Abstract
Background
Presence of donor specific antibodies (Abs) is detrimental to post transplant allograft function. Some sensitized recipients have successfully undergone transplantation following pretransplant conditioning regimen utilizing plasmapheresis and/or intravenous immunoglobulin therapy, but underlying mechanisms that confer such allograft protection are undefined.
Methods
We developed a single HLA mismatched heterotopic murine heart transplant model (HLA-A2 into HLA-A2-sensitized-C57BL/6 ) to determine whether pretreatment of donors with low concentration of HLA class I(W6/32) or control Ab(C1.18.4) will confer protection. Expression levels of survival genes, Bcl2 and HO-1 were analyzed by gene array analysis and quantitative real-time PCR. Expression levels of cytokine panel were analyzed by Luminex. Role of Bcl2 in the induction of allograft protection was analyzed by silencing the Bcl2 expression in the donor hearts using a shRNA specific for Bcl2.
Results
Control Ab pretreated hearts were rejected in <5 days demonstrating hemorrhage, Ab and C4 deposition. In contrast, W6/32 pretreated hearts were rejected at 15 days (P<0.05) that was prolonged to 25 days with anti-lymphocyte serum (ALS) treatment. W6/32 pretreated hearts on day 5 exhibited increased expression of Bcl-2(5.5folds), Bcl-xl(5.5folds) and HO-1(4.4folds); decreased expression of ICAM-1, VCAM-1(3.2 fold), along with reduced levels of cytokines IL-1β(4.4folds), TNF-α(3.7folds), IL-6(7.5folds), IL-12(2.3folds) and chemokines MCP-1(4.5folds), MIG(4.4folds), MIP-1α(3.4folds) and IL-8(3.1folds). Silencing of Bcl2 in accommodated hearts prior to transplant resulted in loss of protection with rejection (9±3Vs.15±2days,p<0.05). 3
Conclusion
Pretreatment of hearts with low levels of anti-HLA Abs increases expression of anti-apoptotic genes that inhibits caspases, leading to decreased inflammatory cytokines and chemokines which promote allograft survival.
Introduction
There is an ever increasing gap between the number of patients requiring solid organ transplantation and number of donor organs available. To address this concern, transplants are being performed across ABO and human leukocyte antigen (HLA) barriers[1,2]. Sensitization detected by presence of donor specific antibodies(Abs) is the major stumbling block for successful transplantation of organs across ABO and HLA incompatibility and results in Ab mediated hyper acute rejection(HAR), evidenced by binding of anti-donor Ab followed by complement activation[3–5]. Removal of Abs by plasmapheresis and intravenous immunoglobulin (IVIG) overcomes Ab mediated rejection[6–11]. Studies have shown that grafts transplanted under these circumstances can undergo rejection upon the return of anti-donor Ab [7,9,12–15]. However, some transplanted allografts continue functioning despite the return of Ab, a phenomenon termed as graft accommodation[7,9,12,14]. As described previously in xenotransplantation, endothelial cell (EC) lining develops resistance to Ab/complement-mediated lysis and this process is mediated by expression of Bcl-xL, Bcl-2 and heme oxygenase-1(HO-1) genes [12].
Although there are reports of accommodation of allografts transplanted into sensitized recipients [7, 9] events that lead to accommodation remain undefined. We have previously demonstrated that ECs exposed to sub-saturating concentrations of HLA class I polyclonal or frame work monoclonal Ab (W6/32), are resistant to activation and Ab/complement-mediated cell death; mediated by an up-regulation of PI-3kinase/Akt/PKA pathway that facilitates Bad phosphorylation and activation of anti-apoptotic genes Bcl-xL, Bcl-2, and HO-1[16,17]. In the present study, we extend these findings to a unique in vivo model. We demonstrate that pretreatment of donor hearts with low levels of W6/32 was able to overcome humoral rejection and prolong graft survival (15days) when transplanted into highly sensitized recipients. W6/32 pretreated hearts exhibited minimal deposition of complement C4, Ab and infiltration of polymorphonuclear cells. They exhibited significant increases in expression of anti-apoptotic genes Bcl-2, Bcl-xl and HO-1 with concomitant reduction in expression of adhesion molecules, inflammatory cytokines and chemokines. Silencing of Bcl2 expression in accommodated hearts prior to transplant resulted in loss of allograft protection thereby indicating a critical role for Bcl2 in this process.
Results
Acute Ab mediated rejection of the HLA-A2 transgenic heart in sensitized recipient
HLA-A2 hearts transplanted into sensitized animals rejected on day 5. H&E analysis exhibited extensive hemorrhage, deposition of Ab and complement C4 and extensive infiltration of neutrophils and macrophages. These results indicate that pre-existing anti-MHC Abs in recipient results in Ab mediated rejection of a single antigen mismatched organ.
Optimal dose and time for pretreatment of donor HLA-A2 hearts with HLA class I Ab W6/32 to prolong survival
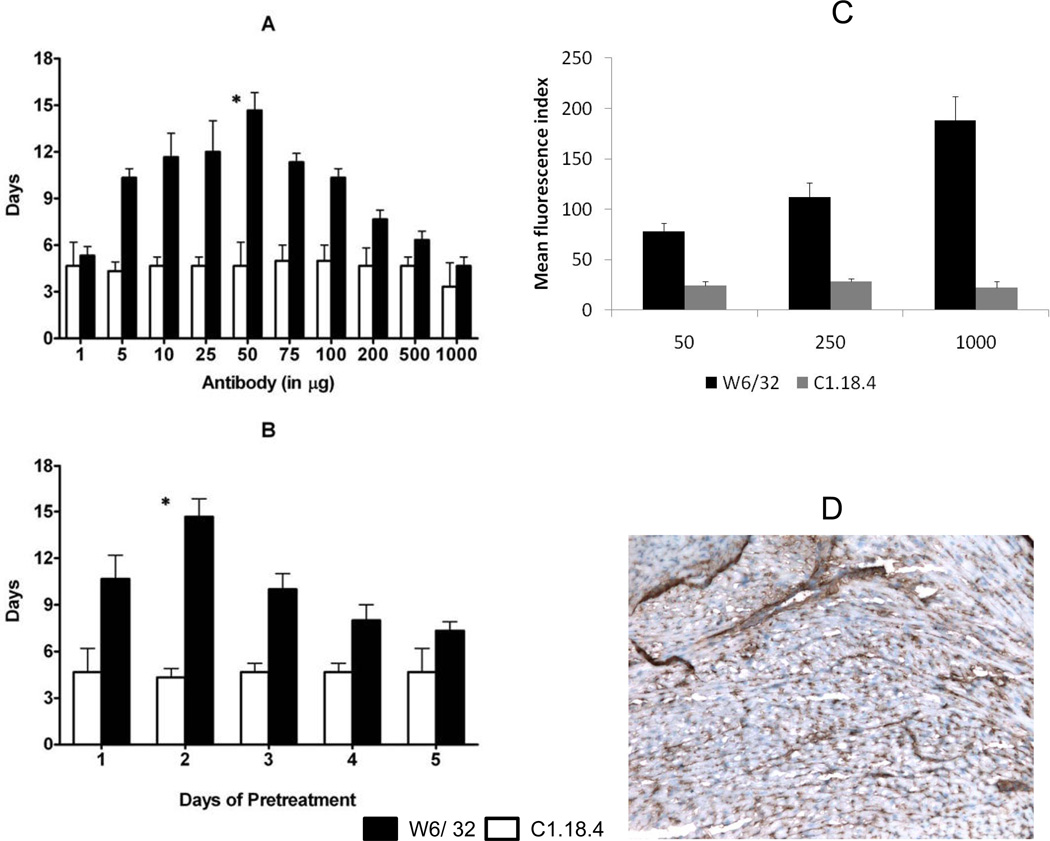
To determine the optimal concentration for pretreatment, we exposed HLA-A2 donor mice with different concentrations of W6/32 or control Ab(C1.18.4) (1,5,10,25,50,75,100,200,500 and 1000µg) and heterotopically transplanted their hearts into sensitized C57BL/6. Pretreatment of donor hearts with 50µg of W6/32 resulted in maximal prolongation of allograft survival (15±2days) (Fig.1A), when compared to hearts that were pretreated with lower and higher concentrations of W6/32 or C1.18.4 (p < 0.05).
Figure 1. Prolongation of the survival of HLA-A2 donor hearts pretreated with 50µg of W6/32.
(A) Donor HLA-A2 mice were pretreated with different concentrations of W6/32 (black bars) or C1.18.4 (white bars) (1, 5, 10, 25, 50, 75, 100, 200, 500 and 1000 µg) on day -2 or (B) Donor HLA-A2 mice were pretreated with 50µg of W6/32(black bars) or C1.18.4 (white bars) for different days (1, 2, 3, 4 and 5 days). The pretreated hearts were heterotopically transplanted into HLA-A2 sensitized C57BL/6 mice (n=3 each) and monitored everyday for rejection (defined as the cessation of heartbeat). The results are expressed as mean survival day’s ± SD of 3 separate experiments. Donor HLA-A2 hearts pretreated with 50µg of W6/32 exhibited a significant prolongation (*p < 0.05) in the mean survival times when compared to donor HLA-A2 hearts pretreated with C1.18.4. (C) 1 X 106 endothelial cells (100μL) isolated from the HLA-A2 transgenic mice were incubated with 1μL of 50, 250 or 1000 μg/ml of W6/32 or control antibody and counter stained with FITC-conjugated anti-mouse antibody. FACS analysis of the cells demonstrated a dose dependent binding of the W6/32 antibody to the endothelial cells from the HLA-A2 transgenic mice. (D) HLA-A2 mice were pretreated with 50μg of W6/32 antibody and the hearts were procured 2 days following treatment. Sections of the hearts were blocked with unconjugated goat anti-mouse antibody and then stained with 1000μg of W6/32 antibody. The sections were then incubated with peroxidase conjugated goat anti-mouse antibody and developed with DAB. Immunohistochemical analysis of the pre-treated hearts demonstrated that there are unbound HLA-A2 molecules on the surface of the hearts pre-treated with subsaturating concentration of W6/32 antibody.
Pretreatment of HLA-A2 hearts with 50µg of W6/32 for two days resulted in maximum prolongation of survival (15±2days) (Fig.1B), when compared to hearts that were untreated or treated for 1,3,4 or 5days (2±1days) or pretreated with 50µg of C1.18.4 (5±1days) regardless of time of pretreatment. FACS analysis of endothelial cells (Fig.1C) with increasing concentrations of antibody demonstrated a dose dependent increase in binding of the antibody to HLA and immunohistochemical staining of pretreated hearts (Fig.1D) with saturating concentration (1000µg) of W6/32 antibody demonstrated significant binding of the antibody.
Increased expression of anti-apoptotic genes and inhibition of adhesion molecules expression in pretreated heart allografts
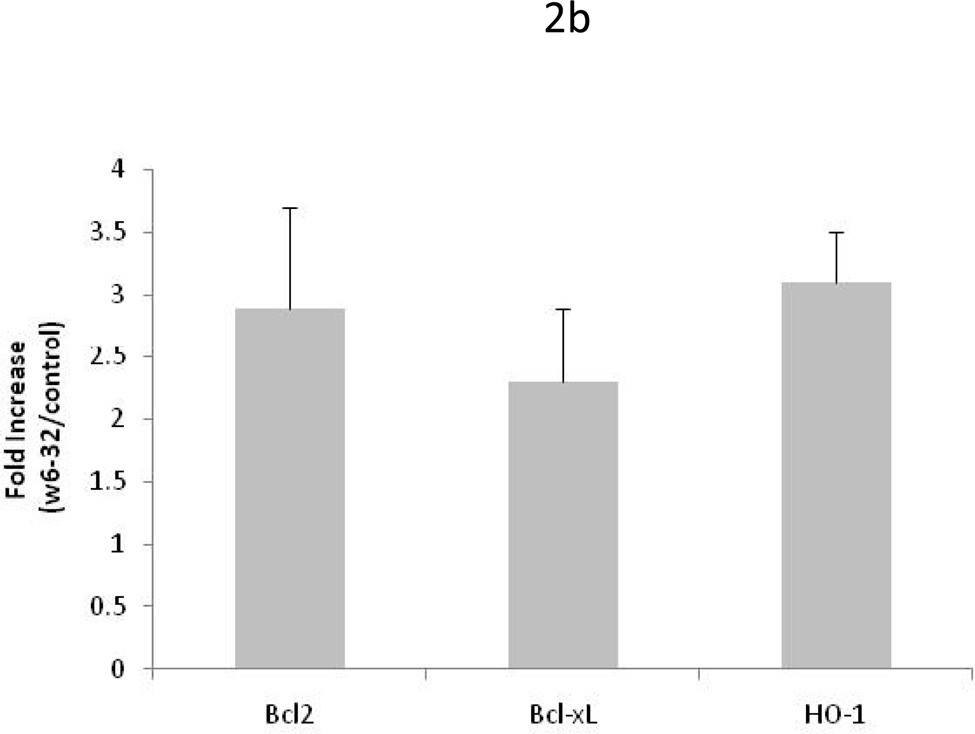
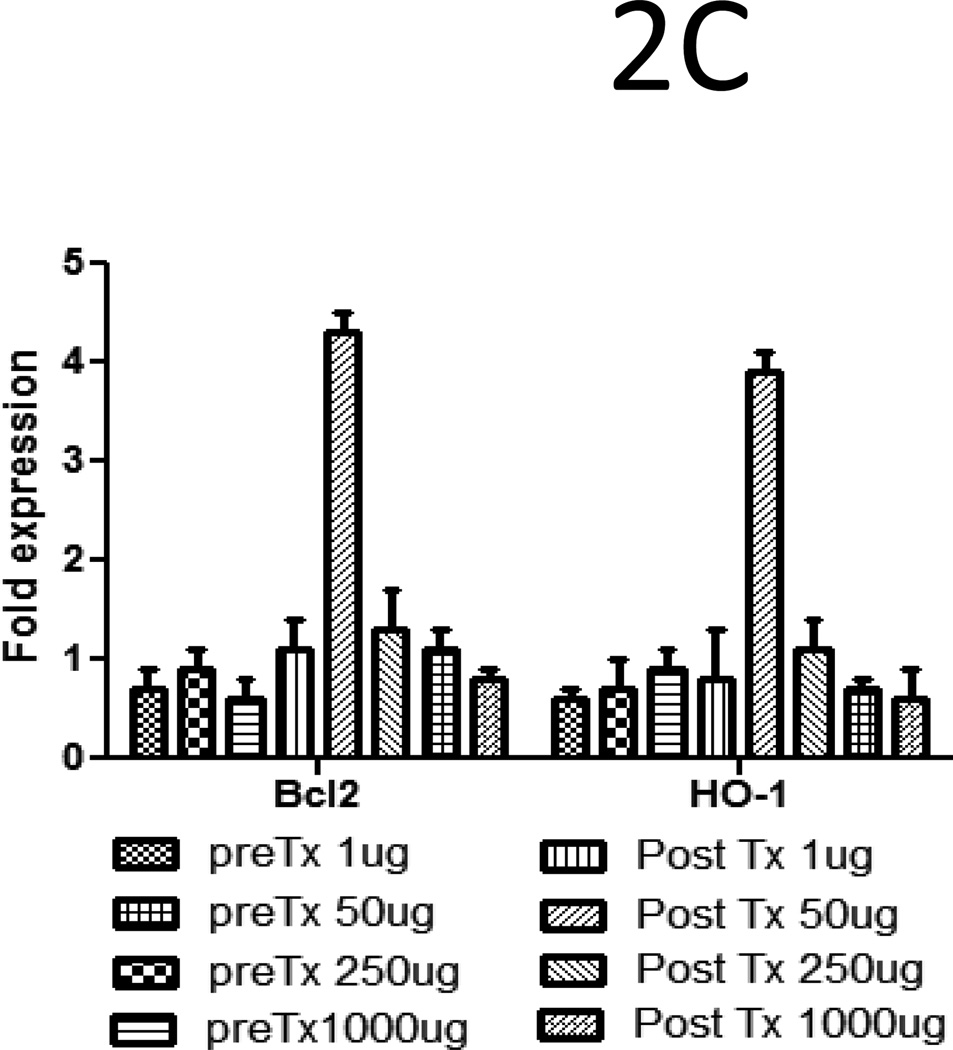
To determine the molecular and cellular changes that lead to prolongation of heart allograft survival, donor hearts pretreated with 50µg of W6/32 or C1.18.4 for two days were transplanted into sensitized C57BL/6. Five days post transplant, the hearts were recovered and gene array analysis and western blotting was performed to determine the expression of apoptotic and inflammatory genes. Gene array analysis revealed a significant increase (p<0.05) in expression of anti-apoptotic genes Bcl-xl (5.5fold), Bcl-2(5.5fold), HO-1(4.4fold), survivin (4.5fold) and significant (p<0.05) inhibition in expression of adhesion molecules ICAM-1(3.2fold), VCAM-1(3.2fold) and PECAM (2.3fold) in donor hearts pretreated with 50µg of W6/32(Fig.2A). Further, the increased presence of proteins expressed by anti-apoptotic genes Bcl-xl, Bcl-2 and HO-1 were confirmed using western blot analysis (Fig.2B). To define the kinetics of expression of anti-apoptotic genes Bcl-2 and HO-1, we analyzed expression of Bcl2 and HO-1 in hearts pretreated for two days with 1µg, 50µg and 1000µg of W6/32. We also analyzed expression of Bcl-2 and HO-1 in hearts with and without accommodation on day 4 post transplant in sensitized recipients. Quantitative RT-PCR analysis of anti-apoptotic gene expression profile demonstrated no significant difference in expression of Bcl-2 and HO-1 genes in hearts treated with 1, 50µg and 1000µg of W6/32 prior to transplant(Fig.2C). However, in donor hearts pretreated with 50µg of W6/32, the levels of Bcl-2 (4.3±1.6folds) and HO-1(3.9±0.7folds) were significantly elevated post transplantation indicating that secondary insults are essential for increased expression of protective genes.
Figure 2. Elevation in the expression of anti apoptotic genes and inhibition in the expression of inflammatory genes in HLA-A2 donor hearts pretreated with 50µg of W6/32.
Donor HLA-A2 mice were pretreated with 50µg of W6/32 (black bars) or C1.18.4 (white bars) on day -2 and the hearts were heterotopically transplanted into HLA-A2 sensitized C57BL/6 mice (n=5 each). 5 days post-transplant into sensitized recipient mice the transplanted hearts were recovered. A: The RNA extracted from the donor hearts using Trizol were reverse-transcribed, radiolabeled and hybridized with mouse Apoptosis Gene Array. The results are shown as a mean ratio ± SD of the expression of apoptotic and inflammatory genes in donor HLA-A2 hearts to the expression of actin in the donor hearts of 3 separate experiments. There was a significant elevation (*p < 0.05) in the expression of anti-apoptotic genes Bcl-xl, Bcl-2, HO-1, survivin and a significant (*p < 0.05) inhibition in the expression of adhesion molecules ICAM-1, VCAM-1 and PECAM in donor hearts pretreated with 50µg of W6/32 for two days. B: The proteins were extracted from the donor HLA-A2 hearts and electrophoresed in a 10% SDS-PAGE gel, transferred to a polyvinylidene difluoride membrane. The proteins were detected by anti-Bcl-xL, anti-BcL-2, and anti-HO-1 antibody. Results are a representative of 3 separate experiments. C: Quantitative RT-PCR analysis (fold increase= expression in W6/32 antibody treated mice /expression in isotype control antibody (C1.18.4) treated mice) of anti-apoptotic gene expression profile demonstrated no significant difference in expression of Bcl2 and HO-1 genes in hearts treated with 1µg, 50µg and 1000µg of W6/32 prior to transplant (Fig 2C). However, in donor hearts pre-treated with 50µg of W6/32 prior to transplant, the levels of Bcl2 (4.3±1.6folds) and HO-1(3.9±0.7folds) were significantly elevated post-transplantation (day 4).
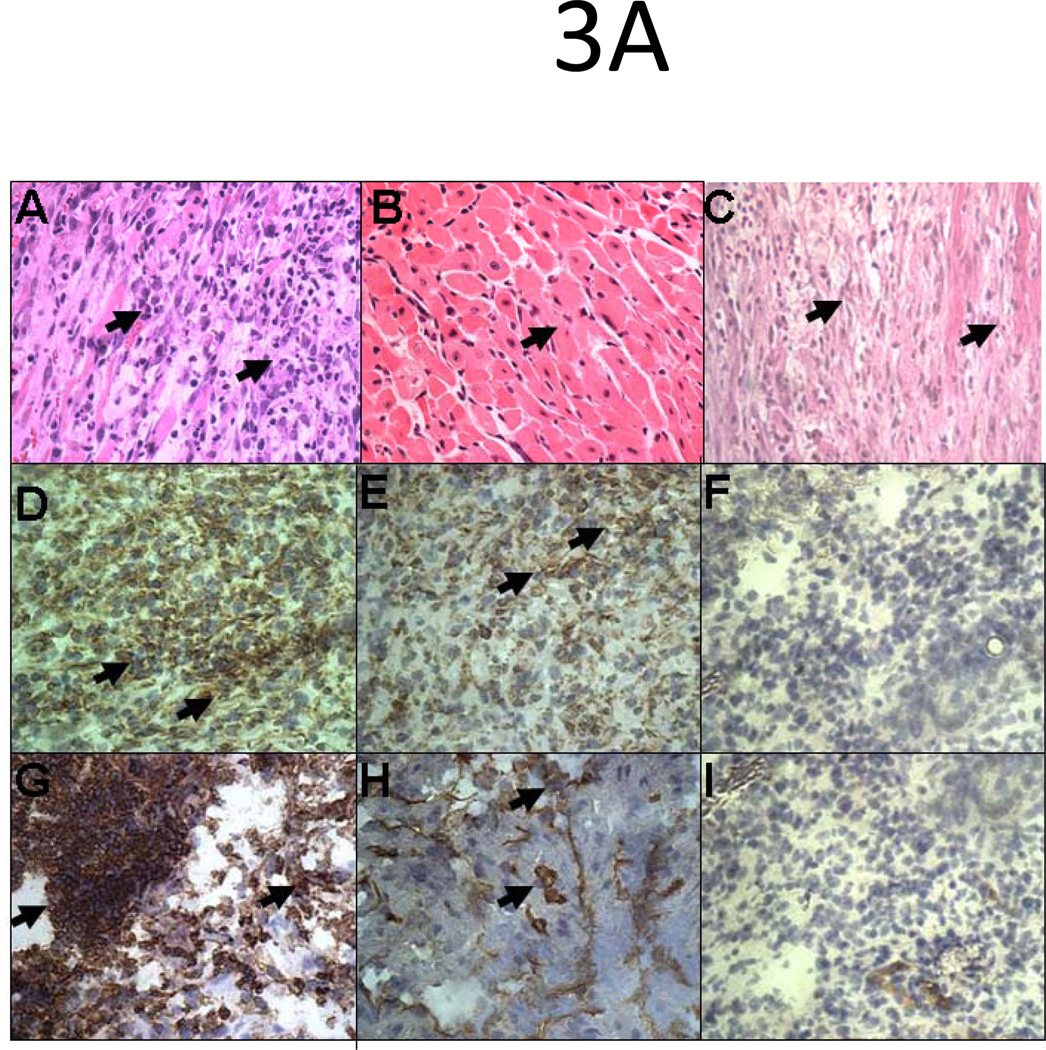
Deposition of Ab, fixation of complement and infiltration of neutrophils and macrophages in HLA-A2 donor hearts pretreated with Isotype control Ab
HLA-A2 allograft pretreated with 50µg of C1.18.4 underwent humoral rejection on day 5 post transplant. Grafts exhibited hemorrhage, deposition of Ab and complement C4 (Fig.3A) and extensive infiltration of neutrophils and macrophages (Fig.3B). In contrast, HLA-A2 allografts pretreated with 50µg of W6/32 exhibited normal morphology on day 5 and necrosis with mononuclear infiltrates on day 15 post transplant (Fig.3A). The hearts also exhibited low levels of Ab and complement C4 deposition on days 5 and 15 post transplant. Further, the allografts did not exhibit infiltration with neutrophils or macrophages (Fig.3B). Analysis of antibody deposition in the grafts demonstrated that there was a significant decrease in the levels of Ab deposited on day 15 compared to day 5. This observation could be attributed to the fact that as with other monoclonal Abs, the half-life of the administered w6/32 mAb is close to 15 days and hence, we would expect a lower Ab deposition in tissue on day 15 when compared to day 5.
Figure 3. Ab mediated rejection and infiltration of neutrophils and macrophages in C1.18.4 pretreated donor hearts five days post-transplant into sensitized recipient C57BL/6 mice.
Donor HLA-A2 mice were pretreated with 50µg of W6/32 or C1.18.4 on day -2 and the hearts were then heterotopically transplanted into HLA-A2 sensitized C57BL/6 mice (n=5 each). C1.18.4 (A, D, G) or W6/32 (B, E, H) pretreated HLA-A2 donor hearts were recovered five days post-transplant into sensitized recipient mice. W6/32 (C, F, I) pretreated hearts were also recovered 15 days post-transplant into sensitized recipient mice. a: The donor HLA-A2 grafts were fixed in 10% paraformaldehyde, embedded in paraffin, cut into 5-μm sections, and stained with hemotoxylin and eosin (A, B, C) or donor HLA-A2 grafts were frozen fresh and sectioned at 5 μm on a cryostat and stained with the rabbit anti-mouse IgG Ab (D, E, F) or rat anti-mouse C4 Ab (G, H, I). A significant increase in the deposition of Ab and activation of complement cascade (arrows) was observed in the hearts of sensitized mice transplanted with grafts pretreated with C1.18.4. The figures shown are a representative of 3 separate experiments. Original magnification×400. b:. The grafts were frozen fresh and sectioned at 5 μm on a cryostat and stained with the rat anti-mouse Ly-6G and LY-6C Ab (A, B, C), rat anti-mouse CD11b Ab (D, E, F). The figures shown are a representative of 3 separate experiments. Original magnification×400. The arrows point to the areas of significant increase in neutrophil and macrophage infiltration in the transplanted hearts.
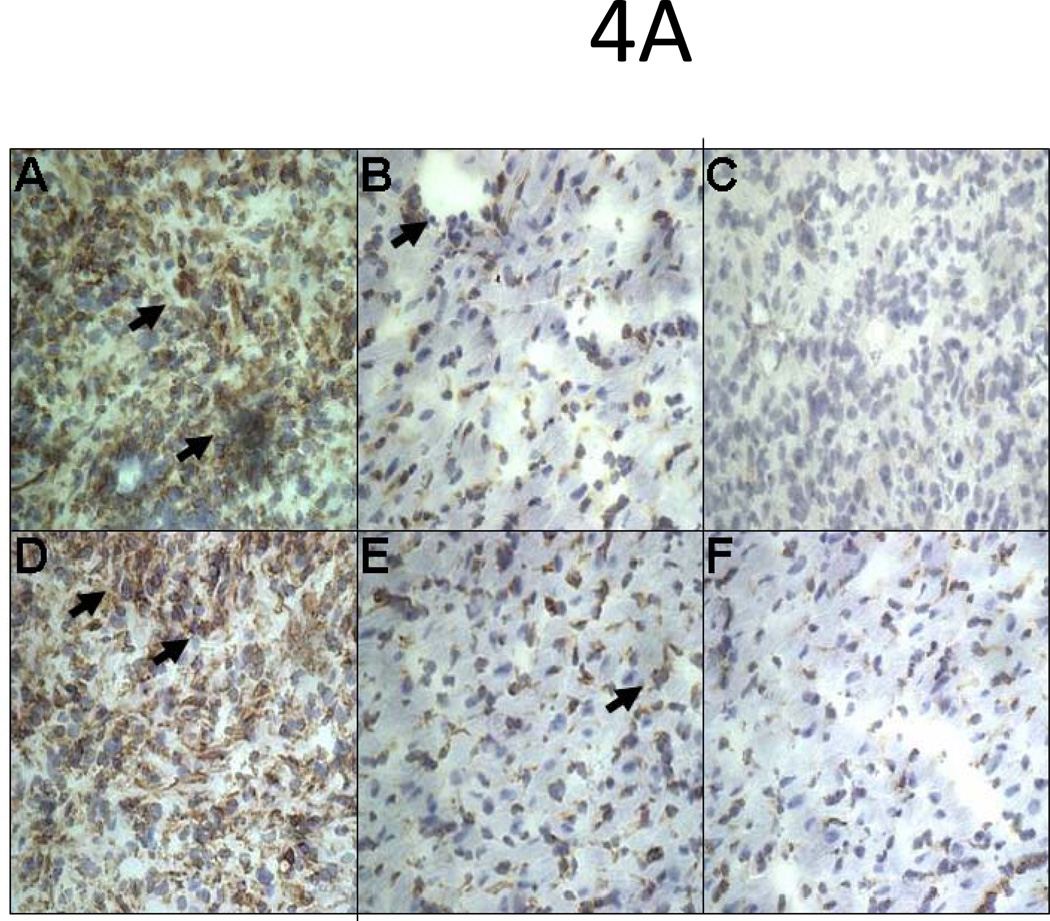
Inhibition in the expression of adhesion molecules and cytokines in HLA-A2 grafts pretreated with HLA class I Ab W6/32
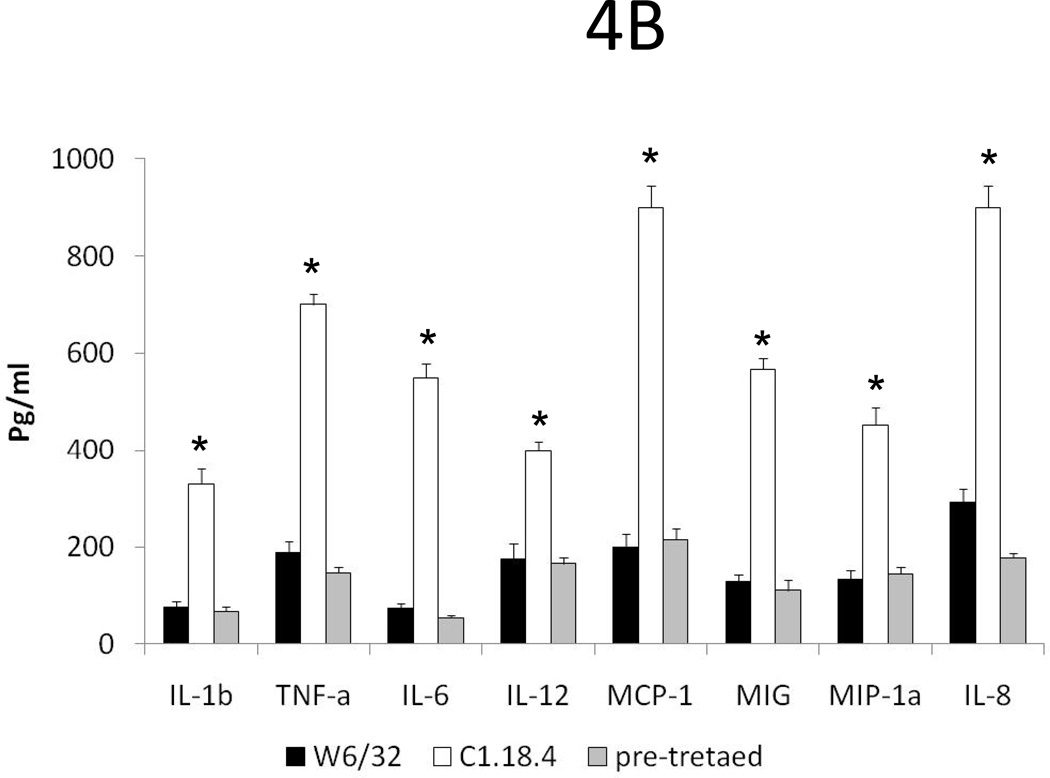
To determine the activation status of the cardiac endothelium, we analyzed the expression of adhesion molecules and cytokines by immunohistochemistry and Luminex. HLA-A2 hearts pretreated with 50µg of W6/32 exhibited low level expression of ICAM-1 and VCAM-1 on day 5 or day 15 post transplant compared to controls (Fig 4A). Analysis of inflammatory cytokines by Luminex in hearts of mice pre-treated with w6/32 Ab did not show any increase in levels of IL-1β, TNF-α, IL-6, and IL-12 when compared to controls. However, as seen in Fig.4B significant (P<0.05) reductions in expression of IL-1β(75vs.330 pg/ml), TNF-α(187vs.700 pg/ml), IL-6 (73vs.550 pg/ml), IL-12 (175vs.400 pg/ml) and chemokines MCP (1200vs.900 pg/ml), MIG (128vs.567 pg/ml), MIP-1α (132vs.453 pg/ml) and IL-8 (291vs900 pg/ml) were observed in HLA-A2 hearts pretreated with 50µg of W6/32 when compared to controls. The data demonstrates that pretreatment of HLA-A2 donor hearts with low levels of W6/32 results in inhibition of inflammatory cytokines, chemokines and adhesion molecules.
Figure 4. Inhibition in the expression of adhesion molecules, cytokines and cellular infiltration in HLA-A2 donor hearts pretreated with 50µg of W6/32.
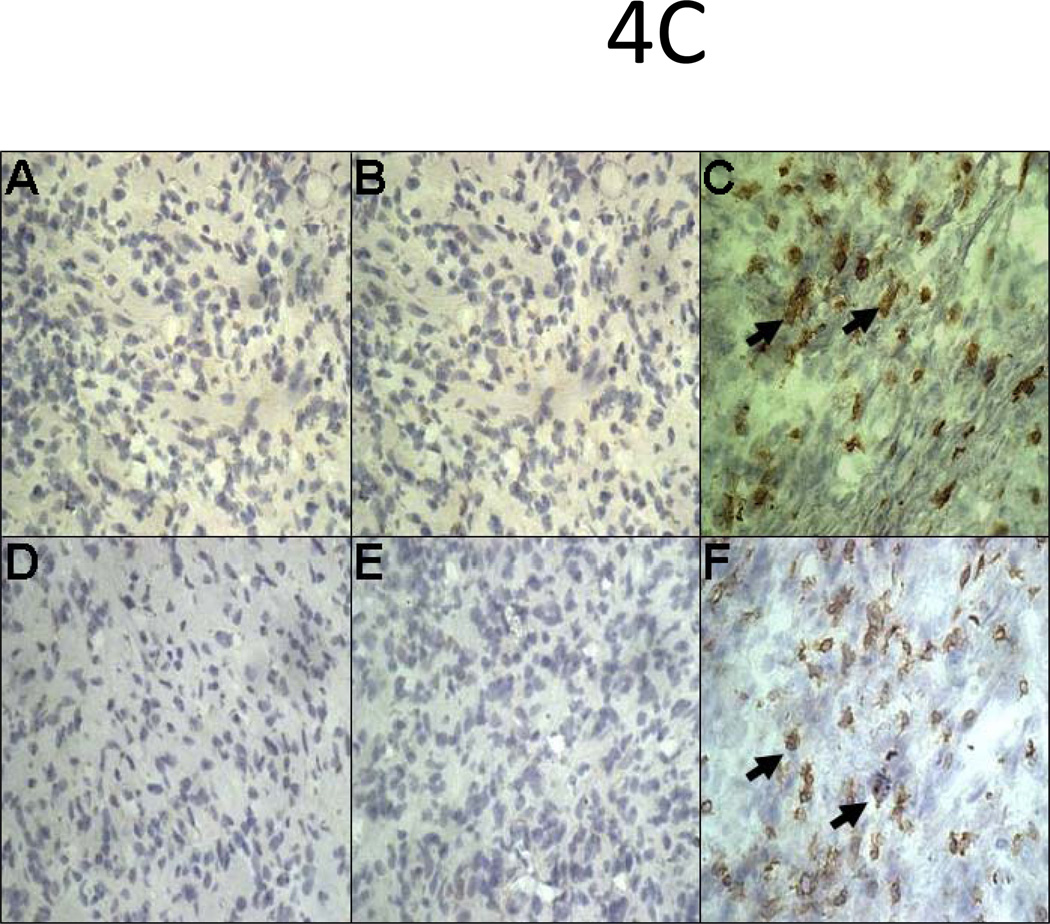
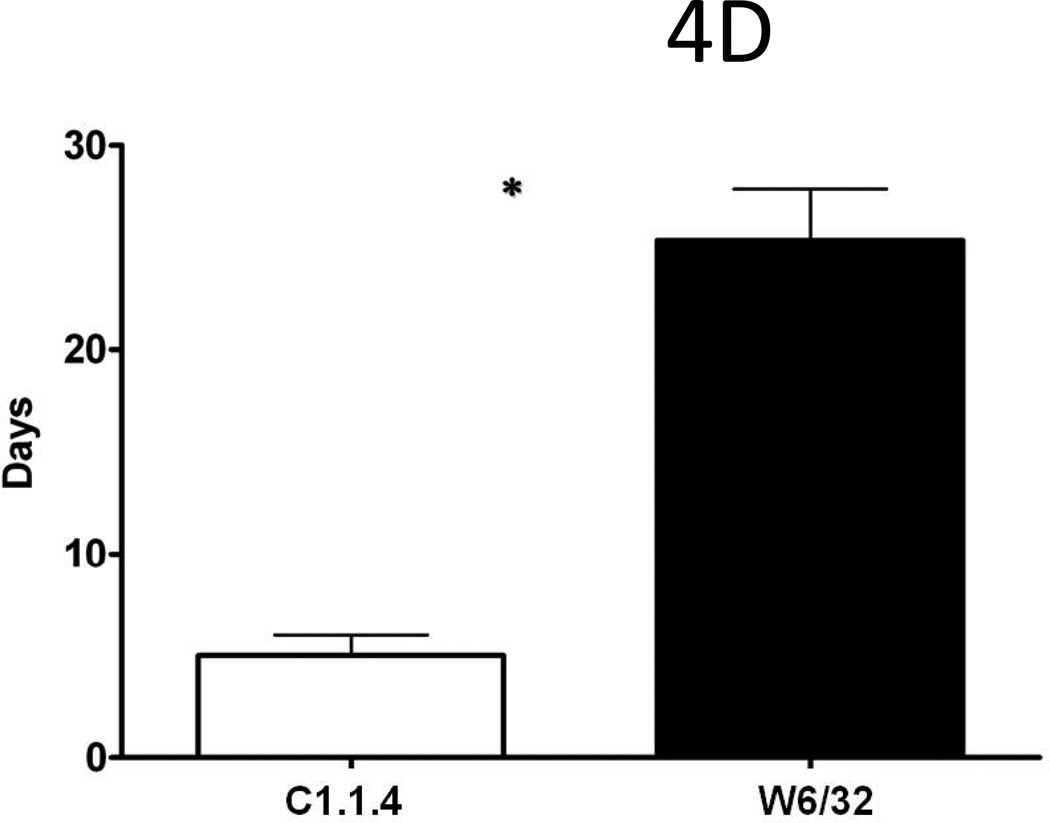
Donor HLA-A2 mice were pretreated with 50µg of W6/32 or C1.18.4 on day -2 and the hearts were then heterotopically transplanted into HLA-A2 sensitized C57BL/6 mice (n=5 each). C1.18.4 (A, D) or W6/32 (B, E) pretreated HLA-A2 donor hearts were recovered five days post-transplant into sensitized recipient mice. W6/32 (C, F) pretreated hearts were also recovered 15 days post-transplant into sensitized recipient mice. a: The donor HLA-A2 grafts were frozen fresh and sectioned at 5 μm on a cryostat and stained with the hamster anti-mouse CD54 Ab (A, B, C), rat anti-mouse CD 106 Ab (D, E, F). The figures shown are a representative of 3 separate experiments. Original magnification×400. Areas of increased expression of VCAM-1 and ICAM-1 expression in the hearts are indicated by arrows. b: Donor HLA-A2 mice were pretreated with 50µg of W6/32 (gray bars) recovered after 2 days and analyzed for expression of cytokines and chemokines The proteins were extracted from the donor HLA-A2 hearts. The cytokines in the protein extracts were analyzed using the 20 plex mouse cytokine solid phase sandwich Multiplex Bead immunoassays (Invitrogen, Carlsbad, CA, USA) as per the manufacturer’s protocols. The results are shown as mean concentration of cytokine in the donor HLA-A2 hearts in pg/ml ± SD of 3 separate experiments. The levels of inflammatory cytokines IL-1β, TNF-α, IL-6, IL-12 and chemokines MCP-1, MIG, MIP-1α and IL-8 were significantly (*p < 0.05) higher in the HLA-A2 donor hearts pretreated with 50µg of C1.18.4, 5 days post-transplant into recipient mice, when compared to the levels in HLA-A2 donor hearts pretreated with 50µg of W6/32 at the same time point. c: The donor HLA-A2 grafts were frozen fresh and sectioned at 5 μm on a cryostat and stained with the rat anti-mouse CD 4 Ab (A,B,C), rat anti-mouse CD8 Ab (D, E, F). The figures shown are a representative of 3 separate experiments. Original magnification×400. d: Sensitized recipient mice were treated with 0.5 ml of rabbit anti-mouse lymphocyte serum on days −3, 0, +3, +6 and +12. The pretreated hearts were heterotopically transplanted into HLA-A2 sensitized C57BL/6 mice and monitored for rejection as defined as the cessation of heartbeat. The results are expressed as mean survival day’s ± SD of 3 separate experiments. Donor HLA-A2 hearts pretreated with 50µg of W6/32 exhibited a significant prolongation (*p < 0.05) in the mean survival times when compared to donor HLA-A2 hearts pretreated with C1.18.4 post-transplant into ALS treated recipient mice.
Infiltration of CD4 and CD8 T cells in HLA-A2 allografts pretreated with 50µg of HLA class I Ab occurs fifteen days post-transplant in sensitized recipients
Immunohistochemical analysis of transplanted hearts pretreated with 50µg of W6/32 or C1.18.4 did not exhibit infiltration of CD4+ and CD8+ T cells on day 5 post transplant. However, on day 15 post transplant, donor HLA-A2 hearts pretreated with 50µg of W6/32 exhibited marked infiltration of CD4+ and CD8+T cells (Fig.4C). These results demonstrate that W6/32 pretreated donor HLA-A2 hearts overcome humoral rejection, but undergo cellular rejection 15 days post-transplant in sensitized recipients.
Pretreatment of donor hearts with 50µg of W6/32 abs did not significantly alter number of passenger leukocytes or their ability to activate immune responses
A confounding factor which would obviate our hypothesis is that Ab pretreatment of donor hearts may result in a decrease in number of passenger leukocytes resulting in decreased immune activation. W6/32 or C1.18.4 treated hearts were analyzed for the number of passenger leukocytes by staining with anti-CD3. There was no significant difference in number of CD3+ cells in hearts harvested from mice with and without pretreatment of anti-HLA Abs (3.2±0.9X106vs2.8 ±1.2X106,p=ns). FACS analysis showed no significant difference in number of CD4(34.6vs38.6%,p=ns), CD8(7.1vs5.5%,p=ns) or CD11(11.8vs13.1%,p=ns) cells in W6/32 treated hearts when compared to controls. These results demonstrate that pretreatment of donor hearts with subsaturating concentrations of anti-HLA Abs has no effect on number of passenger leukocytes.
To determine whether cells from donors treated with subsaturating concentrations of Abs are defective in activating immune responses, we stimulated T cells from C57Bl/6 with irradiated splenocytes from HLA-A2 transgenic C57/Bl6 mice treated for 2 days with 50µg of W6/32 or C1.18.4. Proliferation by thymidine uptake showed no significant difference between the W6.32 and C1.18.4 treated groups (4.6±0.7vs.4.3±0.5, p=NS). These results demonstrate that pretreatment with W6/32 does not significantly alter the ability of cells to activate immune responses.
Prolongation of the survival of HLA-A2 donor hearts pretreated with 50µg of HLA class I Ab transplanted in sensitized C57BL/6 mice treated with anti-mouse lymphocyte serum (ALS)
To determine whether inhibition of cellular immune responses in recipient mice will prolong survival of donor HLA-A2 hearts, recipient mice were treated with ALS. W6/32 pretreated donor hearts transplanted into ALS treated sensitized recipient mice exhibited significant (p<0.05) prolongation of mean survival time (MST, 25±4days) post-transplant (Fig.4C) in contrast to C1.18.1 pretreated donor hearts transplanted into ALS treated sensitized recipient mice (5±1days,Fig.4D). These results suggest that inhibition of cellular immune responses can lead to further prolongation of W6/32 pretreated donor HLA-A2 hearts in sensitized mice.
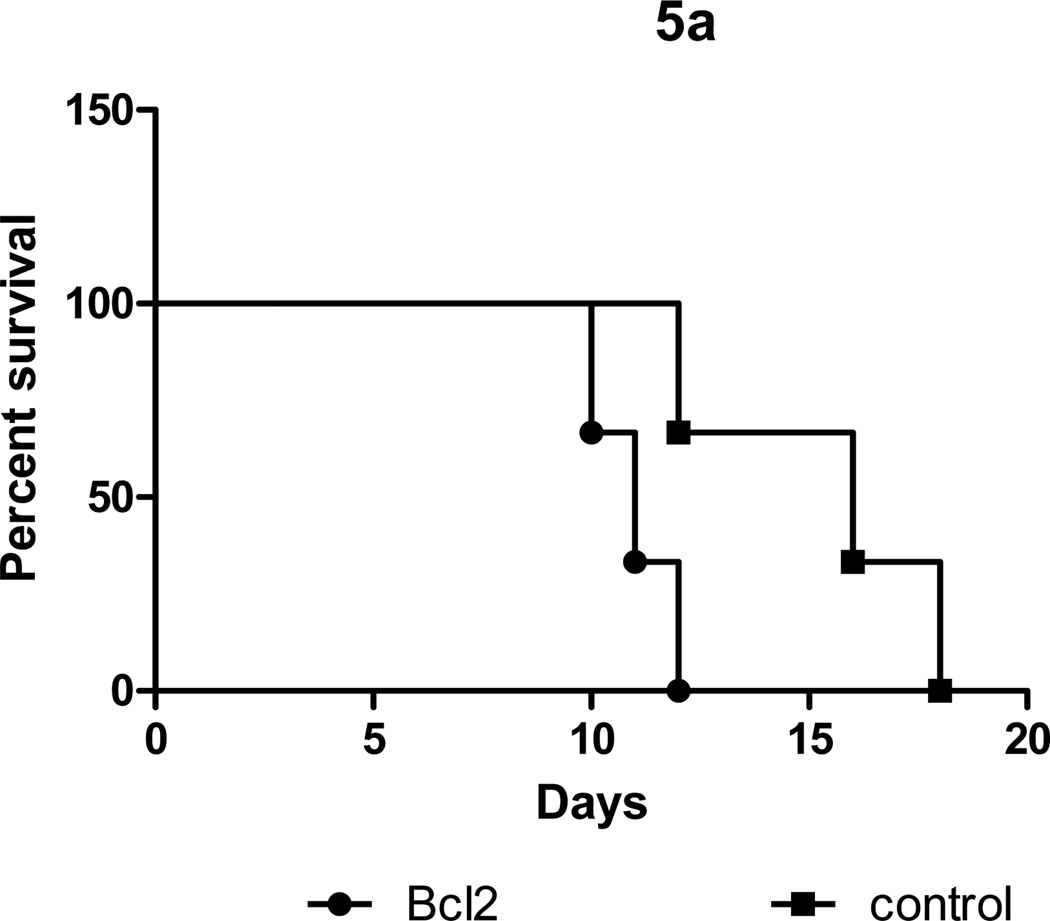
Silencing of Bcl2 expression results in loss of protection induced by pretreatment with subsaturating concentrations of anti-HLA Ab
To determine the role of Bcl-2 in induction of protection following pretreatment with subsaturating concentrations of anti-HLA Abs, we silenced expression of Bcl-2 using a shRNA. We exposed HLA-A2 donor mice with 50µg of W6/32 for two days and heterotopically transplanted their hearts into sensitized C57BL/6. We treated the hearts with 1µg of Bcl-2 specific or control shRNA prior to transplantation. Pretreatment of hearts with Bcl-2 specific shRNA resulted in rejection day 9±3 post transplant when compared to day 15±2 post transplant observed in mice treated with control shRNA (Fig.5,p<0.05). Analysis of Bcl2 expression in the Bcl2 shRNA treated grafts showed a 90% reduction (4.3±1.6 vs. 0.4±0.3, p<0.05) in the expression of Bcl2 when compared to control hearts treated with a nonspecific shRNA or untreated controls (Fig. 5b).These results indicate that Bcl2 plays a critical role in protection against Ab mediated rejection in sensitized recipients.
Figure 5. Silencing of the Bcl2 expression results in loss of protection induced by pretreatment with subsaturating concentrations of anti-HLA antibody.
Two days prior to transplantation, donor HLA-A2 mice were given intraperitoneal injections of 50 µg of HLA class I framework mouse monoclonal Ab (W6/32). One day prior to transplantation the animals were injected with 1µg of Bcl2 specific or control nonspecific shRNA plasmids (Invitrogen) or PBS (control) in inviojet PEI (Polyplus) by intravenous administration. The pretreated hearts were heterotopically transplanted into HLA-A2 sensitized C57BL/6 mice and monitored for rejection as defined as the cessation of heartbeat. A: Survival curves. B: Quantitative real time PCR analysis of Bcl2 expression (fold increase= expression in W6/32 antibody treated mice /expression in untreated mice).
Discussion
In this study we demonstrate that pretreatment of donor hearts with a mAb to HLA prior to transplant in sensitized recipients results in prolongation of the allograft (MST 15±2days) when compared to C1.18.4 pretreated HLA-A2 hearts (MST 5±1days). Histopathologic analysis of C1.18.4 pretreated HLA-A2 rejected grafts revealed hemorrhage, deposition of Ab and complement, and endothelial activation, confirming accelerated Ab mediated rejection. W6/32 pretreated hearts exhibited no hemorrhage, low level of Ab deposition, complement and endothelial activation at day 5, but exhibited infiltration of T cells at 15 days, consistent with cellular rejection (Figure 4). ALS treatment of recipient mice transplanted with W6/32 pretreated hearts resulted in further prolongation (MST 25±4 days, p<0.05). These observations mirror those noted in human kidney recipients where anti-graft Ab levels were reduced pretransplant followed by maintenance of the anti-graft Ab at low levels post-transplant and this sequence enabled donor grafts to survive without rejection even when Ab titers rebounded [9,27]. Similarly, analysis of sensitized renal transplant recipients wherein anti-HLA Abs were removed by immunoadsorption prior to transplant demonstrated that Abs returned in 4/7 patients without evidence of rejection[28]. These findings suggest that exposure of grafts to low levels of anti-MHC Abs prior to transplantation in sensitized hosts could result in prolonged allograft survival.
In our model, maximal prolongation in survival of donor hearts was achieved when donor hearts were exposed to low levels of W6/32 (50 μg) for two days. However, no significant prolongation of survival was noted when the donor hearts were exposed to very low or high concentrations of W6/32. Therefore the results indicate that prolongation of graft survival depends on two critical factors: 1) concentration of HLA class I Ab, and 2) time of exposure. Similar results have been obtained in previously described xenogeneic models of transplantation[14,29] where removal of complement and Ab resulted in inhibition of graft rejection[14,29–31]. However, complete depletion of donor Ab prevented graft accommodation and resulted in rejection of graft with return of Ab titers[14]. The data from these experiments suggested that low levels of anti-graft Ab are required at the time of transplant for accommodation to occur. A major potential benefit of our model is the use of a well characterized mAb that recognizes the frame work of the HLA class I molecule. This enables our study findings to be translated into a clinical setting where the same Ab could be used to pretreat the donors with varying HLA phenotypes. However, the current model has a limitation in that the target antigen recognized for rejection and Ab binding is the only mismatched antigen.
Xenotransplantation models have demonstrated that endothelial cells and smooth muscle cells of accommodated xenografts express “protective genes”, A20, Bcl-xL, Bcl-2 and HO-1 [32]. Anti-HLA allo-Abs as well as monoclonal anti-HLA induce changes in ECs consistent with accommodation [16,17]. Over-expression of anti-apoptotic genes A20, Bcl-xL and Bcl-2 in ECs of transplanted grafts block activation of transcription factor NF-κB and also suppressed activation of pro-inflammatory cytokines and apoptosis [12, 34, 35]. Over-expression of Bcl-2 and HO-1 in xenografts exposed to xenogeneic Ab and complement confer protection against HAR [36,37]. We sought to confirm these findings in our model of allogeneic transplantation. Analysis of cellular and molecular changes that afford protection to W6/32 pretreated HLA-A2 donor hearts (Fig.2) revealed marked increase in expression of Bcl-xl(5.5 fold), Bcl-2(5.5 fold), HO-1(4.4 fold), survivin(4.5 fold) and a significant(p<0.05) inhibition in expression of ICAM-1(3.2 fold), VCAM-1(3.2 fold) and PECAM (2.3 fold). Moreover, inhibition of Bcl2 expression by shRNA in donor hearts treated with subsaturating concentrations of anti-HLA Ab demonstrated that inhibition of protective gene expression resulted in a significant reduction in allograft survival.
Earlier studies in xenotransplantation have demonstrated that long term survival is dependent on expression of anti-apoptotic genes such as A20 and Bcl-2 [27,32,38] and inhibition of proinflammatory cytokines, chemokines and adhesion molecules [12,34,35]. Anti-apoptotic proteins especially the Bcl-2 family has been implicated in inhibition of caspases, a group of enzymes involved in cleavage of pro-form of the cytokine proteins into their active components. We demonstrate a significant reduction in levels of inflammatory cytokines IL-1β, TNF-α, IL-6, IL-12, MCP-1, MIG, MIP-1α and IL-8 in recipient mice transplanted with hearts pretreated with anti-HLA Abs compared to control Ab. These results support the hypothesis that pretreatment of donor grafts with W6/32 results in activation of survival signals in allografts that enable it to overcome humoral rejection.
In summary, we have shown that pretreatment of donor hearts with low levels of HLA class I Ab prior to transplant can confer protection against humoral rejection in highly sensitized recipients. Resistance is conferred by a marked increase in expression of anti-apoptotic genes Bcl-xL, Bcl-2, and HO-1 and reduction in expression of adhesion molecules, inflammatory cytokines and chemokines. We envisage that results from this study should allow development of strategies for using mAb towards HLA class I to promote accommodation in allografts prior to transplantation in clinical settings.
Methods
Mice and treatments
HLA-A2-transgenic (HLA-A2) C57BL/6 mice were a gift from Dr. Victor H. Engelhard (University of Virginia, Charlottesville, VA). (18). C57BL/6 mice were obtained from the National Cancer Institute. All mice were housed under pathogen-free conditions and used at 4–8 weeks of age. All experiments were performed in accordance with guidelines established by the Washington University School of Medicine Animal Studies Committee. C57BL/6 were sensitized by two weekly intraperitoneal (i.p.) injections of 107 splenocytes from HLA-A2 mice and development of anti-HLA-A2 Abs was detected by flow cytometry [18]. Two days prior to transplant, donor HLA-A2 mice were administered 50µg i.p. of HLA class I framework mouse mAb (W6/32) or isotype control Ab (C1.18.4 ). A group of recipient mice were administered 0.5 ml i.p. of rabbit anti-mouse lymphocyte serum (ALS, Sigma, Saint Louis, MO) on day −3, 0, +3, +6 and +12 of transplant.
Heterotopic cardiac transplantation
HLA-A2 C57BL/6 mice were transplanted heterotopically in HLA-A2 sensitized C57Bl/6 as previously described [18]. Rejection was defined by cessation of a palpable heartbeat and was confirmed histologically.
Histology
Tissue sections were stained with primary Abs rat anti-mouse Ly-6G and LY-6C (RB6-8C5) for neutrophil and monocyte lineages, anti-CD11b(M1-70), anti-CD106(MVCAM.A), anti-CD4(H129.19), anti-IgG, anti-CD8(53–6.7) Abs, anti-C4, or hamster anti-mouse CD54(3E2) and developed as described previously [18]. Pre-treated hearts were blocked with unconjugated-anti-mouse antibody and then stained with 1000μg of w6/32 and counterstained with peroxidase conjugated anti-mouse antibodies.
Gene array analysis
Expression profiles of apoptotic and inflammatory genes in hearts pretreated with C1.18.4 or W6/32 were analyzed 5 days post-transplantation, using apoptotic and EC biology gene arrays (SA Biosciences, Frederick, MD).
Western blotting
Recovered hearts were homogenized in T-PER Reagent and used for western blot. Membrane was incubated with rabbit anti-mouse Bcl-2, anti-mouse HO-1, anti-mouse Bcl-xl (cell signaling technologies, Danvers, MA) overnight, washed with PBS-Tween-20, incubated with HRP-conjugated Ab and developed with picoECL kit.
Luminex Assay
Levels of cytokines and in donor HLA-A2 allografts pretreated with 50µg of W6/32 and C1.18.4 were analyzed with 20 plex mouse cytokine luminex assay (Invitrogen, Carlsbad, CA)[19].
Silencing of Bcl2 expression by shRNA administration
Two days prior to transplant, donor HLA-A2 mice were given intraperitoneal injections of 50µg of W6/32. On transplant day, animals were injected with 1µg of Bcl2 specific or control shRNA plasmids (Invitrogen) in inviojet PEI (Polyplus) by intravenous administration.
Statistical analysis
All statistical comparisons were assessed by means of 2 tailed Students t-test using the Prism 4.0 software (Graphpad) with α set at p < 0.05.
Acknowledgments
Funding sources: This work is supported by NIH/NHLBI/NIAID HL66452 & HL092514 (TM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
NF, SR, KN – performed experiments, analyzed date and manuscript preparation
WL- performed experiments
DSN, M J, WC and TM - analyzed date and manuscript preparation
References
- 1.Tanabe K, Takahashi K, Sonda K, Tokumoto T, Ishikawa N, Kawai T, Fuchinoue S, Oshima T, Yagisawa T, Nakazawa H, Goya N, Koga S, Kawaguchi H, Ito K, Toma H, Agishi T, Ota K. Long-term results of ABO-incompatible living kidney transplantation: a single-center experience. Transplantation. 1998;65:224–228. doi: 10.1097/00007890-199801270-00014. [DOI] [PubMed] [Google Scholar]
- 2.Alkhunaizi AM, de Mattos AM, Barry JM, Bennett WM, Norman DJ. Renal transplantation across the ABO barrier using A2 kidneys. Transplantation. 1999;67:1319–1324. doi: 10.1097/00007890-199905270-00005. [DOI] [PubMed] [Google Scholar]
- 3.Alexandre GP, De Bruyere M, Squifflet JP, Moriau M, Latinne D, Pirson Y. Human ABO-incompatible living donor renal homografts. Neth J Med. 1985;28:231–234. [PubMed] [Google Scholar]
- 4.Alexandre GP, Squifflet JP, De Bruyere M, Latinne D, Reding R, Gianello P, Carlier M, Pirson Y. Present experiences in a series of 26 ABO-incompatible living donor renal allografts. Transplant Proc. 1987;19:4538–4542. [PubMed] [Google Scholar]
- 5.Colvin RB, Smith RN. Antibody-mediated organ-allograft rejection. Nat Rev Immunol. 2005;5:807–817. doi: 10.1038/nri1702. [DOI] [PubMed] [Google Scholar]
- 6.Kupin WL, Venkat KK, Hayashi H, Mozes MF, Oh HK, Watt R. Removal of lymphocytotoxic antibodies by pretransplant immunoadsorption therapy in highly sensitized renal transplant recipients. Transplantation. 1991;51:324–329. doi: 10.1097/00007890-199102000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Palmer A, Taube D, Welsh K, Bewick M, Gjorstrup P, Thick M. Removal of anti-HLA antibodies by extracorporeal immunoadsorption to enable renal transplantation. Lancet. 1989;1:10–12. doi: 10.1016/s0140-6736(89)91672-3. [DOI] [PubMed] [Google Scholar]
- 8.Reisaeter AV, Leivestad T, Albrechtsen D, Holdaas H, Hartmann A, Sodal G, Flatmark A, Fauchald P. Pretransplant plasma exchange or immunoadsorption facilitates renal transplantation in immunized patients. Transplantation. 1995;60:242–248. doi: 10.1097/00007890-199508000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Ross CN, Gaskin G, Gregor-Macgregor S, Patel AA, Davey NJ, Lechler RI, Williams G, Rees AJ, Pusey CD. Renal transplantation following immunoadsorption in highly sensitized recipients. Transplantation. 1993;55:785–789. doi: 10.1097/00007890-199304000-00019. [DOI] [PubMed] [Google Scholar]
- 10.Glotz D, Antoine C, Julia P, Suberbielle-Boissel C, Boudjeltia S, Fraoui R, Hacen C, Duboust A, Bariety J. Desensitization and subsequent kidney transplantation of patients using intravenous immunoglobulins (IVIg) Am J Transplant. 2002;2:758–760. doi: 10.1034/j.1600-6143.2002.20809.x. [DOI] [PubMed] [Google Scholar]
- 11.Glotz D, Haymann JP, Francois A, Sansonnetti N, Druet P. Suppression of HLA-specific alloantibodies by high-dose IVIG. Transplantation. 1993;56:335. doi: 10.1097/00007890-199308000-00015. [DOI] [PubMed] [Google Scholar]
- 12.Bach FH, Ferran C, Hechenleitner P, Mark W, Koyamada N, Miyatake T, Winkler H, Badrichani A, Candinas D, Hancock WW. Accommodation of vascularized xenografts: expression of "protective genes" by donor endothelial cells in a host Th2 cytokine environment. Nat Med. 1997;3:196–204. doi: 10.1038/nm0297-196. [DOI] [PubMed] [Google Scholar]
- 13.Fischel RJ, Bolman RM, 3rd, Platt JL, Najarian JS, Bach FH, Matas AJ. Removal of IgM anti-endothelial antibodies results in prolonged cardiac xenograft survival. Transplant Proc. 1990;22:1077–1078. [PubMed] [Google Scholar]
- 14.Hasan R, Van den Bogaerde J, Forty J, Wright L, Wallwork J, White DJ. Xenograft adaptation is dependent on the presence of antispecies antibody, not prolonged residence in the recipient. Transplant Proc. 1992;24:531–532. [PubMed] [Google Scholar]
- 15.Leventhal JR, Sakiyalak P, Witson J, Simone P, Matas AJ, Bolman RM, Dalmasso AP. The synergistic effect of combined antibody and complement depletion on discordant cardiac xenograft survival in nonhuman primates. Transplantation. 1994;57:974–978. [PubMed] [Google Scholar]
- 16.Narayanan K, Phelan D, Jendrisak MD, Mohanakumar T. HLA class I antibody mediated accommodation of endothelial cells via the activation of P13K/cAMP dependent PKA pathway. Transplant Immunol. 2006;15:187–197. doi: 10.1016/j.trim.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Narayanan K, Jaramillo A, Phelan DL, Mohanakumar T. Pre-exposure to sub-saturating concentrations of HLA class I antibodies confers resistance to endothelial cells against antibody complement-mediated lysis by regulating Bad through the phosphatidylinositol 3-kinase/Akt pathway. Eur J Immunol. 2004;34:2303–2312. doi: 10.1002/eji.200324843. [DOI] [PubMed] [Google Scholar]
- 18.Smith CR, Jaramillo A, Liu W, Tu Y, Kaleem Z, Swanson CJ, Mohanakumar T. CD4+ T cell recognition of a single discordant HLA-A2-transgenic molecule through the indirect antigen presentation pathway induces acute rejection of murine cardiac allografts. Transplantation. 2001;71:1640–1648. doi: 10.1097/00007890-200106150-00025. [DOI] [PubMed] [Google Scholar]
- 19.Bharat A, Narayanan K, Street T, Fields RC, Steward N, Aloush A, Meyers B, Schuessler R, Trulock EP, Patterson GA, Mohanakumar T. Early posttransplant inflammation promotes the development of alloimmunity and chronic human lung allograft rejection. Transplantation. 2007;83:150–158. doi: 10.1097/01.tp.0000250579.08042.b6. [DOI] [PubMed] [Google Scholar]
- 20.Glotz D, Haymann J, Naiudet P, Lang P, Druet P, Bairety J. Successful kidney transplantation of immunized patients after desensitization with normal human polyclonal immunoglobulins. Transplant Proc. 1995;27:1038. [PubMed] [Google Scholar]
- 21.Collins AB, Schneeberger EE, Pascual MA, Saidman SL, Williams WW, Tolkoff-Rubin N, Cosimi AB, Colvin RB. Complement activation in acute humoral renal allograft rejection: diagnostic significance of C4d deposits in peritubular capillaries. J Am Soc Nephrol. 1999;10:2208–2214. doi: 10.1681/ASN.V10102208. [DOI] [PubMed] [Google Scholar]
- 22.Cornell LD, Colvin RB. Chronic allograft nephropathy. Curr Opin Nephrol Hypertens. 2005;14:229–234. doi: 10.1097/01.mnh.0000165888.83125.07. [DOI] [PubMed] [Google Scholar]
- 23.Hornick P, Rose M. Chronic rejection in the heart. Methods Mol Biol. 2006;333:131–144. doi: 10.1385/1-59745-049-9:131. [DOI] [PubMed] [Google Scholar]
- 24.Glotz D, Antoine C, Duboust A. Antidonor antibodies and transplantation: how to deal with them before and after transplantation. Transplantation. 2005;79:S30–S32. doi: 10.1097/01.tp.0000153297.11006.13. [DOI] [PubMed] [Google Scholar]
- 25.Bach FH, Ferran C, Candinas D, Miyatake T, Koyamada N, Mark W, Hechenleitner P, Hancock WW. Accommodation of xenografts: expression of "protective genes" in endothelial and smooth muscle cells. Transplant Proc. 1997;29:56–58. doi: 10.1016/s0041-1345(96)00009-7. [DOI] [PubMed] [Google Scholar]
- 26.Platt JL, Lindman BJ, Geller RL, Noreen HJ, Swanson JL, Dalmasso AP, Bach FH. The role of natural antibodies in the activation of xenogenic endothelial cells. Transplantation. 1991;52:1037–1043. doi: 10.1097/00007890-199112000-00019. [DOI] [PubMed] [Google Scholar]
- 27.Semiletova NV, Shen XD, Baibakov B, Feldman DM, Mukherjee K, Frank JM, Stepkowski SM, Busuttil RW, Kupiec-Weglinski JW, Ghobrial RM. Inhibition of chronic rejection by antibody induced vascular accommodation in fully allogeneic heart allografts. Transplantation. 2005;80:1535–1540. doi: 10.1097/01.tp.0000188952.10692.18. [DOI] [PubMed] [Google Scholar]
- 28.Salama AD, Delikouras A, Pusey CD, Cook HT, Bhangal G, Lechler RI, Dorling A. Transplant accommodation in highly sensitized patients: A potential role for Bcl-xL and alloantibody. Amer J Transplant. 2001;1:260–269. doi: 10.1034/j.1600-6143.2001.001003260.x. [DOI] [PubMed] [Google Scholar]
- 29.Williams JM. Acute vascular rejection and accommodation: divergent outcomes of the humoral response to organ transplantation. Transplantation. 2004;78:1471–1478. doi: 10.1097/01.tp.0000140770.81537.64. [DOI] [PubMed] [Google Scholar]
- 30.Cooper DK, Good AH, Koren E, Oriol R, Malcolm AJ, Ippolito RM, Neethling FA, Ye Y, Romano E, Zuhdi N. Identification of alpha-galactosyl and other carbohydrate epitopes that are bound by human anti-pig antibodies: relevance to discordant xenografting in man. Transpl Immunol. 1993;1:198–205. doi: 10.1016/0966-3274(93)90047-c. [DOI] [PubMed] [Google Scholar]
- 31.Cooper DK, Ye Y, Niekrasz M, Kehoe M, Martin M, Neethling FA, Kosanke S, DeBault LE, Worsley G, Zuhdi N, et al. Specific intravenous carbohydrate therapy. A new concept in inhibiting antibody-mediated rejection--experience with ABO-incompatible cardiac allografting in the baboon. Transplantation. 1993;56:769–777. doi: 10.1097/00007890-199310000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Soares MP, Lin Y, Sato K, Stuhlmeier KM, Bach FH. Accommodation. Immunol Today. 1999;20:434–437. doi: 10.1016/s0167-5699(99)01530-3. [DOI] [PubMed] [Google Scholar]
- 33.Jin YP, Fishbein MC, Said JW, Jindra PT, Rajalingam R, Rozengurt E, Reed EF. Anti-HLA class I antibody-mediated activation of the PI3K/Akt signaling pathway and induction of Bcl-2 and Bcl-xL expression in endothelial cells. Hum Immunol. 2004;65:291–302. doi: 10.1016/j.humimm.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 34.Ferran C, Stroka DM, Badrichani AZ, Cooper JT, Wrighton CJ, Soares M, Grey ST, Bach FH. A20 inhibits NF-kappaB activation in endothelial cells without sensitizing to tumor necrosis factor-mediated apoptosis. Blood. 1998;91:2249–2258. [PubMed] [Google Scholar]
- 35.Koch CA, Khalpey ZI, Platt JL. Accommodation: preventing injury in transplantation and disease. J Immunol. 2004;172:5143–5148. doi: 10.4049/jimmunol.172.9.5143. [DOI] [PubMed] [Google Scholar]
- 36.Zhen-Wei X, Jian-Le S, Qi Q, Wen-Wei Z, Xue-Hong Z, Zi-Li Z. Heme oxygenase-1 improves the survival of discordant cardiac xenograft through its anti-inflammatory and anti-apoptotic effects. Pediatr Transplant. 2007;11:850–859. doi: 10.1111/j.1399-3046.2007.00701.x. [DOI] [PubMed] [Google Scholar]
- 37.Soares MP, Lin Y, Anrather J, Csizmadia E, Takigami K, Sato K, Grey ST, Colvin RB, Choi AM, Poss KD, Bach FH. Expression of heme oxygenase-1 can determine cardiac xenograft survival. Nat Med. 1998;4:1073–1077. doi: 10.1038/2063. [DOI] [PubMed] [Google Scholar]
- 38.Wang N, Lee JM, Tobiasch E, Csizmadia E, Smith NR, Gollackes B, Robson SC, Bach FH, Lin Y. Induction of xenograft accommodation by modulation of elicited antibody responses1 2. Transplantation. 2002;74:334–345. doi: 10.1097/00007890-200208150-00008. [DOI] [PubMed] [Google Scholar]