Abstract
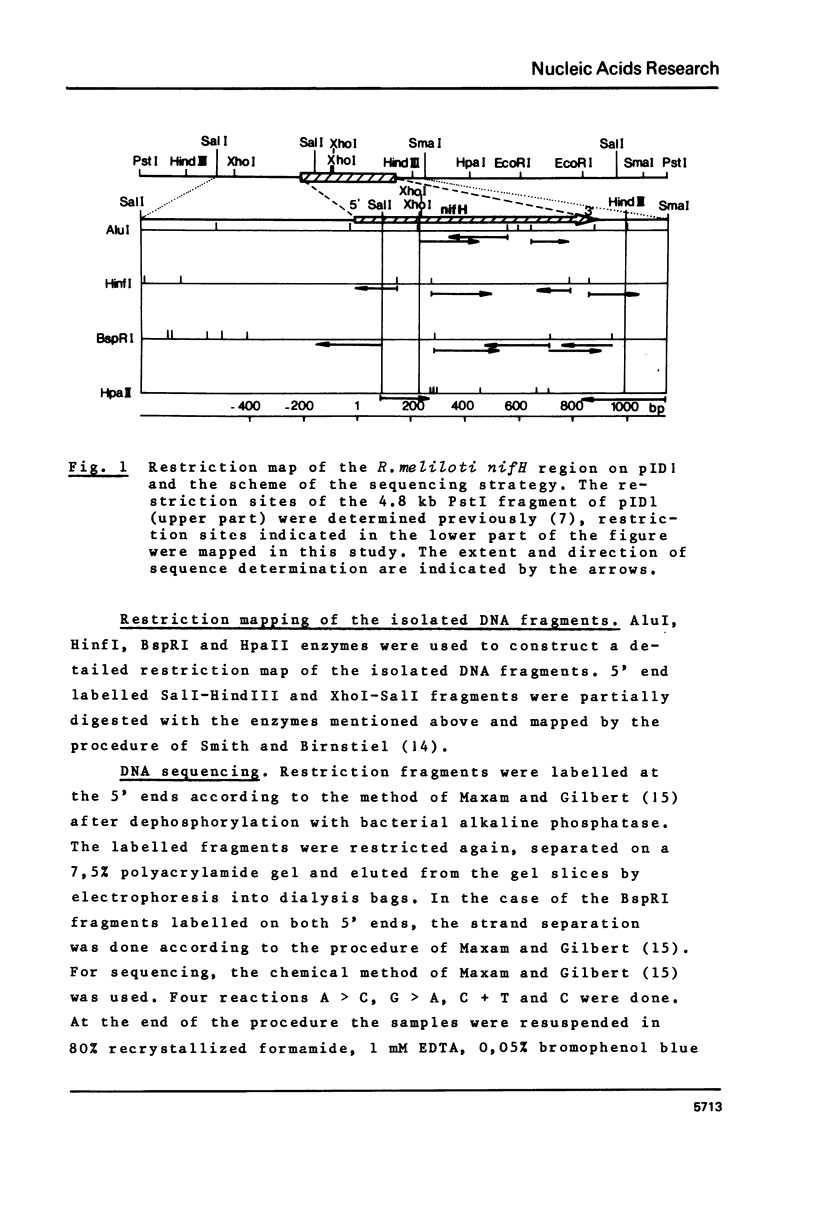
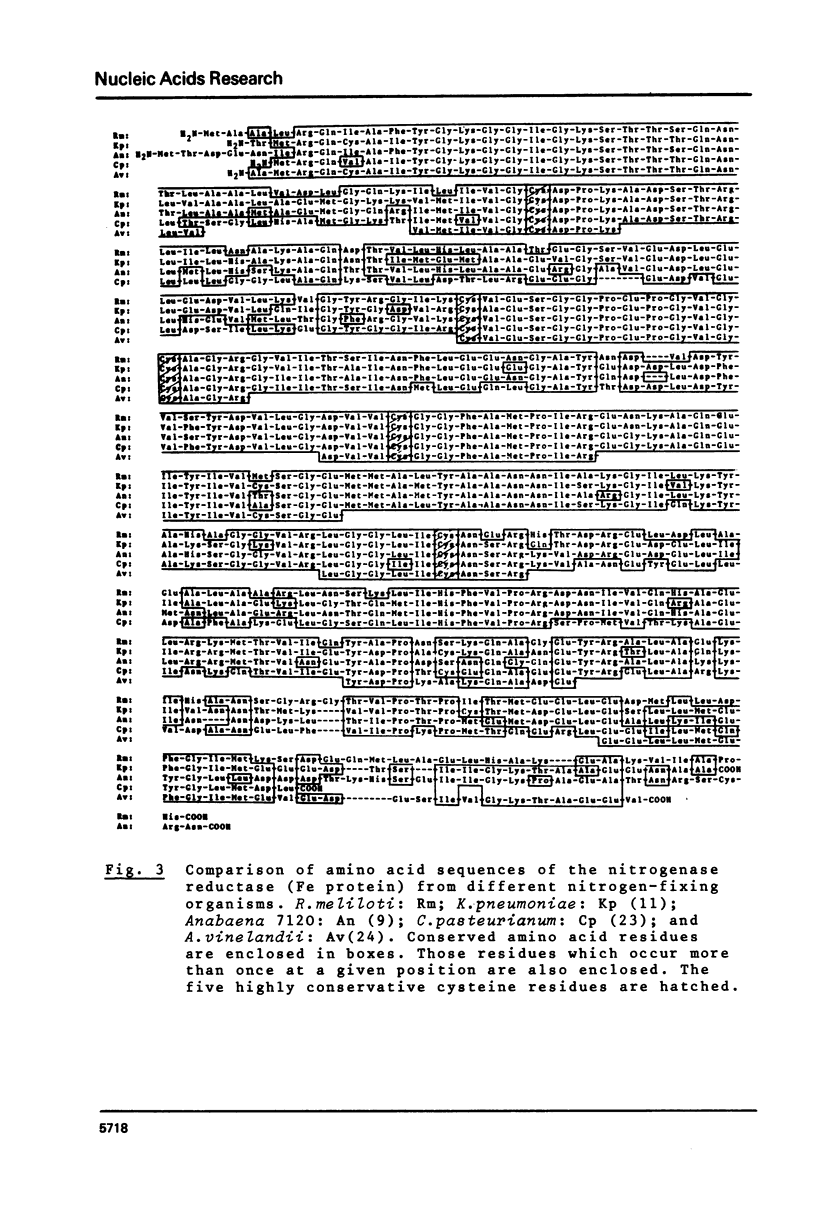
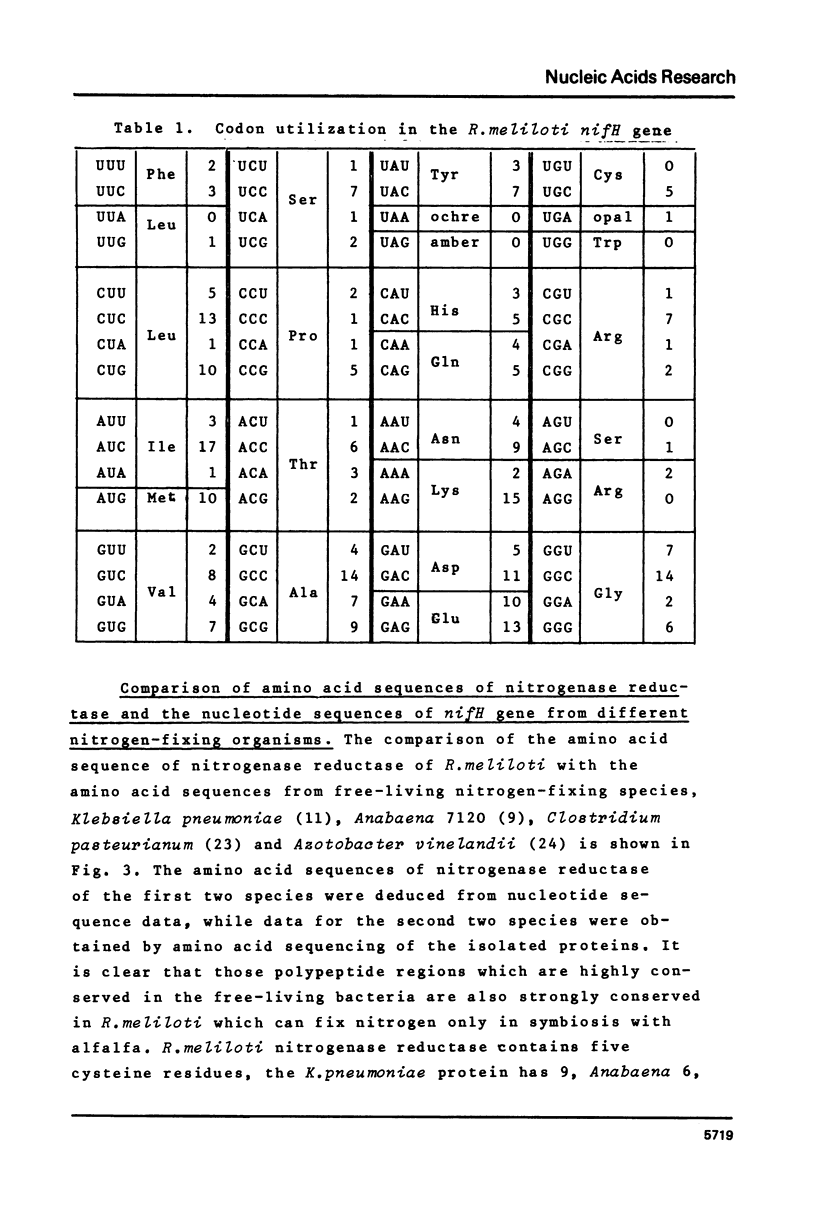
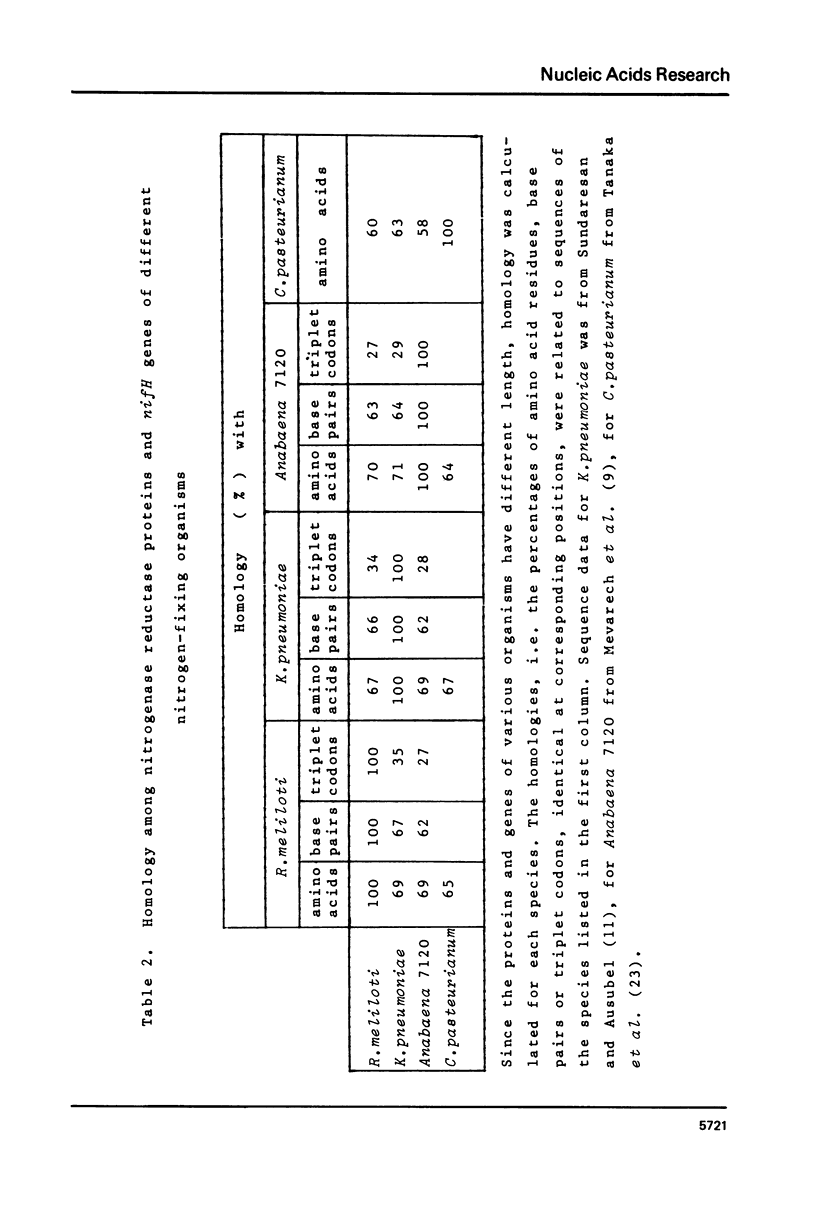
The nucleotide sequence of the structural gene (nifH) of nitrogenase reductase (Fe protein) from R.meliloti 41 with its flanking ends is reported. The amino acid sequence of nitrogenase reductase was deduced from the DNA sequence. The predicted R.meliloti nitrogenase reductase protein consists of 297 amino acid residues, has a molecular weight of 32,740 daltons and contains 5 cysteine residues. The codon usage in the nifH gene is presented. In the 5' flanking region, sequences resembling to consensus sequences of bacterial control regions were found. Comparison of the R.meliloti nifH nucleotide and amino acid sequences with those from different nitrogen-fixing organisms showed that the amino acid sequences are more conserved than the nucleotide sequences. This structural conservation of nitrogenase reductase may be related to its function and may explain the conservation of the nifH gene during evolution.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cannon F. C., Riedel G. E., Ausubel F. M. Overlapping sequences of Klebsiella pneumoniae nifDNA cloned and characterized. Mol Gen Genet. 1979 Jul 2;174(1):59–66. doi: 10.1007/BF00433306. [DOI] [PubMed] [Google Scholar]
- Dixon R., Kennedy C., Kondorosi A., Krishnapillai V., Merrick M. Complementation analysis of Klebsiella pneumoniae mutants defective in nitrogen fixation. Mol Gen Genet. 1977 Nov 29;157(2):189–198. doi: 10.1007/BF00267397. [DOI] [PubMed] [Google Scholar]
- Hausinger R. P., Howard J. B. Comparison of the iron proteins from the nitrogen fixation complexes of Azotobacter vinelandii, Clostridium pasteurianum, and Klebsiella pneumoniae. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3826–3830. doi: 10.1073/pnas.77.7.3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNeil T., MacNeil D., Roberts G. P., Supiano M. A., Brill W. J. Fine-structure mapping and complementation analysis of nif (nitrogen fixation) genes in Klebsiella pneumoniae. J Bacteriol. 1978 Oct;136(1):253–266. doi: 10.1128/jb.136.1.253-266.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur B. J., Rice D., Haselkorn R. Identification of blue-green algal nitrogen fixation genes by using heterologous DNA hybridization probes. Proc Natl Acad Sci U S A. 1980 Jan;77(1):186–190. doi: 10.1073/pnas.77.1.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mevarech M., Rice D., Haselkorn R. Nucleotide sequence of a cyanobacterial nifH gene coding for nitrogenase reductase. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6476–6480. doi: 10.1073/pnas.77.11.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortenson L. E., Thorneley R. N. Structure and function of nitrogenase. Annu Rev Biochem. 1979;48:387–418. doi: 10.1146/annurev.bi.48.070179.002131. [DOI] [PubMed] [Google Scholar]
- Nichols B. P., Blumenberg M., Yanofsky C. Comparison of the nucleoside sequence of trpA and sequences immediately beyond the trp operon of Klebsiella aerogenes. Salmonella typhimurium and Escherichia coli. Nucleic Acids Res. 1981 Apr 10;9(7):1743–1755. doi: 10.1093/nar/9.7.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R. J. Restriction and modification enzymes and their recognition sequences. Nucleic Acids Res. 1980 Jan 11;8(1):r63–r80. doi: 10.1093/nar/8.1.197-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Ruvkun G. B., Ausubel F. M. Interspecies homology of nitrogenase genes. Proc Natl Acad Sci U S A. 1980 Jan;77(1):191–195. doi: 10.1073/pnas.77.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. Determinant of cistron specificity in bacterial ribosomes. Nature. 1975 Mar 6;254(5495):34–38. doi: 10.1038/254034a0. [DOI] [PubMed] [Google Scholar]
- Siebenlist U., Simpson R. B., Gilbert W. E. coli RNA polymerase interacts homologously with two different promoters. Cell. 1980 Jun;20(2):269–281. doi: 10.1016/0092-8674(80)90613-3. [DOI] [PubMed] [Google Scholar]
- Smith H. O., Birnstiel M. L. A simple method for DNA restriction site mapping. Nucleic Acids Res. 1976 Sep;3(9):2387–2398. doi: 10.1093/nar/3.9.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaresan V., Ausubel F. M. Nucleotide sequence of the gene coding for the nitrogenase iron protein from Klebsiella pneumoniae. J Biol Chem. 1981 Mar 25;256(6):2808–2812. [PubMed] [Google Scholar]
- Walseth T. F., Johnson R. A. The enzymatic preparation of [alpha-(32)P]nucleoside triphosphates, cyclic [32P] AMP, and cyclic [32P] GMP. Biochim Biophys Acta. 1979 Mar 28;562(1):11–31. doi: 10.1016/0005-2787(79)90122-9. [DOI] [PubMed] [Google Scholar]



