Abstract
Background
Main pulmonary artery diameter (mPA) and ratio of mPA to ascending aorta diameter (ratio PA) derived from chest CT are commonly reported in clinical practice. We determined the age and sex-specific distribution and normal reference values for mPA and ratio PA by CT in an asymptomatic community-based population.
Methods and Results
In 3171 men and women (mean age 51 ± 10 years, 51% men) from the Framingham Heart Study, a non-contrast ECG gated eight-slice cardiac multi-detector CT was performed. We measured the mPA and transverse axial diameter of the ascending aorta at the level of the bifurcation of the right pulmonary artery and calculated the ratio PA. We defined the healthy referent cohort (n=706) as those without obesity, hypertension, current and past smokers, chronic obstructive pulmonary disease, history of pulmonary embolism, diabetics, cardiovascular disease, and heart valvular surgery. The mean mPA diameter in the overall cohort was 25.1 ± 2.8mm and mean ratio PA was 0.77 ± 0.09. The sex-specific 90th percentile cutoff value for mPA diameter was 28.9 mm in men and 26.9 mm in women and was associated with increase risk for self-reported dyspnea (adjusted odds ratio 1.31, p=0.02). The 90th percentile cutoff value for ratio PA of the healthy referent group was 0.91, similar between gender, but decreased with increasing age (range 0.82 to 0.94), though not associated with dyspnea.
Conclusions
For simplicity, we established 29 mm in men and 27 mm in women as sex-specific normative reference values for mPA and 0.9 for ratio PA.
Keywords: pulmonary artery, Framingham Heart Study, computed tomography
The main pulmonary artery (mPA) and the aorta are routinely imaged and described in every patient undergoing chest computed tomography (CT). Enlarged mPA diameter is a sign of pulmonary hypertension as the mPA adapts to increased pulmonary artery pressure most often due to increase in pulmonary vascular resistance 1–2. Another measure reported to be associated with pulmonary hypertension is an increase size of the mPA relative to the ascending aorta (ratio PA) greater than 1.1, 3–4
Current normative values are based on studies with a small sample size with variable ranges and thus confidence in these values is limited.5–7 To date, there has been no large population study to determine the normal reference range of these two subclinical metrics by CT in asymptomatic patients. In order to establish robust mPA and ratio PA reference values, we analyzed non-contrast electrocardiographically (ECG)-gated chest CT scans from more than 3000 participants of the offspring and third generation participants of the community-based Framingham Heart Study.
Methods
Study population
Participants for this study were from the Offspring and the Third Generation cohorts of the community-based Framingham Heart Study. Selection criteria and study design have been previously described.8 Participants in the analysis attended the Offspring seventh examination cycle (1998–2001) or Third Generation first exam cycle (2002–2005) and have complete risk factor information. Inclusion in the MDCT study was weighted towards participants from larger Framingham Heart Study families and those who resided in the Greater New England area. Men were at least 35 years of age and women at least 40 years of age. In addition, at the time of MDCT scan acquisition, women were non-pregnant, and all participants weighed less than 350 pounds due to MDCT scanner specifications. The Institutional Review Boards of the Boston University Medical Center and Massachusetts General Hospital approved the study and all subjects provided written consent.
Of the 3529 participants scanned (1418 from 2nd generation, 2111 from 3rd generation), 3496 participants attended Offspring Exam 7 or Gen 3 Exam 1. Of the 3496 participants, 325 (9%) had incomplete CT datasets and did not include the main pulmonary artery or uninterpretable CT exams secondary to motion artifact. Thus, analysis of pulmonary and aortic dimensions was performed in 3171 participants of the overall study cohort.
The standard clinic examination at the Offspring seventh cycle or Third Generation first exam cycle included a physician interview, a physical examination, and laboratory tests. Body mass index (BMI) and body surface area (BSA) were calculated from weight and height, which were measured at each index examination. Obesity was defined as a BMI >30kg/m2. Hypertension was defined as systolic blood pressure of at least 140 mm Hg, diastolic blood pressure of at least 90 mm Hg, or use of anti-hypertensive drug treatment. Participants were considered to be current smokers if they smoked at least one cigarette per day for the last year. Chronic obstructive pulmonary disease (COPD) was defined as an FEV1/FVC ratio < 0.70 with FEV1 < 80% of predicted, consistent with Global Initiative for Chronic Obstructive Lung Disease stage 2 or higher, as previously described.9–10 Adult onset diabetes mellitis was defined as a fasting glucose ≥ 126 mg/dL at a Framingham examination or treatment with either insulin or a hypoglycemic agent. Dyslipidemia was defined as total cholesterol of at least 240 mg/dL or use of lipid-lowering drug treatment. The clinical symptom of dyspnea was based on participant self-report.
Cardiovascular disease (CVD) is considered to have developed if there was a definite manifestation of coronary heart disease (CHD), intermittent claudication, congestive heart failure (CHF), or stroke or transient ischemic attack in the absence of a previous manifestation of any of these diseases. Subjects are diagnosed as having developed CHD if one of the following definite manifestations of CHD: myocardial infarction, coronary insufficiency, angina pectoris, sudden death from CHD, non-sudden death from CHD. A definite diagnosis of CHF requires that a minimum of two major or one major and two minor criteria be present concurrently. The presence of other conditions capable of producing the symptoms and signs are considered in evaluating the findings. Major criteria include paroxysmal nocturnal dyspnea or orthopnea; distended neck veins (in other than the supine position); rales; increasing heart size by x-ray; acute pulmonary edema on chest x-ray; ventricular S(3) gallop; increased venous pressure > 16 cm H20; hepatojugular reflux; pulmonary edema, visceral congestion, cardiomegaly shown on autopsy; and weight loss on CHF Rx: 10 lbs./5 days. Minor criteria include bilateral ankle edema; night cough; dyspnea on ordinary exertion; hepatomegaly; pleural effusion by x-ray; decrease in vital capacity by one-third from maximum record; tachycardia (120 beats per minute or more); and pulmonary vascular engorgement on chest x-ray. CVD endpoints were determined prospectively by a three-physician endpoint panel, using criteria as previously described.11 A history of pulmonary embolism (PE) or heart valve surgery was obtained from participant report to the examining physician.
CT Data Acquisition
Subjects were imaged on an eight-slice MDCT scanner (LightSpeed Ultra, General Electric, Milwaukee, WI) with prospective electrocardiographic triggering during a single breath hold in mid-inspiration (typically 18 seconds) using sequential data acquisition. Before the scan a test breath hold was performed to ensure compliance. Scans were prospectively initiated at 50% of the RR interval, which has been widely used for MDCT based measurements of coronary calcium scan and has been shown to provide the best average image quality for MDCT based data acquisition12. Forty-eight contiguous 2.5-mm thick slices (120 kVp, 320/400 mA (for < and > 220 pounds of body weight, respectively), gantry rotation time of 500 ms and corresponding temporal resolution of 250 ms were acquired. The effective radiation exposure was 1.0–1.25 mSv for 320 mA and 400 mA; respectively. Images were reconstructed using a field of view of 35 cm.
CT based Measurements of Pulmonary and Aortic Diameter
The CT scans were read independently by four experienced readers for the pulmonary and aortic dimensions, using a dedicated offline cardiac workstation (Aquarius, Terarecon, San Mateo, CA). Specifically, the transverse axial diameter of the main pulmonary artery and the ascending aorta at the level of the bifurcation of the right pulmonary artery were measured (Figure 1). The ratio PA was calculated as the ratio of the mPA to the ascending aorta diameter (Ao). Measurement reproducibility determined by two independent readers in a random sample of ninety-two subjects were excellent for inter- and intraobserver variability (interobserver intra-class correlation coefficient: mPA= 0.90, Ao= 0.98, and ratio PA= 0.92; intraobserver intra-class correlation coefficient: mPA= 0.97, Ao= 0.99, and ratio PA= 0.96, all p<0.0001; respectively).
Figure 1.
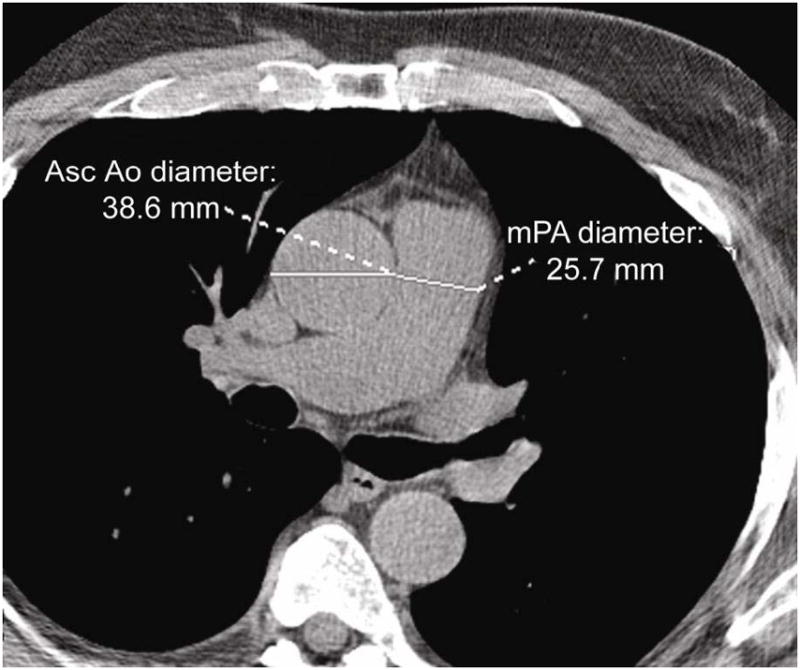
Measurements of main pulmonary artery diameter (mPA) and ratio of the diameters of main PA to ascending aorta (ratio PA) on transaxial image.
Statistical Analysis
Descriptive statistics were expressed as mean ± standard deviation (SD) for continuous variables and as frequency and percentages for nominal variables. A healthy reference sample was created by excluding individuals with any of the following conditions from the overall study sample: obesity, hypertension, current and former smokers, COPD, history of PE, diabetes, CVD, and heart valvular surgery. For each dichotomous risk factor, Student’s t-test was used to compare participants with “cardiopulmonary risk factors” (CPRF) on mean mPA and mean ratio PA. To assess the relationship between mPA and ratio PA with age, we used sex-specific Pearson’s correlation. We used unadjusted and age and sex-adjusted analysis of variance to compare mean mPA and mean ratio PA between the healthy referent cohort and non-referent cohort. We determined the distributions (the 25th, 50th, 75th, and 90th percentiles within age- and sex-specific strata) of mPA and ratio PA measurements in the healthy reference sample and in the overall cohort. We defined abnormally high mPA and ratio PA as exceeding the 90th percentile healthy referent sex-specific cutpoint13 and used Student’s t-test and Chi-square or Fisher’s Exact test to compare the clinical characteristics between groups, as appropriate. We used logistic regression to determine the association of abnormally high mPA and ratio PA to prevalent dyspnea in a cross-sectional manner. A two-sided p-value <0.05 was considered to indicate statistical significance for all tests.
Results
Cohort Characteristics
Table 1 depicts the clinical characteristics of the entire imaged cohort of 3171 men and women in the Framingham Heart Study for whom we performed the pulmonary artery and aortic measurements. The mean age was 51 years, 51% men, with nearly a third of participants found to be obese and have hypertension. Nearly half of the subjects were current or former smokers, but only few had manifest COPD (5.5%) or had a history of PE (0.7%). The mPA diameter of the entire cohort was 25.1 ± 2.8mm and the mean ratio PA was 0.77 ± 0.09.
Table 1.
Clinical Characteristics of the Study Population
| Entire cohort (n=3171) | Healthy referent cohort* (n=706) | |
|---|---|---|
| Age, yrs | 50.8 ± 10.3 | 45.6 ± 8.1 |
| Male gender, % | 1620 (51.1%) | 354 (50.1%) |
| Height, cm | 170.1 ± 9.7 | 171.0 ± 9.6 |
| BSA, m2 | 1.94 ± 0.25 | 1.85 ± 0.21 |
| BMI, kg/m2 | 27.8 ± 5.3 | 24.6 ± 2.9 |
| Obesity, % | 870 (27.5%) | - |
| Systolic blood pressure, mmHg | 122.2 ± 16.4 | 111.5 ± 9.1 |
| Diastolic blood pressure, mmHg | 76.1 ± 9.4 | 71.9 ± 7.2 |
| HTN, % | 941 (29.7%) | - |
| Smoking, % | ||
| Current | 417 (13.2%) | - |
| Former | 1231 (38.8%) | - |
| Never | 1523 (48.0%) | 706 (100.0%) |
| Smoking, pack-years | ||
| Current | 27.9 ± 18.8 | - |
| Former | 16.6 ± 17.1 | - |
| COPD, % | 159 (5.5%) | - |
| Hx of Pulmonary embolism, % | 22 (0.7%) | - |
| DM, % | 196 (6.2%) | - |
| Dyslipidemia, % | 761 (24.0%) | 82 (11.6%) |
| CVD, % | 183 (5.8%) | - |
| CHD, % | 118 (3.7%) | - |
| CHF, % | 9 (0.3%) | - |
| Valvular heart surgery, % | 1 (0.03%) | - |
| Dyspnea, % | 665 (20.9%) | 89 (12.6%) |
| Main pulmonary artery diameter (mPA), mm | 25.1 ± 2.8 | 24.7 ± 2.7 |
| Ratio main PA: ascending aorta diameter (ratio PA) | 0.77 ± 0.09 | 0.80 ± 0.09 |
BSA denotes body surface area; BMI, body mass index; HTN, hypertension; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; CVD, cardiovascular disease; CHD, coronary heart disease; and CHF, congestive heart failure.
Healthy referent cohort consisted of participants in the CT study free of obesity, HTN, current and past smokers, COPD, history of pulmonary embolism, DM, CVD, and prior heart valve surgery.
Also shown in Table 1 are the characteristics of the healthy referent group of 706 participants (mean age of 46 years and 50.1% men).
Relationship of mPA and Ratio PA With Cardiopulmonary Risk Factors
As shown in Table 2, the mPA was larger on average in men than women and in participants who had presence of a CPRF; while the ratio PA, which was slightly increased in men as compared to women, was also lower in participants with risk factors for vascular disease.
Table 2.
Mean (±SD) Values of mPA and Ratio PA as Stratified by Presence or Absence of Dichotomous Cardiopulmonary Risk Factors (CPRF)
| mPA (mm) | P-value | Ratio PA | P-value | |
|---|---|---|---|---|
| Gender | ||||
| Men (n=1620) | 26.0±2.7 | <0.0001 | 0.77±0.09 | 0.03 |
| Women (n=1551) | 24.2±2.7 | 0.76±0.09 | ||
|
| ||||
| Obesity | ||||
| Presence (n=870) | 26.4±2.9 | <0.0001 | 0.77±0.09 | 0.08 |
| Absence (n=2297) | 24.7±2.7 | 0.77±0.09 | ||
|
| ||||
| HTN | ||||
| Presence (n=941) | 25.6±3.1 | <0.0001 | 0.74±0.09 | <0.0001 |
| Absence (n=2227) | 24.9±2.7 | 0.78±0.09 | ||
|
| ||||
| Smoking | ||||
| Current (n=417) | 25.0±2.7 | 0.24 | 0.77±0.08 | 0.52 |
| Former or Never (n=2754) | 25.1±2.9 | 0.77±0.09 | ||
| Former (n=1231) | 25.1±3.0 | 0.71 | 0.76±0.09 | <0.0001 |
| Never (n=1523) | 25.2±2.8 | 0.78±0.09 | ||
| Current or Former (n=1648) | 25.1±2.9 | 0.43 | 0.76±0.09 | <0.0001 |
| Never (n=1523) | 25.2±2.8 | 0.78±0.09 | ||
|
| ||||
| COPD | ||||
| Presence (n=159) | 25.3±3.2 | 0.33 | 0.75±0.09 | 0.0007 |
| Absence (n=2744) | 25.0±2.8 | 0.77±0.09 | ||
|
| ||||
| Hx of Pulmonary embolism | ||||
| Presence (n=22) | 27.0±3.5 | 0.003 | 0.77±0.08 | 0.90 |
| Absence (n=3149) | 25.1±2.9 | 0.77±0.09 | ||
|
| ||||
| DM | ||||
| Presence (n=196) | 26.4±3.1 | <0.0001 | 0.76±0.09 | 0.23 |
| Absence (n=2969) | 25.0±2.8 | 0.77±0.09 | ||
|
| ||||
| Dyslipidemia | ||||
| Presence (n=761) | 25.2±2.7 | 0.43 | 0.75±0.09 | <0.0001 |
| Absence (n=2405) | 25.1±2.9 | 0.77±0.09 | ||
|
| ||||
| CVD | ||||
| Presence (n=183) | 25.8±3.1 | 0.002 | 0.75±0.09 | 0.0003 |
| Absence (n=2988) | 25.1±2.8 | 0.77±0.09 | ||
| CHD | ||||
| Presence (n=118) | 25.7±3.1 | 0.02 | 0.74±0.08 | 0.0001 |
| Absence (n=3053) | 25.1±2.9 | 0.77±0.09 | ||
| CHF | ||||
| Presence (n=9) | 26.5±2.8 | 0.16 | 0.72±0.15 | 0.09 |
| Absence (n=3162) | 25.1±2.9 | 0.77±0.09 | ||
Abbreviations as in Table 1.
For age, a positive but very weak correlation was detected with mPA (men: r=0.10, p<0.0001; women: r=0.07, p=0.004), while a negative modest correlation was seen with ratio PA (men: r= −0.32, p<0.0001; women: r=−0.44, p<0.0001) for the entire cohort. For the healthy referent group, there was weak negative correlation in men (r=−0.11, p=0.04) and no correlation in women (p=0.36) between age and mPA. For age and ratio PA, a negative moderate correlation was observed in the healthy referent group (men: r=−0.47, p<0.0001; women: r=−0.34, p<0.0001).
There is a weak correlation with height and mPA (men: r=0.18, p<0.0001; women r=0.24, p<0.0001), and a moderate correlation was observed with BSA and mPA (men: r=0.41, p<0.0001; women: r=0.42; p<0.0001). The correlations between height and BSA with ratio PA were very weak to none (height: r=0.01, p=0.55 for men and r=0.05, p=0.04 for women; BSA: r=0.08, p=0.001 for men and r=0.12, p<0.0001 for women).
In comparing the healthy referent group (n=706) to the non-referent group (n=2465), the mean mPA was larger in the non-referent group (25.2 ± 2.9 mm) as compared to the healthy referent group (24.7 ± 2.9 mm, p<0.0001). This difference in mPA size between groups persisted even after adjusting for age- and gender (25.2 ± 2.9 mm vs 24.8 ±2.9, p<0.0001). The ratio PA was marginally smaller in the non-referent group as compared to the healthy reference group (unadjusted: 0.76 ± 0.09 vs. 0.80 ± 0.09, age- and gender-adjusted: 0.76 ± 0.08 vs 0.78 ± 0.09, all p<0.0001; respectively).
The distributions of mean mPA and ratio PA for the healthy referent cohort is shown in Table 3.
Table 3. Healthy reference cohort.
Mean and percentiles of mPA diameter and ratio PA by mean age in men and women in the healthy referent sample. The healthy referent cohort excludes obesity, hypertension, current or past smokers, COPD, history of pulmonary embolism, diabetics, CVD, and heart valvular surgery.
| mPA | Ratio PA | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | |||||||||
| Age (years) | <45 | 45–54 | ≥55 | <45 | 45–54 | ≥55 | <45 | 45–54 | ≥55 | <45 | 45–54 | ≥55 |
| N | 211 | 102 | 41 | 142 | 131 | 79 | 211 | 102 | 41 | 142 | 131 | 79 |
| Mean | 25.7 | 25.6 | 25.2 | 23.5 | 23.8 | 24.0 | 0.84 | 0.78 | 0.72 | 0.81 | 0.80 | 0.73 |
| SE | 0.2 | 0.3 | 0.4 | 0.2 | 0.2 | 0.3 | 0.006 | 0.008 | 0.011 | 0.008 | 0.008 | 0.010 |
| 25th | 24.0 | 23.8 | 23.4 | 21.7 | 22.1 | 22.2 | 0.78 | 0.72 | 0.67 | 0.75 | 0.73 | 0.68 |
| 50th | 25.4 | 25.4 | 25.6 | 23.4 | 23.6 | 24.0 | 0.84 | 0.79 | 0.72 | 0.81 | 0.79 | 0.72 |
| 75th | 27.4 | 27.2 | 27.0 | 25.1 | 25.3 | 25.5 | 0.89 | 0.83 | 0.76 | 0.88 | 0.84 | 0.77 |
|
| ||||||||||||
| 90th | 29.1 | 29.2 | 28.0 | 26.6 | 27.4 | 26.9 | 0.93 | 0.89 | 0.82 | 0.94 | 0.90 | 0.84 |
Abbreviations as in Table 1. SE denotes standard error.
In the healthy reference cohort (Table 3), the mPA diameter showed small differences within age strata and were smaller in women than in men (90th percentile cutoff for men ranged from 28.0 to 29.2 mm, and for women ranged from 26.6 to 27.4 mm). The sex (but not age) -specific 90th percentile was 28.9 mm in men and 26.9 mm in women. From the entire imaged cohort, 438 participants (14.0%) had abnormal mPA value above these 90th percentile sex-specific cutpoints. The proportion of patients with enlarged mPA values is illustrated by deciles of age, for both men and women, in Figure 2A. Patients with enlarged mPA values had generally more CVD risk factors, prevalent CVD, and dyspnea than those without enlarged mPA (Table 4). Interestingly, no difference in prevalence of COPD was seen between patients with and without enlarged mPA (p=0.22).
Figure 2.
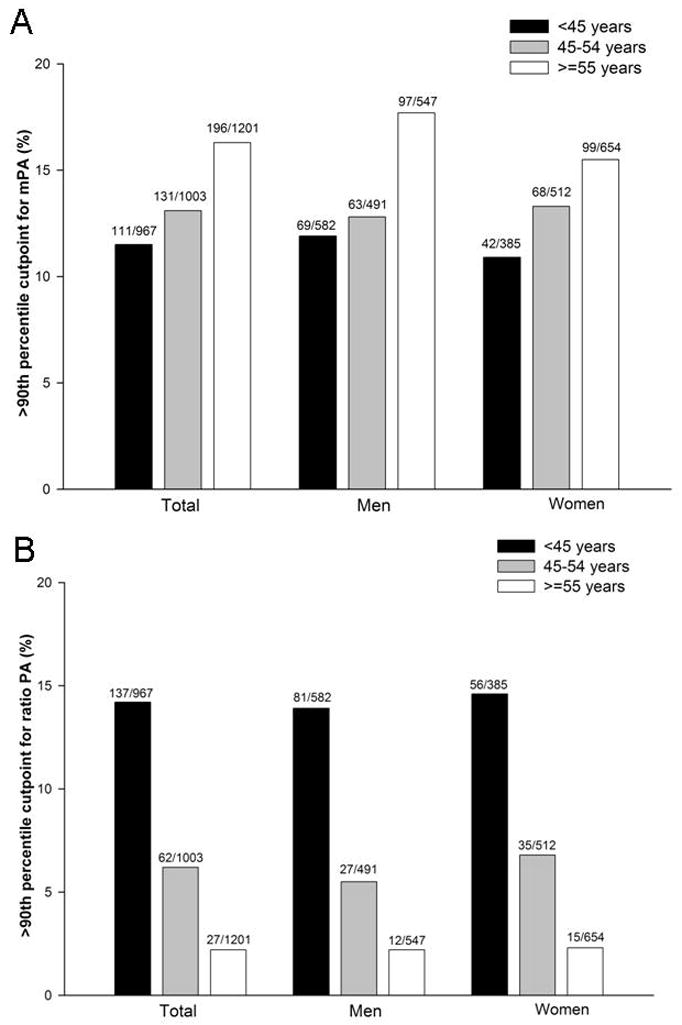
Percentage of participants in the entire FHS above the healthy referent 90th percentile sex-specific cutpoint for mPA (A) and ratio PA >0.9 (B) as stratified by age and sex.
Table 4.
Clinical Characteristics as Stratified by 90th percentile sex-specific cutpoint for mPA and ratio PA >0.9.
| mPA >90th Percentile (n=438) | mPA ≤90th Percentile (n=2733) | P-value | Ratio PA >0.9 (n=226) | Ratio PA ≤0.9 (n=2945) | P-value | |
|---|---|---|---|---|---|---|
| Age, yrs | 52.8 ± 11.5 | 50.4 ± 10.1 | <0.001 | 44.8 ± 8.8 | 51.3 ± 10.3 | <0.001 |
| Male gender, % | 229 (52.3%) | 1391 (50.9%) | 0.59 | 120 (53.1%) | 1500 (50.9%) | 0.53 |
| Height, cm | 172.2 ± 9.9 | 169.8 ± 9.6 | <0.001 | 171.6 ± 9.7 | 170.0 ± 9.6 | 0.02 |
| BSA, m2 | 2.1 ± 0.3 | 1.9 ± 0.2 | <0.001 | 2.0 ± 0.3 | 1.9 ± 0.2 | 0.07 |
| BMI, kg/m2 | 30.6 ± 6.0 | 27.3 ± 5.0 | <0.001 | 27.9 ± 5.3 | 27.8 ± 5.3 | 0.74 |
| Obesity (%) | 198 (45.2%) | 672 (24.6%) | <0.001 | 61 (27.0%) | 809 (27.5%) | 0.87 |
| Systolic blood pressure, mmHg | 124.8 ± 18.0 | 121.8 ± 16.1 | <0.001 | 116.7 ± 14.1 | 122.4 ± 16.5 | <0.001 |
| Diastolic blood pressure, mmHg | 76.6 ± 9.2 | 76.0 ± 9.5 | 0.17 | 74.6 ± 9.0 | 76.2 ± 9.5 | 0.01 |
| HTN (%) | 170 (38.8%) | 771 (28.2%) | <0.001 | 45 (19.9%) | 896 (30.4%) | <0.001 |
| Smoking, % | ||||||
| Current | 48 (11.0%) | 369 (13.5%) | 22 (9.7%) | 395 (13.4%) | ||
| Former | 199 (45.4%) | 1032 (37.8%) | 0.01 | 67 (29.6%) | 1164 (39.5%) | <0.001 |
| Never | 191 (43.6%) | 1332 (48.7%) | 137 (60.6%) | 1386 (47.1%) | ||
| Smoking, pack-years | ||||||
| Current | 26.5 ± 15.7 | 28.1 ± 19.2 | 0.59 | 17.6 ± 11.5 | 28.5 ± 19.0 | 0.01 |
| Former | 18.5 ± 18.5 | 16.3 ± 16.8 | 0.11 | 11.8 ± 13.8 | 16.9 ± 17.2 | 0.02 |
| COPD (%) | 26 (5.9%) | 133 (4.9%) | 0.22 | 6 (2.7%) | 153 (5.2%) | 0.07 |
| Hx of Pulmonary embolism, % | 6 (1.4%) | 16 (0.6%) | 0.07 | 2 (0.9%) | 20 (0.7%) | 0.72 |
| DM, % | 49 (11.2%) | 147 (5.4%) | <0.001 | 12 (5.3%) | 184 (6.2%) | 0.57 |
| Dyslipidemia, % | 101 (23.1%) | 660 (24.1%) | 0.61 | 38 (16.8%) | 723 (24.6%) | 0.01 |
| CVD, % | 36 (8.2%) | 147 (5.4%) | 0.02 | 12 (5.3%) | 171 (5.8%) | 0.76 |
| CHD, % | 25 (5.7%) | 93 (3.4%) | 0.02 | 6 (2.7%) | 112 (3.8%) | 0.38 |
| CHF, % | 2 (0.5%) | 7 (0.3%) | 0.46 | 1 (0.4%) | 8 (0.3%) | 0.64 |
| Valvular Heart Surgery, % | 0 (0.0%) | 1 (0.0%) | 0.69 | 0 (0.0%) | 1 (0.0%) | 0.78 |
| Dyspnea, % | 110 (25.1%) | 555 (20.3%) | 0.02 | 47 (20.8%) | 618 (21.0%) | 0.99 |
Abbreviations as in Table 1.
From the 90th percentile values in the healthy referent cohort (Table 3), there is a decrease in the ratio PA with increasing age with similar values for men and women. Based on the healthy referent group, while the sex-specific 90th percentile cutoff value was 0.91 in both men and women, for simplicity, we used 0.9 as the cutpoint. From the entire cohort, 226 participants (7.1%) had ratio PA value above the 0.9 cutpoint. The proportion of patients with ratio PA values greater than 0.9 is shown by deciles of age, for both men and women, in Figure 2B. Despite more CVD risk factors, especially smoking history, there was no significant difference in prevalent COPD, CVD, or dyspnea between participants the ratio PA above and below 0.9 (Table 4).
Table 5 shows the distribution of mPA and ratio PA for the entire Framingham cohort.
Table 5. Entire Framingham study cohort.
Mean and percentiles of mPA diameter and ratio PA by mean age in men and women of entire study cohort.
| mPA | Ratio PA | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | |||||||||
| Age (years) | <45 | 45–54 | ≥55 | <45 | 45–54 | ≥55 | <45 | 45–54 | ≥55 | <45 | 45–54 | ≥55 |
| N | 582 | 491 | 547 | 385 | 512 | 654 | 582 | 491 | 547 | 385 | 512 | 654 |
| Mean | 25.9 | 26.0 | 26.2 | 23.9 | 24.1 | 24.4 | 0.82 | 0.77 | 0.72 | 0.81 | 0.78 | 0.73 |
| SE | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.003 | 0.004 | 0.003 | 0.005 | 0.004 | 0.003 |
| 25th | 24.0 | 24.1 | 24.0 | 22.2 | 22.3 | 22.5 | 0.76 | 0.71 | 0.67 | 0.74 | 0.71 | 0.68 |
| 50th | 25.7 | 25.9 | 25.9 | 23.8 | 23.9 | 24.2 | 0.81 | 0.77 | 0.72 | 0.80 | 0.78 | 0.73 |
| 75th | 27.5 | 27.5 | 28.1 | 25.5 | 25.7 | 26.0 | 0.87 | 0.82 | 0.77 | 0.87 | 0.83 | 0.78 |
| 90th | 29.1 | 29.4 | 30.0 | 27.0 | 27.4 | 28.0 | 0.91 | 0.88 | 0.83 | 0.93 | 0.88 | 0.84 |
Abbreviations as in Table 1. SE denotes standard error.
Association of mPA and Ratio PA with Dyspnea
Using the sex-specific 90th healthy referent percentile cutoff, more participants with abnormal mPA (26%) had self-reported dyspnea on exertion than those with normal mPA (20%), odds ratio [OR] 1.35, 95% CI: 1.07–1.70, p=0.01. This difference persisted after age and sex adjustment (OR 1.33, 95% CI: 1.05–1.68, p=0.02).
The ratio PA was not significantly associated with self-reported dyspnea for either the cutoff value of 0.9 or the traditionally used value of 1.0 (all p>0.40).1, 3–4
Discussion
The mPA and ratio PA are two measurements that are clinically relevant to cardiopulmonary disease and that are easily and reproducibly obtained in non-contrast chest CT. The use of a large population based samples is essential for establishing accurate normal biometrics for cardiac or chest CT. Using a CT study in the community-based Framingham cohort, we note significant differences in mPA and/or ratio PA according to obesity, hypertension, history of COPD or pulmonary embolism, adult onset diabetes, dyslipidemia and prevalent CVD. Using a healthy referent sample without cardiopulmonary risk factors or disease, we establish a 90th percentile sex-specific cutoff value for mPA for men of 29 mm and for women, 27 mm. We found an association with dyspnea in participants with enlarged mPA using these cutoff values. Similarly, we also establish a cutoff value for ratio PA as 0.9 for both women and men. The ratio PA decreased similarly in both men and women and was smaller with increasing age strata in the healthy referent sample, though no association was found with dyspnea. The strength of our study is the large size of the cohort and uniform patient population.
We chose to measure and report the simple measurement of axial mPA diameter at the level of the bifurcation of the right pulmonary artery because this landmark is easy to define anatomically, rendering it highly reproducible. At that location, the ascending aorta can be easily measured to provide a quick calculation of the ratio PA. A gender-specific but not age-specific cutoff values for the mPA were selected due to the very weak correlation found in men and no correlation in women between age and mPA in the healthy referent group. Moreover, while we observed a positive correlation between mPA diameter with both height and BSA, this data is not routinely available for chest or cardiac imagers at time of image interpretation and would limit its clinical utility.
This simple mPA measurement was larger in the non-healthy referent group as compared to the healthy referent group. A similar pattern is seen in participants with presence of CPRF as compared to those without, Table 2. In contrast, the ratio PA was negatively correlated with age, with very weak to no correlation to height and BSA. The ratio PA was smaller in the non-healthy referent group as compared to the healthy referent group with a similar pattern is seen in participants with presence of CPRF as compared to those without. This pattern is likely driven by the enlarging aortas commonly observed in the elderly population.14
Several small studies have suggested that an enlarged mPA may predict the presence of pulmonary hypertension, with CT-based cutoff values ranging from 28.6 mm to 33.2 mm.1, 3, 7, 15–16 However, diagnostic accuracy is variable depending on the patient selection and cutpoints used, with modest sensitivities ranging from 54–87% and higher specificities of 63–95% for detecting pulmonary hypertension, particularly when higher cutoff values were used.1, 3, 5, 7, 15–16 Our results are consistent with the prior smaller studies and we extend the literature by providing sex-specific CT normative values for mPA diameter. In comparison with other noninvasive modalities, our CT cutoff values are similar to the 28 mm cutoff value of magnetic resonance imaging (MRI),17 while the upper limit of normal is 21 mm by echocardiography.18 Since both CT and MRI are more comparable modalities to visualize the pulmonary artery than echocardiography, our sex-specific CT values may be used for MRI also, if measured at the same slice location. Though we did not directly compare subjects with and without pulmonary hypertension, we did find that participants with self-reported dyspnea were more likely to have abnormal mPA diameter. Abnormal mPA may be used as a positive indicator for identifying possible subclinical disease, though should be prospectively validated.
With respect to the ratio PA, it is generally accepted that at the level of the bifurcation of the main pulmonary artery, the ascending aorta is larger in diameter than the main PA in normal persons. Thus, a ratio PA of greater than 1 is often used to suggest pathology.1, 3–4 In our healthy referent sample, we found that the 90th percentile mean ratio PA ranged from 0.82–0.94, depending on age, and was similar in men and women. Interestingly, the observation that the ratio PA was smaller in older participants may be driven by progressive aortic enlargement with increasing age. While in a small study of 50 patients, the ratio > 1 was found to be predictive of pulmonary hypertension,4 a larger study of 190 patients with pulmonary embolism showed only modest diagnostic accuracy with sensitivity 59% and specificity 82% for predicting moderate to severe pulmonary hypertension (defined as pulmonary artery systolic pressure of > 50 mmHg by echocardiography).16 We did not find an association with dyspnea when using either the 90th percentile cutoff value of 0.9 or the commonly used ratio of 1.0 for ratio PA. Given the lack of association with symptoms, the clinical utility of the ratio PA may be less useful than mPA.
Potential Clinical Significance
These two simple measurements may hold some promise for the preclinical diagnosis for the “silent diseases” of the cardiopulmonary system, such as early pulmonary arterial hypertension or early left heart disease (pulmonary venous hypertension) or a mixed process. Pulmonary hypertension patients may be clinically quiescent for years until diagnosis, when symptoms develop and irreversible changes to the pulmonary vasculature have incurred. It is associated with a high morbidity and mortality and affects a heterogeneous group of patients with increasing prevalence with advancing age.19 However, this disease entity remains largely underreported and the time of symptom onset with dyspnea or shortness of breath to actual diagnosis is often prolonged and delayed (~2 years), occurring in the latter stages of the disease spectrum.19 Once progression of disease results in cor pulmonale and right heart failure, irreversible changes have occurred and prognosis drastically worsens. Definitive diagnosis by invasive right heart catheterization is required for the direct measurement of the pulmonary artery pressures and is the gold standard for detection of this disease.20 The current noninvasive modality used to suggest the presence of pulmonary hypertension is the transthoracic echocardiogram, which can provide an estimate of the pulmonary artery systolic pressure by evaluation of the tricuspid jet velocity.20 Our study is important in that it establishes a standard normative reference value for mPA size and ratio PA by CT. Future studies should aim at determining if these parameters may represent subclinical disease.
Limitations
There are several limitations notable for this study. The CT scan study is limited by its cross-sectional study design and our findings should be prospectively tested. Due to the very limited data regarding the correlation to pathologic conditions, it should be emphasized that at the present time there is only limited evidence for using these data as “cutoff” values. Dyspnea was based on self-report and thus its association should be interpreted as hypothesis generating and cannot be deemed as conclusive. Validation with right heart catheterization for pulmonary artery pressures would be ideal, though such invasive procedures are not feasible in a large population-base asymptomatic cohort. Participants from the Framingham Heart Study are predominant Caucasian in ethnicity and thus the reference values reported in this analysis may not be applicable to other racial groups. Confirmation of these two simple metrics in a more diverse population would be of interest. We recognize that PA and aorta size may be variable depending on body size and while indexing to BSA may provide incremental value,14 most chest CT performed in the clinical setting would not include this data and thus would have less clinical applicability.
Conclusion
In non-contrast MDCT, both mPA and ratio PA measurements are easily obtained and highly reproducible. Using the 90th percentile, the sex-specific cutoff value for mPA for men is 29 mm and for women is 27 mm and for ratio PA is 0.9 for both genders. Enlarged mPA is associated with multiple CVD risk factors and prevalent CVD as well as with dyspnea. Our findings are an important beginning of a more complete understanding of the variability of PA measurements and how they may correlate with cardiopulmonary disease.
Acknowledgments
Sources of Funding
The Framingham Heart Study is supported by the National Heart, Lung and Blood Institute (N01-HC-25195). Drs. Truong and Rogers received support from NIH grant T32HL076136. Dr. Truong also received support from NIH grant K23HL098370 and L30HL093896.
Footnotes
Disclosures
None.
References
- 1.Kuriyama K, Gamsu G, Stern RG, Cann CE, Herfkens RJ, Brundage BH. CT-determined pulmonary artery diameters in predicting pulmonary hypertension. Invest Radiol. 1984;19:16–22. doi: 10.1097/00004424-198401000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Haimovici JB, Trotman-Dickenson B, Halpern EF, Dec GW, Ginns LC, Shepard JA, McLoud TC. Relationship between pulmonary artery diameter at computed tomography and pulmonary artery pressures at right-sided heart catheterization. Massachusetts General Hospital Lung Transplantation Program. Acad Radiol. 1997;4:327–334. doi: 10.1016/s1076-6332(97)80111-0. [DOI] [PubMed] [Google Scholar]
- 3.Edwards PD, Bull RK, Coulden R. CT measurement of main pulmonary artery diameter. Br J Radiol. 1998;71:1018–1020. doi: 10.1259/bjr.71.850.10211060. [DOI] [PubMed] [Google Scholar]
- 4.Ng CS, Wells AU, Padley SP. A CT sign of chronic pulmonary arterial hypertension: the ratio of main pulmonary artery to aortic diameter. J Thorac Imaging. 1999;14:270–278. doi: 10.1097/00005382-199910000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Beiderlinden M, Kuehl H, Boes T, Peters J. Prevalence of pulmonary hypertension associated with severe acute respiratory distress syndrome: predictive value of computed tomography. Intensive Care Med. 2006;32:852–857. doi: 10.1007/s00134-006-0122-9. [DOI] [PubMed] [Google Scholar]
- 6.Lin FY, Devereux RB, Roman MJ, Meng J, Jow VM, Simprini L, Jacobs A, Weinsaft JW, Shaw LJ, Berman DS, Callister TQ, Min JK. The right sided great vessels by cardiac multidetector computed tomography: normative reference values among healthy adults free of cardiopulmonary disease, hypertension, and obesity. Acad Radiol. 2009;16:981–987. doi: 10.1016/j.acra.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Chan AL, Juarez MM, Shelton DK, MacDonald T, Li CS, Lin TC, Albertson TE. Novel computed tomographic chest metrics to detect pulmonary hypertension. BMC Med Imaging. 2011;11:7. doi: 10.1186/1471-2342-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, D’Agostino RB, Sr, Fox CS, Larson MG, Murabito JM, O’Donnell CJ, Vasan RS, Wolf PA, Levy D. The Third Generation Cohort of the National Heart, Lung, and Blood Institute’s Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol. 2007;165:1328–1335. doi: 10.1093/aje/kwm021. [DOI] [PubMed] [Google Scholar]
- 9.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163:1256–1276. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 10.Walter RE, Wilk JB, Larson MG, Vasan RS, Keaney JF, Jr, Lipinska I, O’Connor GT, Benjamin EJ. Systemic inflammation and COPD: the Framingham Heart Study. Chest. 2008;133:19–25. doi: 10.1378/chest.07-0058. [DOI] [PubMed] [Google Scholar]
- 11.Cupples L, D’Agostino RS. Some risk factors related to the annual incidence of cardiovascular disease and death using pooled repeated biennial measurements: Framingham Study, 30-year follow-up. In: Kannel W, Wolf P, RJG, editors. The Framingham Heart Study: an Epidemiologic Investigation of Cardiovascular Disease. Washington, DC: NIH Publication; 1987. pp. 87–203. [Google Scholar]
- 12.Hong C, Bae KT, Pilgram TK. Coronary artery calcium: accuracy and reproducibility of measurements with multi-detector row CT--assessment of effects of different thresholds and quantification methods. Radiology. 2003;227:795–801. doi: 10.1148/radiol.2273020369. [DOI] [PubMed] [Google Scholar]
- 13.Thanassoulis G, Massaro JM, Hoffmann U, Mahabadi AA, Vasan RS, O’Donnell CJ, Fox CS. Prevalence, distribution, and risk factor correlates of high pericardial and intrathoracic fat depots in the Framingham heart study. Circ Cardiovasc Imaging. 2010;3:559–566. doi: 10.1161/CIRCIMAGING.110.956706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davies RR, Gallo A, Coady MA, Tellides G, Botta DM, Burke B, Coe MP, Kopf GS, Elefteriades JA. Novel measurement of relative aortic size predicts rupture of thoracic aortic aneurysms. Ann Thorac Surg. 2006;81:169–177. doi: 10.1016/j.athoracsur.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 15.Tan RT, Kuzo R, Goodman LR, Siegel R, Haasler GB, Presberg KW. Utility of CT scan evaluation for predicting pulmonary hypertension in patients with parenchymal lung disease. Medical College of Wisconsin Lung Transplant Group. Chest. 1998;113:1250–1256. doi: 10.1378/chest.113.5.1250. [DOI] [PubMed] [Google Scholar]
- 16.Sanal S, Aronow WS, Ravipati G, Maguire GP, Belkin RN, Lehrman SG. Prediction of moderate or severe pulmonary hypertension by main pulmonary artery diameter and main pulmonary artery diameter/ascending aorta diameter in pulmonary embolism. Cardiol Rev. 2006;14:213–214. doi: 10.1097/01.crd.0000181619.87084.8b. [DOI] [PubMed] [Google Scholar]
- 17.Kruger S, Haage P, Hoffmann R, Breuer C, Bucker A, Hanrath P, Gunther RW. Diagnosis of pulmonary arterial hypertension and pulmonary embolism with magnetic resonance angiography. Chest. 2001;120:1556–1561. doi: 10.1378/chest.120.5.1556. [DOI] [PubMed] [Google Scholar]
- 18.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise J, Solomon S, Spencer KT, St John Sutton M, Stewart W. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79–108. doi: 10.1016/j.euje.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 19.Hyduk A, Croft JB, Ayala C, Zheng K, Zheng ZJ, Mensah GA. Pulmonary hypertension surveillance--United States, 1980–2002. MMWR Surveill Summ. 2005;54:1–28. [PubMed] [Google Scholar]
- 20.Galie N, Manes A, Branzi A. Evaluation of pulmonary arterial hypertension. Curr Opin Cardiol. 2004;19:575–581. doi: 10.1097/01.hco.0000142066.14966.85. [DOI] [PubMed] [Google Scholar]


