Abstract
Aging is associated with low-grade neuroinflammation including primed microglia that may contribute to deficits in neural plasticity and cognitive function. The current study evaluated whether exercise modulates division and/or activation state of microglia in the dentate gyrus of the hippocampus, as activated microglia can express a classic inflammatory or an alternative neuroprotective phenotype. We also assessed hippocampal neurogenesis to determine whether changes in microglia were associated with new neuron survival. Adult (3.5 months) and aged (18 months) male BALB/c mice were individually housed with or without running wheels for 8 weeks. Mice received bromodeoxyuridine injections during the first or last ten days of the experiment to label dividing cells. Immunofluorescence was conducted to measure microglia division, co-expression of the neuroprotective indicator insulin-like growth factor (IGF-1), and new neuron survival. The proportion of new microglia was increased in aged mice, and decreased from wheel running. Running increased the proportion of microglia expressing IGF-1 suggesting exercise shifts microglia phenotype towards neuroprotection. Additionally, running enhanced survival of new neurons in both age groups. Findings suggest that wheel running may attenuate microglia division and promote a proneurogenic phenotype in aged mice.
Keywords: Exercise, proliferation, hippocampus, neurogenesis, insulin-like growth factor, IGF-1, aging, microglia
INTRODUCTION
Microglia are a highly plastic group of immune cells that reside in the central nervous system (CNS). In the absence of an infection or injury microglia maintain a resting phenotype, in which the cells survey the environment for potential threats. If a threat is detected, microglia undergo morphological changes, proliferate, and begin to release cytoactive agents including proinflammatory cytokines [e.g., interleukin-1β (IL-1β) and IL-6] (Colton, 2009). The expression of this classic inflammatory phenotype facilitates recovery from an insult. Microglia can also express a neuroprotective “alternative” phenotype that participates in regenerative processes, cell growth, and inhibition of inflammation (Colton, 2009). In their alternative state, microglia can produce growth factors, such as insulin-like growth factor (IGF-1) and brain derived neural growth factor (BDNF) (Nakajima, et al., 2002, Wine, et al., 2009), as well as anti-inflammatory cytokines. Signals from the environment likely determine whether microglia remain in a resting state or undergo activation to either the classic inflammatory or alternative neuroprotective phenotype.
Normal aging alters microglia activity, as microglia in the brain of uninjured aged animals are shifted towards the inflammatory (i.e., classic) phenotype (Dilger and Johnson, 2008). There is a basal increase in proinflammatory cytokine production and increased expression of surface markers (e.g., MHC II and CD86) that are associated with classic microglia activation (Dilger and Johnson, 2008, Frank, et al., 2006, Sierra, et al., 2007). The age-related priming of microglia contributes to a prolonged neuroinflammatory response following an immune challenge that results in exaggerated expression of sickness behaviors and cognitive deficits compared to adult animals (Dilger and Johnson, 2008, Godbout, et al., 2005, Kohman, et al., 2007). Another component of the age-related alterations in microglia is an increase in proliferation. Prior reports have shown that microglia from aged animals show increased proliferation in culture as well as in vivo in the retina (Inman and Horner, 2007, Rozovsky, et al., 1998), but the relevance of increased microglia division to their activation status and age-related changes in neural function has yet to be investigated.
One area of the brain important for cognitive performance and sensitive to aging is the hippocampus. The hippocampus is also of interest because it is one of two areas of the brain that unarguably retains the ability to generate new neurons beyond early development. These new neurons are suggested to participate in learning and memory processes (Clark, et al., 2008, Deng, et al., 2010, Shors, et al., 2002, van Praag, et al., 2005). Microglia play a complex role in regulating hippocampal neurogenesis, as depending on their state of activation they can facilitate or inhibit neurogenesis (Yirmiya and Goshen, 2011). For example, administration of the endotoxin lipopolysaccharide (LPS) reduces hippocampal neurogenesis and this effect is mediated by microglia, as administration of the microglia inhibitor, minocycline, blocks the reduction in neurogenesis (Ehninger, et al., 2011). In contrast, microglia in the resting or alternative state support hippocampal neurogenesis, as microglia participate in new cell migration, increase the number of cells that differentiate into neurons and express molecules (e.g., IGF-1) that can enhance neurogenesis (Aarum, et al., 2003, Trejo, et al., 2008, Walton, et al., 2006, Ziv, et al., 2006).
The age-related changes in microglia and proinflammatory cytokine activity are reported to further reduce hippocampal neurogenesis. For example, Gemma et al. (2007) report that administration of a capase-1 inhibitor, that would decrease conversion of pro-IL-1β to the mature form, significantly increased hippocampal neurogenesis in aged, but not young mice. Additionally, administration of the anti-inflammatory chemokine fractalkine (i.e., CX3CL1 or neurotactin), which helps maintain microglia in a resting state, increased hippocampal neurogenesis in aged, but not young, animals (Bachstetter, et al., 2009). Collectively, these data indicate that attenuating age-related changes in microglia activity may enhance new neuron survival.
One intervention known to improve cognition and health, particularly in aged individuals, is exercise. Additionally, exercise has immunomodulatory effects that have been well characterized within the peripheral nervous system (Woods, et al., 2009). However, the ability of exercise to modulate immune activity within the CNS has not been as thoroughly investigated. The existing literature indicates that exercise has direct effects on microglial cell activity. For example, treadmill running was reported to attenuate the age-dependent increase in microglia activation, as measured by staining intensity of CD11b, in a transgenic mouse model of Alzheimer's disease (Nichol, et al., 2008). A recent study found that wheel running attenuated LPS-induced IL-1β production from microglia isolated from aged rats compared to aged sedentary rats (Barrientos, et al., 2011). Additionally, wheel running prevented E.coli-induced reductions in BDNF expression in the hippocampus following learning, indicating that running offers some protection against negative effects of immune activation (Barrientos, et al., 2011). Further, running has been reported to decrease microglia proliferation in the septum and amygdala of adult mice (Ang, et al., 2004, Ehninger, et al., 2011). Whether exercise modulates microglia proliferation in the aged is unknown.
The objective of the current study was to determine whether normal aging increases microglia division within the hippocampus and whether voluntary wheel running would alter the age-related changes in microglia division. Additionally, we assessed the possibility that exercise increases production of IGF-1 from microglia, which may indicate shifting towards the alternative phenotype. Microglia have been shown to promote regeneration through the release of BDNF and IGF-1 (Nakajima, et al., 2002, Wine, et al., 2009). Lastly, we assessed whether running-induced changes in microglia activity were associated with enhancements in hippocampal neurogenesis.
2. METHODS
2.1. Experimental subjects
Subjects were 32 adult (3.5-month-old) and 31 aged (18-month-old) male BALB/c mice from an in-house aging colony. Mice were given ad libitum access to food and water and housed under a reverse 12 hr light/dark cycle in the AAALAC approved animal facility. Prior to the start of the experiment mice were group housed (3-4 mice per cage), but during the experiment mice were individually housed. Animals were treated in compliance with the Guide for the Care and Use of Laboratory Animals and the experiment was conducted in accordance with a protocol approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Illinois at Urbana-Champaign.
2.2. Experimental design
Mice were divided by age into either the exercise (access to a running wheel) or sedentary condition for 8 weeks. Half of the sedentary and exercise mice received daily intraperitoneal (i.p.) injections of bromodeoxyuridine (BrdU: 50 mg/kg), a thymidine analogue that incorporates into dividing cells, during the initial 10 days (BrdU Early) of the study while the others received i.p. injections of saline. The remaining mice received i.p. BrdU injections during the last ten days (BrdU Late) prior to tissue collection while mice given BrdU earlier received saline injections (all mice had a total of 20 i.p. injections). There was a total of eight treatment groups (Age × Exercise condition × BrdU timing). Mice in the sedentary condition were individually housed in standard polypropylene shoebox cages (29 cm L × 19 cm W × 13 cm H). Mice in the exercise condition were individually housed in cages (36 cm L × 20 cm W × 14 cm H) with a 23 cm diameter running wheel (Respironics, Bend, OR). Its important to note that individually housing animals can affect measures of neuroinflammation as well as hippocampal neurogenesis (Ben Menachem-Zidon, et al., 2008, Ibi, et al., 2008). However, individually housing mice was necessary to prevent competition for access to the running wheel and to monitor running behavior in individual mice, as well as to eliminate the influence of social hierarchy and fighting which can also be a form of stress (Kinsey, et al., 2008). Throughout the 8 weeks, wheel rotations were continuously collected in 1 min intervals via magnetic switches interfaced to a computer using the VitalView software (Respironics, Bend, OR). Sedentary mice were deliberately not housed in cages with locked wheels since mice climb in locked wheels (Koteja, et al., 1999, Rhodes, et al., 2000, Rhodes, et al., 2003) and we wanted to limit physical activity in the sedentary group. Animals were weighed weekly, throughout the experiment.
2.3. Immunohistochemistry
2.3.1. Perfusions
Following the 8 weeks of wheel running or sedentary housing all mice were transcardially perfused with 4% paraformaldehyde. Perfusions occurred 24 hours after the final BrdU injection for mice receiving BrdU during the last 10 days of the study. Brains were fixed overnight in 4% paraformaldehyde and then transferred into 30% sucrose solution. Brains were sectioned at 40 micrometers on a cryostat.
2.3.2. BrdU-DAB
A one-in-six series was stained for BrdU to identify newly divided cells. Briefly, free floating sections were rinsed in tissue buffering solution (TBS) and then treated with 0.6% hydrogen peroxide for 30 min. To denature DNA, sections were placed in a solution of 50% de-ionized formamide for 120 min at 65 °C, followed by 10% 20x SCC buffer for 15 min, then 2 N hydrochloric acid for 30 min at 37 °C, and then 0.1 M boric acid (pH 8.5) for 10 min. Sections were blocked with a solution of 0.3% Triton-X and 3% goat serum in TBS (TBS-X plus) for 30 min, and then incubated with primary antibody rat anti-BrdU (1:200; AbD Serotec, Raleigh, NC, USA) at a dilution of 1:200 in TBS-X plus for 72 h at 4 °C. After washing with TBS, sections were treated with TBS-X plus for 30 min and then incubated with a biotinylated goat anti-rat secondary antibody (1:250) in TBS-X plus for 100 min at room temperature. Sections were then treated with the ABC system (Vector, Burlingame, CA, USA) and stained using a diaminobenzidine kit as the chromogen (DAB; Sigma, St. Louis, MO, USA).
2.3.3. Triple labeled immunofluorescence
Separate one-in-six series were triple-labeled with either rat anti-BrdU (1:200), rabbit anti-Iba-1 (1:500; Wako chemicals, Richmond, VA), and goat anti-IGF-1 (1:50, Santa Cruz Biotechnology, Santa Cruz, CA) or rat anti-BrdU, mouse anti-neuronal nuclear protein (NeuN; 1:50; Millipore, Billerica, MA, USA), and rabbit anti-Iba-1 (1:500). Free-floating sections were handled as described above with the exception of the use of a cocktail of primary antibodies. Fluorescent markers (Cy2, Cy3, and Cy5) were conjugated to secondary antibodies, made in goat or donkey, at a dilution of 1:200 and were also delivered as a cocktail. ABC and DAB steps were omitted.
2.4. Image analysis
2.4.1. Hippocampal Neurogenesis
Number of new neurons per cubic millimeter granule layer per mouse was calculated as number of BrdU positive cells per cubic millimeter granule layer multiplied by the average proportion of BrdU positive cells that co-expressed NeuN for a given experimental group. To obtain these estimates, the entire granule layer (bilateral), from the one-in-six series stained for BrdU-DAB, was imaged by systematically advancing the field of view of a Zeiss brightfield light microscope, and taking multiple photographs, via Axiocam interfaced to a computer, under 10x (total 100x) magnification. Images were analyzed by ImageJ software. For each image, the granule layer was outlined, and BrdU-positive nuclei were automatically counted by setting a fixed threshold to remove the background. The threshold selected was validated by comparing automated counts to hands counts. In addition, the area (pixels) within the trace was recorded. Estimates of total number of BrdU positive cells are expressed per cubic micrometer dentate gyrus sampled. Values were further adjusted by removing the fraction of cells predicted to cross the boundary of the section on one side to produce unbiased estimates. These values were then multiplied by the percentage of BrdU cells expressing NeuN estimated from a separate confocal analysis. A confocal Leica SP2 laser scanning confocal microscope (using a 40X oil objective, pinhole size 81.35 m) was used to determine the average group proportion of BrdU positive cells that differentiated into neurons as indicated by co-expression of NeuN in the granule cell layer of the dentate gyrus. This proportion was based on a minimum of 40 BrdU+ cells per group.
2.4.2. Microglia proliferation and phenotype
A confocal microscope was used to identify microglia (Iba-1+) that divided (Iba-1+ and BrdU+) in the dentate gyrus proper. The percentage of Iba-1+ cells that displayed BrdU was calculated by dividing the number of cells that co-labeled with Iba-1 and BrdU by the number of Iba-1 positive cells analyzed and multiplied by 100. On average 210 Iba-1 positive cells were analyzed within the dentate gyrus per animal. Additionally, we determined the percentage of Iba-1 positive cells in the dentate gyrus that co-labeled with the growth factor IGF-1 by dividing the number of Iba-1 positive cells that displayed IGF-1 by the number of Iba-1 positive cells sampled and multiplied by 100. To determine the proportion of recently divided microglia (Iba-1+ and BrdU +) that displayed IGF-1 we divided the number of triple-labeled cells (Iba-1+, BrdU+, and IGF-1+) by the number of Iba-1 positive cells and multiplied by 100.
2.5. Statistical analysis
Body weight was analyzed by repeated measures ANOVA with Age, Exercise condition, and BrdU timing as the between-subjects variables and Day as the within-subjects (i.e., repeated-measure) variable. Distance run was analyzed by repeated measures ANOVA with Age as the between-subjects variable and Day as the within-subjects variable. BrdU-DAB and the number of new neuron data were analyzed by ANOVA with Age, Exercise condition, and BrdU timing as the between-subjects variables. Neuronal differentiation (proportion of BrdU positive cells expressing NeuN), microglia division (proportion of Iba-positive cells expressing BrdU), and IGF expression (proportion of Iba-positive cells expressing IGF-1) data were analyzed by logistic regression. For these analyses, the deviance is reported in place of the F statistic. An alpha level of p<0.05 was considered statistically significant.
3. RESULTS
3.1. Body weight
As expected, aged mice weighed more than adult mice (F(1,61)=407.88;p<0.0001; data not shown). There was also a significant Exercise condition × Day interaction (F(7,427)=3.35;p<0.01; data not shown), that showed mice with access to running wheels weighed less than sedentary mice during the initial weeks, but did not differ from sedentary mice during the later weeks of the study.
3.2. Wheel running
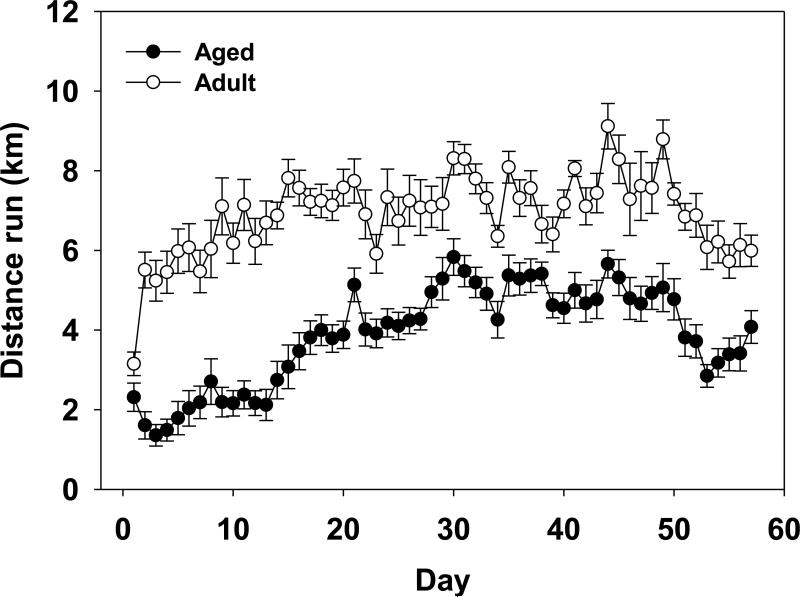
Overall aged mice ran significantly less than adult mice as show by a significant main effect of Age and an Age × Day interaction (F(1,26)=48.36;p<0.0001; F(56,1456)=5.29;p<0.0001, respectively; see Figure 1). Aged mice ran an average of 4.75 km/day and adult mice ran an average of 7.20 km/day. Additionally, there was a significant main effect of Day for distance (km) run per day (F(56,1456)=30.64;p<0.0001). Figure 1 shows that distance run tended to increase daily across both age groups during the first month and then slightly declined the last week.
Figure 1.
Average distance (km) run per day by aged and adult mice over eight weeks of running wheel access. Means ± standard error of the mean (SEM).
3.3. Hippocampal neurogenesis
3.3.1. Neuron differentiation
Table 1 shows the proportion of BrdU+ cells that co-labeled with NeuN within each experimental group. Aged mice had a lower proportion of new cells expressing NeuN compared to adult mice (deviance=67.09, p<0.001). Wheel running increased the proportion of BrdU positive cells that displayed NeuN in all groups except in adults when BrdU was administered the last 10 days of the experiment (BrdU Late). This was reflected in a significant main effect of Exercise condition (deviance=26.13;p<0.001), BrdU timing (deviance=4.32, p<0.05) and Exercise condition × BrdU timing interaction (deviance=4.09,4 p<0.05).
Table 1.
Estimated number of BrdU positive cells in the granular cell layer of the hippocampus per cubic millimeter. The BrdU cell numbers were compared between groups using ANOVA. The proportion of BrdU positive cells that differentiated into new neurons (BrdU+ and NeuN+ cells) was determined by counting a minimum of 40 BrdU positive cells within each of the eight experimental groups and determining the proportion of those cells that co-labeled with NeuN to get an average group proportion. Logistic regression was used to compare the group proportions.
| Experimental group | BrdU positive cells in granule layer × 103/mm3 (SEM) | Percentage of BrdU+ cells displaying NeuN (SEM) |
|---|---|---|
| Adult Runner Early | 12.94 (0.60)* | 89.9 (0.01)* |
| Adult Sedentary Early | 4.18 (0.15) | 77.8 (0.02) |
| Adult Runner Late | 5.35 (0.78) | 73.4 (0.04) |
| Adult Sedentary Late | 3.75 (0.10) | 74.3 (0.04) |
| Aged Runner Early | 1.31 (0.28)+ | 70.6 (0.03)+* |
| Aged Sedentary Early | 1.16 (0.55)+ | 57.0 (0.04)+ |
| Aged Runner Late | 2.43 (0.10)+* | 70.7 (0.07)* |
| Aged Sedentary Late | 1.08 (0.05)+ | 56.3 (0.06)+ |
indicates a significant difference from age-matched sedentary mice.
indicates a significant difference between adult and aged mice within an exercise condition.
3.3.2. Number of new neurons
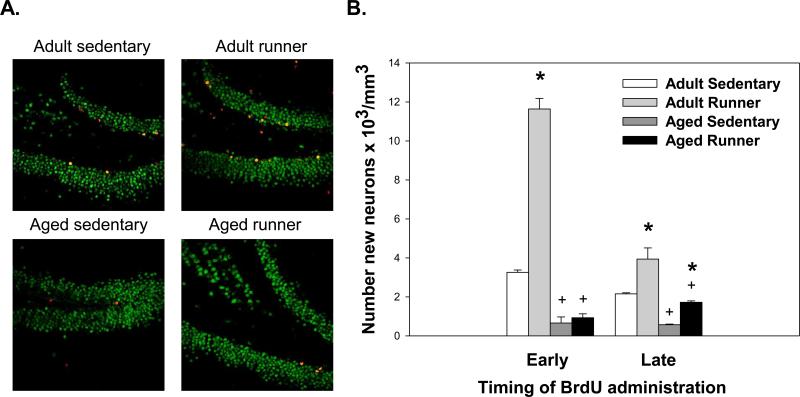
Aged mice showed a reduction in the number of new neurons (i.e., total number of BrdU cells in the granule layer multiplied by the fraction displaying NeuN) as compared to adult mice (F(1,55)=245.77;p<0.0001; see Figure 2). Wheel running significantly increased number of new neurons as compared to sedentary in all groups except aged animals given BrdU injections during the initial 10 days (BrdU Early). This was reflected in a significant effect of Exercise condition (F(1,55)=112.83;p<0.0001), BrdU timing (F(1,55)=55.06;p<0.0001), and three-way interaction between Exercise condition, BrdU timing and Age F(1,55)=46.95;p<0.0001).
Figure 2.
(A) Representative sections triple labeled with antibodies against Iba-1 (macrophage/microglia; blue), NeuN (mature neuron; green), and BrdU (new cell; red) from adult and aged mice injected with BrdU during the first ten days (BrdU Early). (B) Number of new neurons in the granular cell layer of the hippocampus of adult and aged mice housed with or without running wheel access. The number of new neurons was assessed in cells labeled with BrdU during the first (BrdU Early) or last (BrdU Late) ten days of the study. * indicates a significant difference from age-matched sedentary mice. + indicates a significant difference between adult and aged mice within an exercise condition. Means ± SEM.
3.4. Microglia division and phenotype
3.4.1. Microglia division
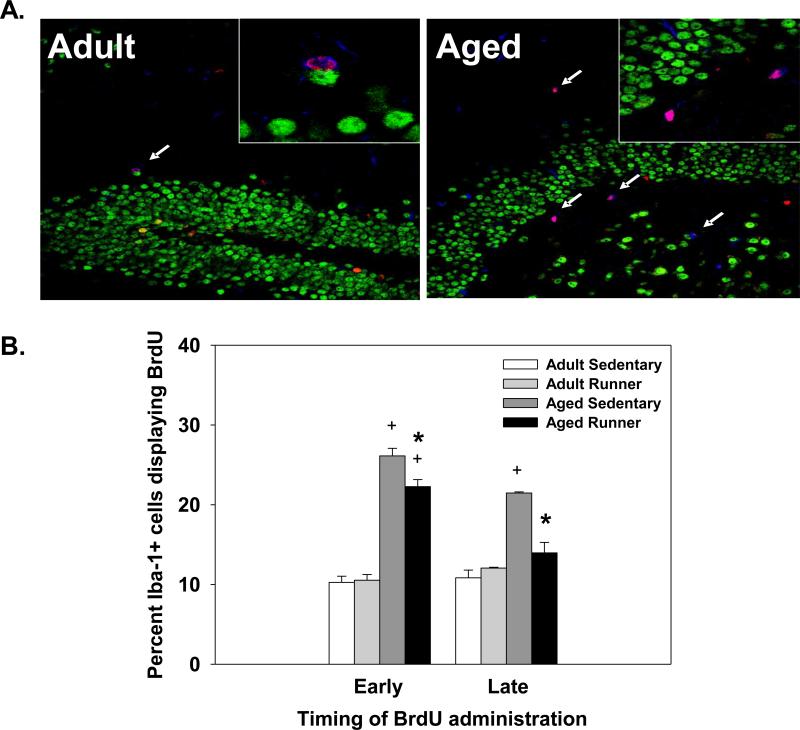
Aged mice had a significantly higher proportion of Iba-1 positive cells that co-labeled with BrdU compared to adult mice. This was reflected in a significant main effect of Age (deviance=286.40, p<0.001; data collapsed across BrdU timing; see Figure 3). Wheel running reduced the proportion of Iba-1 positive cells that expressed BrdU in the aged, but not the adult mice compared to sedentary controls. This was reflected in a significant main effect of Exercise condition and an Age × Exercise interaction (deviance=8.94, p<0.01; deviance=8.53, p<0.01, respectively; see Figure 3).
Figure 3.
(A) Representative hippocampal sections triple labeled with antibodies against Iba-1 (macrophage/microglia; blue), NeuN (mature neuron; green), and BrdU (new cell; red) from adult and aged mice. (B) Average percentage of microglia that underwent division in the dentate gyrus of adult and aged mice. Percentages were calculated by dividing the number of Iba-1 positive cells that expressed BrdU by the number of Iba-1 positive cells and multiplying by 100. * indicates a significant difference from age-matched sedentary mice. + indicates a significant difference between adult and aged mice within an exercise condition. Means ± SEM.
Aged mice given BrdU injections during the initial ten days of wheel access (Early group) showed a greater proportion of Iba-1 positive cells expressing BrdU than aged mice that received BrdU injections during the last ten days of the study (Late group); no difference was observed between the adult mice given BrdU during the initial or last ten days of the study. This was indicated by a significant main effect of BrdU timing and an Age × BrdU timing interaction (deviance=11.43, p<0.001; deviance=17.53, p<0.001, respectively; see Figure 3).
3.4.2. IGF-1 positive microglia
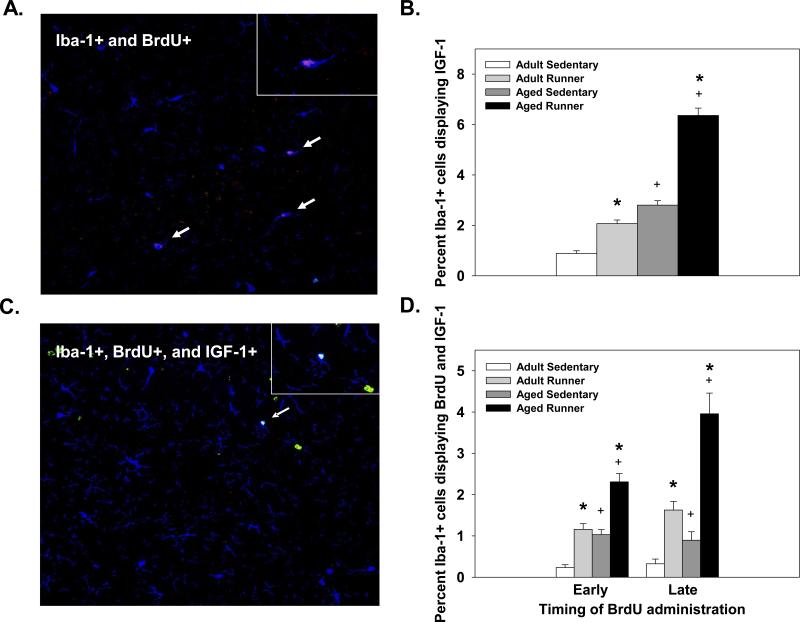
Wheel running significantly increased the proportion of Iba-1 positive cells that co-expressed IGF-1 in both aged and adult mice. This was reflected in significant main effects of Exercise condition (deviance=160.25, p<0.001; see Figure 4B). A significant main of Age (deviance=241.61, p<0.001; see Figure 4B) showed that aged mice had a higher proportion of Iba-1 positive cells that displayed IGF-1 compared to adult mice. The interaction between age and exercise condition was not significant.
Figure 4.
(A) Representative image of a cell co-labeled with antibodies against Iba-1 (macrophage/microglia; blue) and IGF-1 (growth factor; red) from an aged mouse. (B) Average percentage of Iba-1 positive cells that expressed IGF-1 in the dentate gyrus of adult and aged mice. Data are collapsed across BrdU timing. (C) Representative image of a triple-labeled cell with antibodies against Iba-1 (macrophage/microglia; blue), IGF-1 (growth factor; red), and BrdU (new cell; green) from an aged mouse. (D) Average percentage of Iba-1 positive cells that are new (BrdU+) and express IGF-1. * indicates a significant difference from age-matched sedentary mice. + indicates a significant difference between adult and aged mice within an exercise condition. Means ± SEM.
Wheel running significantly increased the proportion of new microglia (Iba-1+ and BrdU+) that expressed IGF-1 compared to sedentary mice. This was reflected in a significant main effect of Exercise condition (deviance=123.84, p<0.001; see Figure 4B). Additionally, aged mice showed a higher proportion of new microglia that displayed IGF-1 than adult mice. This was reflected in a significant main effect of Age (deviance=49.72, p<0.001; see Figure 4B). Runners that received late BrdU injections (last ten days) showed a higher proportion of new microglia that expressed IGF-1 than runners given early BrdU injections (first ten days), which was particularly evident in the aged mice (see Figure 4D). This was reflected in a significant Age × Exercise condition interaction (deviance=5.31, p<0.05) and a significant Exercise condition × BrdU timing interaction (deviance=4.15, p<0.05).
DISCUSSION
Exercise is a valuable intervention to delay or reverse age-related alterations in cognitive decline and disease susceptibility including Alzheimer's and Parkinson's disease (Kramer, et al., 2006). Recent evidence indicates that exercise may have direct effects on microglia, as wheel running attenuates microglia cytokine production (Barrientos, et al., 2011). The results of our studies extend the existing literature by demonstrating that aging increases the proportion of new microglia in the hippocampus. Further, we report that wheel running reduces the proportion of new microglia in aged mice and increases their expression of a proneurogenic phenotype that may contribute to the running-induced increase in hippocampal neurogenesis. Collectively, the results provide novel evidence that wheel running may combat age-related alterations in microglia and increase their expression of IGF-1 a phenotype conducive for neuroprotection, growth and brain plasticity.
Aging is associated with priming of microglia towards the inflammatory phenotype, as shown by increased expression of MHC II (Frank, et al., 2006, Sierra, et al., 2007). Our data extend this observation by revealing that microglia in the hippocampus show an age-related enhancement in division relative to adult mice. This increase in the proportion of new microglia (i.e., BrdU+ and Iba-1+ cells) may result from an increase in both the survival and proliferation of microglia, as aged mice had greater proportions of Iba-1 positive cells displaying BrdU when the cells were labeled either two months or ten days prior to tissue collection. Our findings are consistent with prior in vitro work, as microglia collected from the cortices of aged rats showed increased proliferation in culture compared to young rats (Rozovsky, et al., 1998). Similarly, Inman and Horner (2007) report that a significantly greater proportion of new microglia (BrdU+ and Iba-1+) were present in the retina of middle-aged mice compared to young mice. In conjunction with our findings, the work indicates that aging increases microglia division in various regions of the brain. The mechanisms of the age-related increase in microglia division have yet to be fully elucidated. However, in culture, microglia from aged rats showed decreased responsiveness to the anti-inflammatory cytokine transforming growth factor-β (TGF-β) that normally inhibits microglia proliferation (Rozovsky, et al., 1998) potentially indicating that reduced regulatory control may contribute to the enhanced microglia division.
The observed increase in the proportion of dividing microglia in the aged versus adult mice raises the question of whether the total number of microglia is increased in aged mice. Though not assessed in the current study, a few reports have evaluated whether aging increases the number of microglia within the hippocampus by stereology. The initial report found no significant difference in the total number of microglia labeled with Mac-1 in adult (4 month) compared to aged (27 month) male C57BL/6J mice, as less than a 10% increase in microglia was observed in the aged mice (Long, et al., 1998). However, a separate study that used female C57BL/6J mice, conducted by the same laboratory, found a significant age-related increase in the number of microglia in the dentate gyrus and CA1 region of the hippocampus compared to adult females (Mouton, et al., 2002). Collectively these data indicate a sex difference exists, but whether a strain differences also exists has yet to be assessed. Alternatively, the increase in microglia division we observed in the aged male BALB/c mice may be accompanied by an increase in cell turnover; thereby the total number of microglia would remain similar to the adults. Additional work is needed to clarify these possibilities.
Prior work has shown that exercise influences microglia activity, including microglia proliferation. For instance, exercise decreased microglia proliferation in the septum and amygdala in adult animals (Ang, et al., 2004, Ehninger, et al., 2011). However, Ehninger and Kempermann (2003) report that exercise increased microglia proliferation in the cingulate and motor cortex of adult subjects. In contrast to these findings we found no effect of wheel running on microglia survival or proliferation in the dentate gyrus in the adult mice. We observed similar proportions of BrdU positive microglia in the runner and sedentary adult mice. These differences likely relate to procedural differences (i.e., type and duration of exercise) as well as the brain region assessed.
Our data show that exercise reduces the proportion of microglia in the dentate gyrus that divide in aged mice. This reduction in the proportion of new microglia appears to result from a decrease in both cell survival as well as proliferation. However, wheel running had a greater impact on proliferation, as we observed a larger reduction in the proportion of new microglia when cell were labeled just prior to tissue collection (BrdU Late; see Figure 3B). Whether other brain regions would show a similar reduction following exercise is unknown, as regional variations are reported in young animals (Ang, et al., 2004, Ehninger and Kempermann, 2003, Ehninger, et al., 2011). Given the involvement of the hippocampus in learning and memory, reducing excessive microglia activity may be of particular relevance to cognitive function. Presently, the mechanisms behind the exercise-induced reduction in the proportion of new microglia are unknown, but we can speculate that exercise-induced changes in the microenvironment, such as increasing factors that regulate microglia, may contribute. For instance, Tong et al. (2001) found that wheel running increases expression of fractalkine, a chemokine released by neurons that regulates microglia activation. Alternatively, exercise may reduce inflammatory molecules that could stimulate microglia proliferation. For example, cultured microglia from aged rats that had access to a running wheel had lower basal (unstimulated) expression of IL-1β and TNF-α compared to microglia from sedentary aged rats (Barrientos, et al., 2011). Though more work is needed to identify the mechanism behind these effects, our data provide novel evidence that exercise can attenuate microglia division primarily through reducing microglia proliferation in the aged.
In addition to affecting the proportion of new microglia, our data indicate that wheel running may be capable of altering the phenotype expressed by microglia. Wheel running significantly increased the proportion of microglia that co-labeled with IGF-1 (see Figure 4B). These findings are consistent with prior research that found microglia increased IGF-1 production following environmental enrichment (that included running wheels) (Ziv, et al., 2006). Wheel running significantly increased IGF-1 expression in both new microglia (BrdU+ and Iba-1+) and pre-existing microglia (BrdU- and Iba-1+) in both age groups. These findings indicate that both recently divided and pre-existing microglia are responsive to the changes induced by wheel running. It is interesting that aged mice with access to a running wheel showed a higher percentage of microglia that co-labeled with IGF-1 compared to adult mice. One possibility is that the age-related priming of microglia contributes to this response (Dilger and Johnson, 2008, Frank, et al., 2006). Since microglia in aged animals are in a partially active state they may be more responsive to environmental changes whereas microglia in adults typically express the resting phenotype and may have to undergo activation before IGF-1 or other molecules can be expressed.
Increased IGF-1 expression from microglia is proposed to be beneficial to the brain and to promote recovery. For instance, microglia are reported to express IGF-1 following an ischemic injury, which is thought to facilitate survival of cells in the damaged region (O'Donnell, et al., 2002). Further, Maher et al. (2006) report that intracerebroventricular administration of IGF-1 to aged rats significantly attenuated the interferon-gamma (IFN-γ) induced microglia activation and subsequent IL-1β expression. Collectively, findings indicate that increased IGF-1 levels may help reduce neuroinflammation and facilitate recovery and cell survival. Interestingly, we found an age-related increase in the proportion of Iba-1 positive cells that display IGF-1. This increase in the aged may be a compensatory response to other age related changes occurring within the hippocampus such as an increase in oxidative stress (Piriz, et al., 2010) or increased IGF resistance that is related to reductions in IGF receptor expression (Broughton and Partridge, 2009). However, there is some evidence that increased IGF expression can impair spatial learning ability and increase production of free radicals (Broughton and Partridge, 2009). Further work is needed to elucidate whether the age-related proportional increase in IGF expression from microglia is neuroprotective or detrimental to hippocampal function.
Aging is associated with a substantial decline in hippocampal neurogenesis. Consistent with prior reports (van Praag, et al., 2005, Walter, et al., 2011), we observed a significant reduction in both the proliferation and rate of survival of new cells in aged mice compared to adults. Additionally, fewer new cells differentiated into neurons in the aged (approximately 57%) compared to adults (approximately 77%) under sedentary conditions (see Table 1). In both age groups, wheel running increased hippocampal neurogenesis. Confirming prior reports (Clark, et al., 2008, van Praag, et al., 2005), we observed a significant increase in the number of new cells and increased neuron differentiation in both age groups following wheel running.
Microglia have been reported to contribute to the age-related decrease in hippocampal neurogenesis, as inhibition of microglia activation or IL-1β is known to increase neurogenesis in aged, but not young, animals (Bachstetter, et al., 2009, Gemma, et al., 2007). One possibility is that the increased proportion of microglia expressing of IGF-1 may contribute to the exercise-induced increase in hippocampal neurogenesis, particularly in the aged mice. Prior work suggests that microglia, through expression of IGF-1, may play a role in supporting enhanced neurogenesis following injury (Thored, et al., 2009). Through not assessed in the current study, prior work has shown that exercise increases levels of IGF-1 and BDNF within the brain (Nakajima, et al., 2010, Stranahan, et al., 2009). Therefore other growth factors as well as cell types likely contribute to the exercise-induced increase in neurogenesis. Though additional work is needed to determine the specific contribution of IGF-1 derived from microglia to hippocampal neurogenesis, the present data indicate that exercise increases the proportion of microglia that express a proneurogenic phenotype.
It is important to note that the current study used Iba-1 that labels both microglia and macrophages. Therefore, it is possible that a portion of the cells we identified were macrophages. Though the origin of microglia remains controversial, a recent paper proposed that microglia originate from yolk sac macrophages during early embryonic development (Ginhoux, et al., 2010). Further, the authors propose that perinatal hematopoietic cells, including monocytes, have minimal contribution to maintaining the adult microglia population, as experiments that employed parabiosis or transplantation of donor bone marrow cells revealed a minimal number of donors cells entered the brain (Ginhoux, et al., 2010). These data indicate that microglia precursors that localize in the brain during embryonic development may be primarily responsible for maintaining the microglia population throughout adulthood; potentially indicating that majority of the Iba-1+ cells we identified were microglia. Though others have reported that microglia proliferation and macrophage infiltration may have equal contributions to maintaining the steady-state levels of microglia (Lawson, et al., 1992). Whether the relative contributions of macrophage infiltration and microglia proliferation to homeostatic levels of microglia changes across the lifespan is currently unknown.
The age-related priming of microglia towards the inflammatory phenotype is a central factor in increasing susceptibility to inflammation-related behavioral and cognitive deficits and possibly the progression of neurodegenerative diseases. Our data indicate that exercise may be capable of enhancing features of the alternative neuroprotective phenotype, by increasing the proportion of microglia that produce IGF-1. Further we report that exercise attenuates the age-related increase in microglia division primarily through reducing microglia proliferation that in combination with increased IGF-1 expression may aid in reducing neuroinflammation and subsequently enhance hippocampal neurogenesis and potentially cognitive function. Additional work is needed to determine whether exercise enhances microglia expression of other neuroprotective factors such as BDNF or nerve growth factor (NGF) and whether these changes are associated with improvements in cognitive performance.
RESEARCH HIGHLIGHTS.
Wheel running significantly attenuates the age-related increase in microglia division and increases microglia expression of IGF-1 and hippocampal neurogenesis.
Acknowledgments
Funding: This work was supported by grants from National Institute of Health, MH083807 and DA027487 to J.S.R and from National Institute on Aging K99AG0404184 to R.A.K. Funding sources had no involvement in the experimental design or interpretation of the results.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: All authors declare that there are no conflicts of interest
REFERENCES
- Aarum J, Sandberg K, Haeberlein SL, Persson MA. Migration and differentiation of neural precursor cells can be directed by microglia. Proc Natl Acad Sci U S A. 2003;100:15983–8. doi: 10.1073/pnas.2237050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang ET, Wong PT, Moochhala S, Ng YK. Cytokine changes in the horizontal diagonal band of Broca in the septum after running and stroke: a correlation to glial activation. Neuroscience. 2004;129:337–47. doi: 10.1016/j.neuroscience.2004.06.087. [DOI] [PubMed] [Google Scholar]
- Bachstetter AD, Morganti JM, Jernberg J, Schlunk A, Mitchell SH, Brewster KW, Hudson CE, Cole MJ, Harrison JK, Bickford PC, Gemma C. Fractalkine and CX(3)CR1 regulate hippocampal neurogenesis in adult and aged rats. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Frank MG, Crysdale NY, Chapman TR, Ahrendsen JT, Day HEW, Campeau S, Watkins LR, Patterson SL, Maier SF. Little exercise, big effects: reversing aging and infection-induced memory deficits, and underlying processes. J Neurosci. 2011;31:11578–86. doi: 10.1523/JNEUROSCI.2266-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Menachem-Zidon O, Goshen I, Kreisel T, Ben Menahem Y, Reinhartz E, Ben Hur T, Yirmiya R. Intrahippocampal transplantation of transgenic neural precursor cells overexpressing interleukin-1 receptor antagonist blocks chronic isolation-induced impairment in memory and neurogenesis. Neuropsychopharmacology. 2008;33:2251–62. doi: 10.1038/sj.npp.1301606. [DOI] [PubMed] [Google Scholar]
- Broughton S, Partridge L. Insulin/IGF-like signalling, the central nervous system and aging. Biochem J. 2009;418:1–12. doi: 10.1042/BJ20082102. [DOI] [PubMed] [Google Scholar]
- Clark PJ, Brzezinska WJ, Thomas MW, Ryzhenko NA, Toshkov SA, Rhodes JS. Intact neurogenesis is required for benefits of exercise on spatial memory but not motor performance or contextual fear conditioning in C57BL/6J mice. Neuroscience. 2008;155:1048–58. doi: 10.1016/j.neuroscience.2008.06.051. [DOI] [PubMed] [Google Scholar]
- Colton CA. Heterogeneity of microglial activation in the innate immune response in the brain. J Neuroimmune Pharmacol. 2009;4:399–418. doi: 10.1007/s11481-009-9164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–50. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilger RN, Johnson RW. Aging, microglial cell priming, and the discordant central inflammatory response to signals from the peripheral immune system. J Leukoc Biol. 2008;84:932–9. doi: 10.1189/jlb.0208108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehninger D, Kempermann G. Regional effects of wheel running and environmental enrichment on cell genesis and microglia proliferation in the adult murine neocortex. Cereb Cortex. 2003;13:845–51. doi: 10.1093/cercor/13.8.845. [DOI] [PubMed] [Google Scholar]
- Ehninger D, Wang LP, Klempin F, Romer B, Kettenmann H, Kempermann G. Enriched environment and physical activity reduce microglia and influence the fate of NG2 cells in the amygdala of adult mice. Cell Tissue Res. 2011;345:69–86. doi: 10.1007/s00441-011-1200-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Biedenkapp JC, Rudy JW, Watkins LR, Maier SF. mRNA up-regulation of MHC II and pivotal pro-inflammatory genes in normal brain aging. Neurobiol Aging. 2006;27:717–22. doi: 10.1016/j.neurobiolaging.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Gemma C, Bachstetter AD, Cole MJ, Fister M, Hudson C, Bickford PC. Blockade of caspase-1 increases neurogenesis in the aged hippocampus. Eur J Neurosci. 2007;26:2795–803. doi: 10.1111/j.1460-9568.2007.05875.x. [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–5. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. Faseb J. 2005;19:1329–31. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- Ibi D, Takuma K, Koike H, Mizoguchi H, Tsuritani K, Kuwahara Y, Kamei H, Nagai T, Yoneda Y, Nabeshima T, Yamada K. Social isolation rearing-induced impairment of the hippocampal neurogenesis is associated with deficits in spatial memory and emotion-related behaviors in juvenile mice. J Neurochem. 2008;105:921–32. doi: 10.1111/j.1471-4159.2007.05207.x. [DOI] [PubMed] [Google Scholar]
- Inman DM, Horner PJ. Reactive nonproliferative gliosis predominates in a chronic mouse model of glaucoma. Glia. 2007;55:942–53. doi: 10.1002/glia.20516. [DOI] [PubMed] [Google Scholar]
- Kinsey SG, Bailey MT, Sheridan JF, Padgett DA. The inflammatory response to social defeat is increased in older mice. Physiol Behav. 2008;93:628–36. doi: 10.1016/j.physbeh.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohman RA, Tarr AJ, Byler SL, Boehm GW. Age increases vulnerability to bacterial endotoxin-induced behavioral decrements. Physiol Behav. 2007;91:561–5. doi: 10.1016/j.physbeh.2007.03.032. [DOI] [PubMed] [Google Scholar]
- Koteja P, Garland T, Jr., Sax JK, Swallow JG, Carter PA. Behaviour of house mice artificially selected for high levels of voluntary wheel running. Anim Behav. 1999;58:1307–1318. doi: 10.1006/anbe.1999.1270. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Erickson KI, Colcombe SJ. Exercise, cognition, and the aging brain. J Appl Physiol. 2006;101:1237–42. doi: 10.1152/japplphysiol.00500.2006. [DOI] [PubMed] [Google Scholar]
- Lawson LJ, Perry VH, Gordon S. Turnover of resident microglia in the normal adult mouse brain. Neuroscience. 1992;48:405–15. doi: 10.1016/0306-4522(92)90500-2. [DOI] [PubMed] [Google Scholar]
- Long JM, Kalehua AN, Muth NJ, Calhoun ME, Jucker M, Hengemihle JM, Ingram DK, Mouton PR. Stereological analysis of astrocyte and microglia in aging mouse hippocampus. Neurobiol Aging. 1998;19:497–503. doi: 10.1016/s0197-4580(98)00088-8. [DOI] [PubMed] [Google Scholar]
- Maher FO, Clarke RM, Kelly A, Nally RE, Lynch MA. Interaction between interferon gamma and insulin-like growth factor-1 in hippocampus impacts on the ability of rats to sustain long-term potentiation. J Neurochem. 2006;96:1560–71. doi: 10.1111/j.1471-4159.2006.03664.x. [DOI] [PubMed] [Google Scholar]
- Mouton PR, Long JM, Lei DL, Howard V, Jucker M, Calhoun ME, Ingram DK. Age and gender effects on microglia and astrocyte numbers in brains of mice. Brain Res. 2002;956:30–5. doi: 10.1016/s0006-8993(02)03475-3. [DOI] [PubMed] [Google Scholar]
- Nakajima K, Tohyama Y, Kohsaka S, Kurihara T. Ceramide activates microglia to enhance the production/secretion of brain-derived neurotrophic factor (BDNF) without induction of deleterious factors in vitro. J Neurochem. 2002;80:697–705. doi: 10.1046/j.0022-3042.2001.00752.x. [DOI] [PubMed] [Google Scholar]
- Nakajima S, Ohsawa I, Ohta S, Ohno M, Mikami T. Regular voluntary exercise cures stress-induced impairment of cognitive function and cell proliferation accompanied by increases in cerebral IGF-1 and GST activity in mice. Behav Brain Res. 2010;211:178–84. doi: 10.1016/j.bbr.2010.03.028. [DOI] [PubMed] [Google Scholar]
- Nichol KE, Poon WW, Parachikova AI, Cribbs DH, Glabe CG, Cotman CW. Exercise alters the immune profile in Tg2576 Alzheimer mice toward a response coincident with improved cognitive performance and decreased amyloid. J Neuroinflammation. 2008;5:13. doi: 10.1186/1742-2094-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell SL, Frederick TJ, Krady JK, Vannucci SJ, Wood TL. IGF-I and microglia/macrophage proliferation in the ischemic mouse brain. Glia. 2002;39:85–97. doi: 10.1002/glia.10081. [DOI] [PubMed] [Google Scholar]
- Piriz J, Muller A, Trejo JL, Torres-Aleman I. IGF-I and the aging mammalian brain. Exp Gerontol. 2010;46:96–9. doi: 10.1016/j.exger.2010.08.022. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Koteja P, Swallow JG, Carter PA, Garland T. Body temperatures of house mice artificially selected for high voluntary wheel-running behavior: repeatability and effect of genetic selection. J Therm Biol. 2000;25:391–400. doi: 10.1016/s0306-4565(99)00112-6. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, van Praag H, Jeffrey S, Girard I, Mitchell GS, Garland T, Jr., Gage FH. Exercise increases hippocampal neurogenesis to high levels but does not improve spatial learning in mice bred for increased voluntary wheel running. Behav Neurosci. 2003;117:1006–16. doi: 10.1037/0735-7044.117.5.1006. [DOI] [PubMed] [Google Scholar]
- Rozovsky I, Finch CE, Morgan TE. Age-related activation of microglia and astrocytes: in vitro studies show persistent phenotypes of aging, increased proliferation, and resistance to down-regulation. Neurobiol Aging. 1998;19:97–103. doi: 10.1016/s0197-4580(97)00169-3. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12:578–84. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A, Gottfried-Blackmore AC, McEwen BS, Bulloch K. Microglia derived from aging mice exhibit an altered inflammatory profile. Glia. 2007;55:412–24. doi: 10.1002/glia.20468. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Zhou Y, Martin B, Maudsley S. Pharmacomimetics of exercise: novel approaches for hippocampally-targeted neuroprotective agents. Curr Med Chem. 2009;16:4668–78. doi: 10.2174/092986709789878292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thored P, Heldmann U, Gomes-Leal W, Gisler R, Darsalia V, Taneera J, Nygren JM, Jacobsen SE, Ekdahl CT, Kokaia Z, Lindvall O. Long-term accumulation of microglia with proneurogenic phenotype concomitant with persistent neurogenesis in adult subventricular zone after stroke. Glia. 2009;57:835–49. doi: 10.1002/glia.20810. [DOI] [PubMed] [Google Scholar]
- Tong L, Shen H, Perreau VM, Balazs R, Cotman CW. Effects of exercise on gene-expression profile in the rat hippocampus. Neurobiol Dis. 2001;8:1046–56. doi: 10.1006/nbdi.2001.0427. [DOI] [PubMed] [Google Scholar]
- Trejo JL, Llorens-Martin MV, Torres-Aleman I. The effects of exercise on spatial learning and anxiety-like behavior are mediated by an IGF-I-dependent mechanism related to hippocampal neurogenesis. Mol Cell Neurosci. 2008;37:402–11. doi: 10.1016/j.mcn.2007.10.016. [DOI] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–5. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter J, Keiner S, Witte OW, Redecker C. Age-related effects on hippocampal precursor cell subpopulations and neurogenesis. Neurobiol Aging. 2011;32:1906–14. doi: 10.1016/j.neurobiolaging.2009.11.011. [DOI] [PubMed] [Google Scholar]
- Walton NM, Sutter BM, Laywell ED, Levkoff LH, Kearns SM, Marshall GP, 2nd, Scheffler B, Steindler DA. Microglia instruct subventricular zone neurogenesis. Glia. 2006;54:815–25. doi: 10.1002/glia.20419. [DOI] [PubMed] [Google Scholar]
- Wine RN, McPherson CA, Harry GJ. IGF-1 and pAKT signaling promote hippocampal CA1 neuronal survival following injury to dentate granule cells. Neurotox Res. 2009;16:280–92. doi: 10.1007/s12640-009-9060-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods JA, Vieira VJ, Keylock KT. Exercise, inflammation, and innate immunity. Immunol Allergy Clin North Am. 2009;29:381–93. doi: 10.1016/j.iac.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun. 2011;25:181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9:268–75. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]