Abstract
Background
Glutamate is thought to be involved in the pathophysiology of major depressive disorder and bipolar disorder; however, the molecular changes underlying abnormal glutamatergic signalling remain poorly understood. While previous studies have suggested that the NMDA receptor may be involved in the pathophysiology of mood disorders, it is unclear whether the non-NMDA receptors are also involved. Therefore, we sought to examine whether the expression of the non-NMDA, ionotropic glutamate receptors, AMPA receptor and kainate receptor, is altered in mood disorders.
Methods
We used [3H]AMPA and [3H]kainate to measure the levels of AMPA and kainate receptor, respectively, in the anterior cingulate (BA 24) and dorsolateral prefrontal cortex (BA 46) from post-mortem CNS in 10 subjects with major depressive disorder, 10 subjects with bipolar disorder and 10 control subjects.
Results
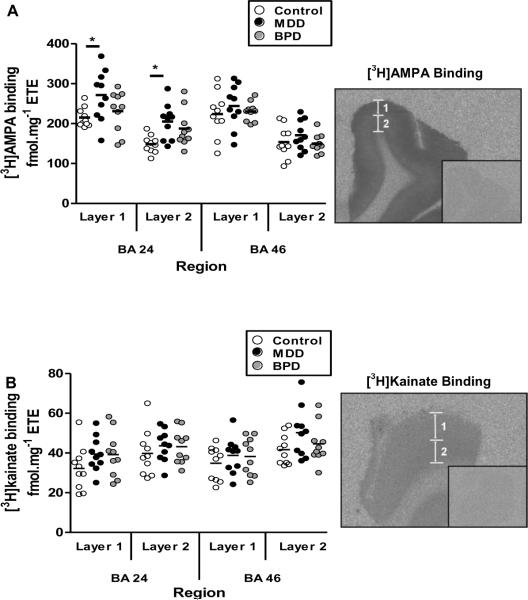
A 20.7% to 27.7% increase in [3H]AMPA binding density was seen in BA 24 (p < 0.05) but not BA 46 (p > 0.05) in major depressive disorder compared to control levels. [3H]AMPA binding density was not changed in bipolar disorder in either BA 24 or BA 46 (p > 0.05) compared to controls. [3H]Kainate binding was not changed in either BA 24 or BA 46 in either disorder compared to controls (p > 0.05).
Limitations
Small sample sizes (n = 10) were used in this study. The subjects were not drug naïve.
Conclusions
Our data suggests increased in AMPA receptor levels in the anterior cingulate are involved in the pathophysiology of major depressive disorder. This data has relevance for the development of new anti-depressant drugs targeted towards the AMPA receptors.
Keywords: AMPA Receptor, Kainate Receptor, Major Depressive Disorder, Bipolar Disorder Frontal Cortex
Introduction
Increased levels of glutamate, the major cortical excitatory neurotransmitter (McCormick, 1992; Tsumoto, 1990), in the blood of patients with major depressive disorder (MDD) suggest the glutamatergic system is involved in the pathophysiology of mood disorders (Mauri et al., 1998). More recently it has been suggested that there is a positive correlation between plasma levels of glutamate and the severity of depressive symptoms (Mitani et al., 2006). Furthermore, increased glutamate levels have been reported in the premotor cortex of post-mortem subjects with MDD and bipolar disorder (BPD) (Hashimoto et al., 2007), whilst brain imaging studies report reduced glutamate levels in the anterior cingulate of subjects with MDD during depressive episodes (Auer et al., 2000).
Several studies suggest a role for the N-methyl-D-aspartate receptor (NMDAR) in the pathophysiology of mood disorders (see Javitt, 2004; Petrie et al., 2000; Skolnick et al., 2009 for reviews). However, there appears to be little data on the possible involvement of the α-amino-3-hydroxy-5-methyl-4-isoxazoleprorionate receptor (AMPAR) and kainate receptor in the pathophysiology of mood disorders. This is a significant lack of knowledge given that animal studies suggest the AMPAR works in concert with NMDAR on the post-synaptic bouton to mediate glutamate's effect on mood (Maeng et al., 2008). Significantly, AMPAR potentiators have been shown to reduce immobility in rodent, behavioural despair models of depression (Li et al., 2001; Li et al., 2003). Notably, the kainate receptor is thought to regulate glutamate release (Jouhanneau et al., 2011). Thus, abnormal kainate receptor expression could have a considerable impact on glutamatergic neurotransmission. Post-mortem studies have reported decreased [3H]kainate binding in the dorsolateral prefrontal cortex and hippocampus from subjects with schizophrenia (Kerwin et al., 1988; Scarr et al., 2005). Comparable data on [3H]kainate binding in the cortex of subjects with mood disorders is not available. However, mRNA expression of the kainate receptor Glu-R5 subunit is decreased in the prefrontal cortex from subjects with MDD and BPD (Knable et al., 2001).
Given the paucity of data on AMPAR and kainate receptors in the CNS of subjects with mood disorders, we sought to measure the binding of AMPAR and kainate receptor-selective radioligands in post-mortem CNS from subjects with MDD and BPD. Binding levels were measured in the dorsolateral prefrontal cortex (DLPFC) (Brodmanns Area [BA] 46) and the anterior cingulate (ACC) (BA 24), two regions high-lighted by neuroimaging studies as being affected in mood disorders (Soares and Mann, 1997).
Methods
Tissue collection
Post-mortem BA 24 and BA 46 tissue from 10 subjects with MDD, 10 subjects with BPD and 10 subjects with no history of psychiatric illness (controls) was obtained from the Victorian Brain Bank Network following approval from the Ethics Committee of the Victorian Institute of Forensic Medicine and the Mental Health Research and Ethics Committee of Melbourne Health. MDD and BPD were diagnosed according to DSM-IV criteria (Hill et al., 1996; Roberts et al., 1998) following a case history review using the Diagnostic Instrument for Brain Studies (DIBS) (Keks et al., 1999). The age, gender, post-mortem interval (PMI), CNS pH and duration of illness (DOI) of the subjects is outlined in Table 1. Cadavers were refrigerated within 5 hr and tissue was frozen to −70°C within 30 min of autopsy. Postmortem interval (PMI) was taken as the time between death and autopsy. Where death was not witnessed, tissue was only collected from subjects who had been seen alive up to 5 hr prior to being found dead. In such cases, PMI was measured from the midpoint between the subject being found and being last seen alive. The pH of the CNS was measured as described previously (Kingsbury et al., 1995).
In Situ Radioligand Binding with Autoradiography
All experiments were performed blind to diagnosis. 5× 20μm frozen sections were cut from BA 24 and BA 46 of each subject and mounted on to gelatinised slides. Single point saturation measurements were used to measure [3H]AMPA and [3H]kainate binding, as previously described (Scarr et al., 2003; Scarr et al., 2005), whereby radioligand binding was measured at a concentration of 3× the Kd of the radioligand for the receptor of interest, as determined in control cortical tissue, to ensure saturation of the receptor binding sites.
To measure [3H]AMPA binding, sections were pre-incubated in 50mM TRIS-HCl, 2.5mM CaCl2, 0.1M KCSN (pH7.4) (buffer 1) for 30 min, rinsed in water and dried. [3H]AMPA binding was performed by incubating 3 sections/subject in 1μM [3H]AMPA (PerkinElmer, Waltham, MA, USA) in buffer 1. Non-specific binding was measured in 2 sections/subject by displacing 1μM [3H]AMPA with 100μM quisqualic acid (Sigma-Aldrich, St. Louis, MO, USA). The sections were incubated for 45 min at 4°C and washed thrice for 15 sec in cold buffer 1.
To measure [3H]kainate binding, sections were pre-incubated in 50mM Tris-acetate (pH7.4) (buffer 2) for 30 min at 4°C, rinsed and dried. 3 sections/subject were incubated in 40nM [3H]kainate (PerkinElmer, Waltham, MA, USA) in buffer 2. Non-specific binding was measured in 2 sections/subject by displacing 40nM [3H]kainic acid with 1mM L-glutamic acid HCl (Sigma-Aldrich, St. Louis, MO, USA). Sections were incubated for 1 hr at 4°C and then washed twice for 2 min in buffer 2. Following the buffer washes, all section were rinsed in water, dried and partially fixed overnight in paraformaldehyde vapour.
The fixed slides were apposed to BAS-TR2025 plates (Fujifilm, Tokyo, Japan) with autoradiographic [3H]microscales™ (Amersham Biosciences, Little Chalfont, UK) for 14 days ([3H]AMPA) and 10 days ([3H]kainate). The plates were scanned in a BAS5000 high resolution phosphoimager (Fujifilm, Tokyo, Japan) and the resulting images analysed using AIS imaging software (Imaging Research, St. Catharines, ON, Canada). The distribution of the binding of each radioligand in each cortical region was carefully assessed, both visually and by measuring changes in the signal intensity along a transept of the cortex and generating a binding density profile across the grey matter, to determine whether binding was restricted to distinct layers within the grey matter. Radioligand binding was measured as an integrated measurement of signal intensity within these layers. Signal intensities were calibrated against the microscales and expressed as the average amount of total bound radioligand/estimated tissue equivalent subtracted from the average non-specific binding for each subject.
Following autoradiography, representative sections were fixed in 10% paraformaldehyde for 45 mins and stained with cresyl violet (0.1% cresyl violet acetate for 25 min and destained in dH20) to localise the layers of binding within the grey matter to cortical laminae.
Statistics
The data was first analysed by the D'Agostino & Pearson omnibus normality test to determine whether they followed a Gaussian distribution. Pearson product moment tests were used to identify relationships between the radioligand binding levels and age, PMI, CNS pH and duration of illness that may have biased our data. R2 > 0.70 was taken as indicative of a strong relationship as this value has been shown to be appropriate for small sample sizes (Gliner et al., 2002). 2-way ANOVA was used to determine whether binding density varied with gender or the incidence of suicide. The impact of any confounding factor that strongly influenced radioligand binding density was further investigated by covariant analysis. 2-way ANOVA followed by Bonferroni post-hoc analyses were used to analyse data with a Gaussian distribution. Where the distribution was non-Gaussian appropriate non-parametric tests were used. Statistical significance was accepted at p < 0.05. Analyses were conducted using Prism 5.01 (Graphpad Software, La Jolla, CA, USA) software.
Results
In both BA 24 and BA 46, [3H]AMPA and [3H]kainate binding were localised to two layers (1 and 2) of differing binding densities within the grey matter. Radioligand binding layer 1 encompassed cortical laminae I-III whilst layer 2 overlayed laminae IV–VI. These binding patterns are consistent with previous findings in the ACC, visual and entorhinal cortices (Palomero-Gallagher et al., 2009; Zilles et al., 2004).
All binding data sets followed a Gaussian distribution (3.68 > K2 > 0.13; P > 0.05). There was no evidence of a strong relationship between the levels of [3H]AMPA or [3H]kainate binding and age, CNS pH, PMI or duration of illness in any diagnostic group ([3H]AMPA: [upper and lower values across all parameters] 0.63 > R2 > 0.01, 0.96 > P > 0.01 ; [3H]kainate: 0.44 > R2 > 0.00, 0.89 > P > 0.04). Binding levels were also unaffected by gender ([3H]AMPA: F = 1.64; df = 1, 112; P = 0.20; [3H]kainate: F = 0.96; df = 1, 112; P = 0.33) or incidence of suicide ([3H]AMPA: F = 0.89; df = 1, 112; P = 0.34; [3H]kainate: F = 0.48; df = 1, 112; P = 0.49)
Analysis of [3H]AMPA binding in MDD, BPD compared to controls revealed a significant effect of diagnosis (F = 9.01; df = 2, 119; P < 0.001). Post-hoc analysis showed that this effect resulted from an increase in [3H]AMPA binding to both layers of BA 24 from subjects with MDD (Layer 1 = 20.7% increase; t = 2.93, p < 0.05: Layer 2 = 27.7% increase; t = 2.96, p < 0.05) compared to controls. [3H]AMPA binding was not different in BA 24 from subjects with BPD and there was no change in the binding of either radioligand in BA46 from subjects with BPD and MDD (2.02 > t > 0.20, p > 0.05) (Figure 1A).
Figure 1.
The binding densities of (A) [3H]AMPA and (B) [3H]kainate in BA 24 and BA 46 from subjects with major depressive disorder (MDD) and bipolar disorder (BPD) compared to controls. Autoradiograms show the total and non-specific (inset) binding for both radioligands. Two discrete layers (layer 1 and layer 2) of radioligand binding were seen in both regions. Each layer was analysed separately. * = P<0.05.
There was a significant effect of diagnosis on the density of [3H]kainate binding in the two cortical regions examined (F = 5.31; df = 2, 119; P = 0.006). However, despite a trend towards increased binding in MDD and BPD in both regions, post-hoc analysis failed to resolve any significant differences in radioligand binding between diagnoses within BA 24 or BA 46 (BA 24, Layer 1: 1.71 > t > 0.37, P > 0.05; Layer 2: 2.45 > t > 0.1, P > 0.05; BA 46, Layer 1: 2.61 > t > 0.37, P > 0.05; Layer 2: 2.61 > t > 0.15, P > 0.05) (Figure 1B).
There was significant variation in [3H]AMPA (F = 31.84, df = 3, 119; p < 0.001) and [3H]kainate (F = 6.05, df = 3, 108; p = 0.001) binding levels between regions and layers. However, there was no significant interaction between regional variation and diagnostic variation in radioligand binding densities ([3H]AMPA: F = 0.86, df = 6, 119, P = 0.53; [3H]kainate: F= 0.27, df= 6, 119, P= 0.95).
Discussion
This study has shown a 20.7%–27.7% increase in [3H]AMPA binding in BA 24, but not BA 46, from subjects with MDD. Increased AMPAR in MDD appears to be regionally specific with previous studies failing to show any change in [3H]AMPA binding in the hippocampus or the entorhinal and perirhinal cortices (Beneyto et al., 2007). There were no differences in [3H]AMPA binding in subjects with BPD compared to controls. These data, plus data from the hippocampus showing unchanged [3H]AMPA binding levels in BPD (Scarr et al., 2003), suggest that widespread changes in AMPAR do not occur in the CNS from subjects with mood disorders. This argument is supported by the finding that levels of AMPAR subunit transcript mRNA was not changed in the DLPFC from subjects with mood disorders (O'Connor et al., 2007).
The MDD and BPD subjects used in this study had been treated with a milieu of antidepressants and mood stabilizers prior to death (see table 1) and there remains a possibility that our data may have been impacted by a drug effect. Both acute and chronic treatment of rats with the antidepressants paroxetine and desipramine does not alter AMPAR protein expression in either membrane fractions or total protein isolates from the frontal cortex (Martinez-Turrillas et al., 2002). However, fluoxetine has been shown to alter the phosphorylation state of the AMPAR subunits (Svenningsson et al., 2002) and this might affect the binding affinity of [3H]AMPA for the AMPAR without affecting expression levels. While we expect our methodology, which measured [3H]AMPA binding at 3× its Kd for the AMPAR, is sufficiently robust to be unaffected by changes in binding affinity, we cannot exclude the possibility that case medication history could have affected our binding data.
Table 1.
Demographic, CNS collection and toxicological data on subjects with MDD, BPD and age / sex matched controls used in this study. Drugs in brackets were prescribed within a month of death but not detected in the blood at autopsy.
| Case | Diagnosis | Gender | Age | PMI | pH | DOI | Cause of death | Antidepressants | Dose (mg/L) | Mood stabilizers | Dose (mg/L) | Other | Dose (mg/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Control | Male | 42 | 30.5 | 6.45 | Ischemic heart disease | |||||||
| 2 | Control | Male | 52 | 33.75 | 6.52 | Cardiomegaly; IHD/CAD | |||||||
| 3 | Control | Female | 47 | 24 | 5.89 | Pulmonary embolus | |||||||
| 4 | Control | Female | 75 | 53 | 6.01 | Multiple organ failure; Septicaemia | |||||||
| 5 | Control | Male | 70 | 59 | 6.11 | RV Rupture following pericardial drainage | |||||||
| 6 | Control | Male | 75 | 69.4 | 6.19 | Cardiogenic shock | |||||||
| 7 | Control | Male | 55 | 30.5 | 6.69 | Coronary artery atherosclerosis | |||||||
| 8 | Control | Male | 66 | 71.75 | 6.47 | Coronary artery atheroma | |||||||
| 9 | Control | Female | 80 | 55 | 6.28 | Ischemic heart disease | |||||||
| 10 | Control | Female | 63 | 50.25 | 6.55 | Coronary artery atherosclerosis | |||||||
|
| |||||||||||||
| 11 | MDD | Male | 37 | 57.75 | 6.84 | 14 | Hanging | Sertraline | 0.2 | Ethanol | Detected | ||
| 12 | MDD | Male | 51 | 41 | 6.71 | 15 | Hanging | Clomipramine Desmethyclomipramine |
0.3 Detected |
||||
| 13 | MDD | Female | 50 | 50.5 | 6.85 | 40 | Mixed drug toxicity | Diazepmam Nordiazepam Codeine Paracetamol Propoxyphene Norpropoxyphene Metoclopramide Verapamil Zolpidem Tramadol |
~0.8 ~1.5 0.1 ~220 7.4 27 ~53 ~3.5 ~0.1 ~15.2 |
||||
| 14 | MDD | Female | 77 | 16.7 | 6.49 | 26 | Doxepin toxicity | Doxepin | 5.4 | ||||
| 15 | MDD | Male | 69 | 44.5 | 6.45 | 20 | Drowning | Diphenhydramine | ~4 | ||||
| 16 | MDD | Male | 79 | 24 | 6.32 | 17 | CO poisoning | Nortriptyline | ~0.7 | Nordiazepam Paracetamol |
~0.1 ~1000 |
||
| 17 | MDD | Male | 55 | 47.75 | 6.60 | 3 | Hanging | Venlafaxine | ~0.2 | ||||
| 18 | MDD | Male | 68 | 60.75 | 6.65 | 9 | Hanging | Citalopram Mianaserin |
Trace ~0.1 |
Temazepam | ~0.2 | ||
| 19 | MDD | Female | 87 | 24.5 | 6.44 | 7 | Chest infection | (Setraline) | |||||
| 20 | MDD | Female | 51 | 23.5 | 6.49 | 25 | Toxicity to Quetiapine | (Mirtazapine) | Diazepam Nordiazepam Quetiapine |
100 ~0.4 ~0.3 |
|||
|
| |||||||||||||
| 21 | BPD | Male | 38 | 24 | 6.42 | 8 | CO poisoning | Ethanol Codeine Paracetamol Doxylamine |
Detected 0.3 46 0.2 |
||||
| 22 | BPD | Male | 59 | 34 | 6.46 | 24 | Ruptured aorta | ||||||
| 23 | BPD | Female | 42 | 25 | 6.54 | 20 | Hanging | Venlafaxine | ~0.5 | (Lithium) | Ethanol Diazepam |
Detected ~0.1 |
|
| 24 | BPD | Female | 64 | 26 | 6.46 | 8 | IHD/CAD | (Venlafaxine) | (Na Valproate) | ||||
| 25 | BPD | Male | 66 | 17 | 6.41 | 12 | Aspirated on food | ||||||
| 26 | BPD | Male | 79 | 8.25 | 6.09 | 17 | Cholecystitis in association with cardiomegaly | Na Valproate | 16 | Zolpidem | 0.2mg | ||
| 27 | BPD | Male | 59 | 37.5 | 5.97 | 8 | Ischemic heart disease; Coronary artery atherosclerosis | (Lithium) | |||||
| 28 | BPD | Male | 61 | 58 | 6.44 | 40 | Acute myocardial infarction | (Na Valproate) | |||||
| 29 | BPD | Female | 74 | 45 | 6.26 | 35 | Combined drug toxicity | Amitriptyline Nortriptyline Dothiepin |
4.9 3.1 4.0 |
Na Valproate | 10 | ||
| 30 | BPD | Female | 56 | 86 | 6.43 | N/A | CO poisoning | Venlafaxine | ~0.6 | Carbamazepine | ~1.8 | Ethanol Paracetamol |
Detected Trace |
Neuroimaging studies have shown that the ACC is involved in depressed mood (Bench et al., 1992). Therefore, the increased [3H]AMPA binding seen in BA 24 may be associated with depressive symptoms in MDD. This is supported by studies reporting that treating mice with the AMPAR potentiator LY392098 improves performance on the forced swim test and tail suspension test models of depression (Li et al., 2001; Li et al., 2003), whilst pretreating ketamine-treated rats with the AMPAR antagonist NBQX abolishes the improved performance on the learned helplessness test and forced swim test that results from ketamine treatment alone (Maeng et al., 2008). Knockout mice lacking the AMPA subunit Glu-R1 gene also display poor performance on the learned helplessness model of depression (Chourbaji et al., 2008), suggesting increased AMPAR signalling may be associated with reduced depression. Elevated AMPAR expression in BA 24, suggested by our data, is likely to increase AMPAR mediated signalling. Therefore, increased AMPAR could be a compensatory response to the molecular changes that result in depression rather than a cause of the illness.
[3H]kainate binding density was not altered in either BA 24 or BA 46 from subjects with MDD and BPD compared to controls. These data add to previous reports that [3H]kainate binding is unchanged in BA 9 of the dorsolateral prefrontal cortex (Dean et al., 2001) or the hippocampus (Scarr et al., 2003) from subjects with BPD. However, mRNA expression of the kainate receptor Glu-R5 subunit is decreased the prefrontal cortex from individuals with MDD and BPD (Knable et al., 2001). Thus, while kainate receptor protein expression appears to be unchanged in mood disorders, the subunit ratio of the kainate receptor tetramer may vary between mood disorders and healthy controls, potentially affecting the kainate signalling.
This study supports a role for the AMPAR in the pathophysiology of MDD and adds to other findings showing other components of the glutamatergic signalling system are altered in mood disorders (e.g. NMDAR (Feyissa et al., 2009), the metabotropic glutamate receptors (Feyissa et al. 2010), the glutamate transporters (Choudary et al., 2005) and glutamate itself (Auer et al., 2000)). Thus, there is a need to continue to increase our understanding of how the glutamatergic synapse is affected by the pathophysiology of mood disorders as a foundation to further probing potential glutamatergic based drug targets as potential new treatment sites.
Acknowledgements
The authors gratefully acknowledge the assistance of Geoffrey Pavey for the preparation of post-mortem tissue and David Copolov, Christine Hill, Nicholas Keks and Kenneth Opeskin for their roles in tissue collection and diagnostic confirmation.
Role of Funding Source The study was supported by Operational Infrastructure Support (OIS) from the Victorian State Government and by the funding grants; NIH RO1 MH069696-01 and NHMRC project grant 3503441. Brian Dean is a NHMRC Senior Research Follow (APP1002240). Elizabeth Scarr is a Royce Abbey Postdoctoral Fellow (Australian Rotary Health Research Fund). The funding sources were not involved in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest There were no conflicts of interest associated with this study.
Contributors Andrew Gibbons was involved in the design of the study statistical analysis of the data and oversaw the preparation of the manuscript. Lucy Brooks was involved in collecting the data and the statistical analysis of the data. Elizabeth Scarr was involved in the design of the study. Brian Dean was involved in the design of the study and heads the laboratory in which the study took place. All authors have made a significant contribution to the preparation of and have approved the manuscript.
References
- Auer DP, Putz B, Kraft E, Lipinski B, Schill J, Holsboer F. Reduced glutamate in the anterior cingulate cortex in depression: An in vivo proton magnetic resonance spectroscopy study. Biol. Psychiatry. 2000;47:305–313. doi: 10.1016/s0006-3223(99)00159-6. [DOI] [PubMed] [Google Scholar]
- Bench CJ, Friston KJ, Brown RG, Scott LC, Frackowiak RSJ, Dolan RJ. The anatomy of melancholia - focal abnormalities of cerebral blood-flow in major depression. Psychol. Med. 1992;22:607–615. doi: 10.1017/s003329170003806x. [DOI] [PubMed] [Google Scholar]
- Beneyto M, Kristiansen LV, Oni-Orisan A, McCullumsmith RE, Meador-Woodruff JH. Abnormal glutamate receptor expression in the medial temporal lobe in schizophrenia and mood disorders. Neuropsychopharmacology. 2007;32:1888–1902. doi: 10.1038/sj.npp.1301312. [DOI] [PubMed] [Google Scholar]
- Choudary PV, Molnar M, Evans SJ, Tomita H, Li JZ, Vawter MP, Myers RM, Bunney WE, Jr., Akil H, Watson SJ, Jones EG. Altered cortical glutamatergic and GABAergic signal transmission with glial involvement in depression. Proc. Natl. Acad. Sci. U.S.A. 2005;102:15653–15658. doi: 10.1073/pnas.0507901102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chourbaji S, Vogt MA, Fumagalli F, Sohr R, Frasca A, Brandwein C, Hortnagl H, Riva MA, Sprengel R, Gass P. AMPA receptor subunit 1 (GluR-A) knockout mice model the glutamate hypothesis of depression. Faseb J. 2008;22:3129–3134. doi: 10.1096/fj.08-106450. [DOI] [PubMed] [Google Scholar]
- Dean B, Pavey G, McLeod M, Opeskin K, Keks N, Copolov D. A change in the density of [H-3]flumazenil, but not [H-3]muscimol binding, in Brodmann's Area 9 from subjects with bipolar disorder. J. Affect. Disord. 2001;66:147–158. doi: 10.1016/s0165-0327(00)00294-9. [DOI] [PubMed] [Google Scholar]
- Feyissa AM, Chandran A, Stockmeier CA, Karolewicz B. Reduced levels of NR2A and NR2B subunits of NMDA receptor and PSD-95 in the prefrontal cortex in major depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2009;33:70–75. doi: 10.1016/j.pnpbp.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feyissa AM, Woolverton WL, Miguel-Hidalgo JJ, Wang ZX, Kyle PB, Hasler G, Stockmeier CA, Iyo AH, Karolewicz B. Elevated level of metabotropic glutamate receptor 2/3 in the prefrontal cortex in major depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2010;34:279–283. doi: 10.1016/j.pnpbp.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gliner JA, Morgan GA, Harmon RJ. Basic associated designs: analysis and interpretation. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41:1256–1258. doi: 10.1097/00004583-200210000-00017. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Sawa A, Iyo M. Increased levels of glutamate in brains from patients with mood disorders. Biol. Psychiatry. 2007;62:1310–1316. doi: 10.1016/j.biopsych.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Hill C, Keks N, Roberts S, Opeskin K, Dean B, MacKinnon A, Copolov D. Problem of diagnosis in postmortem brain studies of schizophrenia. Am. J. Psychiat. 1996;153:533–537. doi: 10.1176/ajp.153.4.533. [DOI] [PubMed] [Google Scholar]
- Javitt DC. Glutamate as a therapeutic target in psychiatric disorders. Mol. Psychiatr. 2004;9:984–997. doi: 10.1038/sj.mp.4001551. [DOI] [PubMed] [Google Scholar]
- Jouhanneau JS, Ball SM, Molnar E, Isaac JTR. Mechanisms of bidirectional modulation of thalamocortical transmission in barrel cortex by presynaptic kainate receptors. Neuropharmacology. 2011;60:832–841. doi: 10.1016/j.neuropharm.2010.12.023. [DOI] [PubMed] [Google Scholar]
- Keks N, Hill C, Opeskin K, Copolov DL, Dean B. Psychiatric diagnosis after death: the problems of accurate diagnosis. In: Dean B, Hyde TM, Klienman JE, editors. Using CNS Tissue in Psychiatry Research: a Practical Guide. J. Gordon & Breach Science Publishers; Sydney: 1999. pp. 19–37. [Google Scholar]
- Kerwin RW, Patel S, Meldrum BS, Czudek C, Reynolds GP. Asymmetrical loss of glutamate receptor subtype in left hippocampus in schizophrenia. Lancet. 1988;1:583–584. doi: 10.1016/s0140-6736(88)91371-2. [DOI] [PubMed] [Google Scholar]
- Kingsbury AE, Foster OJ, Nisbet AP, Cairns N, Bray L, Eve DJ, Lees AJ, Marsden CD. Tissue pH as an indicator of mRNA preservation in human post-mortem brain. Brain Res. Mol. Brain Res. 1995;28:311–318. doi: 10.1016/0169-328x(94)00219-5. [DOI] [PubMed] [Google Scholar]
- Knable MB, Torrey EF, Webster MJ, Bartko JJ. Multivariate analysis of prefrontal cortical data from the Stanley Foundation Neuropathology Consortium. Brain Res. Bull. 2001;55:651–659. doi: 10.1016/s0361-9230(01)00521-4. [DOI] [PubMed] [Google Scholar]
- Li X, Tizzano JP, Griffey K, Clay M, Lindstrom T, Skolnick P. Antidepressant-like actions of an AMPA receptor potentiator (LY392098) Neuropharmacology. 2001;40:1028–1033. doi: 10.1016/s0028-3908(00)00194-5. [DOI] [PubMed] [Google Scholar]
- Li X, Witkin JM, Need AB, Skolnick P. Enhancement of antidepressant potency by a potentiator of AMPA receptors. Cell. Mol. Neurobiol. 2003;23:419–430. doi: 10.1023/A:1023648923447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng S, Zarate CA, Du J, Schloesser RJ, McCammon J, Chen G, Manji HK. Cellular mechanisms underlying the antidepressant effects of ketamine: Role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol. Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Martinez-Turrillas R, Frechilla D, Del Rio J. Chronic antidepressant treatment increases the membrane expression of AMPA receptors in rat hippocampus. Neuropharmacology. 2002;43:1230–1237. doi: 10.1016/s0028-3908(02)00299-x. [DOI] [PubMed] [Google Scholar]
- Mauri MC, Ferrara A, Boscati L, Bravin S, Zamberlan F, Alecci M, Invernizzi G. Plasma and platelet amino acid concentrations in patients affected by major depression and under fluvoxamine treatment. Neuropsychobiology. 1998;37:124–129. doi: 10.1159/000026491. [DOI] [PubMed] [Google Scholar]
- McCormick DA. Neurotransmitter actions in the thalamus and cerebral-cortex and their role in neuromodulation of thalamocortical activity. Prog. Neurobiol. 1992;39:337–388. doi: 10.1016/0301-0082(92)90012-4. [DOI] [PubMed] [Google Scholar]
- Mitani H, Shirayama Y, Yamada T, Maeda K, Ashby CR, Kawahara R. Correlation between plasma levels of glutamate, alanine and serine with severity of depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2006;30:1155–1158. doi: 10.1016/j.pnpbp.2006.03.036. [DOI] [PubMed] [Google Scholar]
- O'Connor JA, Muly EC, Arnold SE, Hemby SE. AMPA receptor subunit and splice variant expression in the DLPFC of schizophrenic subjects and rhesus monkeys chronically administered antipsychotic drugs. Schizophr. Res. 2007;90:28–40. doi: 10.1016/j.schres.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomero-Gallagher N, Vogt BA, Schleicher A, Mayberg HS, Zilles K. Receptor Architecture of Human Cingulate Cortex: Evaluation of the Four-Region Neurobiological Model. Hum. Brain Mapp. 2009;30:2336–2355. doi: 10.1002/hbm.20667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie RXA, Reid IC, Stewart CA. The N-methyl-D-aspartate receptor, synaptic plasticity, and depressive disorder - A critical review. Pharmacol. Ther. 2000;87:11–25. doi: 10.1016/s0163-7258(00)00063-2. [DOI] [PubMed] [Google Scholar]
- Roberts SB, Hill CA, Dean B, Keks NA, Opeskin K, Copolov DL. Confirmation of the diagnosis of schizophrenia after death using DSM-IV: a Victorian experience. Aust. N. Z. J. Psych. 1998;32:73–76. doi: 10.3109/00048679809062709. [DOI] [PubMed] [Google Scholar]
- Scarr E, Beneyto M, Meador-Woodruff JH, Dean B. Cortical glutamatergic markers in schizophrenia. Neuropsychopharmacology. 2005;30:1521–1531. doi: 10.1038/sj.npp.1300758. [DOI] [PubMed] [Google Scholar]
- Scarr E, Pavey G, Sundram S, MacKinnon A, Dean B. Decreased hippocampal NMDA, but not kainate or AMPA receptors in bipolar disorder. Bipolar Disord. 2003;5:257–264. doi: 10.1034/j.1399-5618.2003.00024.x. [DOI] [PubMed] [Google Scholar]
- Skolnick P, Popik P, Trullas R. Glutamate-based antidepressants: 20 years on. Trends Pharmacol. Sci. 2009;30:563–569. doi: 10.1016/j.tips.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Soares JC, Mann JJ. The functional neuroanatomy of mood disorders. J. Psychiatr. Res. 1997;31:393–432. doi: 10.1016/s0022-3956(97)00016-2. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Tzavara ET, Witkin JM, Fienberg AA, Nomikos GG, Greengard P. Involvement of striatal and extrastriatal DARPP-32 in biochemical and behavioral effects of fluoxetine (Prozac) Proc. Natl. Acad. Sci. U. S. A. 2002;99:3182–3187. doi: 10.1073/pnas.052712799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsumoto T. Excitatory amino-acid transmitters and their receptors in neural circuits of the cerebral neocortex. Neurosci. Res. 1990;9:79–102. doi: 10.1016/0168-0102(90)90025-a. [DOI] [PubMed] [Google Scholar]
- Zilles K, Palomero-Gallagher N, Schleicher A. Transmitter receptors and functional anatomy of the cerebral cortex. J. Anat. 2004;205:417–432. doi: 10.1111/j.0021-8782.2004.00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]