOI] [PubMed] [Google Scholar]
- Abe H, Rusak B, Robertson HA (1991) Photic induction of Fos protein in the suprachiasmatic nucleus is inhibited by the NMDA receptor antagonist MK-801. Neurosci Lett 127: 9-12. [DOI] [PubMed] [Google Scholar]
- Abe H, Rusak B, Robertson HA (1992) NMDA and non-NMDA receptor antagonists inhibit photic induction of Fos protein in the hamster suprachiasmatic nucleus. Brain Res Bull 28: 831-835. [DOI] [PubMed] [Google Scholar]
- Aida R, Moriya T, Araki M, Akiyama M, Wada K, Wada E, Shibata S (2002) Gastrin-releasing peptide mediates photic entrainable signals to dorsal subsets of suprachiasmatic nucleus via induction of Period gene in mice. Mol Pharmacol 61: 26-34. [DOI] [PubMed] [Google Scholar]
- Antle MC, Silver R (2005) Orchestrating time: arrangements of the brain's circadian clock. Trends Neurosci, in press. [DOI] [PubMed]
- Antle MC, Foley DK, Foley NC, Silver R (2003) Gates and oscillators: a network model of the brain clock. J Biol Rhythms 18: 339-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant DN, LeSauter J, Silver R, Romero MT (2000) Retinal innervation of calbindin-D28K cells in the hamster suprachiasmatic nucleus: ultrastructural characterization. J Biol Rhythms 15: 103-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher GQ, Dziema H, Collamore M, Burgoon PW, Obrietan K (2002) The p42/44 mitogen-activated protein kinase pathway couples photic input to circadian clock entrainment. J Biol Chem 277: 29519-29525. [DOI] [PubMed] [Google Scholar]
- Butcher GQ, Lee B, Obrietan K (2003) Temporal regulation of light-induced extracellular signal-regulated kinase activation in the suprachiasmatic nucleus. J Neurophysiol 90: 3854-3863. [DOI] [PubMed] [Google Scholar]
- Colwell CS, Ralph MR, Menaker M (1990) Do NMDA receptors mediate the effects of light on circadian behavior? Brain Res 523: 117-120. [DOI] [PubMed] [Google Scholar]
- Colwell CS, Michel S, Itri J, Rodriguez W, Tam J, Lelievre V, Hu Z, Liu X, Waschek JA (2003) Disrupted circadian rhythms in VIP- and PHI-deficient mice. Am J Physiol Regul Integr Comp Physiol 285: R939-R949. [DOI] [PubMed] [Google Scholar]
- Coogan AN, Piggins HD (2003) Circadian and photic regulation of phosphorylation of ERK1/2 and Elk-1 in the suprachiasmatic nuclei of the Syrian hamster. J Neurosci 23: 3085-3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler DJ, Haraura M, Reed HE, Shen S, Sheward WJ, Morrison CF, Marston HM, Harmar AJ, Piggins HD (2003) The mouse VPAC2 receptor confers suprachiasmatic nuclei cellular rhythmicity and responsiveness to vasoactive intestinal polypeptide in vitro Eur J Neurosci 17: 197-204. [DOI] [PubMed] [Google Scholar]
- Dardente H, Poirel VJ, Klosen P, Pevet P, Masson-Pevet M (2002) Per and neuropeptide expression in the rat suprachiasmatic nuclei: compartmentalization and differential cellular induction by light. Brain Res 958: 261-271. [DOI] [PubMed] [Google Scholar]
- de la Iglesia HO, Cambras T, Schwartz WJ, Diez-Noguera A (2004) Forced desynchronization of dual circadian oscillators within the rat suprachiasmatic nucleus. Curr Biol 14: 796-800. [DOI] [PubMed] [Google Scholar]
- Dziema H, Oatis B, Butcher GQ, Yates R, Hoyt KR, Obrietan K (2003) The ERK/MAP kinase pathway couples light to immediate-early gene expression in the suprachiasmatic nucleus. Eur J Neurosci 17: 1617-1627. [DOI] [PubMed] [Google Scholar]
- Earnest DJ, DiGiorgio S, Olschowka JA (1993) Light induces expression of Fos-related proteins within gastrin-releasing peptide neurons in the rat suprachiasmatic nucleus. Brain Res 627: 205-209. [DOI] [PubMed] [Google Scholar]
- Hamada T, LeSauter J, Venuti JM, Silver R (2001) Expression of Period genes: rhythmic and nonrhythmic compartments of the suprachiasmatic nucleus pacemaker. J Neurosci 21: 7742-7750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada T, LeSauter J, Lokshin M, Romero MT, Yan L, Venuti JM, Silver R (2003) Calbindin influences response to photic input in suprachiasmatic nucleus. J Neurosci 23: 8820-8826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada T, Antle MC, Silver R (2004) Temporal and spatial expression patterns of canonical clock genes and clock-controlled genes in the suprachiasmatic nucleus. Eur J Neurosci 19: 1741-1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmar AJ, Marston HM, Shen S, Spratt C, West KM, Sheward WJ, Morrison CF, Dorin JR, Piggins HD, Reubi JC, Kelly JS, Maywood ES, Hastings MH (2002) The VPAC2 receptor is essential for circadian function in the mouse suprachiasmatic nuclei. Cell 109: 497-508. [DOI] [PubMed] [Google Scholar]
- Hughes AT, Fahey B, Cutler DJ, Coogan AN, Piggins HD (2004) Aberrant gating of photic input to the suprachiasmatic circadian pacemaker of mice lacking the VPAC2 receptor. J Neurosci 24: 3522-3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobst EE, Allen CN (2002) Calbindin neurons in the hamster suprachiasmatic nucleus do not exhibit a circadian variation in spontaneous firing rate. Eur J Neurosci 16: 2469-2474. [DOI] [PubMed] [Google Scholar]
- Karatsoreos IN, Yan L, LeSauter J, Silver R (2004) Phenotype matters: identification of light-responsive cells in the mouse suprachiasmatic nucleus. J Neurosci 24: 68-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto K, Nagano M, Kanda F, Chihara K, Shigeyoshi Y, Okamura H (2003) Two types of VIP neuronal components in rat suprachiasmatic nucleus. J Neurosci Res 74: 852-857. [DOI] [PubMed] [Google Scholar]
- Lee HS, Nelms JL, Nguyen M, Silver R, Lehman MN (2003) The eye is necessary for a circadian rhythm in the suprachiasmatic nucleus. Nat Neurosci 6: 111-112. [DOI] [PubMed] [Google Scholar]
- LeSauter J, Kriegsfeld LJ, Hon J, Silver R (2002) Calbindin-D(28K) cells selectively contact intra-SCN neurons. Neuroscience 111: 575-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur AJ, Coogan AN, Ajpru S, Sugden D, Biello SM, Piggins HD (2000) Gastrin-releasing peptide phase-shifts suprachiasmatic nuclei neuronal rhythms in vitro J Neurosci 20: 5496-5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Spasche A, Piggins HD (2004) Vasoactive intestinal polypeptide phase-advances the rat suprachiasmatic nuclei circadian pacemaker in vitro via protein kinase A and mitogen-activated protein kinase. Neurosci Lett 358: 91-94. [DOI] [PubMed] [Google Scholar]
- Moore RY (1996) Entrainment pathways and the functional organization of the circadian system. Prog Brain Res 111: 103-119. [DOI] [PubMed] [Google Scholar]
- Munch IC, Moller M, Larsen PJ, Vrang N (2002) Light-induced c-Fos expression in suprachiasmatic nuclei neurons targeting the paraventricular nucleus of the hamster hypothalamus: phase dependence and immunochemical identification. J Comp Neurol 442: 48-62. [DOI] [PubMed] [Google Scholar]
- Piggins HD, Cutler DJ (2003) The roles of vasoactive intestinal polypeptide in the mammalian circadian clock. J Endocrinol 177: 7-15. [DOI] [PubMed] [Google Scholar]
- Piggins HD, Rusak B (1993) Electrophysiological effects of pressure-ejected bombesin-like peptides on hamster suprachiasmatic nucleus neurons in vitro J Neuroendocrinol 5: 575-581. [DOI] [PubMed] [Google Scholar]
- Piggins HD, Cutler DJ, Rusak B (1994) Effects of ionophoretically applied bombesin-like peptides on hamster suprachiasmatic nucleus neurons in vitro Eur J Pharmacol 271: 413-419. [DOI] [PubMed] [Google Scholar]
- Piggins HD, Antle MC, Rusak B (1995) Neuropeptides phase shift the mammalian circadian pacemaker. J Neurosci 15: 5612-5622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinnock RD, Reynolds T, Woodruff GN (1994) Different types of bombesin receptors on neurons in the dorsal raphe nucleus and the rostral hypothalamus in rat brain slices in vitro Brain Res 653: 119-124. [DOI] [PubMed] [Google Scholar]
- Reed HE, Meyer-Spasche A, Cutler DJ, Coen CW, Piggins HD (2001) Vasoactive intestinal polypeptide (VIP) phase-shifts the rat suprachiasmatic nucleus clock in vitro Eur J Neurosci 13: 839-843. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR (2001) Molecular analysis of mammalian circadian rhythms. Annu Rev Physiol 63: 647-676. [DOI] [PubMed] [Google Scholar]
- Romijn HJ, Sluiter AA, Pool CW, Wortel J, Buijs RM (1996) Differences in colocalization between Fos and PHI, GRP, VIP, and VP in neurons of the rat suprachiasmatic nucleus after a light stimulus during the phase delay versus the phase advance period of the night. J Comp Neurol 372: 1-8. [DOI] [PubMed] [Google Scholar]
- Romijn HJ, Sluiter AA, Wortel J, Van Uum JF, Buijs RM (1998) Immunocytochemical evidence for a diurnal rhythm of neurons showing colocalization of VIP with GRP in the rat suprachiasmatic nucleus. J Comp Neurol 391: 397-405. [PubMed] [Google Scholar]
- Rusak B, Robertson HA, Wisden W, Hunt SP (1990) Light pulses that shift rhythms induce gene expression in the suprachiasmatic nucleus. Science 248: 1237-1240. [DOI] [PubMed] [Google Scholar]
- Silver R, Romero MT, Besmer HR, Leak R, Nunez JM, LeSauter J (1996) Calbindin-D28K cells in the hamster SCN express light-induced Fos. NeuroReport 7: 1224-1228. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Hayashi S, Tamada Y, Ikeda T, Hisa Y, Takamatsu T, Ibata Y (1997) Direct retinal projections to GRP neurons in the suprachiasmatic nucleus of the rat. NeuroReport 8: 2187-2191. [DOI] [PubMed] [Google Scholar]
- Tang KC, Pan JT (1993) Stimulatory effects of bombesin-like peptides on suprachiasmatic neurons in brain slices. Brain Res 614: 125-130. [DOI] [PubMed] [Google Scholar]
- van den Pol AN, Tsujimoto KL (1985) Neurotransmitters of the hypothalamic suprachiasmatic nucleus: immunocytochemical analysis of 25 neuronal antigens. Neuroscience 15: 1049-1086. [DOI] [PubMed] [Google Scholar]
- Yan L, Silver R (2002) Differential induction and localization of mPer1 and mPer2 during advancing and delaying phase shifts. Eur J Neurosci 16: 1531-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]