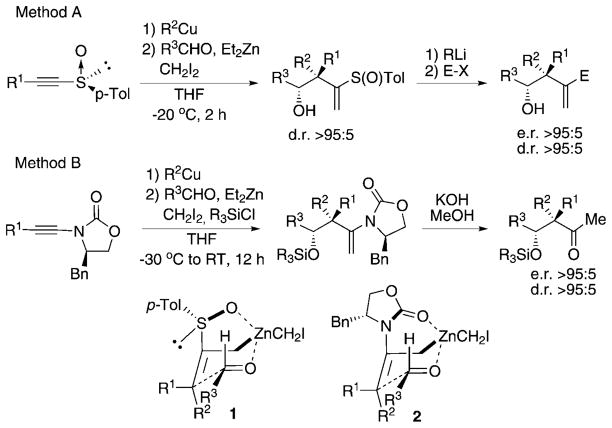
Currently, one of the most dynamic areas in organic synthesis is the asymmetric construction of molecules with quaternary carbon stereocenters, that is, carbon centers with four different alkyl substituents.[1] The state-of-the-art in this field is the asymmetric construction of these stereocenters in acyclic systems.[2] In the last few years, we have been involved in the development of synthetic strategies that have led to the formation of these desired fragments, but we have focused on the concomitant creation of several carbon–carbon bonds in a one-pot operation.[3] Our method consists of a carbometalation reaction of various α-hetero-substituted functionalized alkynes[4] followed by a zinc homologation reaction[5] through the use of a zinc carbenoid, formed in situ,[6] and then an allylation reaction of carbonyl compounds (Scheme 1).
Scheme 1.
Combined carbometalation–zinc homologation and allylation reactions en route to the formation of quaternary stereocenters.
Depending on the nature of the hetero-substituent on the alkyne, alkynyl sulfoxide (Method A) or ynamide (Method B), the formation of either homoallyl alcohol or aldol products, respectively, occurred with very high diastereo- and enantioselectivity. In both cases, the stereochemistry was rationalized through a Zimmerman–Traxler transition state,[7] in which the bulky group in the aldehyde, R3, occupies a pseudoequatorial position. In Method A, since the S–O bond operates as an acceptor site for Lewis acids, the sulfoxide forms a metallacycle that is sterically hindered (1 in Scheme 1). This is also the key element for the excellent diastereoselectivity observed in the transformation of yn-amides into the aldol surrogate adducts as described by transition state 2 (Scheme 1).
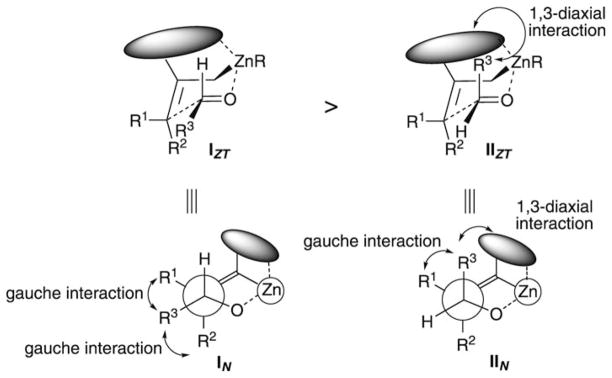
When sterically substantial substituents engaged in the metallacycle are present in position 2, shown as a grey circle in representations IZT and IIZT in Scheme 2, the R3 group of the aldehyde adopts a pseudoequatorial rather than pseudoaxial position to minimize potential 1,3-diaxial interactions, that is, IZT is favored over IIZT. However, by representing the same transition states by using Newman projections, it becomes clear that two gauche interactions exist as in IN, whereas in IIN, in which the R3 group is in a pseudoaxial position, one gauche and one 1,3-diaxial interaction exist (Scheme 2). The stereochemical outcome of the two reactions described in Scheme 1 implies that the combined 1,3-diaxial and gauche interactions occurring in IIN are more destabilizing since only transition state IN (or IZT), possessing two gauche interactions, leads to the products.
Scheme 2.
Zimmerman–Traxler transition states for the allylation reaction of 2,3,3-trisubstituted allylzinc species. Both “chair” and Newman projections are shown.
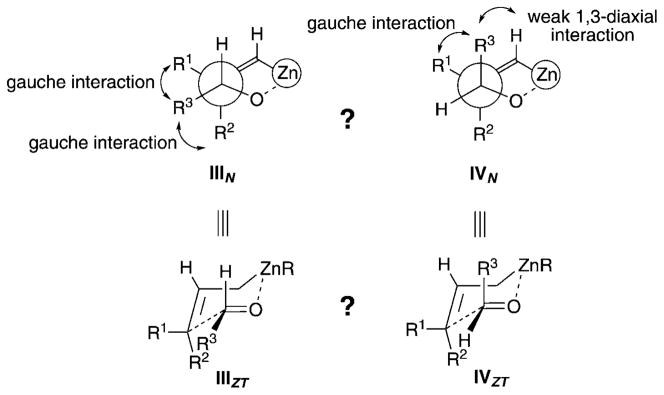
However, what would be the stereochemical outcome of a reaction if the bulky axial groups were replaced by a hydrogen atom? Are the two gauche interactions present in transition state IIIN preferred to transition state IVN, in which the R3 substituent of the aldehyde occupies a pseudoaxial position, as represented in Scheme 3?
Scheme 3.
Transition states for the allylation reaction of 3,3-disubstituted allylzinc species.
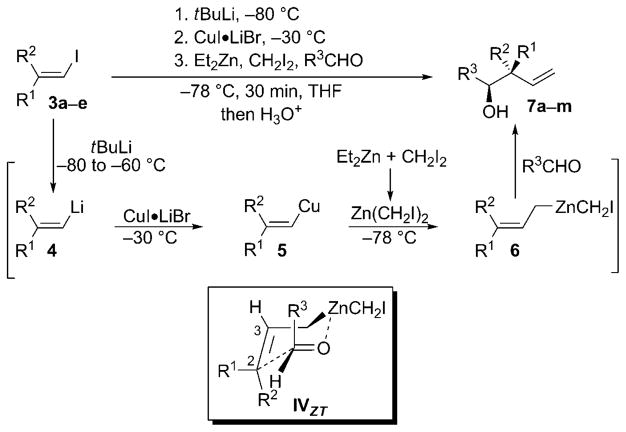
This important stereochemical question can only be solved by control of the constitutional stability of η1-allylmetal compounds, that is, if the haptotropic rearrangement (metalotropic equilibrium) is slower than the reaction with the aldehyde.[8] After extensive experimentation, we found that the successive treatment of vinyl iodides 3, prepared by carbometalation of alkynes[4] with tBuLi followed by the addition of a soluble solution of copper salt led to the formation of vinyl copper 5. Then, at −80°C, the addition of aldehydes, Et2Zn, and CH2I2 to this vinyl copper compound gave the expected homoallylic alcohols 7 through the formation of 3,3-disubstituted allylzinc species 6 (Scheme 4). As shown previously,[3] neither vinyl copper nor Et2Zn reacts with aldehydes at low temperature and as the transmetalation from vinyl copper to vinyl zinc is also a slow process at these temperatures, the reaction between Et2Zn and CH2I2 occurs first to give in situ formation of the zinc carbenoid.[9] This carbenoid then readily homologates vinyl copper 5 into the allyl species 6,[5] which reacts diastereoselectively with aldehydes to give homoallylic alcohols 7 in very high diastereoselectivities (Scheme 4 and Table 1). As shown for 7a (R1=Hex, R2=Et; Table 1, entry 1) and b (R1=Et, R2= Hex; Table 1, entry 2) with aromatic aldehydes, but also for 7j and k with an aliphatic aldehyde, permutation of the alkyl groups at the vinyl iodide allows the independent formation of the two diastereoisomers at the quaternary carbon center. This implies that 1) the haptotropic rearrangement is slower than the reaction of allyl zinc with aldehydes[8] and 2) the reaction does not proceed through an open transition state, but rather occurs via a cyclic transition state. Several different alkyl groups were easily introduced at the all-carbon stereogenic center, which shows the flexibility of the described method. Functionalized aldehydes can also be used in this allylation reaction, such as 4-bromo-benzaldehyde (Table 1, entries 1–5), 4-carbomethoxybenzaldehyde (Table 1, entry 9), and even 4-acetylbenzaldehyde (Table 1, entry 8). In all cases, the reaction proceeds chemo-selectively with respect to the aldehyde. The reaction is not restricted to aromatic aldehydes, since aliphatic aldehydes also lead to the homoallylic alcohols with decent stereoselectivities (Table 1, entries 10–13). The stereochemistry observed in this one-pot reaction was confirmed by comparison with an authentic sample of 7g previously prepared in our research group and analyzed by X-ray crystallographic analysis.[3f] The configurations of other reaction products were assigned by analogy.
Scheme 4.
Diastereoselective allylation reactions of 3,3-disubstituted allylzinc species.
Table 1.
Allylation reactions of 3,3-disubstitued allylzinc species with various aldehydes.[a]
| Starting material | R1 | R2 | R3 | Product | Yield [%][b] | d.r.[c] | |
|---|---|---|---|---|---|---|---|
| 1 | 3a | Hex | Et | p-BrH4C6 | 7a | 71 | 97:3 |
| 2 | 3b | Et | Hex | p-BrH4C6 | 7b | 55 | 90:10 |
| 3 | 3c | Hex | Bu | p-BrH4C6 | 7c | 60 | 98:2 |
| 4 | 3d | Bu | Et | p-BrH4C6 | 7d | 70 | 92:8 |
| 5 | 3e | Me | Et | p-BrH4C6 | 7e | 52 | 90:10 |
| 6 | 3a | Hex | Et | p-MeH4C6 | 7 f | 60 | 98:2 |
| 7 | 3d | Bu | Et | H5C6 | 7g | 70 | 98:2 |
| 8 | 3d | Bu | Et | p-OAcH4C6 | 7h | 63 | 88:12 |
| 9 | 3d | Bu | Et | p-MeO2CH4C6 | 7i | 65 | 88:12 |
| 10[d] | 3b | Hex | Et | nBu | 7j | 70 | 96:4 |
| 11[d] | 3a | Et | Hex | nBu | 7k | 66 | 91:9 |
| 12[d] | 3d | Bu | Et | Ph(CH2)2 | 7l | 62 | 83:17 |
| 13[d] | 3d | Bu | Et | iPr | 7m | 41 | 80:20 |
Hex=hexyl.
Determined after purification by column chromatography on alumina.
Determined by analysis of the crude product by 1H NMR spectroscopy and gas chromatography.
For aliphatic aldehydes, the allylation reaction proceeds at −40°C and the increased temperature can explain the lower diastereoselectivity.
Importantly, the relative configuration of all homoallylic alcohols 7 implies that the R3 group of the aldehyde now occupies a pseudoaxial position in a chair-like transition state when it reacts with 3,3-disubstituted allylzinc species, as shown in IVZT (Scheme 3).[10]
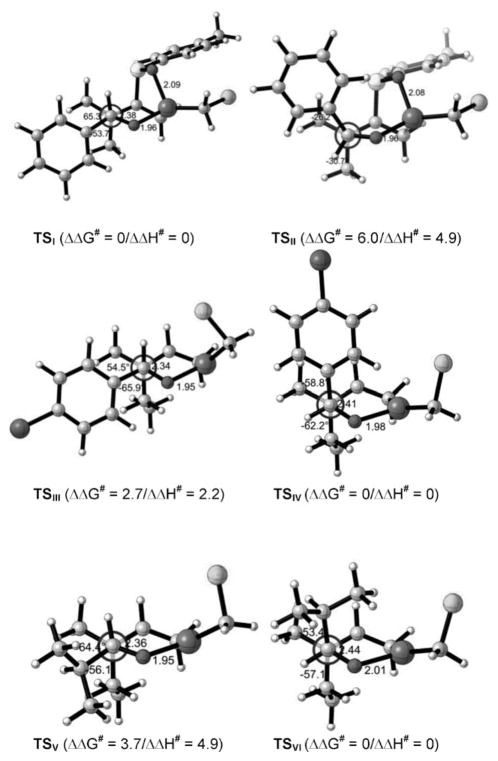
To have a better stereochemical understanding of the reaction of 3,3-disubstituted allylzinc compounds with carbonyl compounds, we have performed theoretical calculations on a model system (Figure 1).[11] We initially investigated the reaction of an allylzinc species possessing a sulfoxide group at C2 with benzaldehyde (as described in 1, Scheme 1). The reaction proceeds via the chair-like transition states shown in TSI and TSII (Figure 1). We also tried to locate possible boat-like transition states, but these could not be found as stationary points. As expected, TSI, in which the aryl group occupies the pseudoequatorial position, is 4.9 kcalmol−1 more stable than TSII, in which the aryl group occupies the pseudoaxial position (the carbon of the aldehyde is circled).[12] However, if the sulfoxide on C2 of the allylzinc is replaced by a hydrogen atom, the reaction still proceeds through a similar chair-like Zimmerman–Traxler transition state, but the stereochemical outcome is reversed since the aryl group of the aldehyde in a equatorial position (TSIII) is now higher in energy (by 2.2 kcalmol−1) than if the same aryl group is in the axial position (TSIV).
Figure 1.
MO5-2x/6-31G(d) optimized structures for all transition states (TS).
These computational results show that two gauche interactions lead to a transition state of higher energy and, to avoid this configuration, the system prefers to have the substituent of the aldehyde in an axial position.[13] Finally, we also checked the case of an aliphatic aldehyde, such as iPrCHO, as the electrophilic partner by computational methods. Again, having the alkyl substituent in the equatorial position is much higher in energy (TSV) than if the substituent is in an axial position (TSVI).
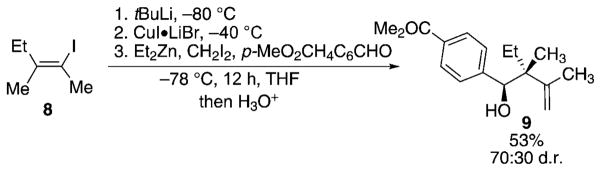
To further probe experimentally the stereochemical outcome resulting from the Zimmerman–Traxler transition state, a 2,3,3-trialkyl-substituted allylzinc compound was prepared and tested in our reaction. In this particular case, the alkyl group at C2 is less sterically demanding than the metallacycle containing the sulfoxide or oxazolidinone moieties and a mixture of diastereoisomers is expected. To check this hypothesis, vinyl iodide 8, easily prepared by zirconium-promoted ethylzination of 2-butyne,[14] was treated under the conditions described in Scheme 4 with 4-carbomethoxybenzaldehyde as the electrophilic partner and homoallyl alcohol 9 was obtained in 53% yield with a diastereoisomeric ratio of 7:3 (Scheme 5). The configuration of the major diastereoisomer was determined by X-ray crystallographic analysis and found to result from a reaction in which the aromatic group of the aldehyde still occupies the pseudoaxial position. However, the formation of the two isomers shows the limitations of the system.
Scheme 5.
Diastereoselective allylation reactions of 2,3,3-trialkyl-substituted allylzinc species.
In summary, the stereochemical outcome of the allylation reaction of 3,3-disubstituted allylzinc species with aldehydes shows that the aryl or alkyl group on the electrophilic carbonyl reactant occupies a pseudoaxial position in the Zimmerman–Traxler transition state to avoid gauche interactions. However, if bulky substituents are present at the C2 center of the allylzinc species, the additional 1,3-diaxial interaction counterbalances the two gauche interactions. We are currently expanding the scope of this new stereochemical feature to other systems. It has become clear from these considerations that the size of the metal and associated ligands in the Zimmerman–Traxler transition state of the 3,3-disubstituted allylmetal[15] should also influence the stereochemistry. This is currently being investigated in our laboratory.
Experimental Section
General procedure for the reaction of vinyl iodides 3
tBuLi (2.2 equiv, 2.2 mmol) was added to a solution of vinyl iodide 3 (1 mmol) in THF (10 mL) at −78°C. The resulting mixture was stirred at this temperature for 10 min. The reaction mixture was then warmed to −40°C and a solution of CuBr·LiBr (1.1 equiv, 1.1 mmol) in THF (2 mL) was added. The mixture was stirred for an additional 40 min. After cooling the reaction mixture to −78°C, a solution of the aldehyde (1 equiv, 1 mmol) in THF (1 mL) was added and the mixture was stirred for 10 min. CH2I2 (6 mmol) and Et2Zn (3 mmol) were then added and the reaction mixture was stirred at −78°C for a further 30 min (for aliphatic aldehydes, the reaction mixture was stirred at −40°C for 4 h). The hydrolysis was performed with an aqueous solution of NH4Cl/NH3 (2:1). After a standard workup, the crude product was purified by column chromatography on alumina to give pure homoallylic alcohol 7.
Acknowledgments
This research was supported by a grant from the Israel Science Foundation, which is administered by the Israel Academy of Sciences and Humanities (70/08), a grant from the Binational Science Foundation (BSF, 2008078), the National Institute of General Medical Sciences, and the National Institutes of Health (GM-36700).
Footnotes
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/chem.201101049.
References
- 1.a) Douglas CJ, Overman LE. Proc Natl Acad Sci USA. 2004;101:5363. doi: 10.1073/pnas.0307113101. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Corey EJ, Guzman-Perez A. Angew Chem. 1998;110:2092. doi: 10.1002/(SICI)1521-3773(19980302)37:4<388::AID-ANIE388>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 1998;37:388. [Google Scholar]; c) Trost BM, Jiang C. Synthesis. 2006:369. [Google Scholar]; d) Christoffers J, Baro A. Adv Synth Catal. 2005;347:1473. [Google Scholar]; e) Christoffers J, Mann A. Angew Chem. 2001;113:4725. [Google Scholar]; Angew Chem Int Ed. 2001;40:4591. [Google Scholar]; f) Denissova I, Barriault L. Tetrahedron. 2003;59:10105. [Google Scholar]; g) Cozzi PG, Hilgraf R, Zimmermann N. Eur J Org Chem. 2007:5969. [Google Scholar]; h) Bella M, Casperi T. Synthesis. 2009:1583. [Google Scholar]; i) Hawner C, Alexakis A. Chem Commun. 2010;46:7295. doi: 10.1039/c0cc02309d. [DOI] [PubMed] [Google Scholar]; j) Das JD, Marek I. Chem Commun. 2011;47:4593. doi: 10.1039/c0cc05222a. [DOI] [PubMed] [Google Scholar]
- 2.For contributions in 2010, see: Gao F, McGrath KP, Lee Y, Hoveyda AH. J Am Chem Soc. 2010;132:14315. doi: 10.1021/ja106829k.Guzman-Martinez A, Hoveyda AH. J Am Chem Soc. 2010;132:10634. doi: 10.1021/ja104254d.Jackowski O, Alexakis A. Angew Chem. 2010;122:3418. doi: 10.1002/anie.201000577.Angew Chem Int Ed. 2010;49:3346.Esumi T, Mori T, Zhao M, Toyota M, Fukuyama Y. Org Lett. 2010;12:888. doi: 10.1021/ol902960n.Denmark SE, Wilson TW. Synlett. 2010:1723.Zhu Q, Lu Y. Chem Commun. 2010;46:2235. doi: 10.1039/b919549a.Ting YF, Chang C, Reddy RJ, Magar DR, Chen K. Chem Eur J. 2010;16:7030. doi: 10.1002/chem.201000483.Tanaka Y, Kanai M, Shibasaki M. J Am Chem Soc. 2010;132:8862. doi: 10.1021/ja1035286.O’Brien JM, Lee K-S, Hoveyda AH. J Am Chem Soc. 2010;132:10630. doi: 10.1021/ja104777u.Chen I-H, Kanai M, Shibasaki M. Org Lett. 2010;12:4098. doi: 10.1021/ol101691p.Shintani R, Takeda M, Nishimura T, Hayashi T. Angew Chem. 2010;122:4061. doi: 10.1002/anie.201000467.Angew Chem Int Ed. 2010;49:3969.Hawner C, Muller D, Gremaud L, Felouat A, Woodward S, Alexakis A. Angew Chem. 2010;122:7935. doi: 10.1002/anie.201003300.Angew Chem Int Ed. 2010;49:7769.Simaan S, Goldberg AFG, Rosset S, Marek I. Chem Eur J. 2010;16:774. doi: 10.1002/chem.200902656.Simaan S, Marek I. J Am Chem Soc. 2010;132:4066. doi: 10.1021/ja100544c.Masarwa A, Marek I. Chem Eur J. 2010;16:9712. doi: 10.1002/chem.201001246.
- 3.a) Dutta B, Gilboa N, Marek I. J Am Chem Soc. 2010;132:5588. doi: 10.1021/ja101371x. [DOI] [PubMed] [Google Scholar]; b) Das JP, Chechik H, Marek I. Nat Chem. 2009;1:128. doi: 10.1038/nchem.131. [DOI] [PubMed] [Google Scholar]; c) Marek I. Chem Eur J. 2008;14:7460. doi: 10.1002/chem.200800580. [DOI] [PubMed] [Google Scholar]; d) Marek I, Sklute G. Chem Commun. 2007:1683. doi: 10.1039/b615042j. [DOI] [PubMed] [Google Scholar]; e) Kolodney G, Sklute G, Perrone S, Knochel P, Marek I. Angew Chem. 2007;119:9451. doi: 10.1002/anie.200702981. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2007;46:9291. [Google Scholar]; f) Sklute G, Marek I. J Am Chem Soc. 2006;128:4642. doi: 10.1021/ja060498q. [DOI] [PubMed] [Google Scholar]; g) Sklute G, Amsallem D, Shibli A, Varghese JP, Marek I. J Am Chem Soc. 2003;125:11776. doi: 10.1021/ja036872t. [DOI] [PubMed] [Google Scholar]
- 4.a) Basheer A, Marek I. Beilstein J Org Chem. 2010;6(77) doi: 10.3762/bjoc.6.77. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Levin A, Basheer A, Marek I. Synlett. 2010:329. [Google Scholar]; c) Sklute G, Bolm C, Marek I. Org Lett. 2007;9:1259. doi: 10.1021/ol070070b. [DOI] [PubMed] [Google Scholar]; d) Chechik-Lankin H, Livshin S, Marek I. Synlett. 2005:2098. [Google Scholar]; e) Chechik-Lankin H, Marek I. Org Lett. 2003;5:5087. doi: 10.1021/ol036154b. [DOI] [PubMed] [Google Scholar]; f) Chinkov N, Majumdar S, Marek I. J Am Chem Soc. 2002;124:10282. doi: 10.1021/ja027027y. [DOI] [PubMed] [Google Scholar]; g) Chinkov N, Majumdar S, Marek I. J Am Chem Soc. 2003;125:13258. doi: 10.1021/ja036751t. [DOI] [PubMed] [Google Scholar]
- 5.a) Knochel P, Chou TS, Chen HG, Yeh MCP, Rozema MJ. J Org Chem. 1989;54:5202. [Google Scholar]; b) Knochel P, Jeong N, Rozema MJ, Yeh MCP. J Am Chem Soc. 1989;111:6474. [Google Scholar]; c) Sidduri AR, Knochel P. J Am Chem Soc. 1992;114:7579. [Google Scholar]; d) Sidduri AR, Rozema MJ, Knochel P. J Org Chem. 1993;58:2694. [Google Scholar]
- 6.Marek I. Tetrahedron. 2002;58:9463. [Google Scholar]
- 7.a) Zimmerman HE, Traxler MD. J Am Chem Soc. 1957;79:1920. [Google Scholar]; b) Li Y, Houk KN. J Am Chem Soc. 1989;111:1236. [Google Scholar]
- 8.Hoffmann RW, Polachowski A. Chem Eur J. 1998;4:1724. [Google Scholar]
- 9.a) Charette AB, Marcoux JF, Molinaro C, Beauchemin A, Brochu C, Isabel E. J Am Chem Soc. 2000;122:4508. [Google Scholar]; b) Denmark SE, O’Connor SP. J Org Chem. 1997;62:3390. doi: 10.1021/jo9702397. [DOI] [PubMed] [Google Scholar]
- 10.a) Evans DA, Siska SJ, Cee VJ. Angew Chem. 2003;115:1803. doi: 10.1002/anie.200350979. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2003;42:1761. [Google Scholar]; b) Nakamura M, Hirai A, Sogi M, Nakamura E. J Am Chem Soc. 1998;120:5846. [Google Scholar]
- 11.a) Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA. Gaussian 03, Revision C.02. Gaussian, Inc; Wallingford CT: 2004. [Google Scholar]; b) Wodrich MD, Corminboeuf C, Schreiner PR, Fokin AA, von P, Schleyer R. Org Lett. 2007;9:1851. doi: 10.1021/ol070354w. [DOI] [PubMed] [Google Scholar]; c) Amin EA, Thruhlar DG. J Chem Theory Comput. 2008;4:75. doi: 10.1021/ct700205n. [DOI] [PubMed] [Google Scholar]; d) Sorkin A, Thrular DG, Amin EA. J Chem Theory Comput. 2009;5:1254. doi: 10.1021/ct900038m. [DOI] [PubMed] [Google Scholar]; e) Schiaffino L, Ercolani G. Chem Eur J. 2010;16:3147. doi: 10.1002/chem.200902543. [DOI] [PubMed] [Google Scholar]
- 12.Tietze LF, Schuffenhauer A, Schreiner PR. J Am Chem Soc. 1998;120:7952. [Google Scholar]
- 13.Despite many attempts, boat transition states could not be located.
- 14.Dumond Y, Negishi E. J Am Chem Soc. 1999;121:11223. [Google Scholar]
- 15.a) Hoffmann RW, Schlapbach A. Liebigs Ann Chem. 1990:1243. [Google Scholar]; b) Hoffmann RW, Schlapbach A. Liebigs Ann Chem. 1991:1203. [Google Scholar]; c) Jubert C, Nowotny S, Kornemann D, Antes I, Tucker CE, Knochel P. J Org Chem. 1992;57:6384. [Google Scholar]; d) Sato M, Yamamoto Y, Hara S, Suzuki A. Tetrahedron Lett. 1993;34:7071. [Google Scholar]; e) Yamamoto Y, Hara S, Suzuki A. Synlett. 1996:883. [Google Scholar]; f) Denmark SE, Fu J. J Am Chem Soc. 2001;123:9488. doi: 10.1021/ja016552e. [DOI] [PubMed] [Google Scholar]; g) Denmark SE, Fu J. Org Lett. 2002;4:1951. doi: 10.1021/ol025971t. [DOI] [PubMed] [Google Scholar]; h) Denmark SE, Fu J, Lawler MJ. J Org Chem. 2006;71:1523. doi: 10.1021/jo052203h. [DOI] [PubMed] [Google Scholar]; i) Denmark SE, Fu J. Chem Commun. 2003:167. doi: 10.1039/b208065f. [DOI] [PubMed] [Google Scholar]; j) Ely RJ, Morken JP. J Am Chem Soc. 2010;132:2534. doi: 10.1021/ja910750b. [DOI] [PMC free article] [PubMed] [Google Scholar]; k) Morgan JB, Morken JP. Org Lett. 2003;5:2573. doi: 10.1021/ol034936z. [DOI] [PubMed] [Google Scholar]; l) Han H, Krische MJ. Org Lett. 2010;12:2844. doi: 10.1021/ol101077v. [DOI] [PMC free article] [PubMed] [Google Scholar]