Abstract
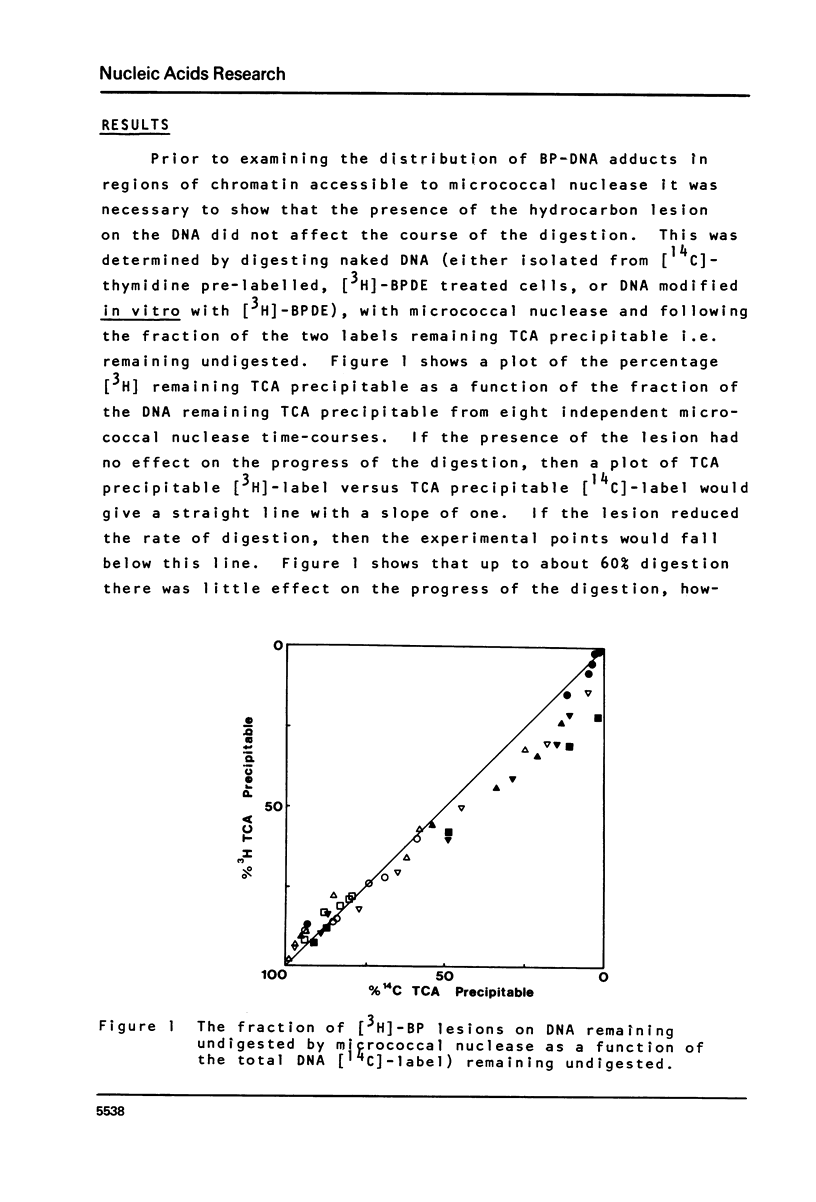
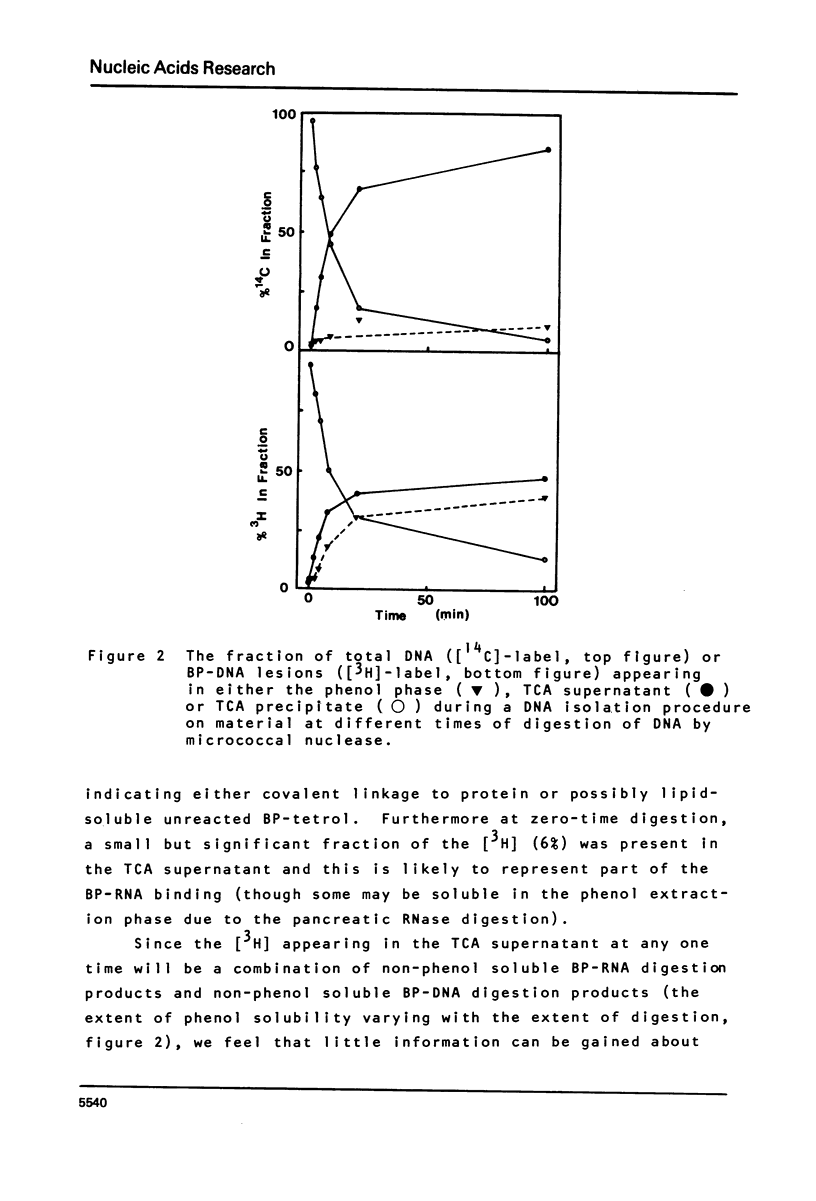
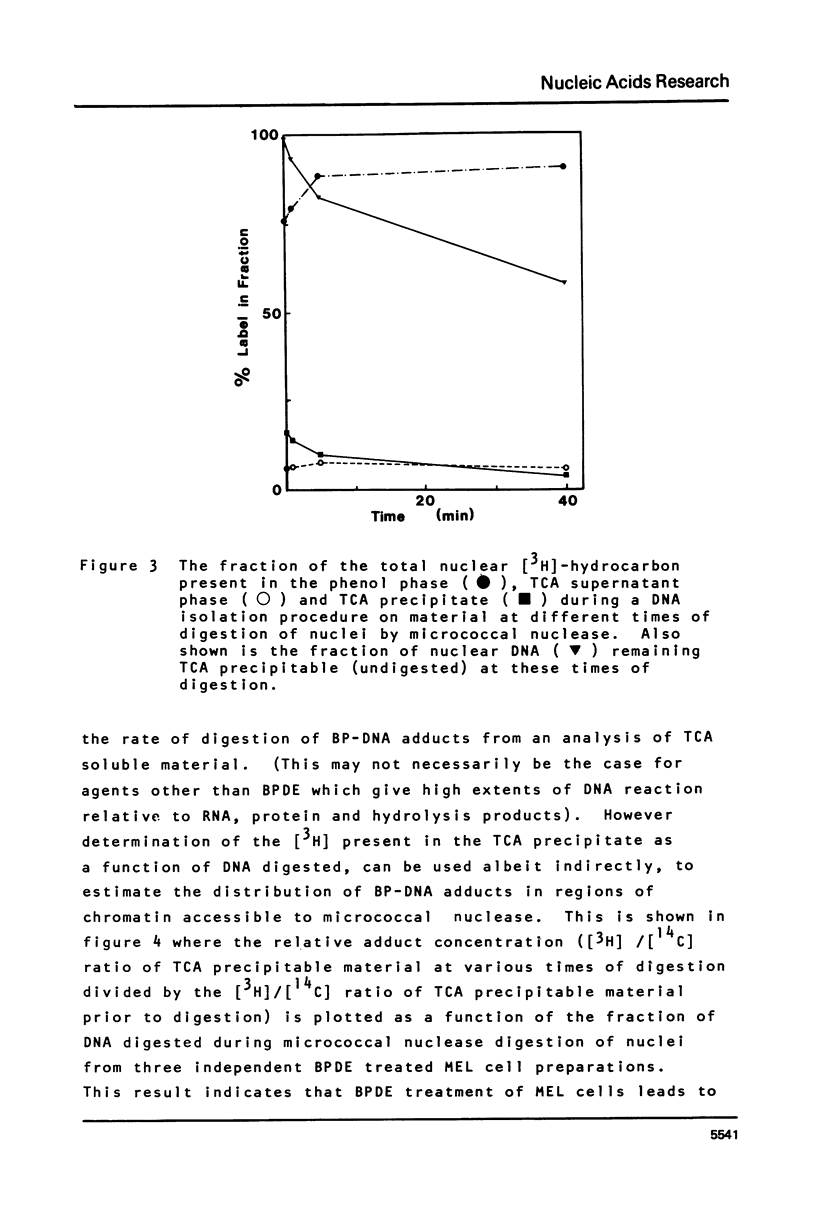
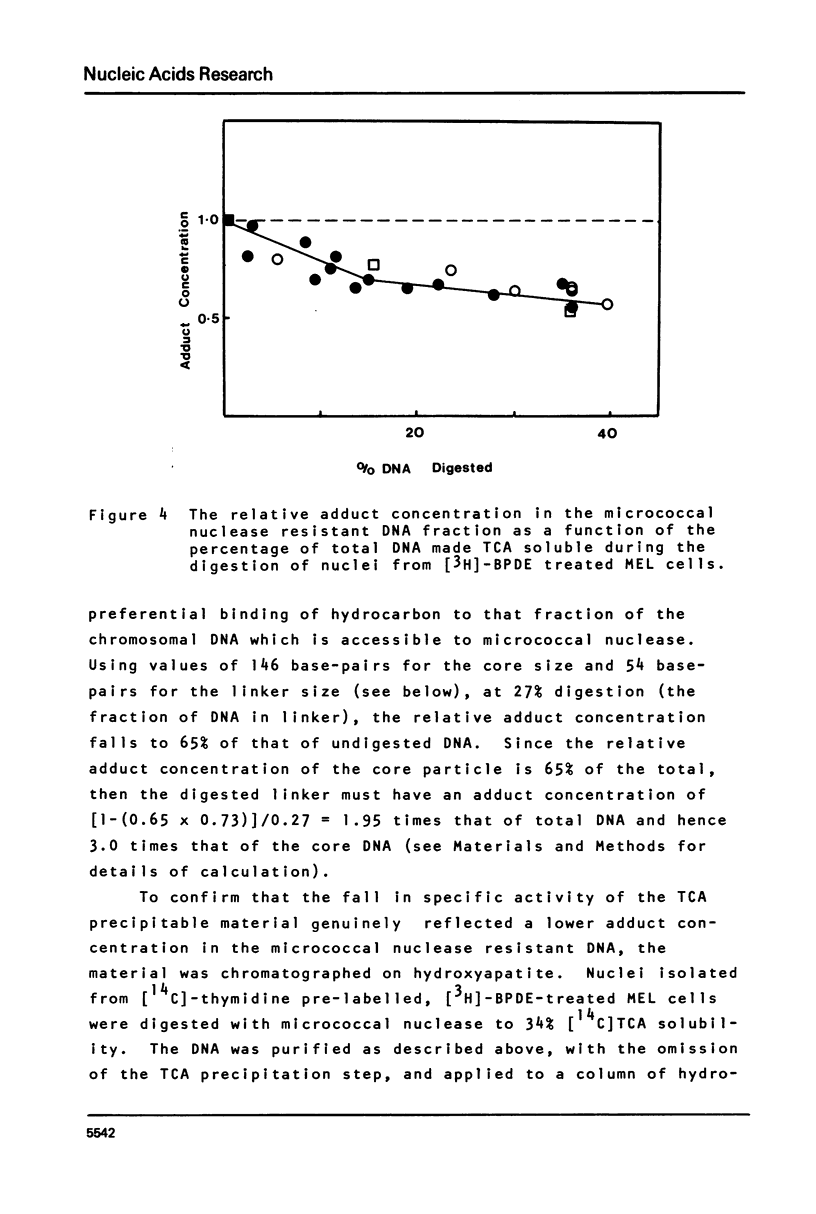
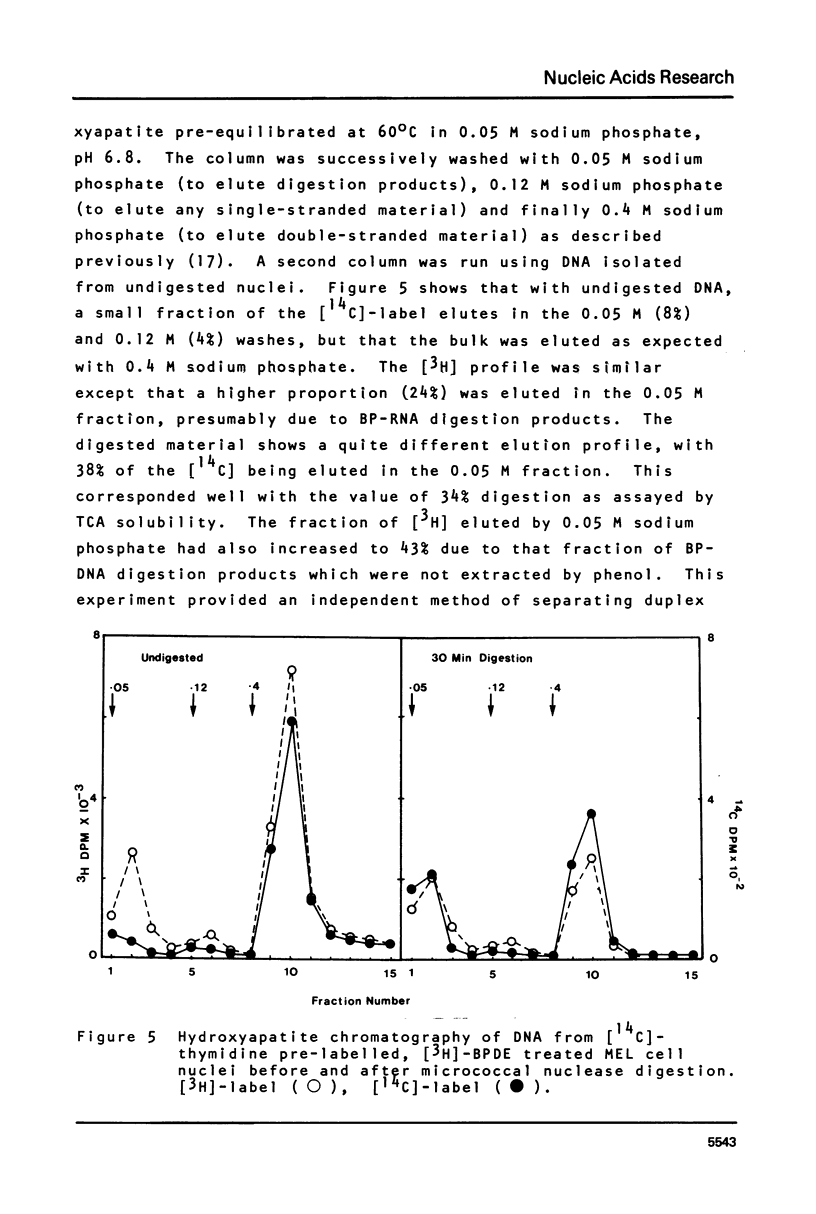
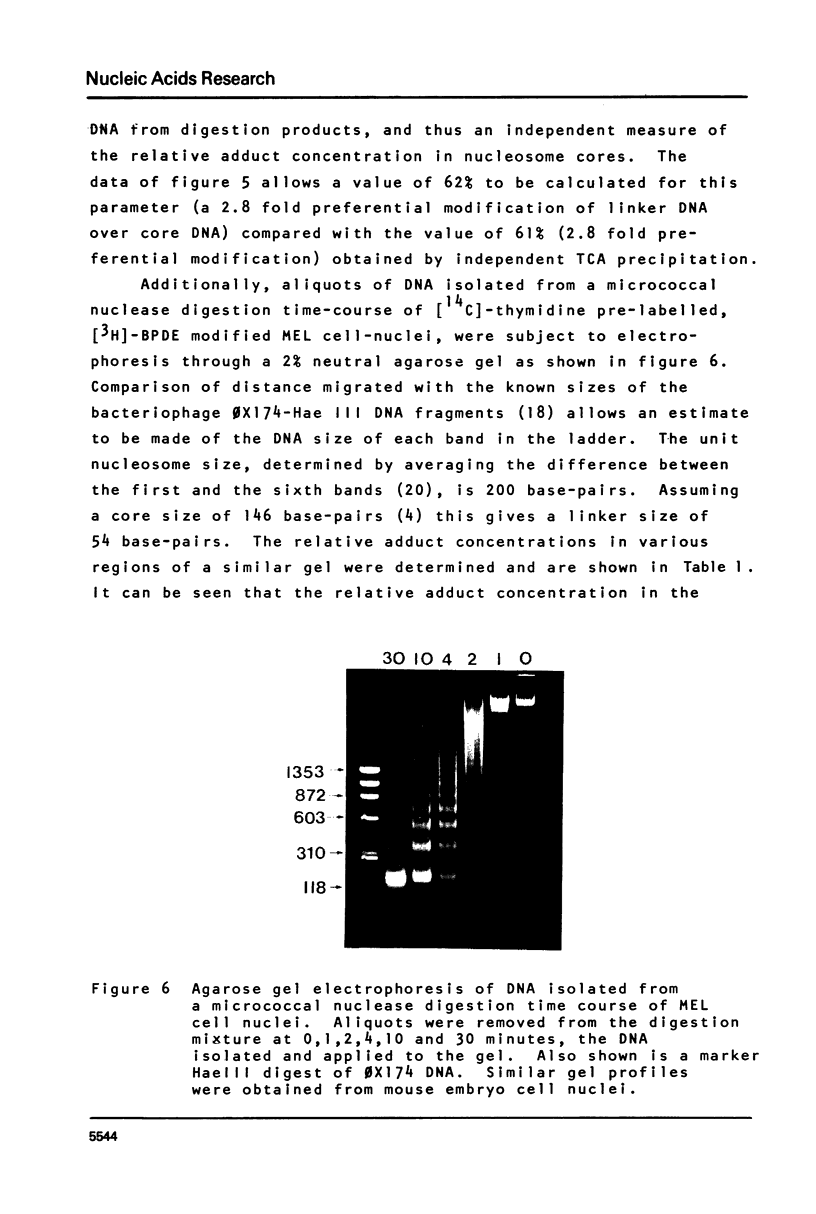
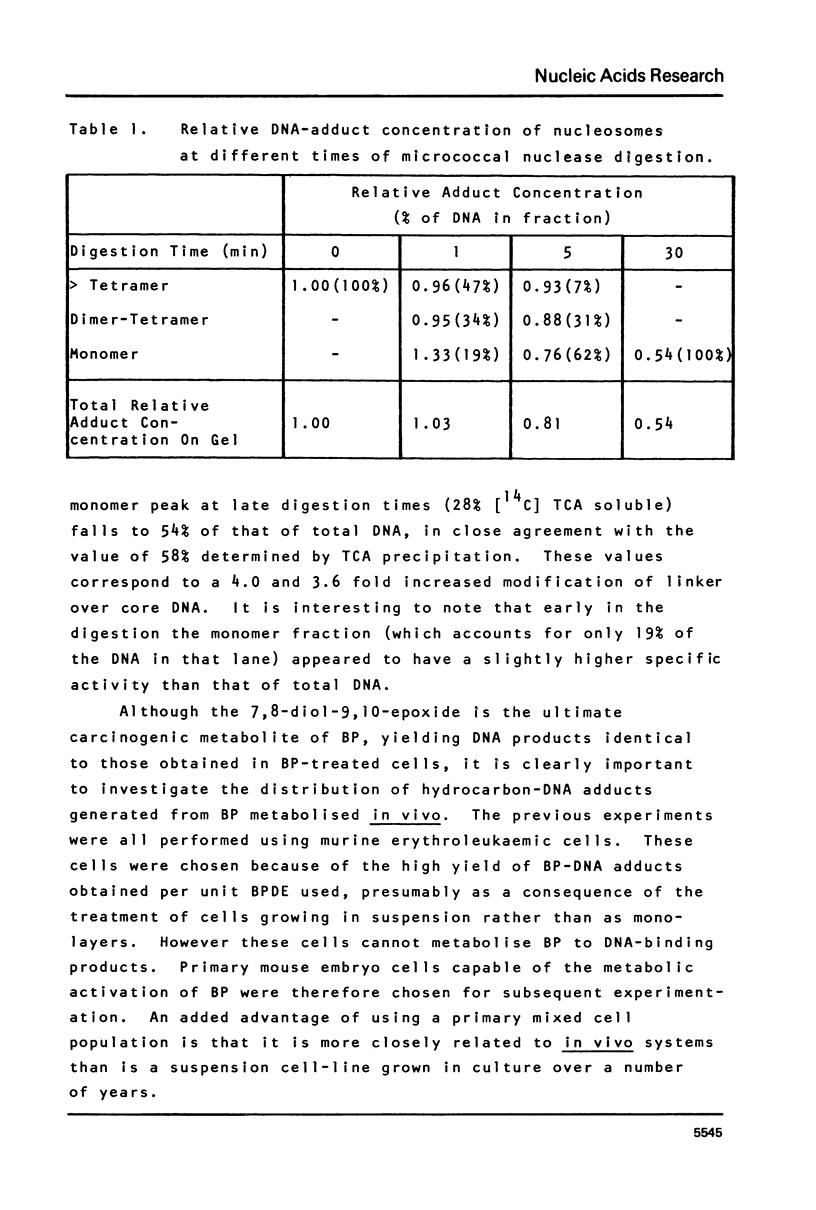
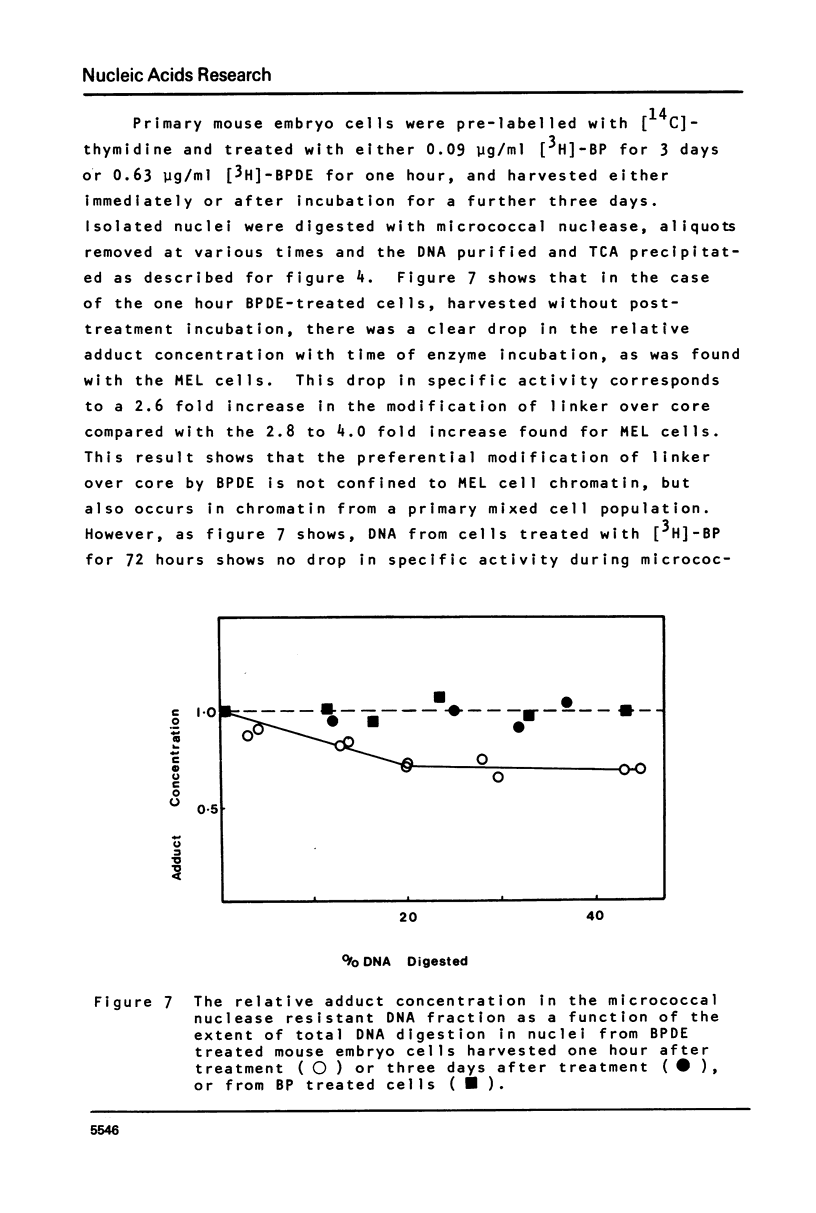
This paper describes the distribution of DNA-lesions generated by the potent carcinogen benzo(a)pyrene (BP) or its ultimate metabolic derivative 7 alpha, 8 8 beta, di-hydroxy-9 beta, 10 beta-epoxy-7,8,9,10-tetrahydrobenzo(a)pyrene (BPDE) within mammalian chromatin using the enzymic probe micrococcal nuclease. We have shown that the progress of the nuclease on naked DNA is unaffected by the presence of the hydrocarbon lesion at moderate extents of digestion. Digestion of nuclei isolated from murine erythroleukaemic cells immediately following BPDE treatment, and analysis of micrococcal nuclease resistant DNA by TCA precipitation, hydroxyapatite chromatography and gel electrophoresis demonstrates a non-random distribution of lesions. Approximately three times more binding occurs on the linker DNA regions between nucleosome cores than on the nucleosome core DNA itself. A similar result was obtained with BPDE treated primary mouse embryo cells; however nuclei isolated from these cells after prolonged treatment with BP (to allow metabolic activation) showed no such preferential binding. Post-treatment incubation of BPDE-treated cells shows that this difference can be accounted for by the loss of preferential localisation with time.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexandrov K., Brookes P., King H. W., Osborne M. R., Thompson M. H. Comparison of the metabolism of benzo[alpha]pyrene and binding to DNA caused by rat liver nuclei and microsomes. Chem Biol Interact. 1976 Mar;12(3-4):269–277. doi: 10.1016/0009-2797(76)90043-0. [DOI] [PubMed] [Google Scholar]
- BROOKES P., LAWLEY P. D. EVIDENCE FOR THE BINDING OF POLYNUCLEAR AROMATIC HYDROCARBONS TO THE NUCLEIC ACIDS OF MOUSE SKIN: RELATION BETWEEN CARCINOGENIC POWER OF HYDROCARBONS AND THEIR BINDING TO DEOXYRIBONUCLEIC ACID. Nature. 1964 May 23;202:781–784. doi: 10.1038/202781a0. [DOI] [PubMed] [Google Scholar]
- Bailey G. S., Nixon J. E., Hendricks J. D., Sinnhuber R. O., Van Holde K. E. Carcinogen aflatoxin B1 is located preferentially in internucleosomal deoxyribonucleic acid following exposure in vivo in rainbow trout. Biochemistry. 1980 Dec 9;19(25):5836–5842. doi: 10.1021/bi00566a027. [DOI] [PubMed] [Google Scholar]
- Bloom K. S., Anderson J. N. Conformation of ovalbumin and globin genes in chromatin during differential gene expression. J Biol Chem. 1979 Oct 25;254(20):10532–10539. [PubMed] [Google Scholar]
- Duncan M., Brookes P., Dipple A. Metabolism and binding to cellular macromolecules of a series of hydrocarbons by mouse embryo cells in culture. Int J Cancer. 1969 Nov 15;4(6):813–819. doi: 10.1002/ijc.2910040610. [DOI] [PubMed] [Google Scholar]
- Feldman G., Remsen J., Shinohara K., Cerutti P. Excisability and persistence of benzo(a)pyrene DNA adducts in epithelioid human lung cells. Nature. 1978 Aug 24;274(5673):796–798. doi: 10.1038/274796a0. [DOI] [PubMed] [Google Scholar]
- Feldman G., Remsen J., Wang T. V., Cerutti P. Formation and excision of covalent deoxyribonucleic acid adducts of benzo[a]pyrene 4,5-epoxide and benzo[a]pyrenediol epoxide I in human lung cells A549. Biochemistry. 1980 Mar 18;19(6):1095–1101. doi: 10.1021/bi00547a008. [DOI] [PubMed] [Google Scholar]
- Gottesfeld J. M., Bloomer L. S. Nonrandom alignment of nucleosomes on 5S RNA genes of X. laevis. Cell. 1980 Oct;21(3):751–760. doi: 10.1016/0092-8674(80)90438-9. [DOI] [PubMed] [Google Scholar]
- Igo-Kemenes T., Omori A., Zachau H. G. Non-random arrangement of nucleosomes in satellite I containing chromatin of rat liver. Nucleic Acids Res. 1980 Nov 25;8(22):5377–5390. doi: 10.1093/nar/8.22.5377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack P., Brookes P. The binding of benzo(a)pyrene to DNA components of differing sequence complexity. Int J Cancer. 1980 Jun 15;25(6):789–795. doi: 10.1002/ijc.2910250615. [DOI] [PubMed] [Google Scholar]
- Jahn C. L., Litman G. W. Accessibility of deoxyribonucleic acid in chromatin to the covalent binding of the chemical carcinogen benzo[a]pyrene. Biochemistry. 1979 Apr 17;18(8):1442–1449. doi: 10.1021/bi00575a009. [DOI] [PubMed] [Google Scholar]
- Jahn C. L., Litman G. W. Distribution of covalently bound benzo(a)pyrene in chromatin. Biochem Biophys Res Commun. 1976 May 23;76(2):534–540. doi: 10.1016/0006-291x(77)90757-4. [DOI] [PubMed] [Google Scholar]
- Jones P. A., Taylor S. M. Cellular differentiation, cytidine analogs and DNA methylation. Cell. 1980 May;20(1):85–93. doi: 10.1016/0092-8674(80)90237-8. [DOI] [PubMed] [Google Scholar]
- Kaneko M., Cerutti P. A. Excision of N-acetoxy-2-acetylaminofluorene-induced DNA adducts from chromatin fractions of human fibroblasts. Cancer Res. 1980 Nov;40(11):4313–4319. [PubMed] [Google Scholar]
- King H. W., Thompson M. H., Brookes P. The benzo(alpha)pyrene deoxyribonucleoside products isolated from DNA after metabolism of benzo(alpha)pyrene by rat liver microsomes in the presence of DNA. Cancer Res. 1975 May;35(5):1263–1269. [PubMed] [Google Scholar]
- Kootstra A., Slaga T. J. Binding of isomers of benzo[a]pyrene diol-epoxide to chromatin. Biochem Biophys Res Commun. 1980 Apr 14;93(3):954–959. doi: 10.1016/0006-291x(80)91168-7. [DOI] [PubMed] [Google Scholar]
- Kootstra A., Slaga T. J., Olins D. E. Interaction of benzo[alpha]pyrene diol-epoxide with nuclei and isolated chromatin. Chem Biol Interact. 1979 Dec;28(2-3):225–236. doi: 10.1016/0009-2797(79)90163-7. [DOI] [PubMed] [Google Scholar]
- Levy A., Noll M. Multiple phases of nucleosomes in the hsp 70 genes of Drosophila melanogaster. Nucleic Acids Res. 1980 Dec 20;8(24):6059–6068. doi: 10.1093/nar/8.24.6059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhee J. D., Felsenfeld G. Nucleosome structure. Annu Rev Biochem. 1980;49:1115–1156. doi: 10.1146/annurev.bi.49.070180.005343. [DOI] [PubMed] [Google Scholar]
- Metzger G., Wilhelm F. X., Wilhelm M. L. Distribution along DNA of the bound carcinogen N-acetoxy-N-2-acetylaminofluorene in chromatin modified in vitro. Chem Biol Interact. 1976 Nov;15(3):257–265. doi: 10.1016/0009-2797(76)90151-4. [DOI] [PubMed] [Google Scholar]
- Metzger G., Wilhelm F. X., Wilhelm M. L. Non-random binding of a chemical carcinogen to the DNA in chromatin. Biochem Biophys Res Commun. 1977 Apr 11;75(3):703–710. doi: 10.1016/0006-291x(77)91529-7. [DOI] [PubMed] [Google Scholar]
- Newbold R. F., Brookes P., Harvey R. G. A quantitative comparison of the mutagenicity of carcinogenic polycyclic hydrocarbon derivatives in cultured mammalian cells. Int J Cancer. 1979 Aug;24(2):203–209. doi: 10.1002/ijc.2910240212. [DOI] [PubMed] [Google Scholar]
- Noll M., Kornberg R. D. Action of micrococcal nuclease on chromatin and the location of histone H1. J Mol Biol. 1977 Jan 25;109(3):393–404. doi: 10.1016/s0022-2836(77)80019-3. [DOI] [PubMed] [Google Scholar]
- Oleson F. B., Mitchell B. L., Dipple A., Lieberman M. W. Distribution of DNA damage in chromatin and its relation to repair in human cells treated with 7-bromomethylbenz(a) anthracene. Nucleic Acids Res. 1979 Nov 10;7(5):1343–1361. doi: 10.1093/nar/7.5.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer W., Zachau H. G. Accessibility of expressed and non-expressed genes to a restriction nuclease. Nucleic Acids Res. 1980 Oct 24;8(20):4621–4638. doi: 10.1093/nar/8.20.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan R., Rajalakshmi S., Sarma D. S., Farber E. Nonrandom nature of in vivo methylation of dimethylnitrosamine and the subsequent removal of methylated products from rat liver chromatin DNA. Cancer Res. 1976 Jun;36(6):2073–2079. [PubMed] [Google Scholar]
- Razin A., Cedar H. Distribution of 5-methylcytosine in chromatin. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2725–2728. doi: 10.1073/pnas.74.7.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Friedmann T., Air G. M., Barrell B. G., Brown N. L., Fiddes J. C., Hutchison C. A., 3rd, Slocombe P. M., Smith M. The nucleotide sequence of bacteriophage phiX174. J Mol Biol. 1978 Oct 25;125(2):225–246. doi: 10.1016/0022-2836(78)90346-7. [DOI] [PubMed] [Google Scholar]
- Sudhakar S., Tew K. D., Schein P. S., Woolley P. V., Smulson M. E. Nitrosourea interaction with chromatin and effect on poly(adenosine diphosphate ribose) polymerase activity. Cancer Res. 1979 Apr;39(4):1411–1417. [PubMed] [Google Scholar]
- Tipping E., Moore B. P., Jones C. A., Cohen G. M., Ketterer B., Bridges J. W. The non-covalent binding of benzo[a]pyrene and its hydroxylated metabolites to intracellular proteins and lipid bilayers. Chem Biol Interact. 1980 Nov;32(3):291–304. doi: 10.1016/0009-2797(80)90096-4. [DOI] [PubMed] [Google Scholar]
- Varshavsky A. J., Sundin O., Bohn M. A stretch of "late" SV40 viral DNA about 400 bp long which includes the origin of replication is specifically exposed in SV40 minichromosomes. Cell. 1979 Feb;16(2):453–466. doi: 10.1016/0092-8674(79)90021-7. [DOI] [PubMed] [Google Scholar]
- Wigley C. B., Newbold R. F., Amos J., Brookes P. Cell-mediated mutagenesis in cultured Chinese hamster cells by polycyclic hydrocarbons: mutagenicity and DNA reaction related to carcinogenicity in a series of compounds. Int J Cancer. 1979 May 15;23(5):691–696. doi: 10.1002/ijc.2910230516. [DOI] [PubMed] [Google Scholar]



