Abstract
The phenotypic diversity of breast carcinoma may be explained by the existence of a sub-population of breast cancer cells, endowed with stem cell-like properties and gene expression profiles, able to differentiate along different pathways. A stem cell-like population of CD44+CD24−/low breast cancer cells was originally identified using cells from metastatic pleural effusions of breast carcinoma patients. We have previously reported that upon in vitro culture as mammospheres under stem cell-like conditions, human MA-11 breast carcinoma cells acquired increased tumorigenicity and lost CD24 expression compared with the parental cell line. We now report that upon passage of MA-11 mammospheres into serum-supplemented cultures, CD24 expression was restored; the rapid increase in CD24 expression was consistent with up-regulation of the antigen, and not with in vitro selection of CD24+ cells. In tumors derived from subcutaneous injection of MA-11 mammospheres in athymic nude mice, 76.1 ± 9.7% of cells expressed CD24, vs. 0.5 ± 1% in MA-11 cells dissociated from mammospheres before injection. The tumorigenicity of sorted CD44+CD24− and CD44+CD24high MA-11 cells was equal. Single cell-sorted CD24− and CD24high MA-11 gave rise in vitro to cell populations with heterogeneous CD24 expression. Also, subcutaneous tumors derived from sorted CD24− sub-populations and single-cell clones had levels of CD24 expression similar to the unsorted cells. To investigate whether the high expression of CD24 contributed to the tumorigenic potential of MA-11 cells, we silenced CD24 by shRNA. CD24 silencing (95%) resulted in no difference in tumorigenicity upon s.c. injection in athymic nude mice compared with mock-transduced MA-11 cells. Since CD24 silencing was maintained in vivo, our data suggest that the level of expression of CD24 is associated with but does not contribute to tumorigenicity. We then compared the molecular profile of the mammospheres with the adherent cell fraction. Gene expression profiling revealed that the increased tumorigenicity of MA-11 mammospheres was associated with changes in ten signal transduction pathways, including MAP kinase, Notch and Wnt, and increased expression of aldehyde dehydrogenase, a cancer-initiating cells-associated marker. Our data demonstrate that (i) the level of CD24 expression is neither a stable feature of mammosphere-forming cells nor confers tumorigenic potential to MA-11 cells; (ii) cancer-initiating cells-enriched MA-11 mammospheres have activated specific signal transduction pathways, potential targets for anti-breast cancer therapy.
Keywords: Breast cancer, cancer stem cells, CD24, microarray
Introduction
Breast carcinomas are characterized by a great degree of heterogeneity: they are comprised of various histological subtypes, have variable clinical manifestations and underlying molecular signatures. Their diversity may be attributed, at least in part, to the presence of a sub-population of stem cell-like cells, named cancer-initiating cells (CIC) or cancer stem cells, able to differentiate along different pathways. The term “CIC” does not imply stem cell origin, but stem cell properties, like the capacity for self-renewal, that position the cancer stem cell at the top of a neoplastic hierarchy, giving rise to differentiated progeny that lack these same properties. Their identification has important diagnostic, prognostic and therapeutic implications. Thus, success of anti-neoplastic therapy may require their complete elimination, in order to stop the indefinite regeneration of the cancer cell population. Their existence has been demonstrated in most types of malignant tumors, including breast carcinoma [1–4].
CIC were thought to be extremely rare on the basis of experiments showing that only a small fraction of cancer cells can seed a tumor in an immune-compromised host. In a recent melanoma study [5], using improved experimental transplantation conditions, the detectable frequency of cells with tumorigenic potential increased by several orders of magnitude. Although cells with tumorigenic potential are likely to be much more frequent in most human cancers than originally estimated, available evidence still supports the conclusion that most human cancers, including breast carcinoma, follow a cancer stem-cell model. The therapeutic implications of CIC remain the same regardless of their absolute frequency: these cells, which may have growth or therapeutic resistance properties that differ from those of the bulk tumor, must be effectively targeted to achieve definitive curative benefits.
A CIC population of CD44+CD24−/low cells was originally identified in breast carcinoma patients [2]. Subsequently, Ponti et al. [6] reported that the large majority of cells (95–98%) of highly tumorigenic mammospheres established from two primary and one recurrent breast cancers were CD44+CD24−/low.
The initial reports that only the CD44+CD24−/low subpopulation of human breast cancer cells contains BCIC have been challenged by subsequent studies [7, 8]. Honeth et al. [8] detected a CD44+CD24−/low sub-population in only 31% of 240 human breast cancer samples analyzed, with a strong association with the basal-like phenotype. In addition, contrasting results have been reported by different groups in regard to the invasiveness of CD44+CD24+ compared with CD44+CD24−/low cells [9–12]. CD24 is a heavily glycosylated, mucin-type protein linked to the cell membrane via glycosyl-phosphatidylinositol [13]. It can bind P-selectin, a lectin expressed by vascular endothelium and platelets. The interaction of breast cancer cells with P-selectin via CD24 may be an important adhesion pathway in the metastatic process [9, 14]. Although the design of therapies that eradicate CIC has the potential to revolutionize the treatment of breast cancer [15], the isolation and expansion of CIC from human primary or metastatic breast lesions to identify molecular therapeutic targets is an extremely difficult task. Recent investigations suggest that most putative CIC markers do not clearly define CIC populations [5, 16]. While the phenotypic identification of CIC is at present uncertain, recent studies from our laboratories and those of others [6, 17–21], show that spheroids formed under stem cell-like culture conditions are highly enriched in CIC. In particular, we have recently reported that spheroid-forming cells, derived from established glioblastoma, mammary carcinoma and melanoma cell lines, displayed increased tumorigenicity compared with their respective adherent parental cell lines, including the human MA-11 breast carcinoma cell line [22], opening the possibility to study CIC in a readily available and abundant biological material. The MA-11 cell line, originated from bone marrow micrometastases of a breast cancer patient [23], displays an heterogeneous level of expression of CD24 on the cell surface. In the present study, we have employed the MA-11 cell line as a model to phenotypically characterize CIC-enriched mammosphere-forming cells and to investigate the relationship between CD24 expression and tumorigenicity.
Materials and Methods
Cell Culture
Human MA-11 breast carcinoma cells were cultured in RPMI-1640 additioned with 10% fetal bovine serum. For mammosphere formation, cells were enzymatically detached and plated at clonal density (300–500/cm2) in serum-free medium (SFM), consisting of D-MEM/F12 low osmolality medium (from Gibco, Grand Island, NY) in the presence of B-27 supplement (Gibco) and growth factors (1000 I.U./ml LIF + 10 ng/ml bFGF and 20 ng/ml EGF).
Flow cytometric analysis
Mouse anti-human CD24 (clone ML5) and anti-human CD44 (clone G44-26) monoclonal antibodies were from BD Biosciences (San Jose, CA). Cell surface CD24 expression was quantitated using the Quantum Simply Cellular System (Bangs Laboratories, Fishers, IN), as previously described [24]. 5×105 cells/sample were incubated with saturating concentrations (10 µg/ml) of antibodies for 30 min at 4°C. Standard curves of beads with fixed antibody-binding capacity and samples were analyzed on a FACSVantage flow cytometer (BD Biosciences, San Jose, CA). Antibody binding capacity for each cell population was calculated using the QuickCal v.2.3. software (Bangs Laboratories), employing median histogram values and linear regression analyses.
Tumor implantation
Animal studies were done under a protocol approved by the Institutional Animal Care and Use Committee. For s.c. injection, cells were resuspended in 100 µl PBS, and injected subcutaneously into the right flank of the animals.
Anti-CD24 short hairpin RNA-retroviral vectors
Control shRNA lentiviral particles (Santa Cruz Biotechnology, Santa Cruz, CA, cat. N. 108080), encoding a scrambled shRNA sequence that will not lead to the specific degradation of any cellular message, and anti-CD24 shRNA lentiviral particles (Santa Cruz Biotechnologies, cat. N. 29978-V), containing the following three expression constructs each encoding human CD24-specific 21 nucleotides (plus hairpin) shRNA
869-CCGATATACTCTAGATGAATTCAAGAGATTCATCTAGAGTATATCGGTTTTT
941-CCTGAGGCTTTGGATTTGATTCAAGAGATCAAATCCAAAGCCTCAGGTTTTT
1136-CGGATTCCAAAGAGTAGAATTCAAGAGATTCTACTCTTTGGAATCCGTTTTT,
were employed to knock-down CD24 expression in MA-11 cells. For transduction of MA-11 cells, lentiviral supernatants were preloaded onto recombinant fibronectin (Retronectin, Takara Shuzo, Japan)-coated plates and centrifuged at 950×g for 30 min at 4°C, thus largely avoiding serum contamination. The operation was repeated a second time with fresh supernatant. The supernatant was then removed and the plates washed with PBS before addition of cells. After transduction, a stable cell lines expressing the shRNA was isolated via selection with 2 µg/ml puromycin.
Adhesion assay
CD24-knockdown, mock transduced, mammospheres and parental control MA-11 cells were plated at 1.5×105 in serum-additioned medium in 24-well plates pre-coated with Retronectin and incubated at 37°C in 5% CO2 for 30 min. The plates were then shaken for 15 sec at 2000 rpm, washed with PBS+0.1% BSA twice, fixed with 4% paraformaldehyde, and stained with 5 mg/ml crystal violet in 2% ethanol. Upon addition of 2%SDS at room temperature for 30 min, absorbance was measured at 550 nm, and compared with the absorbance of control cells plated on Retronectin for 4 hours, fixed and stained under the same conditions omitting the shaking step.
Microarray analysis
Total RNA was isolated from proliferating adherent and mammosphere cultures using Trizol phenol-based extraction. The quality of the RNA was assessed by gel electrophoresis and A260/A280 ratio. Microarray analyses were performed by Ocean Ridge Biosciences (ORB, Palm Beach Gardens, FL) using human exonic evidence-based oligonucleotide (HEEBO) microarrays (http://alizadehlab.stanford.edu/) containing approximately 50,000 70-mer probes complementary to constitutive exons of most human genes, as well as alternatively spliced exons, ESTs, and control sequences. Biotinylated UTP complementary RNA (cRNA) probes were prepared, fragmented and hybridized to the microarrays for 16–18 h with constant rotation. The microarray slides were washed under stringent conditions, stained with Streptavidin-Alexa-647 (Invitrogen), and scanned using an Axon GenePix 4000B scanner. For data analyses, the local background was subtracted and the spot intensities were log2-transformed. The spot intensities were then normalized by subtracting the 70th percentile of spot intensity of the probes against human constitutive exons and adding back a scaling factor (grand mean of 70th percentiles). After removing data for low quality spots, control sequences, and non-human probes, 41,987 human probe intensities remained. The human probes intensities were filtered to identify all probes with intensity above a normalized threshold (log2 (3* standard deviation of raw local background) + mean of log2-transformed negative controls), to arrive at 19,557 probes above threshold in both samples from at least one group. For statistical analysis, samples were binned in two groups (MA-11/adh and MA-11/Ms.). The log2-transformed and normalized spot intensities for the detectable probes were examined for differences between the treatment groups by 1-way ANOVA using National Institute of Aging (NIA) Array Analysis software. This ANOVA was conducted using the Bayesian Error Model and 20 degrees of freedom. Gene ontology analysis was performed using GenMAPP software (Gladstone Institute, San Francisco, CA) for 8989 detectable probes with current Entrez Gene IDs. Specifically, the MAPPfinder module of GenMAPP was first used to map all detectable probes, based on their gene targets, to GO and Local MAPP categories. Then MAPPfinder compared the relative representation in each functional group of genes associated with probes meeting one of 5 differential expression criteria to the relative representation of genes associated with the full set of 19,557 detectable probes. Significance was determined by permutation of Z scores with correction for multiple comparisons as described in the GenMAPP software manual. To shed light on the mammosphere-specific signaling pathways, all 8989 genes linked to GO terms identified in the microarray were subjected to pathway analysis using Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis.
Results
CD24 Expression
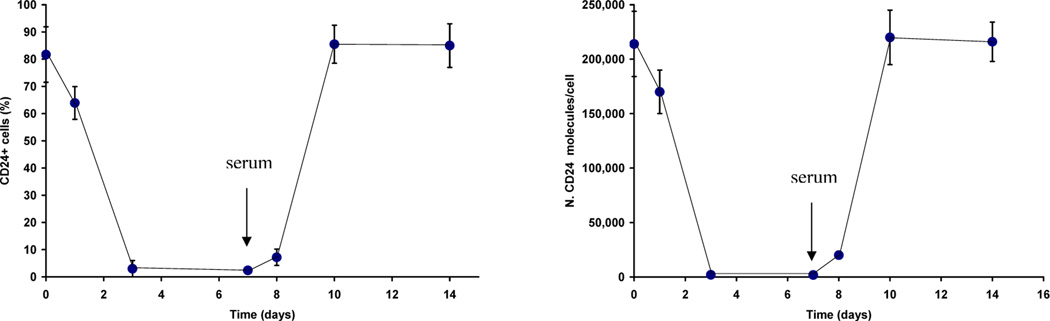
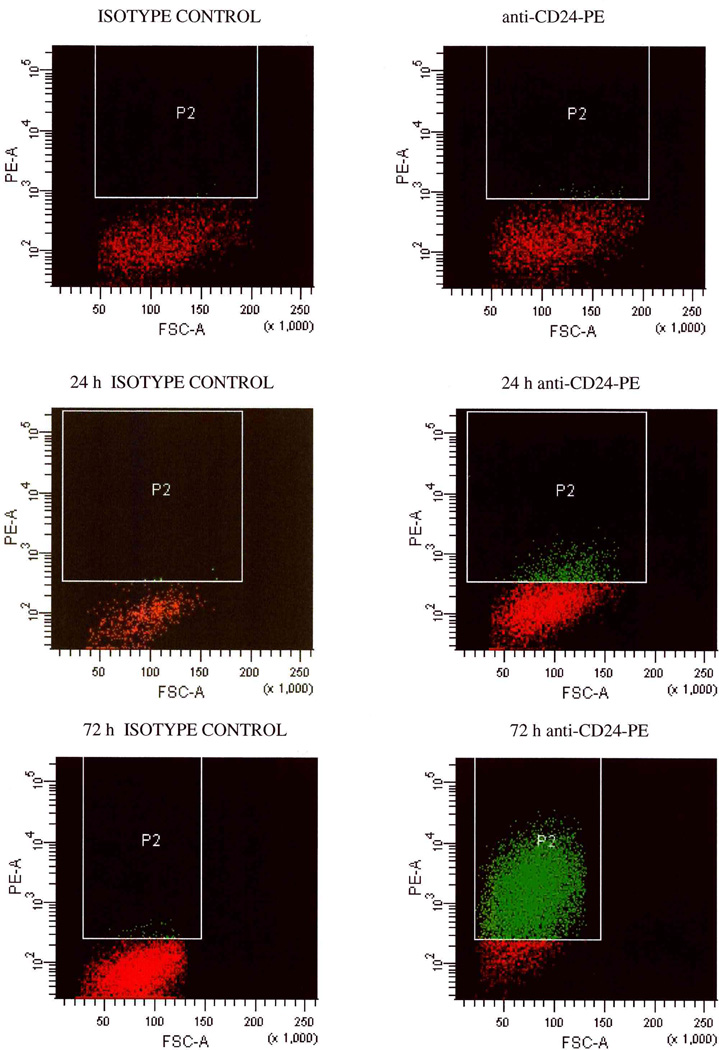
Based on several reports of a CD44+CD24−/low breast CIC population [2, 6, 11], in the present study we investigated the expression of CD24 and CD44 in the human MA-11 breast carcinoma cell line. We measured by flow cytometry, employing phycoerythrin-conjugated anti-human CD24 monoclonal antibodies and the Quantum Simply Cellular System, the number of CD24 molecules on the surface of MA-11 cells. CD24 expression ranged from 0 to 1,120,000 molecules/cell, with an average number of 214,000 molecules/cell. All cells in the MA-11 line expressed CD44, ranging from 900,000 to 8,800,000/cell, with an average number of 4,100,000 molecules/cell. Therefore, the MA-11 line is composed of both CD44+CD24−/low and CD44+CD24high sub-populations. We have previously reported that upon in vitro culture as mammospheres under stem cell-like conditions, MA-11 cells acquired increased tumorigenicity and displayed reduced levels of CD24 compared with parental adherent cells [22]. To investigate whether MA-11 cells grown as mammospheres had irreversibly lost CD24 expression, we dissociated the mammospheres into single cells and cultured them on adherent plastic in serum-supplemented medium. Fig. 1 shows the percentage of CD24+ cells and the average number of CD24 molecules per cell upon growth as mammospheres in serum-free medium and upon dissociation (day 7) and re-plating in adherence in the presence of serum. Both the percentage of CD24+ cells and the number of CD24 molecules per cell decreased very rapidly during mammosphere culture, and then rapidly increased in serum-supplemented cultures. Fig. 2 shows a representative flow cytometry experiment of CD24 re-expression upon culture of cells dissociated from MA-11 mammospheres in serum-supplemented medium. After 24 and 72h, 10 and 88% MA-11 cells expressed CD24; the rapid increase in CD24 expression was consistent with up-regulation of the antigen, and not with in vitro selection of CD24+ cells.
Figure 1.
Changes in CD24 expression upon culture of MA-11 cells as mammospheres and subsequent re-plating under adherent serum-supplemented culture conditions. MA-11 cells were cultured in DMEM additioned with B27 supplement, EGF, bFGF and LIF for 7 days on non-adherent plastic. On day 7 (arrows), mammospheres were disaggregated by incubation with Trypsin-EDTA and plated under adherent conditions in serum-supplemented DMEM. CD24 expression was measured after incubation with PE-conjugated anti-CD24 antibody by flow cytometry. Data are expressed as percentage of CD24+ cells compared with isotype control (left panel) and mean number of molecules per cell (right panel). CD24 molecules were quantitated using the Quantum Simply Cellular System as described in Materials and Methods. Points are the mean of three to four separate experiments; bars, SD.
Figure 2.
CD24 re-expression upon culture of cells dissociated from MA-11 mammospheres in serum-supplemented medium. MA-11 cells were cultured under stem cell-like conditions for seven days, then dissociated and analyzed by flow cytometry immediately (top panels) or 24 and 72h after plating under adherent conditions in serum-supplemented medium. FSC-A, forward scatter-amplitude.
CD24− MA-11 mammospheres generate CD24+ tumors
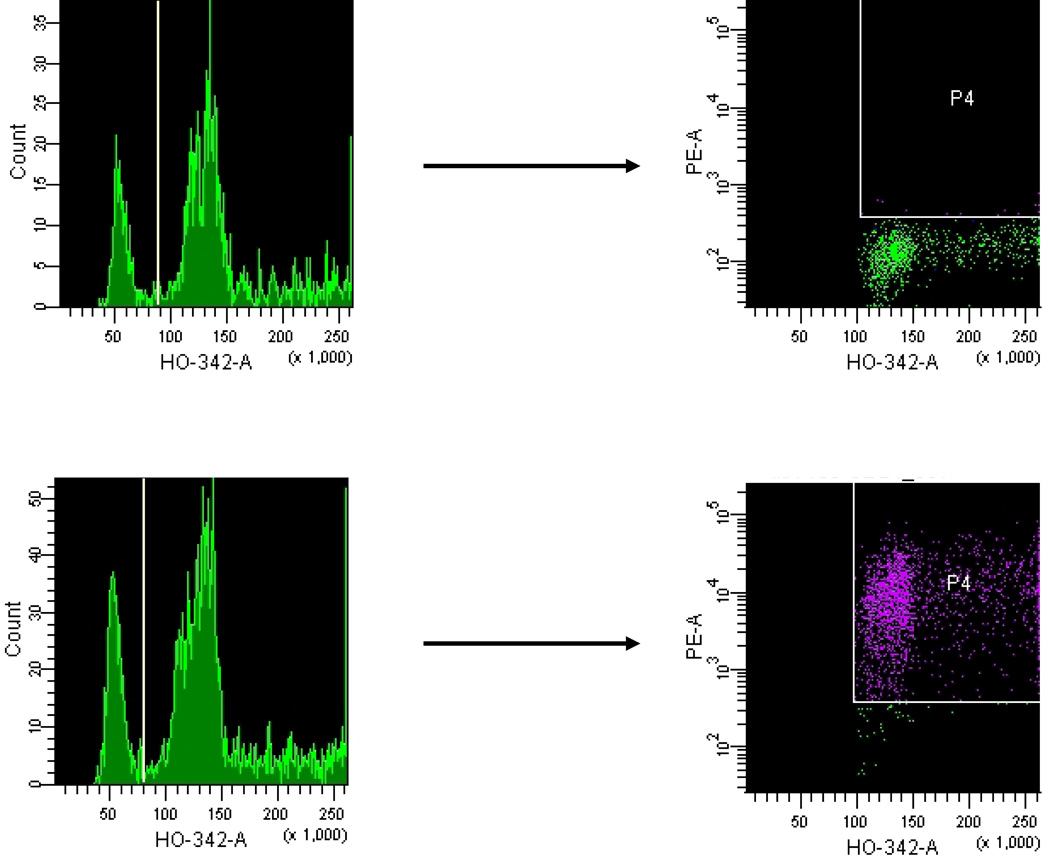
When implanted s.c. in the flank of athymic nude mice, MA-11 mammospheres, that did not express CD24, generated tumors with high expression of CD24, similar to the level of the parental cell line growing under adherent conditions. Fig. 3 shows a representative flow cytometry experiment of cells dissociated from s.c. tumors, employing the vital Hoechst 33342 DNA stain to distinguish between hyperdiploid MA-11 cells, and contaminating mouse diploid cells present in the tumor. An overview of CD24+ cells before and after in vivo growth of adherent MA-11 and of mammospheres is reported in Table 1: the percentage of CD24+ cells did not vary for adherent cells, and increased dramatically for mammospheres. Upon re-growth in adherent culture, CD24 expression remained constant, while in serum-free medium CD24 expression was rapidly lost.
Figure 3.
Expression of CD24 in MA-11 cells from a subcutaneous tumor growing in a Balb/c athymic nude mouse 30 days after local implant of MA-11 cells. To distinguish the diploid normal cell population of the host from the hyperdiploid MA-11 cells, we employed the supravital DNA stain Hoechst 33342 (HO-342-A). After mechanical dissociation of the excised tumor and passage through a 70-µm filter, cells were stained with 5 µg/ml Hoechst, and with fluorescently conjugated anti-CD24 monoclonal antibody. Cells were analyzed using a 3-laser FACSVantage. 5 min before analysis, 5 µg/ml 7-AAD was added to each sample to exclude non-viable cells (7-AAD positive). P4 gates include only the CD24+PE+ cells based on their respective isotype controls. Over 90% of tumor cells were positive for CD24-PE.
Table 1.
CD24 expression in adherent (adh) and mammospheres (Ms.) cultures of MA-11 cells in vitro, in vivo and ex-vivo. Upon culture for a week under adherent conditions in serum-supplemented medium (adh) or as mammospheres under stem cell-like conditions (Ms.), the percentage of CD24+ cells was assessed by flow cytometry (mean ± SE). MA-11 cells were then implanted in the flank of Balb/c athymic nude mice. The resulting tumors were dissociated and analyzed by flow cytometry for percentage of CD24+ cells immediately upon dissociation or after culture under adh or Ms. conditions for one week.
| Percent of CD24+ cells | |||
|---|---|---|---|
| Cell line | In vitro | In vivo | Ex-vivo (one week) |
| MA-11/adh MA-11/Ms. |
75.8 ± 10 (n=18) 0.5 ± 1 (n=10) |
73.6 ± 15.5 (n=18) 76.1 ± 9.7 (n=10) |
75 ± 8 (n=18) 0.7 ± 0.8 (n=10) |
CD24+ and CD24− sorted MA-11 cell sub-populations
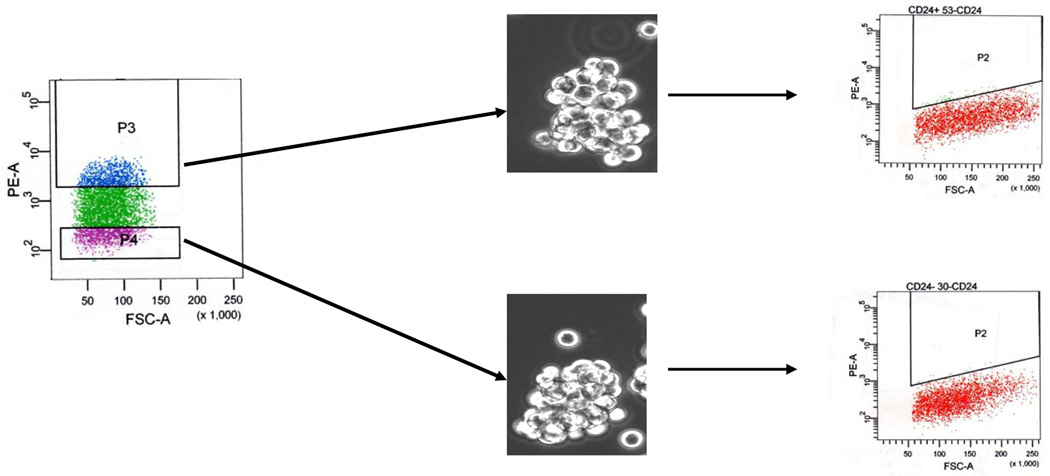
We then stained MA-11 with PE-conjugated anti-CD24 antibodies, and sorted by flow cytometry CD24high (highest 20%; mean number of molecules/cell=744,000) and CD24−/low cells (lowest 20%; mean number of molecules/cell=0). At post-sort analysis, the populations were over 99.5% pure. CD24high and CD24−/low were cultured on adherent plastic in serum-supplemented medium. After 48h in culture, 25 % of sorted CD24− cells re-expressed CD24. After 10 days, CD24high and CD24−/low cells had 293,000 and 81,000 CD24 molecules/cell, respectively. A representative flow cytometry experiment for CD24- sorted MA-11 cells is shown in Fig. 4. We then cloned by limiting dilution CD24+ and CD24− sorted cells, and selected three clones each with an average of 800,000 and 0 molecules/cell for CD24+ and CD24−, respectively. Also single CD24− and CD24high cells cultured after sorting in serum-supplemented medium under adherent conditions gave rise to cell populations with heterogeneous CD24 expression (Fig. 5). No difference in CD44 expression was observed between the sorted populations (not shown). Both CD24+ and CD24− cells, when grown under serum-free conditions, formed mammospheres with similar efficiency (0.15 ± 0.05 (SD) vs. 0.14 ± 0.03 (SD) for CD24+ and CD24−, respectively) and had low or undetectable expression of CD24 (Fig. 6).
Figure 4.
Re-expression of CD24 in sorted CD24− MA-11 cells upon culture under adherent conditions. After labeling of MA-11 cells with anti-CD24-PE antibody, CD24- cells were sorted by flow cytometry, cultured up to ten days on adherent plastic in serum-supplemented medium, and re-analyzed by flow cytometry for CD24 expression at 2 and 10 days after sorting.
Figure 5.
Changes in CD24 expression upon culture of CD24+ and CD24− MA-11 cells. MA-11 cells were sorted by flow cytometry on the basis of CD24 expression and subsequently cultured in DMEM additioned with 10% fetal bovine serum for 15 days under adherent conditions in serum-supplemented medium. CD24 expression was measured after incubation with PE-conjugated anti-CD24 antibody by flow cytometry. Data are expressed as mean number of molecules per cell. CD24 molecules were quantitated using the Quantum Simply Cellular System as described in Materials and Methods. Solid circles, sorted CD24+ cells; solid squares, average of three sorted CD24+ cell clones; open circles, sorted CD24− cells; open squares, average of three sorted CD24− cell clones. Bars, SD.
Figure 6.
Fluorescence-activated cell sorting based on level of CD24 expression in MA-11 cells. After binding of phycoerythrin (PE)-conjugated anti-CD24 antibody, the 20% most PE-bright (P3) and the 20% most PE-dim cells (P4) (purity greater than 99.5%) were sorted and cultured under serum-free conditions. Representative mammospheres formed after 7 days of culture and their level of CD24 expression, measured by flow cytometry, are shown.
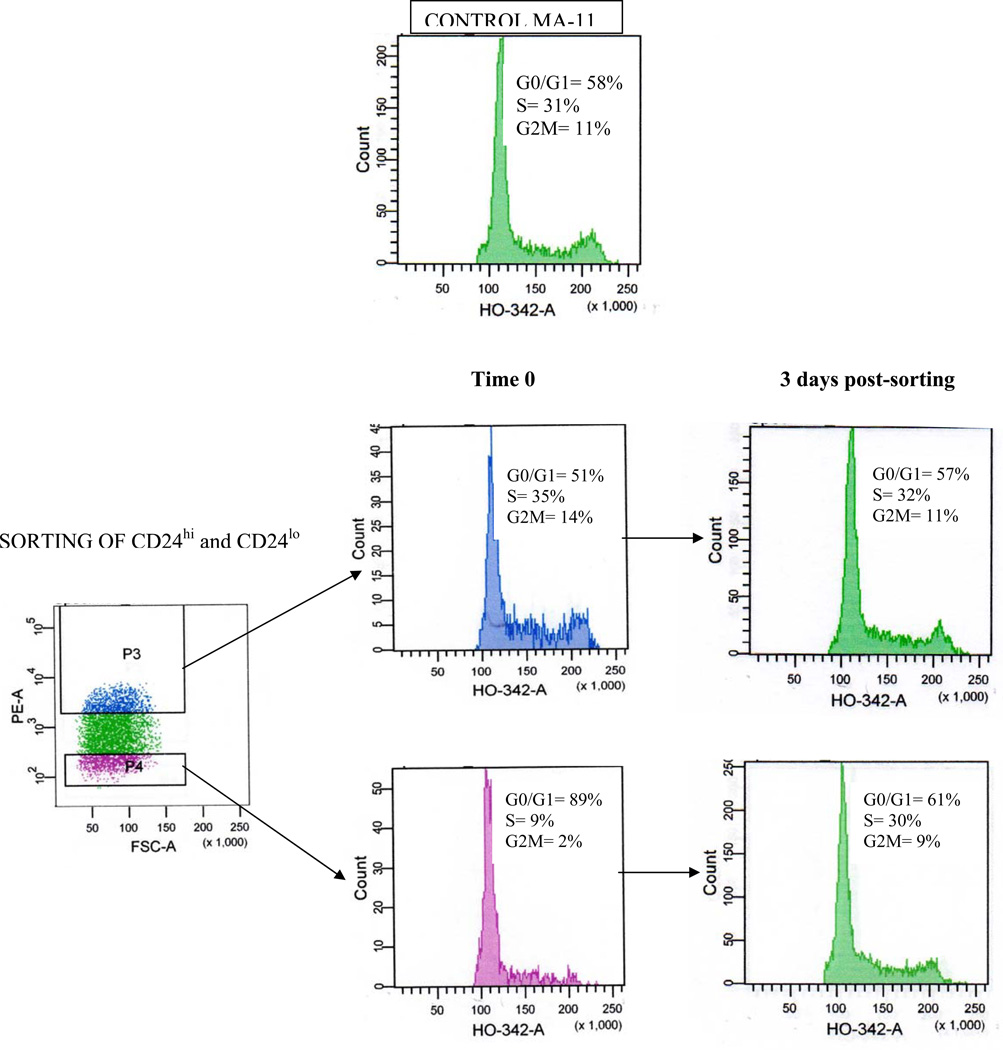
Next, we investigated the relative contribution of CD24+ and CD24− cells to tumorigenicity. Sorted CD24− cell populations and single cell clones injected s.c. into athymic nude mice generated CD24+ tumors (Table 2), with expression levels similar to those of the unsorted cells. The take rates and the growth rates of CD24+ and CD24− cells were equal (Table 2). Thus, implant of 5×105 CD24+ and CD24− bulk cell populations or of CD24+ and CD24− sorted clones resulted in 100% take rate, while implant of 5×104 CD24+ and CD24− bulk cell populations resulted in 43 and 46% take rates, respectively (Wilcoxon two-tailed test, p>0.05). Inocula of 5×103 cells in six mice per group did not result in tumor formation during the 6 months-observation period (not shown). Upon dissociation of MA-11 cells derived from a s.c. tumor and simultaneous staining with PE-conjugated anti-CD24 and Hoechst 33342 DNA stain, we observed that the cells with the highest expression of CD24 were predominantly in the S and G2-M phase of the cell cycle, while those with the lowest or no expression of CD24 were predominantly in G0/G1 (Fig. 7). The proportions of CD24high and CD24low/− cells in G0/G1 phases were 51% and 89%, respectively. The proportions in S phase were 35% and 9%, respectively. Finally, the proportions in G2/M phases were 14% and 2%, respectively. These results indicated that, compared to CD24− cells, more of the CD24high cells were in the synthetic phase and less were in growth arrest phases. However, after 3 days in culture, the doubling time of CD24− was the same as CD24+ cells, their cell cycle distribution was similar to that of CD24+ cells (Fig. 7) and their capacity to grow as mammospheres (Fig. 6) and their tumorigenicity were unchanged (Table 2).
Table 2.
Subcutaneous growth of sorted CD24+ and CD24− MA-11 breast carcinoma cells in athymic nude mice.
| Parameter | 5×104 CD24+ (n = 12) |
5×104 CD24− (n = 8) |
5×105 CD24+ (n = 8) |
5×105 CD24− (n = 8) |
5×105CD24+ clone (n=4) |
5×105 CD24-clone (n = 6) |
|---|---|---|---|---|---|---|
| CD24/cell pre-implant | 755,000 | 0 | 755,000 | 0 | 650000 | 19000 |
| CD24/cell post-implant | 310,000 ± 70,000 | 150,000 ± 55,000 | 290,000 ± 53,000 | 165,000 ± 40,000 | 350,000 ± 50,000 | 170,000 ± 30,000 |
| Take rate (%) | 42 | 50a | 100 | 100 | 100 | 100 |
| Time to 1 cm3 tumor (days) | 43.2 ± 9.2 | 46.2 ± 5.7a | 30 ± 8 | 29 ± 9a | 29 ± 2.6 | 29.5 ± 11.4a |
NS, not significant.. Statistical significance between groups was determined by Wilcoxon two-tailed test. P < 0.05 was considered significant.
Figure 7.
Cell cycle analysis of CD24−/low and CD24hi cells. MA-11 cells were stained with phycoerythrin (PE)-conjugated anti-CD24 antibody. The 20% most PE-bright and the 20% most PE-dim cells were sorted (purity greater than 99.5% at post-sort analysis), stained with the supravital DNA stain Hoechst 33342 for cell cycle analysis and analyzed by flow cytometry. After sorting, cells were cultured for three additional days, detached, re-stained with Hoechst 33342 and analyzed by flow cytometry.
CD24 down-regulation in MA-11 cells
To investigate whether CD24, rather than being a marker of CIC, was positively associated with tumor growth, we knocked down CD24 expression in MA-11 cells by transduction with anti-CD24 shRNA lentiviral particles, and with lentiviral particles encoding a scrambled shRNA sequence as control. Transduced cells were selected by puromycin. We employed the Quantum Simply Cellular System and PE-labeled anti-CD24 mAb to quantitate the number of CD24/cell: CD24 expression decreased by 95%, from 214,000 ± 55,000 (SD)/cell to 11,000 ± 5,000 (SD)/cell (p<0.001). No apparent changes in CD44 levels of expression were observed in CD24-knockdown cells (Suppl. Fig. 1 online). Upon s.c. injection of 5×105 cells in Balb/c athymic nude mice, no difference in tumor growth rate was observed between control and CD24− knockdown cells (Fig. 8). A role of CD24 in cell adhesion processes has been suggested by several studies. To investigate whether CD24 knockdown resulted in changes in adhesion properties of MA-11 cells, we plated control and CD24 knockdown cells on recombinant fibronectin for 30 min at 37°C, followed by shaking of the plate at 2000 rpm for 15 sec. After appropriate washing, fixation of the cells and staining with crystal violet as described in Materials and Methods, the absorbance of the individual wells was compared with that of control cells cultured on recombinant fibronectin for four h, fixed and stained without the shaking step. The adhesive capacity of CD24-knockdown MA-11 cells was unchanged (Table 3). Instead, CD24-negative mammospheres had decreased capacity of substrate adhesion compared with parental adherent cells when plated upon dissociation on fibronectin in serum-additioned medium (Table 3).
Figure 8.
Growth rate of tumors derived from s.c. implant of control and CD24- knockdown MA-11 cells. Cells were transduced with anti-CD24 shRNA lentiviral particles (□), containing three expression constructs each encoding human CD24-specific 21 nucleotides (plus hairpin) shRNA or with control shRNA lentiviral particles (○), encoding a scrambled shRNA sequence that will not lead to the specific degradation of any cellular message, and selected by puromycin as described in Materials and Methods. Balb/c athymic nude mice were implanted s.c. in the flank with 5×105 cells. Tumor volumes were calculated according to the formula V=1/2(a×b2). Points, mean of six experimental mice; bars, SE. No significant difference between groups was observed by Wilcoxon two-tailed test (considering P < 0.05 as significant).
Table 3.
Adhesion of MA-11 cells to fibronectin in vitro.
| Parameter | Percent of plated cells ± SD |
|---|---|
| Mock-transduced | 68.4 ± 4.6 |
| CD24-knockdown | 65.1 ± 1.4* |
| Parental control MA-11 | 67.1 ± 2.0 |
| Mammospheres | 42.2 ± 4.3a |
Cells were plated at 1.5×105 in serum-additioned medium in 24-well plates pre-coated with a recombinant fibronectin fragment (Retronectin) and incubated at 37°C in 5% CO2 for 30 min before measuring cell adhesion as described in Materials and Methods. Data are the mean of three independent experiments. SD, standard deviation.
p>0.05, ANOVA test;
p=0.0008,
ANOVA test
HEEBO gene expression array suggests genes and pathways involved in the increased tumorigenicity of MA-11 mammospheres
To identify the genes that are preferentially expressed in the mammospheres compared with the adherent cell population, we performed microarray analysis of the gene expression profiles of the two cell types, using the HEEBO array on RNA isolated from two independent cultures. A total of 863 annotated genes showed significant differences with >2-fold differences and FDR (false discovery rate) <0.05, including 663 up-regulated and 200 down-regulated in MA-11/Ms vs. MA-11/adh (Suppl. Table 1 online). In all cases where multiple probes were available for the 863 genes, the changes in expression were very similar. To shed light on CIC-specific signaling pathways, all genes differentially expressed in the microarray between mammospheres and adherent cells were subjected to pathway analysis using the KEGG database and assigned to pathway networks.
Ten signaling pathways, MAPK, Wnt, Notch, PPAR, TGFbeta, PIP, Insulin, calcium, GnRH, and focal adhesion were identified containing more than five differentially expressed genes with FDR (false discovery rate) <0.05 (Suppl. Fig. 2). In particular, 21 genes of the MAPK pathway were differentially expressed (12 up-regulated and 9 down-regulated in mammospheres), 5 genes of the Notch pathway and 7 genes of the Wnt pathway were respectively up- and down-regulated in mammospheres. An overview of the MAPK, Notch and Wnt pathways derived from the KEGG database with the differentially regulated genes on the microarray encircled are presented in Suppl. Figs. 3–6. We also found higher expression of the CIC marker, aldehyde dehydrogenase, in the mammosphere than in the adherent population.
Discussion
Because of limited supply and difficult identification of breast CIC from patient samples, in vitro models are critical for the study of breast CIC biology and to design targeted therapeutic interventions. We have previously reported that upon in vitro culture as mammospheres under stem cell-like conditions, human MA-11 breast carcinoma cells acquired increased tumorigenicity and lost CD24 expression compared with the parental cell line. In this study, employing as CIC model the MA-11 cell line, we have demonstrated that the level of CD24 expression was not a stable feature of mammosphere-forming cells, and CD24 rather appeared to be a marker that is down-regulated in the highly tumorigenic MA-11 mammospheres. Our findings that CD24 is rapidly and transiently down-regulated under certain culture conditions reconcile the apparent discrepancy of the pro-malignant and pro-invasiveness role of CD24 with the CD24−/low phenotype of breast CIC [2, 6–9, 11, 12]. The rapid changes in CD24 expression did not allow to define a specific CD44+CD24− sub-population associated with increased tumorigenicity. Clonal studies on cells sorted on the basis of CD24 expression revealed that CD24−/low are generated from CD24hi, and CD24hi are generated from CD24−/low cells. The rapid up- and down-regulation of putative stem cell markers is not novel: Monzani et al. [25] have recently shown that after injecting CD133+ melanoma cells in NOD-SCID mice, most of the tumors became CD133-negative. Furthermore, growing these cells in vitro after few passages they re-expressed CD133. These data support the hypothesis that individual cancer cells can recapitulate the heterogeneity of the tumors from which they derive and that, according to the cancer stem cell model, epigenetic differences in tumorigenic potential among cancer cells have to be irreversible [26].
CD44 in MA-11 cells is expressed in 100% of the cells at homogeneous levels. Also, the observation that CD44 is almost ubiquitously expressed, the report of CD44 antagonism to breast cancer metastasis [27], and the existence of several CD44 variants derived from alternative splicing suggest that CD44 is not an ideal marker for breast CIC. Differently from other established human breast carcinoma cell lines, such as MCF-7 and MDA-MB-231 (Rappa, G., unpublished data), MA-11 cells have an heterogeneous level of expression of CD24 on their surface. This difference may be due to the fact that MA-11 cells have been propagated in culture for less than MCF-7 and MDA-MB-231 and may have undergone less extensive clonal selection than the other cell lines. This heterogeneity has been previously shown in glioblastoma cell lines for another putative CIC marker, CD133 [28]. Our data are also in agreement with the recent finding that the CD44+CD24−/low phenotype can be induced by epigenetic events, such as exposure to TGF-beta, a factor secreted by tumor-associated stroma [29].
We observed high CD24 levels in mouse xenografts derived from s.c. injection of CD44+CD24hi as well as CD44+CD24− cells, suggesting an important role for CD24 in tumor growth. To investigate whether CD24 expression was connected to tumor growth rather than to differentiation of CIC, we studied the effects of CD24 knock-down on MA-11 tumorigenicity. CD24 silencing did not determine any change in the tumorigenic potential of MA-11 cells, suggesting that CD24 expression is associated with in vivo tumor growth, but it is not a determinant of the tumorigenic capacity of the cells. However, since CD24 silencing would have little or no effect on CD44+CD24−/low MA-11 cells, the in vivo contribution of CD44+CD24−/low cells to tumor growth could not be established by this type of experiments. Interestingly, although MA-11 cells dissociated from mammospheres displayed decreased cell adhesion to fibronectin in vitro compared with parental cells, CD24 silencing did not change cell adhesiveness to fibronectin, suggesting that additional phenotypic changes are responsible for the decreased adhesive properties of mammospheres.
cDNA microarray analysis was performed to shed light on the gene expression profile specific to breast CIC. Current cancer drugs, which are developed extensively based on their activity to inhibit bulk replicating cancer cells, may not effectively inhibit CIC (14–16). It is conceivable that targeting CIC will be helpful in eradicating tumors more efficiently (14–16). One promising approach is to target CIC survival signaling pathways (14), where leukemia stem cell research has already made some progress (32). We found that several signal transduction pathways, potential targets for anti-breast cancer therapy, were differentially expressed in the CIC-enriched mammosphere population vs. the adherent cell line. Microarray analysis of the signaling pathway genes identified ten pathways, including MAPK, Notch, and Wnt/β-Catenin, altered in MA-11 mammospheres compared with parental MA-11 cells.
That changes in some of these pathways are implicated in the biological phenomena described in this study is suggested by recent literature data: thus, several pieces of evidence suggest that Notch is relevant to the survival of breast cancer stem cells [30, 31]; Wnt signaling reportedly plays a critical role in regulating stem/progenitor cells in the mammary gland as well as other tissue compartments; and aberrant activation of Wnt signaling induces mammary tumors from stem/progenitor cells [32]. Also, activation of the classical MAPK pathway, leading to c-ras activation, has been associated with breast cancer stem cells and breast tumor epithelial-mesenchymal transition [33, 34].
Conclusion
In this report we show that the level of CD24 expression is not a permanent feature of mammosphere-forming cells or of a cellular subset; it depends on the in vitro growth conditions, and, presumably, on the in vivo microenvironment (niche). Also, the level of CD24 expression is not a determinant of tumorigenicity for MA-11 cells. We are aware that this study has some limitations: (i) MA-11 cells may not accurately reflect the behavior of human breast cancer in patients; (ii) MA-11 mammospheres may be heterogeneous, and this study may actually underestimate the differences between breast CIC and bulk cancer. Further validation of CIC isolated from breast cancer patient specimens and study of the long-term tumorigenic potential of CD24+ and CD24− cells isolated from mouse xenografts (e.g., in serial transplantation experiments) is needed in future studies. We have also found that CIC-enriched MA-11 mammospheres have activated specific signal transduction pathways, potential targets for anti-breast cancer therapy. These data suggest that, employing appropriate cell models, short-term selection and propagation of breast CIC as mammosphere cultures in a defined medium, coupled with gene expression profiling, represents a suitable tool to study and therapeutically target signal transduction pathways of CIC: the notion of which pathways are activated by CIC may render them specific therapeutic targets.
Supplementary Material
Suppl. Fig. 1. CD44 expression does not change with CD24 knock-down. CD24-knockdown and control MA-11 cells were co-stained with nati-CD44-PE and anti-CD24-FITC, and analyzed by flow cytometry. Left panel, control MA-11 cells; right panel, CD24-knockdown MA-11 cells.
Suppl. Fig. 2. KEGG Pathway analysis for cDNA microarray of MA-11 mammospheres vs. MA-11 bulk cell population. Criteria 0 = genes up-regulated in mammospheres; criteria 1 = genes down-regulated in mammospheres.
Suppl. Fig. 3. KEGG MAP kinase Pathway analysis for cDNA microarray of MA-11 mammospheres vs. MA-11 bulk cell population. Genes up-regulated in mammospheres are encircled.
Suppl. Fig. 4. KEGG MAP kinase Pathway analysis for cDNA microarray of MA-11 mammospheres vs. MA-11 bulk cell population. Genes down-regulated in mammospheres are encircled.
Suppl. Fig. 5. KEGG Wnt Pathway analysis for cDNA microarray of MA-11 mammospheres vs. MA-11 bulk cell population. Genes down-regulated in mammospheres are encircled.
Suppl. Fig. 6. KEGG Notch Pathway analysis for cDNA microarray of MA-11 mammospheres vs. MA-11 bulk cell population. Genes up-regulated in mammospheres are encircled.
Acknowledgements
We thank Dr. Giuseppe Pizzorno for reviewing the manuscript and Mr. Fabio Anzanello for his skillful technical assistance. This work was supported by a grant from the National Cancer Institute (R01-CA133797).
Footnotes
Competing interests.
The authors declare that they have no competing interests.
Authors’ contributions.
GR performed the in vitro comparison of adherent cultures vs. mammospheres and the in vivo experiments. AL performed the flow cytometry experiments, CD24 silencing and microarray analysis. Both GR and AL were involved in designing all experiments, and writing the manuscript.
References
- 1.Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 2.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 4.Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 5.Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–598. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, Coradini D, Pilotti S, Pierotti MA, Daidone MG. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65:5506–5511. doi: 10.1158/0008-5472.CAN-05-0626. [DOI] [PubMed] [Google Scholar]
- 7.Abraham BK, Fritz P, McClellan M, Hauptvogel P, Athelogou M, Brauch H. Prevalence of CD44+/CD24−/low cells in breast cancer may not be associated with clinical outcome but may favor distant metastasis. Clin Cancer Res. 2005;11:1154–1159. [PubMed] [Google Scholar]
- 8.Honeth G, Bendahl PO, Ringner M, Saal LH, Gruvberger-Saal SK, Lovgren K, Grabau D, Ferno M, Borg A, Hegardt C. The CD44+/CD24− phenotype is enriched in basal-like breast tumors. Breast Cancer Res. 2008;10:R53. doi: 10.1186/bcr2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim HJ, Kim JB, Lee KM, Shin I, Han W, Ko E, Bae JY, Noh DY. Isolation of CD24(high) and CD24(low/−) cells from MCF-7: CD24 expression is positively related with proliferation, adhesion and invasion in MCF-7. Cancer Lett. 2007;258:98–108. doi: 10.1016/j.canlet.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 10.Fogel M, Friederichs J, Zeller Y, Husar M, Smirnov A, Roitman L, Altevogt P, Sthoeger ZM. CD24 is a marker for human breast carcinoma. Cancer Lett. 1999;143:87–94. doi: 10.1016/s0304-3835(99)00195-0. [DOI] [PubMed] [Google Scholar]
- 11.Sheridan C, Kishimoto H, Fuchs RK, Mehrotra S, Bhat-Nakshatri P, Turner CH, Goulet R, Jr, Badve S, Nakshatri H. CD44+/CD24− breast cancer cells exhibit enhanced invasive properties: an early step necessary for metastasis. Breast Cancer Res. 2006;8:R59. doi: 10.1186/bcr1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schindelmann S, Windisch J, Grundmann R, Kreienberg R, Zeillinger R, Deissler H. Expression profiling of mammary carcinoma cell lines: correlation of in vitro invasiveness with expression of CD24. Tumour Biol. 2002;23:139–145. doi: 10.1159/000064030. [DOI] [PubMed] [Google Scholar]
- 13.Kay R, Takei F, Humphries RK. Expression cloning of a cDNA encoding M1/69-J11d heat-stable antigens. J Immunol. 1990;145:1952–1959. [PubMed] [Google Scholar]
- 14.Aigner S, Ramos CL, Hafezi-Moghadam A, Lawrence MB, Friederichs J, Altevogt P, Ley K. CD24 mediates rolling of breast carcinoma cells on P-selectin. FASEB J. 1998;12:1241–1251. doi: 10.1096/fasebj.12.12.1241. [DOI] [PubMed] [Google Scholar]
- 15.Huff CA, Matsui WH, Douglas Smith B, Jones RJ. Strategies to eliminate cancer stem cells: clinical implications. Eur J Cancer. 2006;42:1293–1297. doi: 10.1016/j.ejca.2006.01.045. [DOI] [PubMed] [Google Scholar]
- 16.Joo KM, Kim SY, Jin X, Song SY, Kong DS, Lee JI, Jeon JW, Kim MH, Kang BG, Jung Y, Jin J, Hong SC, Park WY, Lee DS, Kim H, Nam DH. Clinical and biological implications of CD133-positive and CD133-negative cells in glioblastomas. Lab Invest. 2008;88:808–815. doi: 10.1038/labinvest.2008.57. [DOI] [PubMed] [Google Scholar]
- 17.Zhang S, Balch C, Chan MW, Lai HC, Matei D, Schilder JM, Yan PS, Huang TH, Nephew KP. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008;68:4311–4320. doi: 10.1158/0008-5472.CAN-08-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kondo T, Setoguchi T, Taga T. Persistence of a small subpopulation of cancer stem-like cells in the C6 glioma cell line. Proc Natl Acad Sci U S A. 2004;101:781–786. doi: 10.1073/pnas.0307618100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pellegatta S, Poliani PL, Corno D, Menghi F, Ghielmetti F, Suarez-Merino B, Caldera V, Nava S, Ravanini M, Facchetti F, Bruzzone MG, Finocchiaro G. Neurospheres enriched in cancer stem-like cells are highly effective in eliciting a dendritic cell-mediated immune response against malignant gliomas. Cancer Res. 2006;66:10247–10252. doi: 10.1158/0008-5472.CAN-06-2048. [DOI] [PubMed] [Google Scholar]
- 20.Grimshaw MJ, Cooper L, Papazisis K, Coleman JA, Bohnenkamp HR, Chiapero-Stanke L, Taylor-Papadimitriou J, Burchell JM. Mammosphere culture of metastatic breast cancer cells enriches for tumorigenic breast cancer cells. Breast Cancer Res. 2008;10:R52. doi: 10.1186/bcr2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vlashi E, Kim K, Lagadec C, Donna LD, McDonald JT, Eghbali M, Sayre JW, Stefani E, McBride W, Pajonk F. In vivo imaging, tracking, and targeting of cancer stem cells. J Natl Cancer Inst. 2009;101:350–359. doi: 10.1093/jnci/djn509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rappa G, Mercapide J, Anzanello F, Prasmickaite L, Xi Y, Ju J, Fodstad O, Lorico A. Growth of cancer cell lines under stem cell-like conditions has the potential to unveil therapeutic targets. Exp Cell Res. 2008;314:2110–2122. doi: 10.1016/j.yexcr.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Engebraaten O, Fodstad O. Site-specific experimental metastasis patterns of two human breast cancer cell lines in nude rats. Int J Cancer. 1999;82:219–225. doi: 10.1002/(sici)1097-0215(19990719)82:2<219::aid-ijc12>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 24.Rappa G, Fodstad O, Lorico A. The stem cell-associated antigen CD133 (Prominin-1) is a molecular therapeutic target for metastatic melanoma. Stem Cells. 2008;26:3008–3017. doi: 10.1634/stemcells.2008-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monzani E, Facchetti F, Galmozzi E, Corsini E, Benetti A, Cavazzin C, Gritti A, Piccinini A, Porro D, Santinami M, Invernici G, Parati E, Alessandri G, La Porta CA. Melanoma contains CD133 and ABCG2 positive cells with enhanced tumourigenic potential. Eur J Cancer. 2007;43:935–946. doi: 10.1016/j.ejca.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 26.Shackleton M, Quintana E, Fearon ER, Morrison SJ. Heterogeneity in cancer: cancer stem cells versus clonal evolution. Cell. 2009;138:822–829. doi: 10.1016/j.cell.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 27.Lopez JI, Camenisch TD, Stevens MV, Sands BJ, McDonald J, Schroeder JA. CD44 attenuates metastatic invasion during breast cancer progression. Cancer Res. 2005;65:6755–6763. doi: 10.1158/0008-5472.CAN-05-0863. [DOI] [PubMed] [Google Scholar]
- 28.Bexell D, Gunnarsson S, Siesjo P, Bengzon J, Darabi A. CD133+ and nestin+ tumor-initiating cells dominate in N29 and N32 experimental gliomas. Int J Cancer. 2009;125:15–22. doi: 10.1002/ijc.24306. [DOI] [PubMed] [Google Scholar]
- 29.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sansone P, Storci G, Giovannini C, Pandolfi S, Pianetti S, Taffurelli M, Santini D, Ceccarelli C, Chieco P, Bonafe M. p66Shc/Notch-3 interplay controls self-renewal and hypoxia survival in human stem/progenitor cells of the mammary gland expanded in vitro as mammospheres. Stem Cells. 2007;25:807–815. doi: 10.1634/stemcells.2006-0442. [DOI] [PubMed] [Google Scholar]
- 31.Farnie G, Clarke RB. Mammary stem cells and breast cancer--role of Notch signalling. Stem Cell Rev. 2007;3:169–175. doi: 10.1007/s12015-007-0023-5. [DOI] [PubMed] [Google Scholar]
- 32.Lindvall C, Bu W, Williams BO, Li Y. Wnt signaling, stem cells, and the cellular origin of breast cancer. Stem Cell Rev. 2007;3:157–168. doi: 10.1007/s12015-007-0025-3. [DOI] [PubMed] [Google Scholar]
- 33.Dreesen O, Brivanlou AH. Signaling pathways in cancer and embryonic stem cells. Stem Cell Rev. 2007;3:7–17. doi: 10.1007/s12015-007-0004-8. [DOI] [PubMed] [Google Scholar]
- 34.Liu M, Casimiro MC, Wang C, Shirley LA, Jiao X, Katiyar S, Ju X, Li Z, Yu Z, Zhou J, Johnson M, Fortina P, Hyslop T, Windle JJ, Pestell RG. p21CIP1 attenuates Ras- and c-Myc-dependent breast tumor epithelial mesenchymal transition and cancer stem cell-like gene expression in vivo. Proc Natl Acad Sci U S A. 2009;106:19035–19039. doi: 10.1073/pnas.0910009106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Suppl. Fig. 1. CD44 expression does not change with CD24 knock-down. CD24-knockdown and control MA-11 cells were co-stained with nati-CD44-PE and anti-CD24-FITC, and analyzed by flow cytometry. Left panel, control MA-11 cells; right panel, CD24-knockdown MA-11 cells.
Suppl. Fig. 2. KEGG Pathway analysis for cDNA microarray of MA-11 mammospheres vs. MA-11 bulk cell population. Criteria 0 = genes up-regulated in mammospheres; criteria 1 = genes down-regulated in mammospheres.
Suppl. Fig. 3. KEGG MAP kinase Pathway analysis for cDNA microarray of MA-11 mammospheres vs. MA-11 bulk cell population. Genes up-regulated in mammospheres are encircled.
Suppl. Fig. 4. KEGG MAP kinase Pathway analysis for cDNA microarray of MA-11 mammospheres vs. MA-11 bulk cell population. Genes down-regulated in mammospheres are encircled.
Suppl. Fig. 5. KEGG Wnt Pathway analysis for cDNA microarray of MA-11 mammospheres vs. MA-11 bulk cell population. Genes down-regulated in mammospheres are encircled.
Suppl. Fig. 6. KEGG Notch Pathway analysis for cDNA microarray of MA-11 mammospheres vs. MA-11 bulk cell population. Genes up-regulated in mammospheres are encircled.