Abstract
Hormone secretion is highly organized temporally, achieving optimal biological functioning and health. The master clock located in the suprachiasmatic nucleus (SCN) of the hypothalamus coordinates the timing of circadian rhythms, including daily control of hormone secretion. In the brain, the SCN drives hormone secretion. In some instances, SCN neurons make direct synaptic connections with neurosecretory neurons. In other instances, SCN signals set the phase of “clock genes” that regulate circadian function at the cellular level within neurosecretory cells. The protein products of these clock genes can also exert direct transcriptional control over neuroendocrine releasing factors. Clock genes and proteins are also expressed in peripheral endocrine organs providing additional modes of temporal control. Finally, the SCN signals endocrine glands via the autonomic nervous system, allowing for rapid regulation via multisynaptic pathways. Thus, the circadian system achieves temporal regulation of endocrine function by a combination of genetic, cellular, and neural regulatory mechanisms to ensure that each response occurs in its correct temporal niche. The availability of tools to assess the phase of molecular/cellular clocks and of powerful tract tracing methods to assess connections between “clock cells” and their targets provides an opportunity to examine circadian-controlled aspects of neurosecretion, in the search for general principles by which the endocrine system is organized.
Keywords: Circadian, Diurnal, Endocrinel, Neurosecretion, Clock genes, Suprachiasmatic
Circadian aspects of reproduction
The importance of circadian (about a day) timing in hormone production/secretion has been known since the 1950s when Everett and Sawyer determined that a stimulatory signal occurring during a narrow temporal window on the afternoon of proestrus is necessary for induction of ovulation later that night (Everett and Sawyer, 1950). Such close temporal organization is important for successful reproduction, as numerous hormone-dependent behavioral and physiological processes must be coordinated. If optimal temporal relationships are disrupted, pronounced deficits in fertility can result. For example, ovulation, behavioral estrus, fertilization, and pregnancy maintenance require a specific temporal pattern of hormone secretion in spontaneous ovulators such as rats, hamsters, and mice (Blaustein et al., 1994; McEwen et al., 1987; Mong et al., 2003). Prior to behavioral estrus, rising levels of estrogen both trigger a precisely timed preovulatory surge in luteinizing hormone (LH) and stimulate the production of brain progesterone receptors in preparation for progesterone effects on neurons. The timing of progesterone receptor regulation relative to estrogen ensures that behavioral receptivity is coordinated with the time of ovulation, thereby increasing the likelihood of pregnancy (Hansen et al., 1979). Following ovulation, a prolactin surge is necessary to support the corpus luteum to maintain progesterone secretion necessary for pregnancy and its maintenance (Egli et al., 2004). This sequence is under circadian control.
In the present review, we summarize evidence indicating that the timing of endocrine secretions is coordinated by a brain “clock” located in the suprachiasmatic nucleus (SCN) of the hypothalamus (Fig. 1). Over the last decade, there have been substantial, rapid advances in our understanding of the circadian modulation of brain and peripheral organ activity. Current data indicate that a neural signal from the SCN is necessary for the circadian timing of hormone secretion, while a diffusible signal is sufficient to modulate non-endocrine events such as daily behavioral activities. The identification of core clock genes, clock-controlled genes (CCG), and their localization in neurosecretory cells (Kriegsfeld et al., 2003; Olcese et al., 2003), the pituitary gland (Shieh, 2003; Von Gall et al., 2002) and a number of peripheral endocrine glands (Bittman et al., 2003; Morse et al., 2003; Zylka et al., 1998) each provide new opportunities for evaluating the loci and mechanisms of temporal gating of hormone secretion.
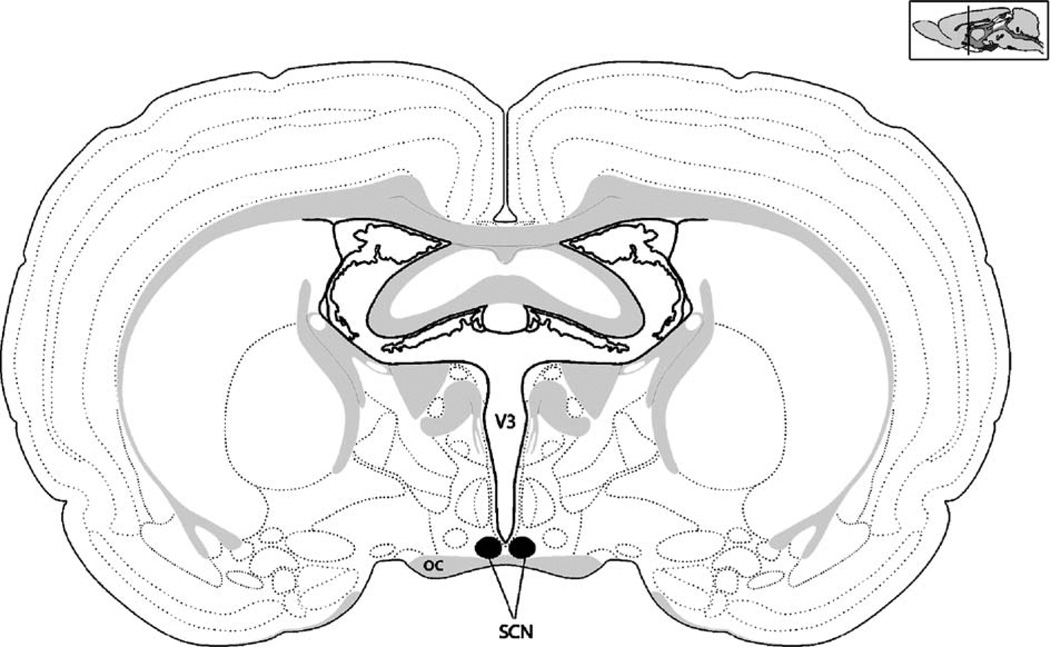
Fig. 1.
The mammalian circadian clock is located in the suprachiasmatic nucleus (SCN) of the anterior hypothalamus. The SCN pictured here in this schematic is a coronal section through a rodent brain. The SCN is situated at the base of the brain of the brain directly above the optic chiasm (oc) and surrounding the third ventricle (V3). The sagittal schematic in the upper right corner depicts the approximate rostral–caudal location depicted in the coronal section.
We review evidence that hormone secretion is regulated not only by the feedback loops long studied by endocrinologists but also by the SCN and SCN-derived temporal signals acting directly on neurosecretory cells, on the autonomic nervous system, and on clock genes and clock-controlled genes. To this end, we present an abbreviated introduction to the core negative feedback loop controlling cellular circadian clock function to provide a basis for understanding how time can be tracked within a cell and to set the foundation for understanding the possible role of clock genes in endocrine regulation. For detailed summaries of research on cellular/molecular clock genes and proteins, the reader is referred to the following reviews (Albrecht, 2004; Ashmore and Sehgal, 2003; Du and Tong, 2002; Duffield, 2003; Gachon et al., 2004; Glossop and Hardin, 2002; Green, 2003; Hastings et al., 2003; Liu, 2003; Okamura, 2003a,b; Okamura et al., 2002; Piggins, 2002; Roenneberg and Merrow, 2003; Schibler and Sassone-Corsi, 2002; Schultz and Kay, 2003; Zordan et al., 2003).
Circadian control of endocrine secretions
Biological events typically exhibit marked, predictable cycles ranging in time from seconds to years. In the endocrine system, virtually every factor measured to date shows a circadian (endogenous) or diurnal (driven) rhythm. These alterations are achieved by modulation of pulse amplitude (i.e., amount of hormone released), pulse frequency (i.e., rate of hormone release), or by a combination of both of these processes. For example, studies in male rhesus macaques in which animals were sampled at 20-min intervals in an LD cycle revealed diurnal rhythms in luteinizing hormone (LH), testosterone, prolactin, and cortisol (Plant, 1981). Likewise, studies in rats, Syrian hamsters, and humans indicate circadian variation in gonadotropins and gonadal steroids around the onset of puberty (Andrews and Ojeda, 1981; Boyar et al., 1976; de la Iglesia et al., 1999; Jakacki et al., 1982; Smith and Stetson, 1980). It has been suggested that diurnal variation in hormone concentrations may simply be modulated by sleep. However, sleep reversal (subjects sleep during the day rather than night) and sleep interruption do not affect the daily pattern of most hormones, confirming regulation by an endogenous clock independent of sleep (Desir et al., 1982; Kapen et al., 1974; Van Cauter and Refetoff, 1985), although interactions between sleep and the circadian system exist (Kriegsfeld et al., 2002a). The present review highlights the current understanding of circadian system regulation of endocrine rhythms via multiple means of modulation and proposes a novel mechanism of hierarchical control beginning with the master brain clock. For a complete description of daily hormone secretion patterns, the reader is referred to the following reviews (Gore, 1998; Hastings, 1991; Kriegsfeld et al., 2002a; Turek and Van Cauter, 1994).
Endocrine influences on the circadian system
Not only does the SCN regulate endocrine rhythms but hormones also feed back to the SCN, presumably to “finetune” the temporal pattern of endocrine secretion given current conditions. For example, high-affinity melatonin receptors are localized to the SCN, and administration of melatonin can alter SCN phase (Dubocovich et al., 1996; Hastings et al., 1997; Lewy et al., 1992; Slotten et al., 1999; Vanecek and Watanabe, 1999). In addition, exogenous melatonin alters the phase of SCN electrical activity measured in a slice preparation in vitro in a manner predicted by the phase response curve (McArthur et al., 1991). The sensitivity of the SCN to melatonin may be a function of daily variation in the density of melatonin binding sites within the SCN (Schuster et al., 2001). In humans, melatonin administration causes phase delays during late night or early morning and phase advances in late morning to early afternoon (Lewy and Sack, 1997). This finding has significant implications for shift workers, jet lag, and the blind.
Estrogen has pronounced effects on circadian activity rhythms, likely through both direct effects on the SCN and indirect mechanisms. In females, estrogen modulates both period and activity consolidation, suggesting actions on the circadian clock rather than transient effects on SCN targets. Cycling female hamsters and rats show a phase advance in locomotor activity on the day of estrous (scalloping), when estradiol levels are highest, and continuous administration of estradiol in silastic capsules shortens the free-running period of ovariectomized hamsters (Morin et al., 1977). When hamsters are maintained in constant light, the normally stable activity phase frequently splits into two activity components that stabilize approximately 12 h apart. Continuous administration of estradiol in silastic capsules to ovariectomized hamsters prevents these changes (Morin, 1980).
Direct effects of estrogen on the circadian clock are suggested by the expression of α and β estrogen receptors in the SCN across mammals, including humans (Gundlah et al., 2000; Kruijver and Swaab, 2002; Su et al., 2001). These receptors may be important for its normal development and synchronization to the environment (Abizaid et al., 2004; Gundlah et al., 2000). In humans, the presence of estrogen receptor expression, along with sex differences in SCN structure, suggests that estrogen may act on the SCN during development (Hofman et al., 1988, 1996; Kruijver and Swaab, 2002). Indirect effects in estrogen on the circadian clock are indicated by studies in which simultaneous injection of anterograde and retrograde tract tracers into the SCN reveal that ERα-expressing cells in the preoptic area, amygdala, BNST, and arcuate provide input to the SCN, but the SCN does not project directly to these ERα-expressing cells (de la Iglesia et al., 1999).
In males, testosterone also affects consolidation of locomotor activity rhythms. Extended exposure to short day lengths induces a decrease in testicular size and a decline in plasma testosterone concentrations in male hamsters (Ellis and Turek, 1983). Following testicular regression (or after castration), there is an increase in lability of activity onset, an expansion of the daily activity duration, with a decrease in wheel revolutions per cycle; testosterone replacement prevents these changes (Morin and Cummings, 1981). Testosterone may act through SCN androgen receptors. To date, androgen receptors have been identified in the SCN of several species (Clancy et al., 1994; Fernandez-Guasti et al., 2000; Kashon et al., 1996; Michael and Rees, 1982; Rees and Michael, 1982). Alternatively, testosterone may exert its effects through conversion to estradiol, which may act either directly on receptors in the SCN (e.g., Shughrue et al., 1997), or indirectly in ER-expressing cells in other brain areas that, in turn, communicate with the SCN (de la Iglesia et al., 1999). In rats, conversion of testosterone to estradiol may be important for the activity-stimulating effects of testosterone (Roy andWade, 1975). Estradiol is nearly 100 times as effective as testosterone at increasing activity, while dihydrotesosterone (a non-aromatizable androgen) has no effect on wheel-running activity of rats (Roy and Wade, 1975). Taken together, these findings suggest that the conversion of testosterone to estrogen, by aromatase, may be important for the effects of testosterone on circadian rhythms.
Identification of a brain “clock”: from tissue to gene
A highly localized brain clock in mammals was first suggested in 1972, with the demonstration that lesions ablating the SCN abolish circadian rhythmicity in adrenal corticoid secretion and locomotor behavior (Moore and Eichler, 1972; Stephan and Zucker, 1972). SCN-lesioned animals continue to show the full range of normal behaviors, but their temporal organization is lost and never recovers, irrespective of how early in development the lesions are performed (Mosko and Moore, 1979). The initial conclusion that the SCN serves as a brain master clock has been confirmed in the subsequent 30 years by converging lines of research involving in vivo, ex vivo, and in vitro studies carried out in many different laboratories. For example, transplants of donor SCN tissue into the brains of arrhythmic, SCN-lesioned hosts restore circadian rhythmicity in behavior (Lehman et al., 1987; Ralph et al., 1990). Importantly, rhythms are restored with the period of the donor SCN, indicating that the transplanted tissue does not act by restoring host brain function but that the “clock” is contained in the transplanted tissue. Further evidence that clock function is contained within the SCN comes from studies demonstrating that circadian rhythms in neural firing rate persist in isolated SCN tissue maintained in culture (Green and Gillette, 1982; Groos and Hendriks, 1982; Shibata et al., 1982). An excellent overview of these studies in historical perspective is available (Weaver, 1998).
While the foregoing work demonstrated that the SCN tissue as a whole served as a clock, the finding that circadian rhythmicity is a property of individual SCN neurons set the stage for the next breakthrough. The demonstration that dispersed, cultured SCN cells exhibit circadian rhythms of electrical activity indicated that circadian timing is a cellular property rather than an emergent property of a neural network (Welsh et al., 1995). These studies allowed for the exploration and subsequent discovery of the cellular molecular machinery responsible for circadian function.
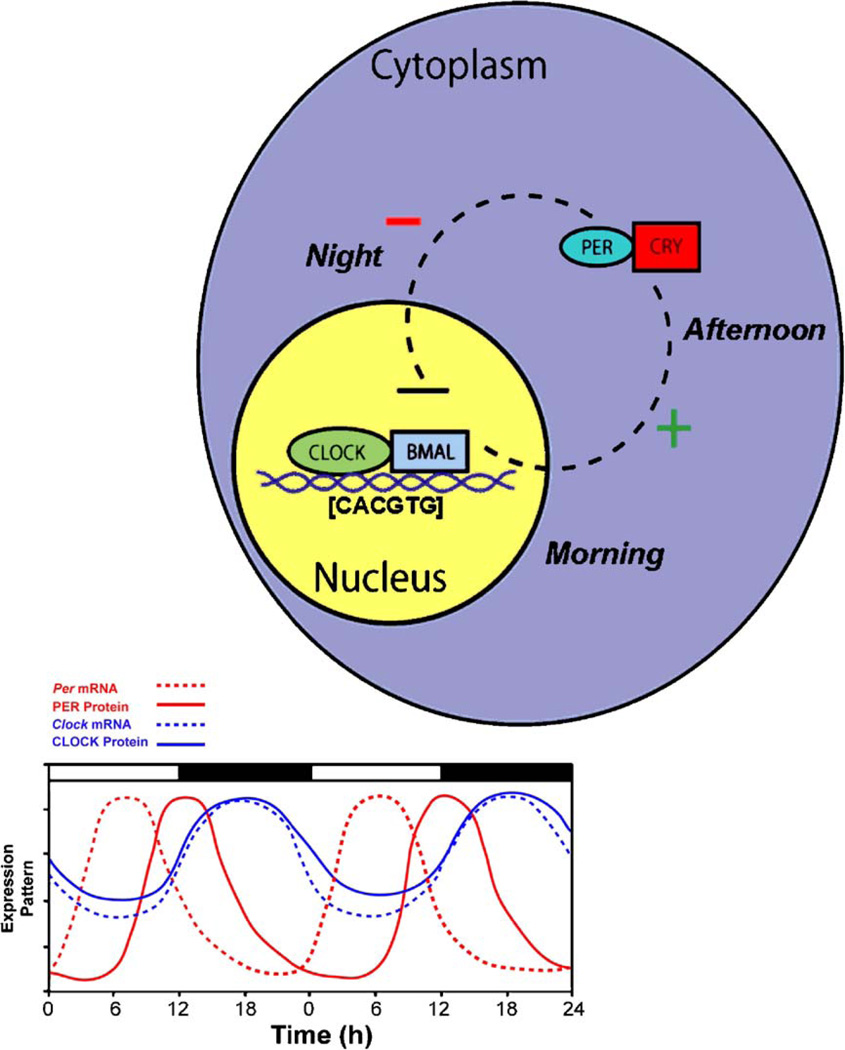
Within a cell, circadian rhythms are produced by an autoregulatory transcriptional/translational negative feedback loop that takes approximately 24 h, whereas the general mechanism for circadian oscillations at the cellular level is common among organisms, the components comprising the feedback loop differ. For the purpose of clarity, only the core mammalian feedback loop is described. To date, it is thought that two proteins, CLOCK and BMAL1, bind to one another and drive the transcription of messenger RNA (mRNA) of the Period (Per) and Cryptochrome (Cry) genes by binding to the E-box (CACGTG) domain on these gene promoters. Three Period (Per1, Per2, and Per3) and two cryptochrome genes (Cry1 and Cry2) have been identified. The mRNA for these genes is translated into PER and CRY proteins in the cytoplasm of the cell over the course of the day. Throughout the day, these proteins build up within the cytoplasm, and when they reach high enough levels, they form hetero- and homo-dimers. These newly formed dimers then feed back to the nucleus where they bind to the CLOCK:BMAL1 protein complex to turn off their own transcription (Fig. 2). Numerous other “clock” genes and regulatory enzymes have been identified but will not be reviewed for the sake of brevity. Future studies on the specific genes and their interactions that result in circadian timekeeping at the cellular level will likely yield exciting new information on other regulatory elements and their interactions.
Fig. 2.
A simplified model of the intracellular mechanisms responsible for mammalian circadian rhythm generation. The process begins when CLOCK and BMAL1 proteins dimerize to drive the transcription of the Per (Per1, Per2, and Per3) and Cry (Cry1 and Cry2) genes. In turn, Per and Cry are translocated to the cytoplasm and translated into their respective proteins. Throughout the day, PER and CRY proteins rise within the cell cytoplasm. When levels of PER and CRY reach a threshold, they form heterodimers, feed back to the cell nucleus and negatively regulate CLOCK:BMAL1 mediated transcription of their own genes. This feedback loop takes approximately 24 h, thereby leading to an intracellular circadian rhythm.
Discovery of the genes regulating circadian rhythmicity led to breakthroughs identifying clock genes and their protein products in numerous sites, including extra-SCN brain loci and in the periphery (Abe et al., 2002; Balsalobre et al., 1998; Kriegsfeld et al., 2003; Yamazaki et al., 2000). These findings, in turn, led to questions about the unique nature of the master oscillator in the SCN, the functional significance of extra-SCN oscillators, and mechanisms of coordination of these widely dispersed clocks.
To compare cellular mechanisms of clock gene expression in the SCN and in the periphery, embryonic fibroblasts from wild-type and (behaviorally arrhythmic) Cry−/− mice were used (Yagita et al., 2001). Clock properties of cell lines derived from peripheral cells of each strain were similar to those of the strain-specific SCN, supporting the conclusion of common core clock gene function in all tissues (Yagita et al., 2001). It remains controversial, however, whether peripheral oscillators are similar to those of the SCN in their ability to sustain endogenous rhythmicity for long durations (Balsalobre et al., 1998; Yoo et al., 2004).
When a tissue, either SCN or peripheral, loses coherent rhythmicity, it is important to determine whether this is due to dampening of rhythms in individual cells or to loss of synchrony among a population of cells in the tissue. Use of Per1-luciferase transgenic animals indicates that rhythms in peripheral tissues damp then disappear over time due to uncoupling (desynchronization) among oscillators that retain their individual rhythms (Nagoshi et al., 2004; Welsh et al., 2004). Presumably peripheral clock cells normally get phase information (directly or indirectly) from the SCN to synchronize individual oscillators to each other. In this view, the SCN sets the phase of peripheral circadian clocks daily, coordinating the activity of tissues and organs of the body relative to one another, thereby maintaining homeostasis.
Circadian output and orchestration of endocrine function
Diffusible signals controlling behavioral rhythms
Rhythmic electrical activity and oscillation of clock genes within the SCN neurons ultimately lead to rhythmicity in the whole organism. Compelling evidence for a diffusible output signal derives from neural tissue transplantations in which the SCN from a fetal donor is implanted into the third ventricle of an adult, SCN-lesioned host. As mentioned previously, these grafts restore activity-related behaviors such as locomotor, drinking, and gnawing rhythms (Lehman et al., 1987; Ralph et al., 1990; Silver et al., 1990). That a diffusible signal is sufficient to restore locomotor rhythmicity in SCN-lesioned hosts was demonstrated by encapsulating donor SCN tissue in a membrane that prevented neural outgrowth while allowing the diffusion of signals between graft and host (Silver et al., 1996).
One candidate diffusible signal is prokineticin-2 (PK2) (Cheng et al., 2002). This protein is expressed rhythmically in the SCN, and its receptor is present in all major SCN targets (Cheng et al., 2002, 2005). Likewise, PK2 administration during the night (when levels are low) inhibits wheel running behavior. Whether or not this signal normally operates in a diffusible manner and/or is released synaptically requires further examination. A second candidate diffusible signal is transforming growth factor-alpha (TGF-alpha) (Kramer et al., 2001). As with PK2, TGF-alpha is expressed rhythmically in the SCN, and its administration inhibits wheel-running behavior. The receptor for TGF-alpha is also expressed in the SPVZ, the major target of the SCN. The degree which TGF-alpha is released in a diffusible manner under normal conditions is not known. Studies in which the contribution of neural efferents and diffusible signals can be distinguished are necessary to begin to answer this question.
Although it is intriguing to speculate on the role of these signals in communicating information from the SCN, the problem of unequivocally identifying an endogenous, physiologically relevant diffusible SCN signal is complex and parallel in scope to the task faced by Sir Geoffrey Harris' in providing evidence for hypothalamic control over pituitary function in the 1950s. The necessary and sufficient criteria to confirm the existence of a diffusible signal in a fluid volume have been summarized previously (Nicholson, 1999). First, the removal or replacement of the signaling substance must result in a change in the response being controlled. The substance should be present or increased, or both, in a well-defined temporal relationship to the response (and similarly declines when the response disappears). In addition, evidence must be obtained that a fluid compartment is the conduit for a diffusible or transported signal. The signal must have access to and enter the compartment where the fluid dynamics and turnover in the compartment should allow appropriate movement of the signal. While PK2 and TGF-alpha meet some of these criteria, further research is necessary to clarify the role of these molecules in communicating circadian information.
Neural control of neurosecretory factors
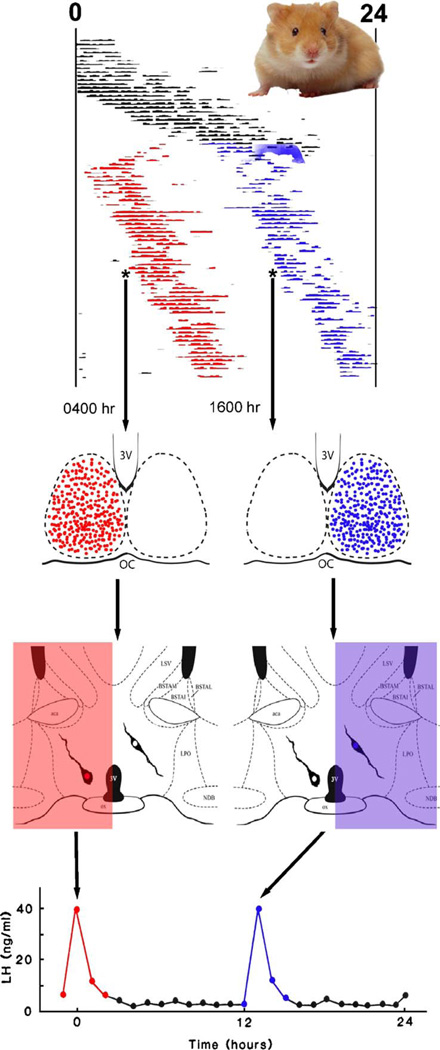
In contrast to behavioral rhythms (e.g., locomotion, drinking, gnawing), endocrine rhythms require neural projections from the SCN to endocrine targets; endocrine rhythms are abolished after knife cuts severing SCN efferents (Hakim et al., 1991; Nunez and Stephan, 1977) and are not restored in SCN-lesioned transplanted animals (Meyer-Bernstein et al., 1999; Nunez and Stephan, 1977; Silver et al., 1996), presumably due to inadequate neural innervation of the host brain by the graft. Further evidence for a neural SCN output signal regulating hormone secretion is seen in studies of female hamsters. When housed in constant light, the activity of a subset of hamsters “splits” into two separate activity bouts within a 24-h interval. These split females display two daily LH surges, each approximately half the concentration of a single surge in a non-split female (Swann and Turek, 1985) (Fig. 3). Under normal conditions, both halves of the bilaterally symmetrical SCN are active in synchrony. In ovariectomized, estrogen-implanted split hamsters killed during one of their activity bouts, however, activation of the SCN occurs on one side of the brain (monitored by FOS expression) but not on the other, suggesting that each half of the SCN can control an activity bout (de la Iglesia et al., 2000). Remarkably, FOS activation in GnRH neurons was only seen on the side of the brain in which SCN FOS expression occurred (de la Iglesia et al., 2003). These findings suggest that the precise timing of the LH surge is derived from a neural signal originating in the SCN and communicated to ipsilateral GnRH neurons, as a diffusible output signal would reach both sides of the brain. Importantly, some hypothalamic sites are activated ipsilaterally, while others are activated either ipsilaterally or bilaterally in the split animal, again supporting the notion of multiple SCN output pathways (Tavakoli-Nezhad and Schwartz, 2005; Yan et al., 2005).
Fig. 3.
Circadian control of gonadotropin secretion. Syrian hamsters normally exhibit one consolidated bout of activity every 24 h. Under conditions of constant light, the hamsters activity splits into two components separated by about 12 h. In one ingenious study (de la Iglesia et al., 2003), the investigators killed animals prior to each of the activity bouts (see asterisk on activity records). Brains were analyzed for FOS activity in the SCN and in neurons of the GnRH neuronal system. Splitting behavior resulted in only one half of the SCN being active during a given time of day. GnRH was only activated (expressed FOS) on the side of the brain in which the SCN was active. Given that ovariectomized, estrogen-implanted hamsters with split behavior experience two LH surges (Swann and Turek, 1985), we are speculating that the neural mechanism underlying this phenomenon can be explain by differential left–right activation of the GnRH system at two times of day.
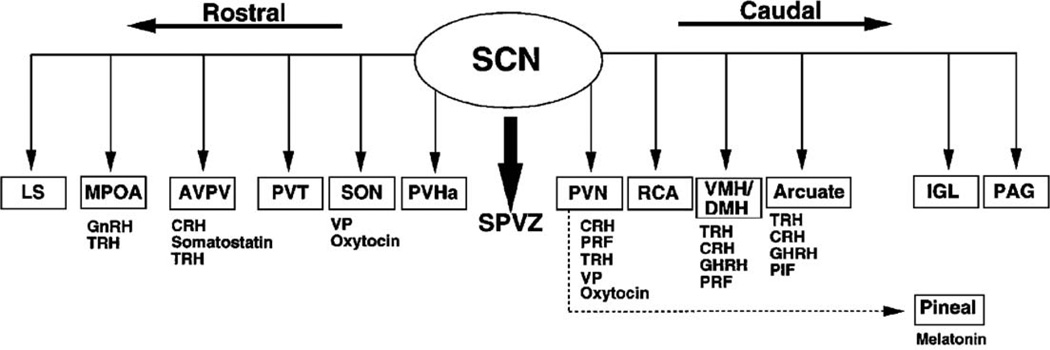
Neural output from the SCN has been extensively investigated in rats and hamsters using tract tracing techniques (Kalsbeek et al., 1993; Kriegsfeld et al., 2004; Leak and Moore, 2001; Morin et al., 1994; Stephan et al., 1981; Watts and Swanson, 1987; Watts et al., 1987). Importantly, many of these monosynaptic projections target brain regions containing neuroendocrine cells producing hypothalamic releasing hormones (Fig. 4). Direct projections have been traced from the SCN to the medial preoptic area (MPOA), supraoptic nucleus (SON), anteroventral periventricular nucleus (AVPV), the paraventricular nucleus (PVN), the dorsomedial nucleus of the hypothalamus (DMH), and the lateral septum and the arcuate (Arc). The SCN also projects to the pineal through a multisynaptic pathway (Klein, 1985; Klein et al., 1983). There is abundant evidence for direct neural SCN control of neuroendocrine cell populations (Buijs et al., 1998, 2003; Egli et al., 2004; Gerhold et al., 2001; Horvath, 1997; Horvath et al., 1998; Kalsbeek and Buijs, 2002; Kalsbeek et al., 1996a,b, 2000; Kriegsfeld et al., 2002a, b; Van der Beek et al., 1993, 1997b; Vrang et al., 1995). Because these cell populations can regulate neurochemicals that are secreted into the CSF (Reiter and Tan, 2002; Skinner and Caraty, 2002; Skinner and Malpaux, 1999; Tricoire et al., 2003) or general circulation, SCN-derived signals can control widespread systems in the brain and body.
Fig. 4.
Efferent projections of the rodent SCN to its targets in the brain. Below each target area is a list of neuroendocrine cells that lie in that region of the brain and could potentially be regulated by direct projections from the SCN. Solid lines represent monosynaptic projections, while the dotted line represents a multisynaptic projection to the pineal gland. The pronounced overlap between neuroendocrine cells and SCN efferent terminals, combined with reports demonstrating direct neuronal projections from the SCN to neuroendocrine cells (e.g., GnRH and CRH cells), provides suggestive evidence for a global mechanism of circadian hormonal regulation. Adapted from Kriegsfeld et al. (2002a) with permission.
Together, the findings summarized above suggest several possibilities: behavioral rhythms may be controlled by a diffusible signal(s), while endocrine rhythms may require neural output. Alternatively, behavioral and endocrine rhythms can both be supported by diffusible signals, but the threshold for supporting behavioral rhythms is lower. Finally, behavioral rhythms are controlled by both neural and diffusible signals, and either can maintain rhythmic function, while endocrine rhythms can only be supported via neural connections. Definitive identification of biologically significant endogenous diffusible signal(s) and the precise mode of SCN control is a current line of inquiry.
Neural SCN output and estrus regulation
SCN control of the rodent estrous cycle has been investigated extensively (Barbacka-Surowiak et al., 2003), providing an excellent model system for investigations of circadian and neuroendocrine interactions. The SCN sends projections directly to GnRH neurons in female rodents (Horvath et al., 1998; Van der Beek et al., 1997a). These efferents express the SCN peptide, vasoactive intestinal polypeptide (VIP). GnRH neurons particularly important for the regulation of the estrous cycle are activated at the time of proestrus and receive SCN input (van der Beek et al., 1994). Also, sex differences in the daily expression of SCN VIP mRNA are seen in rats, with females exhibiting a peak 12 h out of phase with that of males (Krajnak et al., 1998). Presumably, the signal regulating the estrous cycle is sexually dimorphic, thereby lending further support for VIP regulation of estrus. Furthermore, antisense oligonucleotides directed against VIP lead to a delayed and attenuated LH surge (Harney et al., 1996), reminiscent of that seen in middle-aged rats (Gore, 1998). Sex differences also exist in the pattern of projections from the SCN (Horvath et al., 1998; Van der Beek et al., 1997a,b). These sex differences in SCN projections upon GnRH cells, and in the production of neurochemicals in SCN cells projecting to the GnRH system, may underlie the absence of an LH surge in males.
In addition to direct connections to GnRH neurons, the SCN projects extensively to the anteroventral periventricular nucleus (AVPV), a brain region associated with the induction of the preovulatory LH surge (Le et al., 1997; Levine, 1997). The cells to which the SCN projects are estrogen-responsive (Watson et al., 1995), suggesting that the AVPV may be an important integration point for circadian and steroidal signals. Given the widespread projections of the SCN throughout the CNS, along with extensive input to the GnRH system, the potential for additional indirect modulation of the reproductive axis is considerable.
Vasopressin, another SCN peptide important in the regulation of the estrous cycle, is synthesized and released in a circadian manner. SCN vasopressin is rhythmically secreted with a peak during a sensitive time window prior to the LH surge (Kalsbeek et al., 1996a,b). Vasopressin administration into the MPOA induces an LH surge in SCN-lesioned, ovariectomized rats treated with estradiol (Palm et al., 2001a, b). Electrical stimulation of the MPOA and VP administration into the MPOA induces an LH surge in SCN-lesioned rats (Palm et al., 1999). Finally, in co-cultures of POA and SCN tissue, the rhythm of GnRH release is in phase with the rhythm of VP release, but not with that of VIP (Funabashi et al., 2000). Paradoxically, Brattleboro rats incapable of synthesizing vasopressin are fertile, although abnormal estrous cyclicity and reduced fertility have been noted (Boer et al., 1982). Taken together, these data indicate a potential role for VP in inducing the LH surge, although it is likely not the sole mediator.
Positive feedback effects of estrogen serve a permissive role in initiating the LH surge upon the arrival of the signal from the circadian pacemaker (Barbacka-Surowiak et al., 2003; Levine, 1997); implants of an anti-estrogen into the POA block the LH surge (Petersen and Barraclough, 1989). This dependence on estrogen ensures the maturation of the follicle during the time of the surge, while the circadian dependence ensures that receptive behavior coincides with ovulation. It remains unclear, however, how these two signals converge at the cellular level to allow integration at the appropriate time of day. One possibility is that estrogen alters neurochemical secretion by the SCN or the signaling efficacy of these chemicals via second messenger systems and kinases (Chappell and Levine, 2000; Levine, 1997). An alternative hypothesis is that estrogen stimulates ligand-independent progesterone receptor production, and the timed neuronal signal acts on progesterone receptors (Chappell et al., 2000; Levine, 1997; Levine et al., 2001). This scheme suggests that the effects of estrogen are integrated with the SCN signal at the level of progesterone receptors to ensure that the GnRH system is sensitive to the daily signal only during the preovulatory estrogen surge. The fact that few estrogen receptors have been localized to GnRH neurons (Shivers et al., 1983) suggests that estrogen acts to produce progesterone in neurons upstream of the GnRH system and hints at important, unidentified, SCN projections to these upstream components. Given the potential importance of estrogen/progesterone receptor expressing neurons upstream of the GnRH system, it will be interesting to establish the means by which the circadian system communicates with and modulates these regulatory elements.
The importance of a functional molecular clock in driving GnRH cells is seen in mice with a mutant form of the Clock gene. These mice have long, irregular estrous cycles and fail to exhibit an LH surge following estradiol treatment. Furthermore, this mutation also leads to an increase in fetal reabsorption during pregnancy and a decline in full-term parturition (Miller et al., 2004). These deficits are associated with a decline in midterm levels of progesterone, suggesting abnormal secretion patterns of prolactin (Miller et al., 2004). Together, these findings highlight the importance of the circadian system in regulating the temporal pattern of hormone secretion necessary for mating, pregnancy, and its maintenance, although results using mutant models must be interpreted cautiously until converging approaches consistently support these conclusions.
Not only does the SCN regulate GnRH during the estrous cycle, but other aspects of the estrous cycle are also regulated by the SCN. During proestrus, rising levels of estradiol reach a critical point and trigger the release of prolactin at a specific time of day. This release of prolactin is dependent upon the estradiol-induced increase in tuberoinfundibular dopaminergic (TIDA) neuron activity (Neill et al., 1971). Administration of an estradiol antiserum on the morning of diestrus-2 blocks the proestrous surge of prolactin (Neill et al., 1971). The proper timing of prolactin is achieved via SCN projections to TIDA neurons in the arcuate (Horvath, 1997). Additionally, TIDA neurons rhythmically express the clock gene, Per1, providing a potential additional means of temporal control (Kriegsfeld et al., 2003)—and see below). SCN lesions result in an abolition of a daily prolactin rhythm (Mai et al., 1994) and abolish the preovulatory prolactin surge (Pan and Gala, 1985). Given the importance of prolactin timing in maintaining the corpus luteum, these findings further suggest an essential role for the circadian clock in reproduction and may explain the increased fetal reabsorption in Clock mutant mice described above (Miller et al., 2004).
It has been well established that environmental factors act to fine-tune endogenous regulation of the reproductive cycle. For example, the timing of the prolactin surge is regulated by the phase of the light–dark (LD) cycle. A change in the LD cycle leads to predictable changes in the timing of the estrogen-induced prolactin surge and the mating-induced prolactin surge (Blake, 1976; Pieper and Gala, 1979). Thus, environmental time of day information is transmitted to the circadian system to precisely coordinate reproductive events relative to local time. Cervically stimulated prolactin surges have a free-running cycle in ovariectomized, estradiol-treated rats held in constant conditions. This pattern is abolished after ablation of the SCN (Bethea and Neill, 1980). As with the LH surge, this finding suggests that both the timing and production of the prolactin surge require a signal from the circadian clock. The means by which environmental stimuli other than light (e.g., social signals, local conditions, nutrition, etc.) are integrated into this system to fine-tune the timing of hormonal and behavioral events are unknown and represent an opportunity for exploration.
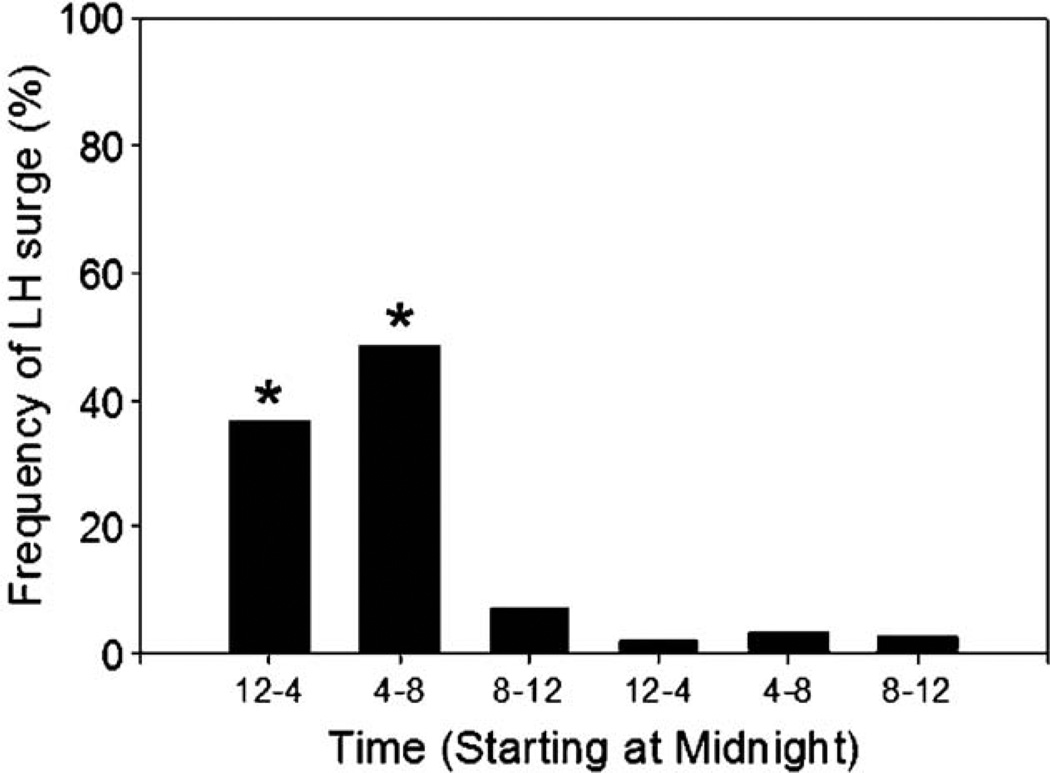
As with estrous behavior, the preovulatory LH surge occurs at regular 4- or 5-day intervals in rats, on the day of proestrus at a specific time of day coupled to the LD cycle (Colombo et al., 1974). Interestingly, despite the fact that the temporal pattern of SCN neural activity is similar in nocturnal and diurnal rodents (Smale et al., 2003), with a peak during the day and a trough at night, the timing of the preovulatory LH surge is reversed (Mahoney et al., 2004; McElhinny et al., 1999). Although the mechanisms by which this reversal occurs remain elusive, comparisons between diurnal and nocturnal species may provide insight into how circadian control is accomplished in humans (i.e., a diurnal species). Studies in rhesus initially indicated that the LH surge could be induced at any circadian phase in primates (Knobil, 1974). However, frequent urinary LH monitoring of women with regular menstrual cycles suggests a pronounced influence of circadian timing on the preovulatory LH surge, with most exhibiting the LH surge between midnight and 8:00 AM (Cahill et al., 1998; Edwards, 1981) (Fig. 5). Of 155 regular cycles monitored, 146 surges occurred during this 8-h time window (Cahill et al., 1998). Given that the daily timing of the LH surge in women can be unmasked under carefully monitored and controlled conditions, it is likely that the human ovulatory cycle also requires interactions between the circadian and reproductive systems.
Fig. 5.
Frequency of onset of the LH surge by time of day. A total of 155 cycles from 35 women were monitored. The graph represents the percentage of preovulatory LH surges occurring during each time interval. Adapted from Cahill et al. (1998).
Direct and indirect transcriptional control as a clock output
The circadian system exerts a widespread influence over numerous bodily functions. DNA microarray studies in mice indicate that ~5–9% of the genome, excluding genes involved in the core clock loop, are under circadian control (Akhtar et al., 2002; Panda et al., 2002; Storch et al., 2002). However, these so-called clock-controlled genes (CCGs) differ among tissues, with any two tissues likely sharing less than 10% of CCGs under circadian control. Together, these findings suggest that circadian control is ubiquitous throughout the body, and tissue-specific processes may be controlled by differential activation of downstream genes in individual systems.
Direct transcriptional control
CCGs maintain a predictable phase relationship with the core clock genes (Ueda et al., 2002), indicating that the CCGs are either directly or indirectly regulated via the circadian transcriptional machinery. The expression of some CCGs is directly controlled by the CLOCK:BMAL1 heterodimer binding to an E-box enhancer (CACGTG) in their promoter (Jin et al., 1999). An example of direct transcriptional control by the circadian system is seen with vasopressin regulation. Vasopressin is present in the SCN where it acts locally to regulate rhythm generation (Mihai et al., 1994a,b). It cannot be the sole regulatory factor, as the SCN of Brattleboro rats maintains rhythms in electrical activity (Ingram et al., 1998), and these animals exhibit only slight disruptions in rhythm amplitude and entrainability (Brown and Nunez, 1989; Murphy et al., 1993, 1996). In addition to a role within the nucleus, SCN, vasopressin-expressing neurons signal distant hypothalamic targets and SCN vasopressin signaling has been implicated in the control of estrus (Buijs et al., 2003a,b; Kalsbeek et al., 1996a, b; Mihai et al., 1994a,b; Palm et al., 2001a,b). The SCN rhythm in vasopressin (a rhythm that is not present in other vasopressinergic cell population—see below) is dependent on an E-box element in the 5′ flanking region of the vasopressin gene to which the CLOCK:BMAL complex binds. The vasopressin rhythm is abolished in mutant mice with aberrant Clock gene expression (Jin et al., 1999; Silver et al., 1999).
E-box elements in the promoter have the potential for direct control by circadian clock genes, but this alone is not sufficient. In the SON (unlike the SCN), vasopressin is dependent on osmotic balance and is not rhythmic (Jin et al., 1999). While expression of Clock is robust in the SCN and SON, Bmal1 is expressed robustly only in the SCN and is barely detectable in the SON. These findings indicate some of the conditions necessary for direct control of genes regulating neuroendocrine function by the circadian clock transcriptional machinery.
A gene with an E-box in its promoter region is a potential target for direct control by the transcriptional machinery of the circadian clock. We screened hypothalamic and pituitary endocrine factors for E-box elements in the promoter of their published gene sequences to determine their potential for this means of control (Table 1). We found that some releasing hormones (TSH, GHRH, and vasopressin) do have E-box enhancers. We have shown in the adult mouse brain that a subset of neuroendocrine cells in the preoptic area, paraventricular nucleus of the hypothalamus, and the arcuate nucleus express the clock gene Per1 (Kriegsfeld et al., 2003), suggesting that this direct transcriptional regulation likely plays a key role in the organization of endocrine timing.
Table 1.
List of mammalian neuroendocrine factors containing an E-box (CACGTG) enhancer in their promoter
| Gene | E-box (#) | Reference |
|---|---|---|
| Hypothalamic factors: | ||
| CRH | No | (Muglia et al., 1994) |
| GHRH | Yes (1) | (Laird, 2001–direct submission) |
| GnRH-I | No | (Hayflick et al., 1989) |
| GnRH-II | No | (White et al., 1998) |
| Ghrelin | No | (Kanamoto et al., 2004) |
| Oxytocin | No | (Hara et al., 1990) |
| Vasopressin | Yes (1) | (Hara et al., 1990; Jin et al., 1999) |
| TRH | No | (Satoh et al., 1996) |
| Pituitary: | ||
| POMC (ACTH, MSH) | No | (Drouin et al., 1985) |
| FSH | No | (Kumar, 1994–direct submission) |
| GH | No | (Das et al., 1996) |
| LH | No | (Kaiser et al., 1998) |
| Prolactin | No | (Gubbins et al., 1980) |
| TSH (beta subunit) | Yes (3) | (Croyle and Maurer, 1984) |
Indirect transcriptional control
In studies of the liver, it has been demonstrated that D-element binding protein (DBP) is another CCG under direct transcriptional regulation by the core circadian feedback loop (Ripperger et al., 2000). Importantly, DBP binds to other gene promoters to temporally regulate their transcription. This process is important in the control of hepatic metabolic processes; DBP activates the transcription of albumin, cholesterol 7α hydroxylase, and cytochrome P450 (Lavery et al., 1999). This second order system can provide ubiquitous control via the circadian system and temporal control of key enzymatic pathways involved in hormone production.
A process similar to hepatic regulation by DBP may modulate the reproductive axis, as the gene for GnRH does not have an E-box but appears to be under circadian control. A series of studies using GT1 cells, an immortalized line of GnRH cells, demonstrated the rhythmic expression of numerous circadian clock genes (Chappell et al., 2003; Gillespie et al., 2003; Olcese et al., 2003). Importantly, GnRH release occurs episodically approximately every 90 min in most species, and GT1 cells also express this ultradian pattern of GnRH production. When clock genes are disrupted in GT1 cells, not only is the daily rhythm disrupted, but both pulse frequency and amplitude are also dramatically altered (Chappell et al., 2003). These findings suggest that, in addition to 24-h cycles, clock genes expressed within neuroendocrine cells may regulate the episodic pattern of hormone secretion outside of the circadian range, even when the regulated neuroendocrine gene lacks an E-box enhancer. While these data are intriguing, a direct link between clock genes and ultradian rhythmicity awaits further supporting evidence.
While the forgoing studies using embryonic cell lines provide insights into cellular mechanisms, how these data generalize to functioning in vivo needs to be determined, as immortalized cell lines may exhibit properties different from those of the living animal. In addition, cell lines lack the innumerable regulatory systems that act directly or indirectly on GnRH cells in vivo. To address the question of whether or not GnRH cells in adult mice express Per1 message, we used mice with a green fluorescent protein (GFP) reporter driven by Per1 promoter. In these mice, GFP expression was observed in neurons near to GnRH-immunoreactive cells, but the two proteins were not co-localized. We have confirmed these negative findings using double-label immunofluroscence in mice and rats using several different antibodies against Per1 and GnRH (Kriegsfeld and Silver, unpublished data). We conclude that there are key differences between clock gene expression between cultured GT1 cells and GnRH neurons in the brains of adult animals.
System-level control and coordination of endocrine function
The task in understanding the orchestration of hormonal systems is best understood by recognizing that most processes in the body exhibit a circadian rhythm, and that activity in various systems exhibits different phases (i.e., peak and trough times) relative to each other. One explanation for how this feat is accomplished suggests that the SCN secretes one neurochemical to control each rhythmic process at its appropriate phase. In this view, the SCN secretes numerous substances, each precisely timed. Alternatively, control may be accomplished by the specificity of SCN projections and combinations of transmitter release at each target (Kalsbeek and Buijs, 2002). As an alternative hypothesis, we have suggested that SCN timing signals have different consequences at each targeted effector system (Kriegsfeld et al., 2003).
According to this view, a small number of rhythmic SCN signals must be differentially interpreted by a large number of targets to accomplish precise phase control by the SCN over an ensemble of rhythmic processes. Different targets respond differentially to the same message based on time of day (or local conditions), with some systems responding maximally to a signal(s) at a particular time of day, while another system might respond to this same signal(s) with inhibition. This hypothesis requires that target systems of the SCN have a mechanism for keeping time.
Seasonal as well as circadian timing are dependent upon the SCN. Several hypotheses have been proposed to account for how the SCN and its targets track time on a seasonal basis (Carr et al., 2003; Hofman, 2004; Lincoln et al., 2003). In the SCN of Syrian and Siberian hamsters, photoperiod alters the duration of clock and clock-controlled gene expression, while the amplitude of gene expression is influenced by photoperiod in the pars tuberalis (Johnston et al., 2003; Messager et al., 2000). In sheep, however, the relative timing of clock genes is altered by photoperiod in the pars tuberalis, providing a mechanism of temporal encoding and downstream control (Hazlerigg et al., 2004; Lincoln et al., 2002, 2003, 2005). These correlational results are intriguing and suggest that phase and/or amplitude of clock and CCGs in SCN brain targets and endocrine glands may predict their responsiveness to upstream signals on a daily schedule.
Numerous systems display time-gated sensitivity to stimuli, with the same stimulus or neurochemical producing different effects at different times of day, suggesting temporal control at effector sites rather than passive regulation by the SCN. The preoptic area, for example, exhibits robust clock gene expression (Palm et al., 2001a,b; Tei et al., 1997; Yamamoto et al., 2001. Importantly, stimulation of the POA of ovariectomized rats with the SCN peptide, vasopressin, induces an LH surge during the second portion of the light period, but not the first (Palm, 2001 #122-001), indicating important temporal control at the level of the POA.
Clocks in the neuroendocrine system
The fact that time-keeping machinery is functional in numerous central neurosecretory cells (Kriegsfeld et al., 2003) and peripheral endocrine tissues (Bittman et al., 2003; Morse et al., 2003; Shieh, 2003; Von Gall et al., 2002; Zylka et al., 1998) represents a mechanism by which SCN targets can anticipate the reception of SCN signals and respond based upon local needs and time of day. In addition, because peripheral systems are controlled hierarchically by multiple upstream components, temporal modification in each “link” along the hypothalamo– pituitary–endocrine gland axis could provide additional control over daily patterns of individual rhythms. At the top of this circadian hierarchy of control, the SCN sends signals to neuroendocrine cells and tissues to maintain synchronization among cellular oscillators in phenotypically distinct neuroendocrine cell populations. Although capable of oscillating independently, these neuroendocrine cells respond to periodic SCN input to maintain a synchronized rhythm in the local cell population. Such mechanism could account for the loss of coherent circadian endocrine rhythms following lesions or transection of outputs from the SCN (Meyer-Bernstein et al., 1999; Moore and Eichler, 1972; Nunez and Stephan, 1977). Loss of coherence among elements would result in a blunted overall output and absence of rhythmicity when individual cells drift out of phase with each other.
If the SCN coordinates cell populations, why do individual cells need their own clocks? According to this view, clocks in SCN target populations provide responsiveness to local conditions and temporal fine-tuning in a local population, not possible with a single driving master clock. These local clocks could selectively respond depending on time of day and appropriately drive the expression of cell/tissue-dependent CCGs that act as output to affect target systems or regulate local conditions. Coordinated rhythmic output from neuroendocrine cells can then be communicated to the pituitary, which also exhibits circadian clock gene expression indicating a zone for further temporal modification (Messager et al., 2000). Finally, rhythmic information from the pituitary can be communicated humorally to target glands in the periphery that themselves express clock genes (Bittman et al., 2003; Zylka et al., 1998). As mentioned previously, multisynaptic projections from the SCN to several endocrine glands have been identified using viral tracers (Buijs et al., 1998, 1999; Gerendai and Halasz, 2000; Kalsbeek et al., 2000). These connections provide a mechanism for the SCN to coordinate peripheral cellular oscillators to optimize responses to slower endocrine signals. Several lines of evidence support the notion that neural communication from the SCN to the periphery is responsible for the timing of clock gene expression in targets organs and glands (Guo et al., 2005; Shibata, 2004; Terazono et al., 2003).
The top of the hierarchy: neural SCN output
An organization of this type requires that the SCN communicate (directly or indirectly) with neuroendocrine cells expressing clock genes. There is substantial evidence of direct projections from the SCN to neuroendocrine cells (Buijs et al., 1993; Horvath et al., 1998; Kriegsfeld et al., 2002a,b; Teclemariam-Mesbah et al., 1997; Van der Beek et al., 1997a, b; Vrang et al., 1995). Expression of the clock gene, Per1, is rhythmically expressed in the Arc (Abe et al., 2002) and exhibits a stress-induced increase in the PVH (Abe et al., 2002; Takahashi et al., 2001). Per1 has been localized to CRH-ir cells in the PVH (Takahashi et al., 2001), while the neurochemical phenotype of Per1-expressing cells in the Arc has been identified as dopaminergic providing a potential mechanism of control of prolactin rhythms and the preovulatory prolactin surge (Kriegsfeld et al., 2003).
Hormonal and neural communication to glands
Relative to the neural communication by the SCN to neuroendocrine cells, hormonal communication is slow. However, by using the bloodstream as a route of communication, hormones modulated by the circadian system can communicate rhythmic information throughout the body. Additional temporal control occurs at target glands and organs by using circadian clock machinery to modulate responsiveness to hormonal signals. If this means of temporal control is implemented at peripheral targets, necessary alterations in the timing of organ/gland responsiveness due to changes in local conditions can be communicated rapidly to hormone-sensitive targets to adjust the timing of their circadian clocks. Given that numerous organs (e.g., liver, pancreas) and endocrine glands (e.g., testes, adipose tissue, adrenal gland) investigated to date receive autonomic innervation from the SCN (Bamshad et al., 1998; Bartness et al., 2001; Buijs et al., 1999, 2003; Kalsbeek et al., 2000; Olcese et al., 2003), these multisynaptic connections may provide a rapid means of clock resetting in peripheral tissues to ensure proper reception of slower diffusible communication (Fig. 6). A role of autonomic control in peripheral clock resetting comes from investigations in which manipulations of autonomic connections to liver reset clock gene expression in this organ (Terazono et al., 2003).
Fig. 6.
Overall organization of the circadian system. This organization is based on the postulation that rhythmic system physiology is controlled by a combination of neural and diffusible signals originating from the SCN. In this view, specific systems may be differentially regulated by SCN signals via local clocks allowing for more specific responsiveness based upon local needs and time of day (see text for additional details).
In summary, global clock resetting may be accomplished via hormonal signals, as glucocorticoids can adjust the phase of peripheral circadian clock genes (Balsalobre et al., 2000). Whereas the role of hormones other than glucocorticoids in resetting peripheral oscillators has not been investigated, the marked effects of hormones on circadian function reviewed herein suggest a potentially crucial role for endocrine factors in orchestrating this multioscillatory arrangement.
Conclusions and perspectives
In general, we are not aware of the precision in the timing and coordination of numerous events in our bodies, unless it is disrupted (e.g., jet lag). However, processes as fundamental as the timing of sleep and its coordination with feelings of hunger are a manifestation of numerous physiological and biochemical events that change systematically and predictably over the course of the day. Given the numerous salient time cues in the environment, one might intuit that these daily changes are passive responses to environmental change. However, as reviewed here, daily rhythms are endogenously generated and are synchronized to external time cues in order to ensure that bodily processes are carried out at the appropriate, optimal time of day or night.
Because most brain and bodily processes require a significant amount of time to achieve appropriate regulation the body must anticipate these changes and prepare accordingly in advance. For example, genomic actions of steroid hormones can take several hours to have their effects, and these hormones must be secreted prior to the time during which the behavior is best performed. Likewise, timed peak and trough hormone secretion may be required to prevent receptor down-regulation and desensitization. Because generating new receptors requires significant metabolic energy and time, episodic hormone secretion may be required to allow for adequate receptor turnover. Thus, an endogenous timekeeping system is necessary to anticipate environmental change and initiate internal adjustments in advance of the appropriate environmental time in order to coordinate innumerable bodily processes.
The present overview shows how the circadian system controls the timing of hormone secretion using a number of mechanisms, including direct transcriptional and SCN neural control of neurosecretory factors, and control of glands by hormones, clock genes, and autonomic innervation. Because hormones can have a widespread influence over physiology and behavior, and provide a means by which circadian information can be communicated systemically, it is important to determine how these rhythms are regulated. Not only are hormones modulated by the circadian system, but hormonal feedback to the SCN also influences circadian function (Dubocovich et al., 1996; Ellis and Turek, 1983; Hastings et al., 1997; Jechura et al., 2003; Labyak and Lee, 1995; Lewy and Sack, 1997; Morin et al., 1977). Together, these mechanisms controlling endocrine timing entail regulatory actions by the circadian system and provide extensive opportunities for empirical investigations of behaviorally relevant systems.
Acknowledgments
We thank Dr. Lily Yan for the discussions and suggestions during the preparation of this review. We also thank Sean Duffy for the editorial and technical assistance. The work described in our laboratory was supported by NIH grants NS37919 (RS) and MH-12408 (LJK).
References
- Abe M, Herzog ED, Yamazaki S, Straume M, Tei H, Sakaki Y, Menaker M, Block GD. Circadian rhythms in isolated brain regions. J. Neurosci. 2002;22(1):350–356. doi: 10.1523/JNEUROSCI.22-01-00350.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abizaid A, Mezei G, Horvath TL. Estradiol enhances light-induced expression of transcription factors in the SCN. Brain Res. 2004;1010(1–2):35–44. doi: 10.1016/j.brainres.2004.01.089. [DOI] [PubMed] [Google Scholar]
- Akhtar RA, Reddy AB, Maywood ES, Clayton JD, King VM, Smith AG, Gant TW, Hastings MH, Kyriacou CP. Circadian cycling of the mouse liver transcriptome, as revealed by cDNA microarray, is driven by the suprachiasmatic nucleus. Curr. Biol. 2002;12(7):540–550. doi: 10.1016/s0960-9822(02)00759-5. [DOI] [PubMed] [Google Scholar]
- Albrecht U. The mammalian circadian clock: a network of gene expression. Front. Biosci. 2004;9:48–55. doi: 10.2741/1196. [DOI] [PubMed] [Google Scholar]
- Andrews WW, Ojeda SR. A detailed analysis of the serum luteinizing hormone secretory profile in conscious, free-moving female rats during the time of puberty. Endocrinology. 1981;109(6):2032–2039. doi: 10.1210/endo-109-6-2032. [DOI] [PubMed] [Google Scholar]
- Ashmore LJ, Sehgal A. A fly’s eye view of circadian entrainment. J. Biol. Rhythms. 2003;18(3):206–216. doi: 10.1177/0748730403018003003. [DOI] [PubMed] [Google Scholar]
- Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93(6):929–937. doi: 10.1016/s0092-8674(00)81199-x. [DOI] [PubMed] [Google Scholar]
- Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schutz G, Schibler U. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289(5488):2344–2347. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- Bamshad M, Aoki VT, Adkison MG, Warren WS, Bartness TJ. Central nervous system origins of the sympathetic nervous system outflow to white adipose tissue. Am. J. Physiol. 1998;275(1 Pt. 2):R291–R299. doi: 10.1152/ajpregu.1998.275.1.R291. [DOI] [PubMed] [Google Scholar]
- Barbacka-Surowiak G, Surowiak J, Stoklosowa S. The involvement of suprachiasmatic nuclei in the regulation of estrous cycles in rodents. Reprod. Biol. 2003;3(2):99–129. [PubMed] [Google Scholar]
- Bartness TJ, Song CK, Demas GE. SCN efferents to peripheral tissues: implications for biological rhythms. J. Biol. Rhythms. 2001;16(3):196–204. doi: 10.1177/074873040101600302. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Neill JD. Lesions of the suprachiasmatic nuclei abolish the cervically stimulated prolactin surges in the rat. Endocrinology. 1980;107(1):1–5. doi: 10.1210/endo-107-1-1. [DOI] [PubMed] [Google Scholar]
- Bittman EL, Doherty L, Huang L, Paroskie A. Period gene expression in mouse endocrine tissues. Am. J. Physiol.: Regul., Integr. Comp. Physiol. 2003;285(3):R561–R569. doi: 10.1152/ajpregu.00783.2002. [DOI] [PubMed] [Google Scholar]
- Blake CA. Effects of intravenous infusion of catecholamines on rat plasma luteinizing hormone and prolactin concentrations. Endocrinology. 1976;98(1):99–104. doi: 10.1210/endo-98-1-99. [DOI] [PubMed] [Google Scholar]
- Blaustein JD, Tetel MJ, Ricciardi KH, Delville Y, Turcotte JC. Hypothalamic ovarian steroid hormone-sensitive neurons involved in female sexual behavior. Psychoneuroendocrinology. 1994;19(5–7):505–516. doi: 10.1016/0306-4530(94)90036-1. [DOI] [PubMed] [Google Scholar]
- Boer GJ, Boer K, Swaab DF. On the reproductive and developmental differences within the Brattleboro strain. Ann. N. Y. Acad. Sci. 1982;394:37–45. doi: 10.1111/j.1749-6632.1982.tb37409.x. [DOI] [PubMed] [Google Scholar]
- Boyar RM, Wu RH, Roffwarg H, Kapen S, Weitzman ED, Hellman L, Finkelstein JW. Human puberty: 24-hour estradiol in pubertal girls. J. Clin. Endocrinol. Metab. 1976;43(6):1418–1421. doi: 10.1210/jcem-43-6-1418. [DOI] [PubMed] [Google Scholar]
- Brown MH, Nunez AA. Vasopressin-deficient rats show a reduced amplitude of the circadian sleep rhythm. Physiol. Behav. 1989;46(4):759–762. doi: 10.1016/0031-9384(89)90364-8. [DOI] [PubMed] [Google Scholar]
- Buijs RM, Markman M, Nunes-Cardoso B, Hou YX, Shinn S. Projections of the suprachiasmatic nucleus to stress-related areas in the rat hypothalamus: a light and electron microscopic study. J. Comp. Neurol. 1993;335(1):42–54. doi: 10.1002/cne.903350104. [DOI] [PubMed] [Google Scholar]
- Buijs RM, Hermes MH, Kalsbeek A. The suprachiasmatic nucleus-paraventricular nucleus interactions: a bridge to the neuroendocrine and autonomic nervous system. Prog. Brain Res. 1998;119:365–382. doi: 10.1016/s0079-6123(08)61581-2. [DOI] [PubMed] [Google Scholar]
- Buijs RM, Wortel J, Van Heerikhuize JJ, Feenstra MG, Ter Horst GJ, Romijn HJ, Kalsbeek A. Anatomical and functional demonstration of a multisynaptic suprachiasmatic nucleus adrenal (cortex) pathway. Eur. J. Neurosci. 1999;11(5):1535–1544. doi: 10.1046/j.1460-9568.1999.00575.x. [DOI] [PubMed] [Google Scholar]
- Buijs RM, la Fleur SE, Wortel J, Van Heyningen C, Zuiddam L, Mettenleiter TC, Kalsbeek A, Nagai K, Niijima A. The suprachiasmatic nucleus balances sympathetic and parasympathetic output to peripheral organs through separate preautonomic neurons. J. Comp. Neurol. 2003a;464(1):36–48. doi: 10.1002/cne.10765. [DOI] [PubMed] [Google Scholar]
- Buijs RM, van Eden CG, Goncharuk VD, Kalsbeek A. The biological clock tunes the organs of the body: timing by hormones and the autonomic nervous system. J. Endocrinol. 2003b;177(1):17–26. doi: 10.1677/joe.0.1770017. [DOI] [PubMed] [Google Scholar]
- Cahill DJ, Wardle PG, Harlow CR, Hull MG. Onset of the preovulatory luteinizing hormone surge: diurnal timing and critical follicular prerequisites. Fertil. Steril. 1998;70(1):56–59. doi: 10.1016/s0015-0282(98)00113-7. [DOI] [PubMed] [Google Scholar]
- Carr AJ, Johnston JD, Semikhodskii AG, Nolan T, Cagampang FR, Stirland JA, Loudon AS. Photoperiod differentially regulates circadian oscillators in central and peripheral tissues of the Syrian hamster. Curr. Biol. 2003;13(17):1543–1548. doi: 10.1016/s0960-9822(03)00619-5. [DOI] [PubMed] [Google Scholar]
- Chappell PE, Levine JE. Stimulation of gonadotropin-releasing hormone surges by estrogen: I. Role of hypothalamic progesterone receptors. Endocrinology. 2000;141(4):1477–1485. doi: 10.1210/endo.141.4.7428. [DOI] [PubMed] [Google Scholar]
- Chappell PE, Lee J, Levine JE. Stimulation of gonadotropin-releasing hormone surges by estrogen: II. Role of cyclic adenosine 3′5′-monophosphate. Endocrinology. 2000;141(4):1486–1492. doi: 10.1210/endo.141.4.7427. [DOI] [PubMed] [Google Scholar]
- Chappell PE, White RS, Mellon PL. Circadian gene expression regulates pulsatile gonadotropin-releasing hormone (GnRH) secretory patterns in the hypothalamic GnRH-secreting GT1-7 cell line. J. Neurosci. 2003;23(35):11202–11213. doi: 10.1523/JNEUROSCI.23-35-11202.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng MY, Bullock CM, Li C, Lee AG, Bermak JC, Belluzzi J, Weaver DR, Leslie FM, Zhou QY. Prokineticin 2 transmits the behavioural circadian rhythm of the suprachiasmatic nucleus. Nature. 2002;417(6887):405–410. doi: 10.1038/417405a. [DOI] [PubMed] [Google Scholar]
- Cheng MY, Bittman EL, Hattar S, Zhou QY. Regulation of prokineticin 2 expression by light and the circadian clock. BMC Neurosci. 2005;6(1):17. doi: 10.1186/1471-2202-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy AN, Whitman C, Michael RP, Albers HE. Distribution of androgen receptor-like immunoreactivity in the brains of intact and castrated male hamsters. Brain Res. Bull. 1994;33(3):325–332. doi: 10.1016/0361-9230(94)90201-1. [DOI] [PubMed] [Google Scholar]
- Colombo JA, Baldwin DM, Sawyer CH. Timing of the estrogen-induced release of LH in ovariectomized rats under an altered lighting schedule. Proc. Soc. Exp. Biol. Med. 1974;145(3):1125–1127. doi: 10.3181/00379727-145-37965. [DOI] [PubMed] [Google Scholar]
- Croyle ML, Maurer RA. Thyroid hormone decreases thyrotropin subunit mRNA levels in rat anterior pituitary. DNA. 1984;3(3):231–236. doi: 10.1089/dna.1.1984.3.231. [DOI] [PubMed] [Google Scholar]
- Das P, Meyer L, Seyfert HM, Brockmann G, Schwerin M. Structure of the growth hormone-encoding gene and its promoter in mice. Gene. 1996;169(2):209–213. doi: 10.1016/0378-1119(95)00815-2. [DOI] [PubMed] [Google Scholar]
- de la Iglesia HO, Blaustein JD, Bittman EL. Oestrogen receptor-alpha-immunoreactive neurones project to the suprachiasmatic nucleus of the female Syrian hamster. J. Neuroendocrinol. 1999;11(7):481–490. doi: 10.1046/j.1365-2826.1999.00341.x. [DOI] [PubMed] [Google Scholar]
- de la Iglesia HO, Meyer J, Carpino A, Jr, Schwartz WJ. Antiphase oscillation of the left and right suprachiasmatic nuclei. Science. 2000;290(5492):799–801. doi: 10.1126/science.290.5492.799. [DOI] [PubMed] [Google Scholar]
- de la Iglesia HO, Meyer J, Schwartz WJ. Lateralization of circadian pacemaker output: activation of left- and right-sided luteinizing hormone-releasing hormone neurons involves a neural rather than a humoral pathway. J. Neurosci. 2003;23(19):7412–7414. doi: 10.1523/JNEUROSCI.23-19-07412.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desir D, Van Cauter E, L'Hermite M, Refetoff S, Jadot C, Caufriez A, Copinschi G, Robyn C. Effects of “jet lag” on hormonal patterns: III. Demonstration of an intrinsic circadian rhythmicity in plasma prolactin. J. Clin. Endocrinol. Metab. 1982;55(5):849–857. doi: 10.1210/jcem-55-5-849. [DOI] [PubMed] [Google Scholar]
- Drouin J, Chamberland M, Charron J, Jeannotte L, Nemer M. Structure of the rat pro-opiomelanocortin (POMC) gene. FEBS Lett. 1985;193(1):54–58. doi: 10.1016/0014-5793(85)80078-8. [DOI] [PubMed] [Google Scholar]
- Du YZ, Tong J. Genetic regulation of circadian clock. Sheng Li Ke Xue Jin Zhan. 2002;33(4):343–345. [PubMed] [Google Scholar]
- Dubocovich ML, Benloucif S, Masana MI. Melatonin receptors in the mammalian suprachiasmatic nucleus. Behav. Brain Res. 1996;73(1–2):141–147. doi: 10.1016/0166-4328(96)00086-1. [DOI] [PubMed] [Google Scholar]
- Duffield GE. DNA microarray analyses of circadian timing: the genomic basis of biological time. J. Neuroendocrinol. 2003;15(10):991–1002. doi: 10.1046/j.1365-2826.2003.01082.x. [DOI] [PubMed] [Google Scholar]
- Edwards RG. Test-tube babies, 1981. Nature. 1981;293(5830):253–256. doi: 10.1038/293253a0. [DOI] [PubMed] [Google Scholar]
- Egli M, Bertram R, Sellix MT, Freeman ME. Rhythmic secretion of prolactin in rats: action of oxytocin coordinated by vasoactive intestinal polypeptide of suprachiasmatic nucleus origin. Endocrinology. 2004:3386–3394. doi: 10.1210/en.2003-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis GB, Turek FW. Testosterone and photoperiod interact to regulate locomotor activity in male hamsters. Horm. Behav. 1983;17(1):66–75. doi: 10.1016/0018-506x(83)90016-8. [DOI] [PubMed] [Google Scholar]
- Everett JW, Sawyer CH. A 24-hour periodicity in the “LH-release apparatus” of female rat disclosed by barbiturate administration. Endocrinology. 1950;47:198–218. doi: 10.1210/endo-47-3-198. [DOI] [PubMed] [Google Scholar]
- Fernandez-Guasti A, Kruijver FP, Fodor M, Swaab DF. Sex differences in the distribution of androgen receptors in the human hypothalamus. J. Comp. Neurol. 2000;425(3):422–435. doi: 10.1002/1096-9861(20000925)425:3<422::aid-cne7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Funabashi T, Shinohara K, Mitsushima D, Kimura F. Gonadotropin-releasing hormone exhibits circadian rhythm in phase with arginine-vasopressin in co-cultures of the female rat preoptic area and suprachiasmatic nucleus. J. Neuroendocrinol. 2000;12(6):521–528. doi: 10.1046/j.1365-2826.2000.00481.x. [DOI] [PubMed] [Google Scholar]
- Gachon F, Nagoshi E, Brown SA, Ripperger J, Schibler U. The mammalian circadian timing system: from gene expression to physiology. Chromosoma. 2004:103–112. doi: 10.1007/s00412-004-0296-2. [DOI] [PubMed] [Google Scholar]
- Gerendai I, Halasz B. Central nervous system structures connected with the endocrine glands. Findings obtained with the viral transneuronal tracing technique. Exp. Clin. Endocrinol. Diabetes. 2000;108(6):389–395. doi: 10.1055/s-2000-8134. [DOI] [PubMed] [Google Scholar]
- Gerhold LM, Horvath TL, Freeman ME. Vasoactive intestinal peptide fibers innervate neuroendocrine dopaminergic neurons. Brain Res. 2001;919(1):48–56. doi: 10.1016/s0006-8993(01)02993-6. [DOI] [PubMed] [Google Scholar]
- Gillespie JM, Chan BP, Roy D, Cai F, Belsham DD. Expression of circadian rhythm genes in gonadotropin-releasing hormone-secreting GT1-7 neurons. Endocrinology. 2003;144(12):5285–5292. doi: 10.1210/en.2003-0802. [DOI] [PubMed] [Google Scholar]
- Glossop NR, Hardin PE. Central and peripheral circadian oscillator mechanisms in flies and mammals. J. Cell Sci. 2002;115(Pt. 17):3369–3377. doi: 10.1242/jcs.115.17.3369. [DOI] [PubMed] [Google Scholar]
- Gore AC. Circadian rhythms during aging. In: Mobbs CV, Hof PR, editors. Functional Endocrinology of Aging. vol. 29. Basel: Karger; 1998. pp. 127–165. [Google Scholar]
- Green CB. Molecular control of Xenopus retinal circadian rhythms. J. Neuroendocrinol. 2003;15(4):350–354. doi: 10.1046/j.1365-2826.2003.00999.x. [DOI] [PubMed] [Google Scholar]
- Green DJ, Gillette R. Circadian rhythm of firing rate recorded from single cells in the rat suprachiasmatic brain slice. Brain Res. 1982;245(1):198–200. doi: 10.1016/0006-8993(82)90361-4. [DOI] [PubMed] [Google Scholar]
- Groos G, Hendriks J. Circadian rhythms in electrical discharge of rat suprachiasmatic neurones recorded in vitro. Neurosci. Lett. 1982;34(3):283–288. doi: 10.1016/0304-3940(82)90189-6. [DOI] [PubMed] [Google Scholar]
- Gubbins EJ, Maurer RA, Lagrimini M, Erwin CR, Donelson JE. Structure of the rat prolactin gene. J. Biol. Chem. 1980;255(18):8655–8662. [PubMed] [Google Scholar]
- Gundlah C, Kohama SG, Mirkes SJ, Garyfallou VT, Urbanski HF, Bethea CL. Distribution of estrogen receptor beta (ERbeta) mRNA in hypothalamus, midbrain and temporal lobe of spayed macaque: continued expression with hormone replacement. Brain Res. Mol. Brain Res. 2000;76(2):191–204. doi: 10.1016/s0006-8993(99)02475-0. [DOI] [PubMed] [Google Scholar]
- Guo H, Brewer JM, Champhekar A, Harris RB, Bittman EL. Differential control of peripheral circadian rhythms by suprachiasmatic-dependent neural signals. Proc. Natl. Acad. Sci. U. S. A. 2005;102(8):3111–3116. doi: 10.1073/pnas.0409734102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakim H, DeBernardo AP, Silver R. Circadian locomotor rhythms, but not photoperiodic responses, survive surgical isolation of the SCN in hamsters. J. Biol. Rhythms. 1991;6(2):97–113. doi: 10.1177/074873049100600201. [DOI] [PubMed] [Google Scholar]
- Hansen S, Sodersten P, Eneroth P, Srebro B, Hole K. A sexually dimorphic rhythm in oestradiol-activated lordosis behaviour in the rat. J. Endocrinol. 1979;83(2):267–274. doi: 10.1677/joe.0.0830267. [DOI] [PubMed] [Google Scholar]
- Hara Y, Battey J, Gainer H. Structure of mouse vasopressin and oxytocin genes. Brain Res. Mol. Brain Res. 1990;8(4):319–324. doi: 10.1016/0169-328x(90)90045-f. [DOI] [PubMed] [Google Scholar]
- Harney JP, Scarbrough K, Rosewell KL, Wise PM. In vivo antisense antagonism of vasoactive intestinal peptide in the suprachiasmatic nuclei causes aging-like changes in the estradiol-induced luteinizing hormone and prolactin surges. Endocrinology. 1996;137(9):3696–3701. doi: 10.1210/endo.137.9.8756535. [DOI] [PubMed] [Google Scholar]
- Hastings MH. Neuroendocrine rhythms. In: Redfern PH, Waterhouse J, editors. Pharmacological Therapy. vol. 50. Great Britain: Pergamon Press; 1991. pp. 35–71. [Google Scholar]
- Hastings MH, Duffield GE, Ebling FJ, Kidd A, Maywood ES, Schurov I. Non-photic signalling in the suprachiasmatic nucleus. Biol. Cell. 1997;89(8):495–503. doi: 10.1016/s0248-4900(98)80005-1. [DOI] [PubMed] [Google Scholar]
- Hastings MH, Reddy AB, Maywood ES. A clockwork web: circadian timing in brain and periphery, in health and disease. Nat. Rev. Neurosci. 2003;4(8):649–661. doi: 10.1038/nrn1177. [DOI] [PubMed] [Google Scholar]
- Hayflick JS, Adelman JP, Seeburg PH. The complete nucleotide sequence of the human gonadotropin-releasing hormone gene. Nucleic Acids Res. 1989;17(15):6403–6404. doi: 10.1093/nar/17.15.6403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlerigg DG, Andersson H, Johnston JD, Lincoln G. Molecular characterization of the long-day response in the Soay sheep, a seasonal mammal. Curr. Biol. 2004;14(4):334–339. doi: 10.1016/j.cub.2004.01.057. [DOI] [PubMed] [Google Scholar]
- Hofman MA. The brain’s calendar: neural mechanisms of seasonal timing. Biol. Rev. Camb. Philos. Soc. 2004;79(1):61–77. doi: 10.1017/s1464793103006250. [DOI] [PubMed] [Google Scholar]
- Hofman MA, Fliers E, Goudsmit E, Swaab DF. Morphometric analysis of the suprachiasmatic and paraventricular nuclei in the human brain: sex differences and age-dependent changes. J. Anat. 1988;160:127–143. [PMC free article] [PubMed] [Google Scholar]
- Hofman MA, Zhou JN, Swaab DF. Suprachiasmatic nucleus of the human brain: an immunocytochemical and morphometric analysis. Anat. Rec. 1996;244(4):552–562. doi: 10.1002/(SICI)1097-0185(199604)244:4<552::AID-AR13>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Horvath TL. Suprachiasmatic efferents avoid phenestrated capillaries but innervate neuroendocrine cells, including those producing dopamine. Endocrinology. 1997;138(3):1312–1320. doi: 10.1210/endo.138.3.4976. [DOI] [PubMed] [Google Scholar]
- Horvath TL, Cela V, van der Beek EM. Gender-specific apposition between vasoactive intestinal peptide-containing axons and gonadotrophin-releasing hormone-producing neurons in the rat. Brain Res. 1998;795(1–2):277–281. doi: 10.1016/s0006-8993(98)00208-x. [DOI] [PubMed] [Google Scholar]
- Ingram CD, Ciobanu R, Coculescu IL, Tanasescu R, Coculescu M, Mihai R. Vasopressin neurotransmission and the control of circadian rhythms in the suprachiasmatic nucleus. Prog. Brain Res. 1998;119:351–364. doi: 10.1016/s0079-6123(08)61580-0. [DOI] [PubMed] [Google Scholar]
- Jakacki RI, Kelch RP, Sauder SE, Lloyd JS, Hopwood NJ, Marshall JC. Pulsatile secretion of luteinizing hormone in children. J. Clin. Endocrinol. Metab. 1982;55(3):453–458. doi: 10.1210/jcem-55-3-453. [DOI] [PubMed] [Google Scholar]
- Jechura TJ, Walsh JM, Lee TM. Testosterone suppresses circadian responsiveness to social cues in the diurnal rodent Octodon degus. J. Biol. Rhythms. 2003;18(1):43–50. doi: 10.1177/0748730402239675. [DOI] [PubMed] [Google Scholar]
- Jin X, Shearman LP, Weaver DR, Zylka MJ, de Vries GJ, Reppert SM. A molecular mechanism regulating rhythmic output from the suprachiasmatic circadian clock. Cell. 1999;96(1):57–68. doi: 10.1016/s0092-8674(00)80959-9. [DOI] [PubMed] [Google Scholar]
- Johnston JD, Cagampang FR, Stirland JA, Carr AJ, White MR, Davis JR, Loudon AS. Evidence for an endogenous per1- and ICER-independent seasonal timer in the hamster pituitary gland. FASEB J. 2003;17(8):810–815. doi: 10.1096/fj.02-0837com. [DOI] [PubMed] [Google Scholar]
- Kaiser UB, Sabbagh E, Chen MT, Chin WW, Saunders BD. Sp1 binds to the rat luteinizing hormone beta (LHbeta) gene promoter and mediates gonadotropin-releasing hormone-stimulated expression of the LHbeta subunit gene. J. Biol. Chem. 1998;273(21):12943–12951. doi: 10.1074/jbc.273.21.12943. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Buijs RM. Output pathways of the mammalian suprachiasmatic nucleus: coding circadian time by transmitter selection and specific targeting. Cell Tissue Res. 2002;309(1):109–118. doi: 10.1007/s00441-002-0577-0. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Teclemariam-Mesbah R, Pevet P. Efferent projections of the suprachiasmatic nucleus in the golden hamster (Mesocricetus auratus) J. Comp. Neurol. 1993;332(3):293–314. doi: 10.1002/cne.903320304. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, van der Vliet J, Buijs RM. Decrease of endogenous vasopressin release necessary for expression of the circadian rise in plasma corticosterone: a reverse microdialysis study. J. Neuroendocrinol. 1996a;8(4):299–307. doi: 10.1046/j.1365-2826.1996.04597.x. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, van Heerikhuize JJ, Wortel J, Buijs RM. A diurnal rhythm of stimulatory input to the hypothalamo-pituitary-adrenal system as revealed by timed intrahypothalamic administration of the vasopressin V1 antagonist. J. Neurosci. 1996b;16(17):5555–5565. doi: 10.1523/JNEUROSCI.16-17-05555.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalsbeek A, Fliers E, Franke AN, Wortel J, Buijs RM. Functional connections between the suprachiasmatic nucleus and the thyroid gland as revealed by lesioning and viral tracing techniques in the rat. Endocrinology. 2000;141(10):3832–3841. doi: 10.1210/endo.141.10.7709. [DOI] [PubMed] [Google Scholar]
- Kanamoto N, Akamizu T, Tagami T, Hataya Y, Moriyama K, Takaya K, Hosoda H, Kojima M, Kangawa K, Nakao K. Genomic structure and characterization of the 5′-flanking region of the human ghrelin gene. Endocrinology. 2004:4144–4153. doi: 10.1210/en.2003-1718. [DOI] [PubMed] [Google Scholar]
- Kapen S, Boyar RM, Finkelstein JW, Hellman L, Weitzman ED. Effect of sleep–wake cycle reversal on luteinizing hormone secretory pattern in puberty. J. Clin. Endocrinol. Metab. 1974;39(2):293–299. doi: 10.1210/jcem-39-2-293. [DOI] [PubMed] [Google Scholar]
- Kashon ML, Arbogast JA, Sisk CL. Distribution and hormonal regulation of androgen receptor immunoreactivity in the forebrain of the male European ferret. J. Comp. Neurol. 1996;376(4):567–586. doi: 10.1002/(SICI)1096-9861(19961223)376:4<567::AID-CNE6>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Klein DC. Photoneural regulation of the mammalian pineal gland. Ciba Found. Symp. 1985;117:38–56. doi: 10.1002/9780470720981.ch4. [DOI] [PubMed] [Google Scholar]
- Klein DC, Smoot R, Weller JL, Higa S, Markey SP, Creed GJ, Jacobowitz DM. Lesions of the paraventricular nucleus area of the hypothalamus disrupt the suprachiasmatic leads to spinal cord circuit in the melatonin rhythm generating system. Brain Res. Bull. 1983;10(5):647–652. doi: 10.1016/0361-9230(83)90033-3. [DOI] [PubMed] [Google Scholar]
- Knobil E. On the control of gonadotropin secretion in the rhesus monkey. Recent Prog. Horm. Res. 1974;30(0):1–46. doi: 10.1016/b978-0-12-571130-2.50005-5. [DOI] [PubMed] [Google Scholar]
- Krajnak K, Kashon ML, Rosewell KL, Wise PM. Sex differences in the daily rhythm of vasoactive intestinal polypeptide but not arginine vasopressin messenger ribonucleic acid in the suprachiasmatic nuclei. Endocrinology. 1998;139(10):4189–4196. doi: 10.1210/endo.139.10.6259. [DOI] [PubMed] [Google Scholar]
- Kramer A, Yang FC, Snodgrass P, Li X, Scammell TE, Davis FC, Weitz CJ. Regulation of daily locomotor activity and sleep by hypothalamic EGF receptor signaling. Science. 2001;294(5551):2511–2515. doi: 10.1126/science.1067716. [DOI] [PubMed] [Google Scholar]
- Kriegsfeld LJ, LeSauter JL, Hamada T, Pitts SM, Silver R. Circadian rhythms in the endocrine system. In: Pfaff D, Etgen A, editors. Hormones, Brain, and Behavior. New York: Academic Press; 2002a. [Google Scholar]
- Kriegsfeld LJ, Silver R, Gore AC, Crews D. Vasoactive intestinal polypeptide contacts on gonadotropin-releasing hormone neurones increase following puberty in female rats. J. Neuroendocrinol. 2002b;14(9):685–690. doi: 10.1046/j.1365-2826.2002.00818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Korets R, Silver R. Expression of the circadian clock gene Period 1 in neuroendocrine cells: an investigation using mice with a Per1:GFP transgene. Eur. J. Neurosci. 2003;17(2):212–220. doi: 10.1046/j.1460-9568.2003.02431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Leak RK, Yackulic CB, LeSauter J, Silver R. Organization of suprachiasmatic nucleus projections in Syrian hamsters (Mesocricetus auratus): an anterograde and retrograde analysis. J. Comp. Neurol. 2004;468(3):361–379. doi: 10.1002/cne.10995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruijver FP, Swaab DF. Sex hormone receptors are present in the human suprachiasmatic nucleus. Neuroendocrinology. 2002;75(5):296–305. doi: 10.1159/000057339. [DOI] [PubMed] [Google Scholar]
- Labyak SE, Lee TM. Estrus- and steroid-induced changes in circadian rhythms in a diurnal rodent, Octodon degus. Physiol. Behav. 1995;58(3):573–585. doi: 10.1016/0031-9384(95)00096-2. [DOI] [PubMed] [Google Scholar]
- Lavery DJ, Lopez-Molina L, Margueron R, Fleury-Olela F, Conquet F, Schibler U, Bonfils C. Circadian expression of the steroid 15 alpha-hydroxylase (Cyp2a4) and coumarin 7-hydroxylase (Cyp2a5) genes in mouse liver is regulated by the PAR leucine zipper transcription factor DBP. Mol. Cell. Biol. 1999;19(10):6488–6499. doi: 10.1128/mcb.19.10.6488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le WW, Attardi B, Berghorn KA, Blaustein J, Hoffman GE. Progesterone blockade of a luteinizing hormone surge blocks luteinizing hormone-releasing hormone Fos activation and activation of its preoptic area afferents. Brain Res. 1997;778(2):272–280. doi: 10.1016/s0006-8993(97)00971-2. [DOI] [PubMed] [Google Scholar]
- Leak RK, Moore RY. Topographic organization of suprachiasmatic nucleus projection neurons. J. Comp. Neurol. 2001;433(3):312–334. doi: 10.1002/cne.1142. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Silver R, Gladstone WR, Kahn RM, Gibson M, Bittman EL. Circadian rhythmicity restored by neural transplant. Immunocytochemical characterization of the graft and its integration with the host brain. J. Neurosci. 1987;7(6):1626–1638. doi: 10.1523/JNEUROSCI.07-06-01626.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JE. New concepts of the neuroendocrine regulation of gonadotropin surges in rats. Biol. Reprod. 1997;56(2):293–302. doi: 10.1095/biolreprod56.2.293. [DOI] [PubMed] [Google Scholar]
- Levine JE, Chappell PE, Schneider JS, Sleiter NC, Szabo M. Progesterone receptors as neuroendocrine integrators. Front. Neuroendocrinol. 2001;22(2):69–106. doi: 10.1006/frne.2001.0210. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Ahmed S, Jackson JM, Sack RL. Melatonin shifts human circadian rhythms according to a phase-response curve. Chronobiol. Int. 1992;9(5):380–392. doi: 10.3109/07420529209064550. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Sack RL. Exogenous melatonin’s phase-shifting effects on the endogenous melatonin profile in sighted humans: a brief review and critique of the literature. J. Biol. Rhythms. 1997;12(6):588–594. doi: 10.1177/074873049701200614. [DOI] [PubMed] [Google Scholar]
- Lincoln G, Messager S, Andersson H, Hazlerigg D. Temporal expression of seven clock genes in the suprachiasmatic nucleus and the pars tuberalis of the sheep: evidence for an internal coincidence timer. Proc. Natl. Acad. Sci. U. S. A. 2002;99(21):13890–13895. doi: 10.1073/pnas.212517599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln GA, Andersson H, Loudon A. Clock genes in calendar cells as the basis of annual timekeeping in mammals-a unifying hypothesis. J. Endocrinol. 2003;179(1):1–13. doi: 10.1677/joe.0.1790001. [DOI] [PubMed] [Google Scholar]
- Lincoln GA, Johnston JD, Andersson H, Wagner G, Hazlerigg DG. Photorefractoriness in mammals: dissociating a seasonal timer from the circadian-based photoperiod response. Endocrinology. 2005;146(9):3782–3790. doi: 10.1210/en.2005-0132. [DOI] [PubMed] [Google Scholar]
- Liu Y. Molecular mechanisms of entrainment in the Neurospora circadian clock. J. Biol. Rhythms. 2003;18(3):195–205. doi: 10.1177/0748730403018003002. [DOI] [PubMed] [Google Scholar]
- Mahoney MM, Sisk C, Ross HE, Smale L. Circadian regulation of gonadotropin-releasing hormone neurons and the preovulatory surge in luteinizing hormone in the diurnal rodent, Arvicanthis niloticus, and in a nocturnal rodent, Rattus norvegicus. Biol. Reprod. 2004;70(4):1049–1054. doi: 10.1095/biolreprod.103.021360. [DOI] [PubMed] [Google Scholar]
- Mai LM, Shieh KR, Pan JT. Circadian changes of serum prolactin levels and tuberoinfundibular dopaminergic neuron activities in ovariectomized rats treated with or without estrogen: the role of the suprachiasmatic nuclei. Neuroendocrinology. 1994;60(5):520–526. doi: 10.1159/000126789. [DOI] [PubMed] [Google Scholar]
- McArthur AJ, Gillette MU, Prosser RA. Melatonin directly resets the rat suprachiasmatic circadian clock in vitro. Brain Res. 1991;565(1):158–161. doi: 10.1016/0006-8993(91)91748-p. [DOI] [PubMed] [Google Scholar]
- McElhinny TL, Sisk CL, Holekamp KE, Smale L. A morning surge in plasma luteinizing hormone coincides with elevated Fos expression in gonadotropin-releasing hormone-immunoreactive neurons in the diurnal rodent, Arvicanthis niloticus. Biol. Reprod. 1999;61(4):1115–1122. doi: 10.1095/biolreprod61.4.1115. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Jones KJ, Pfaff DW. Hormonal control of sexual behavior in the female rat: molecular, cellular and neurochemical studies. Biol. Reprod. 1987;36(1):37–45. doi: 10.1095/biolreprod36.1.37. [DOI] [PubMed] [Google Scholar]
- Messager S, Hazlerigg DG, Mercer JG, Morgan PJ. Photoperiod differentially regulates the expression of Per1 and ICER in the pars tuberalis and the suprachiasmatic nucleus of the Siberian hamster. Eur. J. Neurosci. 2000;12(8):2865–2870. doi: 10.1046/j.1460-9568.2000.00174.x. [DOI] [PubMed] [Google Scholar]
- Meyer-Bernstein EL, Jetton AE, Matsumoto SI, Markuns JF, Lehman MN, Bittman EL. Effects of suprachiasmatic transplants on circadian rhythms of neuroendocrine function in golden hamsters. Endocrinology. 1999;140(1):207–218. doi: 10.1210/endo.140.1.6428. [DOI] [PubMed] [Google Scholar]
- Michael RP, Rees HD. Autoradiographic localization of 3H-dihydrotestosterone in the preoptic area, hypothalamus, and amygdala of a male rhesus monkey. Life Sci. 1982;30(24):2087–2093. doi: 10.1016/0024-3205(82)90450-7. [DOI] [PubMed] [Google Scholar]
- Mihai R, Coculescu M, Wakerley JB, Ingram CD. The effects of [Arg8]vasopressin and [Arg8]vasotocin on the firing rate of suprachiasmatic neurons in vitro. Neuroscience. 1994a;62(3):783–792. doi: 10.1016/0306-4522(94)90476-6. [DOI] [PubMed] [Google Scholar]
- Mihai R, Juss TS, Ingram CD. Suppression of suprachiasmatic nucleus neurone activity with a vasopressin receptor antagonist: possible role for endogenous vasopressin in circadian activity cycles in vitro. Neurosci. Lett. 1994b;179(1–2):95–99. doi: 10.1016/0304-3940(94)90943-1. [DOI] [PubMed] [Google Scholar]
- Miller BH, Olson SL, Turek FW, Levine JE, Horton TH, Takahashi JS. Circadian clock mutation disrupts estrous cyclicity and maintenance of pregnancy. Curr. Biol. 2004;14(15):1367–1373. doi: 10.1016/j.cub.2004.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mong J, Easton A, Kow LM, Pfaff D. Neural, hormonal and genetic mechanisms for the activation of brain and behavior. Eur. J. Pharmacol. 2003;480(1–3):229–231. doi: 10.1016/j.ejphar.2003.08.109. [DOI] [PubMed] [Google Scholar]
- Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972;42(1):201–206. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- Morin LP. Effect of ovarian hormones on synchrony of hamster circadian rhythms. Physiol. Behav. 1980;24(4):741–749. doi: 10.1016/0031-9384(80)90406-0. [DOI] [PubMed] [Google Scholar]
- Morin LP, Cummings LA. Effect of surgical or photoperiodic castration, testosterone replacement or pinealectomy on male hamster running rhythmicity. Physiol. Behav. 1981;26(5):825–838. doi: 10.1016/0031-9384(81)90106-2. [DOI] [PubMed] [Google Scholar]
- Morin LP, Goodless-Sanchez N, Smale L, Moore RY. Projections of the suprachiasmatic nuclei, subparaventricular zone and retrochiasmatic area in the golden hamster. Neuroscience. 1994;61(2):391–410. doi: 10.1016/0306-4522(94)90240-2. [DOI] [PubMed] [Google Scholar]
- Morin LP, Fitzgerald KM, Zucker I. Estradiol shortens the period of hamster circadian rhythms. Science. 1977;196(4287):305–307. doi: 10.1126/science.557840. [DOI] [PubMed] [Google Scholar]
- Morse D, Cermakian N, Brancorsini S, Parvinen M, Sassone-Corsi P. No circadian rhythms in testis: Period1 expression is clock independent and developmentally regulated in the mouse. Mol. Endocrinol. 2003;17(1):141–151. doi: 10.1210/me.2002-0184. [DOI] [PubMed] [Google Scholar]
- Mosko SS, Moore RY. Neonatal suprachiasmatic nucleus lesions: effects on the development of circadian rhythms in the rat. Brain Res. 1979;164:17–38. doi: 10.1016/0006-8993(79)90003-9. [DOI] [PubMed] [Google Scholar]
- Muglia LJ, Jenkins NA, Gilbert DJ, Copeland NG, Majzoub JA. Expression of the mouse corticotropin-releasing hormone gene in vivo and targeted inactivation in embryonic stem cells. J. Clin. Invest. 1994;93(5):2066–2072. doi: 10.1172/JCI117201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy HM, Wideman CH, Nadzam GR. Vasopressin deficiency and circadian rhythms during food-restriction stress. Peptides. 1993;14(6):1215–1220. doi: 10.1016/0196-9781(93)90178-j. [DOI] [PubMed] [Google Scholar]
- Murphy HM, Wideman CH, Nadzam GR. The interaction of vasopressin and the photic oscillator in circadian rhythms. Peptides. 1996;17(3):467–475. doi: 10.1016/0196-9781(95)02129-9. [DOI] [PubMed] [Google Scholar]
- Nagoshi E, Saini C, Bauer C, Laroche T, Naef F, Schibler U. Circadian gene expression in individual fibroblasts: cell-autonomous and self-sustained oscillators pass time to daughter cells. Cell. 2004;119(5):693–705. doi: 10.1016/j.cell.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Neill JD, Freeman ME, Tillson SA. Control of the proestrus surge of prolactin and luteinizing hormone secretion by estrogens in the rat. Endocrinology. 1971;89(6):1448–1453. doi: 10.1210/endo-89-6-1448. [DOI] [PubMed] [Google Scholar]
- Nicholson C. Signals that go with the flow. Trends Neurosci. 1999;22(4):143–145. doi: 10.1016/s0166-2236(98)01388-5. [DOI] [PubMed] [Google Scholar]
- Nunez AA, Stephan FK. The effects of hypothalamic knife cuts on drinking rhythms and the estrus cycle of the rat. Behav. Biol. 1977;20(2):224–234. doi: 10.1016/s0091-6773(77)90786-6. [DOI] [PubMed] [Google Scholar]
- Okamura H. Integration of mammalian circadian clock signals: from molecule to behavior. J. Endocrinol. 2003a;177(1):3–6. doi: 10.1677/joe.0.1770003. [DOI] [PubMed] [Google Scholar]
- Okamura H. Molecular mechanisms of biological clock: from molecular rhythms to physiological rhythms. No To Shinkei. 2003b;55(1):5–11. [PubMed] [Google Scholar]
- Okamura H, Yamaguchi S, Yagita K. Molecular machinery of the circadian clock in mammals. Cell Tissue Res. 2002;309(1):47–56. doi: 10.1007/s00441-002-0572-5. [DOI] [PubMed] [Google Scholar]
- Olcese J, Domagalski R, Bednorz A, Weaver DR, Urbanski HF, Reuss S, Middendorff R. Expression and regulation of mPer1 in immortalized GnRH neurons. NeuroReport. 2003;14(4):613–618. doi: 10.1097/00001756-200303240-00018. [DOI] [PubMed] [Google Scholar]
- Palm IF, Van Der Beek EM, Wiegant VM, Buijs RM, Kalsbeek A. Vasopressin induces a luteinizing hormone surge in ovariectomized, estradiol-treated rats with lesions of the suprachiasmatic nucleus. Neuroscience. 1999;93(2):659–666. doi: 10.1016/s0306-4522(99)00106-2. [DOI] [PubMed] [Google Scholar]
- Palm IF, van der Beek EM, Swarts HJ, van der Vliet J, Wiegant VM, Buijs RM, Kalsbeek A. Control of the estradiol-induced prolactin surge by the suprachiasmatic nucleus. Endocrinology. 2001a;142(6):2296–2302. doi: 10.1210/endo.142.6.8219. [DOI] [PubMed] [Google Scholar]
- Palm IF, van der Beek EM, Wiegant VM, Buijs RM, Kalsbeek A. The stimulatory effect of vasopressin on the luteinizing hormone surge in ovariectomized, estradiol-treated rats is time-dependent. Brain Res. 2001b;901(1–2):109–116. doi: 10.1016/s0006-8993(01)02309-5. [DOI] [PubMed] [Google Scholar]
- Pan JT, Gala RR. Central nervous system regions involved in the estrogen-induced afternoon prolactin surge: I. Lesion studies. Endocrinology. 1985;117(1):382–387. doi: 10.1210/endo-117-1-382. [DOI] [PubMed] [Google Scholar]
- Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109(3):307–320. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- Petersen SL, Barraclough CA. Suppression of spontaneous LH surges in estrogen-treated ovariectomized rats by microimplants of antiestrogens into the preoptic brain. Brain Res. 1989;484(1–2):279–289. doi: 10.1016/0006-8993(89)90371-5. [DOI] [PubMed] [Google Scholar]
- Pieper DR, Gala RR. The effect of light on the prolactin surges of pseudopregnant and ovariectomized, estrogenized rats. Biol. Reprod. 1979;20(4):727–732. doi: 10.1095/biolreprod20.4.727. [DOI] [PubMed] [Google Scholar]
- Piggins HD. Human clock genes. Ann. Med. 2002;34(5):394–400. doi: 10.1080/078538902320772142. [DOI] [PubMed] [Google Scholar]
- Plant TM. Time courses of concentrations of circulating gonadotropin, prolactin, testosterone, and cortisol in adult male rhesus monkeys (Macaca mulatta) throughout the 24 h light–dark cycle. Biol. Reprod. 1981;25(2):244–252. doi: 10.1095/biolreprod25.2.244. [DOI] [PubMed] [Google Scholar]
- Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247(4945):975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- Rees HD, Michael RP. Brain cells of the male rhesus monkey accumulate 3H-testosterone or its metabolites. J. Comp. Neurol. 1982;206(3):273–277. doi: 10.1002/cne.902060307. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Tan DX. Role of CSF in the transport of melatonin. J. Pineal Res. 2002;33(1):61. doi: 10.1034/j.1600-079x.2002.2e001.x. [DOI] [PubMed] [Google Scholar]
- Ripperger JA, Shearman LP, Reppert SM, Schibler U. CLOCK, an essential pacemaker component, controls expression of the circadian transcription factor DBP. Genes Dev. 2000;14(6):679–689. [PMC free article] [PubMed] [Google Scholar]
- Roenneberg T, Merrow M. The network of time: understanding the molecular circadian system. Curr. Biol. 2003;13(5):R198–R207. doi: 10.1016/s0960-9822(03)00124-6. [DOI] [PubMed] [Google Scholar]
- Roy EJ, Wade GN. Role of estrogens in androgen-induced spontaneous activity in male rats. J. Comp. Physiol. Psychol. 1975;89(6):573–579. doi: 10.1037/h0077436. [DOI] [PubMed] [Google Scholar]
- Satoh T, Yamada M, Iwasaki T, Mori M. Negative regulation of the gene for the preprothyrotropin-releasing hormone from the mouse by thyroid hormone requires additional factors in conjunction with thyroid hormone receptors. J. Biol. Chem. 1996;271(44):27919–27926. doi: 10.1074/jbc.271.44.27919. [DOI] [PubMed] [Google Scholar]
- Schibler U, Sassone-Corsi P. A web of circadian pacemakers. Cell. 2002;111(7):919–922. doi: 10.1016/s0092-8674(02)01225-4. [DOI] [PubMed] [Google Scholar]
- Schultz TF, Kay SA. Circadian clocks in daily and seasonal control of development. Science. 2003;301(5631):326–328. doi: 10.1126/science.1085935. [DOI] [PubMed] [Google Scholar]
- Schuster C, Gauer F, Malan A, Recio J, Pevet P, Masson-Pevet M. The circadian clock, light/dark cycle and melatonin are differentially involved in the expression of daily and photoperiodic variations in mt(1) melatonin receptors in the Siberian and Syrian hamsters. Neuroendocrinology. 2001;74(1):55–68. doi: 10.1159/000054670. [DOI] [PubMed] [Google Scholar]
- Shibata S. Neural regulation of the hepatic circadian rhythm. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2004;280(1):901–909. doi: 10.1002/ar.a.20095. [DOI] [PubMed] [Google Scholar]
- Shibata S, Oomura Y, Kita H, Hattori K. Circadian rhythmic changes of neuronal activity in the suprachiasmatic nucleus of the rat hypothalamic slice. Brain Res. 1982;247(1):154–158. doi: 10.1016/0006-8993(82)91041-1. [DOI] [PubMed] [Google Scholar]
- Shieh KR. Distribution of the rhythm-related genes rPERIOD1, rPERIOD2, and rCLOCK, in the rat brain. Neuroscience. 2003;118(3):831–843. doi: 10.1016/s0306-4522(03)00004-6. [DOI] [PubMed] [Google Scholar]
- Shivers BD, Harlan RE, Morrell JI, Pfaff DW. Absence of oestradiol concentration in cell nuclei of LHRH-immunoreactive neurones. Nature. 1983;304(5924):345–347. doi: 10.1038/304345a0. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and-beta mRNA in the rat central nervous system. J. Comp. Neurol. 1997;388(4):507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Silver R, Lehman MN, Gibson M, Gladstone WR, Bittman EL. Dispersed cell suspensions of fetal SCN restore circadian rhythmicity in SCN-lesioned adult hamsters. Brain Res. 1990;525(1):45–58. doi: 10.1016/0006-8993(90)91319-c. [DOI] [PubMed] [Google Scholar]
- Silver R, LeSauter J, Tresco PA, Lehman MN. A diffusible coupling signal from the transplanted suprachiasmatic nucleus controlling circadian locomotor rhythms. Nature. 1996;382(6594):810–813. doi: 10.1038/382810a0. [DOI] [PubMed] [Google Scholar]
- Silver R, Sookhoo AI, LeSauter J, Stevens P, Jansen HT, Lehman MN. Multiple regulatory elements result in regional specificity in circadian rhythms of neuropeptide expression in mouse SCN. NeuroReport. 1999;10(15):3165–3174. doi: 10.1097/00001756-199910190-00008. [DOI] [PubMed] [Google Scholar]
- Skinner DC, Caraty A. Measurement and possible function of GnRH in cerebrospinal fluid in ewes. Reprod. Suppl. 2002;59:25–39. [PubMed] [Google Scholar]
- Skinner DC, Malpaux B. High melatonin concentrations in third ventricular cerebrospinal fluid are not due to Galen vein blood recirculating through the choroid plexus. Endocrinology. 1999;140(10):4399–4405. doi: 10.1210/endo.140.10.7074. [DOI] [PubMed] [Google Scholar]
- Slotten H, Pitrosky B, Pevet P. Entrainment of rat circadian rhythms by daily administration of melatonin. Influence of the mode of administration. Adv. Exp. Med. Biol. 1999;460:279–281. doi: 10.1007/0-306-46814-x_30. [DOI] [PubMed] [Google Scholar]
- Smale L, Lee T, Nunez AA. Mammalian diurnality: some facts and gaps. J. Biol. Rhythms. 2003;18(5):356–366. doi: 10.1177/0748730403256651. [DOI] [PubMed] [Google Scholar]
- Smith SG, III, Stetson MH. Maturation of the clock-timed gonadotropin release mechanism in hamsters: a key event in the pubertal process? Endocrinology. 1980;107(5):1334–1337. doi: 10.1210/endo-107-5-1334. [DOI] [PubMed] [Google Scholar]
- Stephan FK, Zucker I. Rat drinking rhythms: central visual pathways and endocrine factors mediating responsiveness to environmental illumination. Physiol. Behav. 1972;8(2):315–326. doi: 10.1016/0031-9384(72)90379-4. [DOI] [PubMed] [Google Scholar]
- Stephan FK, Berkley KJ, Moss RL. Efferent connections of the rat suprachiasmatic nucleus. Neuroscience. 1981;6(12):2625–2641. doi: 10.1016/0306-4522(81)90108-1. [DOI] [PubMed] [Google Scholar]
- Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, Wong WH, Weitz CJ. Extensive and divergent circadian gene expression in liver and heart. Nature. 2002;417(6884):78–83. doi: 10.1038/nature744. [DOI] [PubMed] [Google Scholar]
- Su JD, Qiu J, Zhong YP, Chen YZ. Expression of estrogen receptor-alpha and-beta immunoreactivity in the cultured neonatal suprachiasmatic nucleus: with special attention to GABAergic neurons. NeuroReport. 2001;12(9):1955–1959. doi: 10.1097/00001756-200107030-00036. [DOI] [PubMed] [Google Scholar]
- Swann JM, Turek FW. Multiple circadian oscillators regulate the timing of behavioral and endocrine rhythms in female golden hamsters. Science. 1985;228(4701):898–900. doi: 10.1126/science.4001926. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Yokota S, Hara R, Kobayashi T, Akiyama M, Moriya T, Shibata S. Physical and inflammatory stressors elevate circadian clock gene mPer1 mRNA levels in the paraventricular nucleus of the mouse. Endocrinology. 2001;142(11):4910–4917. doi: 10.1210/endo.142.11.8487. [DOI] [PubMed] [Google Scholar]
- Tavakoli-Nezhad M, Schwartz WJ. c-Fos expression in the brains of behaviorally “split” hamsters in constant light: calling attention to a dorsolateral region of the suprachiasmatic nucleus and the medial division of the lateral habenula. J. Biol. Rhythms. 2005;20(5):419–429. doi: 10.1177/0748730405278443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teclemariam-Mesbah R, Kalsbeek A, Pevet P, Buijs RM. Direct vasoactive intestinal polypeptide-containing projection from the suprachiasmatic nucleus to spinal projecting hypothalamic paraventricular neurons. Brain Res. 1997;748(1–2):71–76. doi: 10.1016/s0006-8993(96)01246-2. [DOI] [PubMed] [Google Scholar]
- Tei H, Okamura H, Shigeyoshi Y, Fukuhara C, Ozawa R, Hirose M, Sakaki Y. Circadian oscillation of a mammalian homologue of the Drosophila period gene. Nature. 1997;389(6650):512–516. doi: 10.1038/39086. [DOI] [PubMed] [Google Scholar]
- Terazono H, Mutoh T, Yamaguchi S, Kobayashi M, Akiyama M, Udo R, Ohdo S, Okamura H, Shibata S. Adrenergic regulation of clock gene expression in mouse liver. Proc. Natl. Acad. Sci. U. S. A. 2003;100(11):6795–6800. doi: 10.1073/pnas.0936797100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricoire H, Moller M, Chemineau P, Malpaux B. Origin of cerebrospinal fluid melatonin and possible function in the integration of photoperiod. Reprod. Suppl. 2003;61:311–321. [PubMed] [Google Scholar]
- Turek FW, Van Cauter E. Rhythms in reproduction. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. New York: Raven Press; 1994. pp. 487–540. [Google Scholar]
- Ueda HR, Chen W, Adachi A, Wakamatsu H, Hayashi S, Takasugi T, Nagano M, Nakahama K, Suzuki Y, Sugano S, Iino M, Shigeyoshi Y, Hashimoto S. A transcription factor response element for gene expression during circadian night. Nature. 2002;418(6897):534–539. doi: 10.1038/nature00906. [DOI] [PubMed] [Google Scholar]
- Van Cauter E, Refetoff S. Multifactorial control of the 24-hour secretory profiles of pituitary hormones. J. Endocrinol. Invest. 1985;8(4):381–391. doi: 10.1007/BF03348519. [DOI] [PubMed] [Google Scholar]
- van der Beek EM, Wiegant VM, van der Donk HA, van den Hurk R, Buijs RM. Lesions of the suprachiasmatic nucleus indicate the presence of a direct vasoactive intestinal polypeptide-containing projection to gonadotrophin-releasing hormone neurons in the female rat. J. Neuroendocrinol. 1993;5(2):137–144. doi: 10.1111/j.1365-2826.1993.tb00373.x. [DOI] [PubMed] [Google Scholar]
- van der Beek EM, van Oudheusden HJ, Buijs RM, van der Donk HA, van den Hurk R, Wiegant VM. Preferential induction of c-fos immunoreactivity in vasoactive intestinal polypeptide-innervated gonadotropin-releasing hormone neurons during a steroid-induced luteinizing hormone surge in the female rat. Endocrinology. 1994;134(6):2636–2644. doi: 10.1210/endo.134.6.8194489. [DOI] [PubMed] [Google Scholar]
- Van der Beek EM, Horvath TL, Wiegant VM, Van den Hurk R, Buijs RM. Evidence for a direct neuronal pathway from the suprachiasmatic nucleus to the gonadotropin-releasing hormone system: combined tracing and light and electron microscopic immunocytochemical studies. J. Comp. Neurol. 1997a;384(4):569–579. doi: 10.1002/(sici)1096-9861(19970811)384:4<569::aid-cne6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- van der Beek EM, Wiegant VM, van Oudheusden HJ, van der Donk HA, van den Hurk R, Buijs RM. Synaptic contacts between gonadotropin-releasing hormone-containing fibers and neurons in the suprachiasmatic nucleus and perichiasmatic area: an anatomical substrate for feedback regulation? Brain Res. 1997b;755(1):101–111. doi: 10.1016/s0006-8993(97)00086-3. [DOI] [PubMed] [Google Scholar]
- Vanecek J, Watanabe K. Mechanisms of melatonin action in the pituitary and SCN. Adv. Exp. Med. Biol. 1999;460:191–198. doi: 10.1007/0-306-46814-x_21. [DOI] [PubMed] [Google Scholar]
- von Gall C, Garabette ML, Kell CA, Frenzel S, Dehghani F, Schumm-Draeger PM, Weaver DR, Korf HW, Hastings MH, Stehle JH. Rhythmic gene expression in pituitary depends on heterologous sensitization by the neurohormone melatonin. Nat. Neurosci. 2002;5(3):234–238. doi: 10.1038/nn806. [DOI] [PubMed] [Google Scholar]
- Vrang N, Larsen PJ, Mikkelsen JD. Direct projection from the suprachiasmatic nucleus to hypophysiotrophic corticotropin-releasing factor immunoreactive cells in the paraventricular nucleus of the hypothalamus demonstrated by means of Phaseolus vulgaris-leucoagglutinin tract tracing. Brain Res. 1995;684(1):61–69. doi: 10.1016/0006-8993(95)00425-p. [DOI] [PubMed] [Google Scholar]
- Watson RE, Jr, Langub MC, Jr, Engle MG, Maley BE. Estrogen-receptive neurons in the anteroventral periventricular nucleus are synaptic targets of the suprachiasmatic nucleus and peri-suprachiasmatic region. Brain Res. 1995;689(2):254–264. doi: 10.1016/0006-8993(95)00548-5. [DOI] [PubMed] [Google Scholar]
- Watts AG, Swanson LW. Efferent projections of the suprachiasmatic nucleus: II. Studies using retrograde transport of fluorescent dyes and simultaneous peptide immunohistochemistry in the rat. J. Comp. Neurol. 1987;258(2):230–252. doi: 10.1002/cne.902580205. [DOI] [PubMed] [Google Scholar]
- Watts AG, Swanson LW, Sanchez-Watts G. Efferent projections of the suprachiasmatic nucleus: I. Studies using anterograde transport of Phaseolus vulgaris leucoagglutinin in the rat. J. Comp. Neurol. 1987;258(2):204–229. doi: 10.1002/cne.902580204. [DOI] [PubMed] [Google Scholar]
- Weaver DR. The suprachiasmatic nucleus: a 25-year retrospective. J. Biol. Rhythms. 1998;13(2):100–112. doi: 10.1177/074873098128999952. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Logothetis DE, Meister M, Reppert SM. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron. 1995;14(4):697–706. doi: 10.1016/0896-6273(95)90214-7. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Yoo SH, Liu AC, Takahashi JS, Kay SA. Bioluminescence imaging of individual fibroblasts reveals persistent, independently phased circadian rhythms of clock gene expression. Curr. Biol. 2004;14(24):2289–2295. doi: 10.1016/j.cub.2004.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RB, Eisen JA, Kasten TL, Fernald RD. Second gene for gonadotropin-releasing hormone in humans. Proc. Natl. Acad. Sci. U. S. A. 1998;95(1):305–309. doi: 10.1073/pnas.95.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagita K, Tamanini F, van Der Horst GT, Okamura H. Molecular mechanisms of the biological clock in cultured fibroblasts. Science. 2001;292(5515):278–281. doi: 10.1126/science.1059542. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Shigeyoshi Y, Ishida Y, Fukuyama T, Yamaguchi S, Yagita K, Moriya T, Shibata S, Takashima N, Okamura H. Expression of the Per1 gene in the hamster: brain atlas and circadian characteristics in the suprachiasmatic nucleus. J. Comp. Neurol. 2001;430(4):518–532. [PubMed] [Google Scholar]
- Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288(5466):682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- Yan L, Foley NC, Bobula JM, Kriegsfeld LJ, Silver R. Two antiphase oscillations occur in each suprachiasmatic nucleus of behaviorally split hamsters. J. Neurosci. 2005;25(39):9017–9026. doi: 10.1523/JNEUROSCI.2538-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, Takahashi JS. PERIOD2:LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc. Natl. Acad. Sci. U. S. A. 2004;101(15):5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zordan M, Sandrelli F, Costa R. A concise overview of circadian timing in Drosophila. Front. Biosci. 2003;8:d870–d877. doi: 10.2741/1036. [DOI] [PubMed] [Google Scholar]
- Zylka MJ, Shearman LP, Weaver DR, Reppert SM. Three period homologs in mammals: differential light responses in the suprachiasmatic circadian clock and oscillating transcripts outside of brain. Neuron. 1998;20(6):1103–1110. doi: 10.1016/s0896-6273(00)80492-4. [DOI] [PubMed] [Google Scholar]