Abstract
BACKGROUND
Genetic influences may be discerned in families that have multiple affected members and may manifest as an earlier age of cancer diagnosis. In this study we determine whether cancers develop at an earlier age in multiplex Familial Barrett’s Esophagus (FBE) kindreds, defined by 3 or more members affected by Barrett’s esophagus (BE) or esophageal adenocarcinoma (EAC).
METHODS
Information on BE/EAC risk factors and family history was collected from probands at eight tertiary care academic hospitals. Age of cancer diagnosis and other risk factors were compared between non-familial (no affected relatives), duplex (two affected relatives), and multiplex (three or more affected relatives) FBE kindreds.
RESULTS
The study included 830 non-familial, 274 duplex and 41 multiplex FBE kindreds with 274, 133 and 43 EAC and 566, 288 and 103 BE cases, respectively. Multivariable mixed models adjusting for familial correlations showed that multiplex kindreds were associated with a younger age of cancer diagnosis (p = 0.0186). Median age of cancer diagnosis was significantly younger in multiplex compared to duplex and non-familial kindreds (57 vs. 62 vs. 63 yrs, respectively, p = 0.0448). Mean body mass index (BMI) was significantly lower in multiplex kindreds (p = 0.0033) as was smoking (p < 0.0001), and reported regurgitation (p = 0.0014).
CONCLUSIONS
Members of multiplex FBE kindreds develop EAC at an earlier age compared to non-familial EAC cases. Multiplex kindreds do not have a higher proportion of common risk factors for EAC, suggesting that this aggregation might be related to a genetic factor.
IMPACT
These findings indicate that efforts to identify susceptibility genes for BE and EAC will need to focus on multiplex kindreds.
Keywords: Esophageal adenocarcinoma, Barrett’s esophagus, genetics, family history
INTRODUCTION
Increasing age, male gender, white race, chronic gastro-esophageal reflux disease (GERD), obesity, and smoking are recognized risk factors for Barrett’s esophagus (BE) and esophageal adenocarcinoma (EAC) -including esophagogastric junctional adenocarcinoma (EGJAC)-.(1, 2) Consensus guidelines for screening of BE are based on these risk factors.(3) We and others have also reported the aggregation of BE and EAC in families and this syndrome has been termed Familial Barrett’s esophagus (FBE).(4, 5) This aggregation suggests a biological relationship between BE and EAC. Less than 10% of probands with these diseases are members of FBE kindreds.(6)
Familial aggregation of diseases may be caused by common environmental exposures in family members, a genetic predisposition to disease, or both. A younger age of disease diagnosis in families is often an indication that family members share a genetic susceptibility. Although associated with gastroesophageal reflux, the development of BE itself is asymptomatic, and therefore the incident age of BE cannot be determined. In a previous study, we found that the age of EAC diagnosis in FBE kindreds was the same as in non-familial kindreds.(7) However, a subsequent segregation analysis demonstrated that the pattern of aggregation of BE and its associated cancers in FBE pedigrees was clearly more consistent with a complex genetic model than a purely environmental one.(8) For complex diseases such as BE and cancer, genetic influences such as a younger age of disease onset may be more evident in pedigrees that have 3 or more affecteds. Thus, we decided to perform an updated analysis of age of cancer diagnosis concentrating on the small subset of FBE kindreds with 3 or more family members affected with BE, or EAC. The primary aim of this study was to compare age of EAC diagnosis and other known risk factors in kindreds with 3 or more affected members termed multiplex FBE, kindreds with 2 affected members termed duplex FBE, and non-familial kindreds.
METHODS
Recruitment
The recruitment methodology for probands with BE, and EAC and their families for this ongoing prospective multi-center FBE study has been previously described.(4, 6, 9, 10) The Familial Barrett’s Esophagus Consortium is an ongoing collaboration started in 1998. The trial, registered at clinicaltrials.gov with the identifier NCT00288119 aims to ultimately identify putative susceptibility genes for BE and its associated cancers. Individuals with BE, EAC, or EGJAC have been recruited at 8 tertiary care academic hospitals in the United States at varying time periods over the past 8 years.(10) Eligible patients were identified in the endoscopy suite of participating institutions with IRB approval. These include individuals with newly-identified BE, those undergoing surveillance endoscopy for BE, patients with newly-identified EAC and patients with known EAC undergoing endoscopy for palliation or staging. After providing informed consent, subjects are given a self-administerd FBE questionnaire. Probands, defined as the first known affected member in a family, have the opportunity to discuss and clarify family history with their relatives before mailing back the questionnaire. The questionnaire is structured to systematically collect data on gastro-esophageal reflux symptoms, risk factors for BE and, EAC such as age, gender, race, gastroesophageal reflux symptoms (patterned on the validated Mayo GERQ) (11), and self reported obesity measures at 1, 5, and 10 years prior to diagnosis and family history of BE, EAC, stomach and throat cancer (which may be incorrect ways of reporting esophageal cancer). Pedigrees are then constructed from the questionnaire. Permission is obtained to contact eligible relatives, defined as a first-degree relative of a proband (or confirmed affected relative). Attempts are made to confirm the diagnosis for all family members reported to have a history of BE or a suspected esophageal cancer. Eligible family members are invited to participate over the mail, with a second attempt if there is no reply after 1 month.. For relatives who are deceased, the next of kin are asked to complete the questionnaire, specifically the age of cancer diagnosis. Institutional review boards for human investigation at each individual hospital participating in the study have approved this protocol.
Definitions
For probands as well as relatives, the definition of BE required clear documentation of endoscopic evidence of BE in the tubular esophagus in the endoscopy report, as well as histological evidence of intestinal metaplasia on surgical pathology report to satisfy study criteria. Biopsies reported to be obtained from the gastroesophageal junction, an irregular Z-line, or at the cardia were not considered to be diagnostic of BE. EAC was defined as a mass predominantly involving the tubular esophagus or the esophagogastric junction with histological evidence of an adenocarcinoma. A designated single expert gastrointestinal pathologist reviewed all biopsies from within an institution. For relatives reported with esophageal cancer or BE, records were requested from outside hospitals.
Kindreds were classified as confirmed FBE when the diagnosis of BE or EAC was confirmed in the proband and at least one affected relative by review of records. Kindreds were classified as possible FBE diagnosis when the proband reported affected family members but medical records on the family member could not be obtained. Multiplex FBE kindreds were defined as kindreds with confirmation of diagnosis in at least two members of the kindred plus additional family members who were reported to be affected with BE or cancer in whom diagnosis was confirmed or suspected. Individuals were classified as having non-familial disease if the proband reported no affected family member or the proband reported an affected family member but the family member did not meet ascertainment criteria for BE or EAC upon review of medical records. Duplex FBE kindreds were classified as confirmed affection status in two members of the kindred or confirmed affection status in one member plus suspected affection status in second member and no report of affection status in any other members of the kindred. Only individuals with a confirmed diagnosis where included in the analysis.
Statistical Analysis
We assessed differences in the age of diagnosis and other variables of interest between the three family groups (non-familial, duplex and multiplex). For this analysis, data was available o all subjects in the duplex and multiplex kindreds, and the probands in the non-familial group (as unaffected relatives do not complete the questionnaire). The specific variables of interest were age at diagnosis, gender, obesity, race, acid regurgitation, heartburn, smoking and alcohol consumption. Obesity was examined by comparing the BMI (in kg/m2) calculated 1 year before diagnosis. When comparing the differences by family type in the proportion of other cancers, all subjects for all family types were used, utilizing the information reported by the probands from non-familial pedigrees as a control. Fisher’s exact tests or Chi-square tests were used to compare categorical variables generating 2-sided p-values and the Wilcoxon test was used to compare average age at diagnosis and body mass index (BMI) generating two-sided p-values among different family history types (non-familial, duplex and multiplex). P-values < 0.05 were considered statistically significant. We also used multivariable mixed linear models to explore the association between age of diagnosis and family type, allowing for adjustment for familial correlations of EAC. Statistical Analysis Software (Version 9.2, SAS, Inc., Cary, NC) was used for all analyses.
RESULTS
A total of 1146 kindreds from the same number of probands were included in the study, 830 (72.4%) non-familial kindreds, 275 (23.9%) duplex kindreds and 41 (3.7%) multiplex kindreds. (Table 1). The cohort included a total of 1397 individuals affected with either BE or EAC, of which 1146 were probands and 251 were relatives. There were 947 cases of BE, of these 566 were in the non-familial kindreds, 288 in duplex kindreds and 103 in multiplex kindreds. A total of 450 cases of EAC are included, 274 were identified in non-familial kindreds, 133 in duplex kindreds and 43 in multiplex kindreds. Of the affected individuals, 80.8% were male and 19.2% were female, and the racial distribution was 98% white and 2% other.
Table 1.
Individuals with BE and esophageal cancer, by family type
| Nonfamilial | Duplex | Multiplex | p-value | |
|---|---|---|---|---|
| Probands | 830 | 275 | 41 | |
| Relatives | N/A | 146 | 105 | |
| Total individuals | 830 | 421 | 146 | |
|
| ||||
| BE | 556 | 288 | 103 | |
| Esophageal Cancer | 274 | 133 | 43 | |
|
| ||||
| Gender | ||||
| Men | 693/830(83%) | 326/421(77%) | 110/146(75%) | 0.0076^ |
| Women | 137/830(17%) | 95/421(23%) | 36/146(25%) | |
|
| ||||
| White Race | 803/828(97%) | 390/392(99 %) | 137/137(100%) | 0.0018* |
Fishers exact test p-value;
Chi-square test p-value
Cancer stage
The cancer stage was not significantly different between the different types of kindreds, although there was an insignificantly higher proportion of stage III cancer (62.5%) and stage IV cancer (25%) in the multiplex group. (Table 2).
Table 2.
Age of diagnosis and cancer stage for individuals with esophageal cancer
| Nonfamilial | Duplex | Multiplex | ||
|---|---|---|---|---|
| Average Age at Cancer | ||||
| Diagnosis | 62.9 (n=274) | 62.6 (n=133) | 57.6 (n=43) | 0.0448# |
|
| ||||
| Cancer Stage | ||||
| I | 55/195(28.2%) | 13/57(22.8%) | 2/16(12.5%) | 0.0535* |
| II | 49/195(25.1%) | 15/57(26.3%) | 0(0%) | |
| III | 66/195(33.8%) | 19/57(33.3%) | 10/16(62.5%) | |
| IV | 25/195(12.8%) | 10/57(17.5%) | 4/16(25%) | |
Wilcoxon test p-value;
Fishers exact test p-value;
Chi-square test p-value
Age at diagnosis
The median age at the time of diagnosis of EAC was 63 years for non-familial cases, 62 years for individuals in duplex kindreds, and 57 years for those in multiplex kindreds (p=0.0448). (Table 2). Figure 1 shows a composite histogram for the age at the time of diagnosis by family type, displaying a leftward shift in the age at diagnosis distribution for multiplex kindreds when compared to the other groups.
Figure 1.
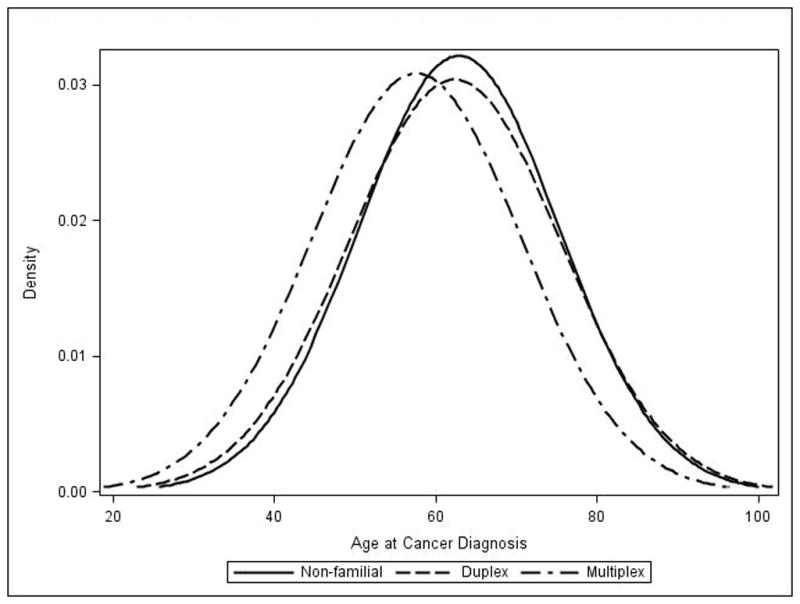
Overlapping histograms for age at the time of diagnosis, according to family type.
Mixed modeling further supported the association between family history and age at diagnosis of cancer after adjusting for familial correlations and controlling for multiple potential confounders (Table 3). This model only includes subjects with a diagnosis of EAC. Being an individual from a multiplex kindred was associated with a younger age of diagnosis (p=0.018). A higher body mass index was also associated a younger age of diagnosis of cancer (p=0.007). All other variables of interest were not significant in this model. Hence, the final model only included adjustment for BMI and familial correlations and showed that being a member of a multiplex kindred was still inversely associated (p=0.0280, Table 3) with the age of diagnosis.
Table 3.
Multivariable Mixed models for the association between age at diagnosis of esophageal cancer and family type.
| Multivariable model (adjusted for familial correlations) (n=357) | p-value | ||
|---|---|---|---|
| Family type | Multiplex vs. others | 0.0180 | |
| Duplex vs. others | 0.7220 | ||
| Gender | 0.1996 | ||
| Race | 0.2101 | ||
| BMI | 0.0070 | ||
| Smoking | 0.6736 | ||
| Heartburn | 0.9490 | ||
| Regurgitation | 0.0850 | ||
| Family Size | 0.3408 | ||
|
| |||
| Multivariable model (adjusted for familial correlations, BMI) (n=279) | p-value | ||
|
| |||
| Family type | Multiplex vs. others | 0.0280 | |
| Duplex vs. others | 0.3966 | ||
Symptoms and exposures
Information on symptoms and exposures was available for probands with BE or EAC and affected relatives (Table 4). There was a statistically significant difference in the number of individuals that had regurgitation, with a rate of 54.6% from multiplex kindreds, 66.25% from non-familial kindreds and 57% from duplex kindreds (p = 0.0014). There was no discernable difference by kindred type in the number of subjects that reported heartburn, with approximately 70% of subjects in all three groups endorsing the symptom.
Table 4.
Symptoms and exposures according to family type (includes affected individuals in the non-familial group and all individuals in the duplex and multiplex kindreds)
| Nonfamilial | Duplex | Multiplex | p-value | |
|---|---|---|---|---|
| BE/EAC/EGJAC | ||||
| Affected | 830 | 421 | 146 | |
| Unaffected | N/A | 3071 | 1065 | |
| Total | 830 | 3438 | 1211 | |
|
| ||||
| Heartburn Present | ||||
| Yes | 507/724(70.03%) | 339/528(64.20%) | 178/263(67.68%) | 0.2294* |
| No | 211/724(29.14%) | 183/528(34.66%) | 84/263(31.94%) | |
| Unknown | 6/724(0.83%) | 6/528(1.14%) | 1/263(0.38%) | |
|
| ||||
| Regurgitation Present | ||||
| Yes | 475/717(66.25%) | 301/525(57.33%) | 144/264(54.55%) | 0.0014* |
| No | 233/717(32.50%) | 216/525(41.14%) | 118/264(44.70%) | |
| Unknown | 9/717(1.26%) | 8/525(1.52%) | 2/264(0.76%) | |
|
| ||||
| Smoking | ||||
| Yes | 466/725(64.28%) | 271/529(51.23%) | 120/263(45.63%) | 6.3E-8* |
| No | 251/725(34.62%) | 253/529(47.83%) | 140/263(53.23%) | |
| Unknown | 8/725(1.10%) | 5/529(0.95%) | 3/263(1.14%) | |
|
| ||||
| Alcohol | ||||
| Yes | 581/723(80.36%) | 446/527(84.63%) | 238/261(91.19%) | 8.2E-4* |
| No | 126/723(17.43%) | 74/527(14.04%) | 22/261(8.43%) | |
| Unknown | 16/723(2.21%) | 7/527(1.33%) | 1/261(0.38%) | |
|
| ||||
| Average BMI | 29.6 | 28.9 | 28.6 | 0.0033# |
Wilcoxon test p-value;
Fishers exact test p-value;
Chi-square test p-value
The proportion of those that reported smoking was higher in the non-familial kindreds (64.28%) than in the duplex and multiplex kindreds (51.23% and 45.63% respectively; p <0.0001). In contrast, more individuals reported alcohol consumption in the multiplex kindreds (91.19%) when compared to the non-familial and duplex kindreds (80.36% and 84.63%, respectively; p=0.0013). The median body mass index (BMI) was 28.24 for non-familial cases, 27.8 for duplex kindreds and 27.26 for multiplex kindreds (p=0.0033). Restricting the analysis to only patients diagnosed with cancer, there were no differences by family type for heartburn, regurgitation, smoking status, alcohol status or BMI.
Other types of cancer
Self-reported cancer information was obtained from probands regarding their relative’s cancer status via the questionnaire (Table 5). In multiplex kindreds, 13.88% of individuals reported a cancer other than esophageal, this proportion was 16.5% in the duplex kindreds and 16.62% in the non-familial cases (p=0.2407). In this latter category, the most common cancers reported were in the breast, skin, ovary and prostate. Among those with a family history of esophageal cancer, the most common other types of cancer reported were breast, lung, skin and prostate. Multiplex kindreds had a statistically significant greater chance of lung (p=0.0021), bone (p=0.0027) and liver (p=0.0234) cancer as compared to the other family types.
Table 5. Cancers other than esophageal for all individuals (includes relatives without esophageal cancer), according to family type.
(Note: Family type refers to the number of individuals in the family with esophageal cancer).
| Family Type | Non-familial | Duplex | Multiplex | p-value | ||||
|---|---|---|---|---|---|---|---|---|
| Other Cancer | Yes | 523 | 16.62% | 227 | 16.50% | 68 | 13.88% | 0.2407* |
|
| ||||||||
| Colon | No | 2940 | 98.10% | 1189 | 98.26% | 434 | 99.31% | 0.1868# |
| Yes | 57 | 1.90% | 21 | 1.74% | 3 | 0.69% | ||
|
| ||||||||
| Pancreatic | No | 2985 | 99.67% | 1208 | 99.59% | 437 | 99.77% | 0.9234# |
| Yes | 10 | 0.33% | 5 | 0.41% | 1 | 0.23% | ||
|
| ||||||||
| Breast (female) | No | 1236 | 93.78% | 498 | 94.50% | 190 | 94.53% | 0.8935* |
| Yes | 79 | 5.99% | 29 | 5.50% | 11 | 5.47% | ||
|
| ||||||||
| Ovarian (female) | No | 1273 | 96.73% | 517 | 98.48% | 192 | 97.46% | 0.1162* |
| Yes | 43 | 3.27% | 8 | 1.52% | 5 | 2.54% | ||
|
| ||||||||
| Prostate (male) | No | 1631 | 97.03% | 665 | 96.66% | 234 | 97.10% | 0.8841* |
| Yes | 50 | 2.97% | 23 | 3.34% | 7 | 2.90% | ||
|
| ||||||||
| Skin | No | 2897 | 96.63% | 1171 | 96.38% | 429 | 97.06% | 0.7891* |
| Yes | 101 | 3.37% | 44 | 3.62% | 13 | 2.94% | ||
|
| ||||||||
| Lung | No | 2944 | 98.26% | 1171 | 96.78% | 424 | 96.36% | 0.0021* |
| Yes | 52 | 1.74% | 39 | 3.22% | 16 | 3.64% | ||
|
| ||||||||
| Bone | No | 2989 | 99.83% | 1201 | 99.26% | 434 | 99.09% | 0.0027# |
| Yes | 5 | 0.17% | 9 | 0.74% | 4 | 0.91% | ||
|
| ||||||||
| Liver | No | 2982 | 99.60% | 1206 | 99.59% | 432 | 98.63% | 0.0234* |
| Yes | 12 | 0.40% | 5 | 0.41% | 6 | 1.37% | ||
DISCUSSION
Earlier age of disease onset is generally considered to be an indicator of genetic susceptibility to disease. In our previous study of a smaller cohort, when we examined age of cancer diagnosis in our entire FBE and non-familial cohorts, we failed to identify a difference in age of cancer diagnosis between familial and non-familial cancers.(7) However, in this study, once we restricted ourselves to investigating the smaller subset of multiplex FBE kindreds with 3 or more members with BE, EAC, or EGJAC, we discovered that EAC is diagnosed at a significantly earlier age in these FBE kindreds when compared to duplex and non-familial kindreds. This finding is supported by mixed modeling accounting for familial correlations, with a p-value of 0.028. These results, which support a genetic basis for FBE, at least in multiplex kindreds, are also consistent with our previous segregation analysis.(8) The results of this study also suggest that individuals who are members of multiplex FBE pedigree and are candidates for endoscopic screening (3) may be considered for screening at an earlier age than the general population.
Multiplex kindreds also differed significantly from duplex and non-familial kindreds in terms of risk factors associated with BE and EAC. The proportion of individuals reporting heartburn, which is the most common symptom of GERD, was not different, although proportion of individuals with regurgitation was lower. Other risk factors such as high BMI and smoking, which have been positively associated with BE and EAC in previous studies, were inversely associated with multiplex FBE kindreds, suggesting that familial aggregation of BE and its associated cancers is not related to a common exposure to these environmental factors in family members. Absence of other known risk factors in subjects with FBE also makes it more likely that a currently unrecognized, and possibly genetic, factor is operative in these subjects.
The definition of BE in this cohort was quite rigorous and excluded intestinal metaplasia associated with carditis. Conversely, our definition of the FBE trait was broad and included BE and EAC because evidence suggests that nearly all EAC develop from BE. (12–17) We followed the recent TNM Classification that now stages EGJAC similar to EAC. (18) Interestingly, all but one of the cancers in the multiplex FBE kindreds were EAC’s suggesting that for the purpose of genetic studies it may be prudent to restrict the trait to rigorously defined BE and EAC. This study also found that cancers in multiplex FBE kindreds were not diagnosed at an earlier stage and in fact the results suggest that these cancers may be more advanced at diagnosis. Although we did not have direct data on which cancers were diagnosed as a result of endoscopic screening or surveillance, this finding reassures us that the early age of cancer diagnosis in these multiplex kindreds is not related to awareness of disease and early medical care seeking behavior in these families.
In other complex diseases, genetic predisposition is manifested strongly in families with 3 or more affected individuals. For example, the risk of pancreatic cancer is not significantly increased in families with 2 members with pancreatic cancer but is significantly increased in families with 3 or more members with the disease.(19) Similarly, an individual’s risk for colon neoplasias is increased depending on the number of relatives with colon adenomas or colon cancers.(20, 21) Familial cancer syndromes are often also associated with cancers of other organs. This study did not find an association between FBE and other cancers in general. There was a significant association between multiplex FBE and lung, bone and liver cancer, but the number of subjects reporting these cancers in the present study was small. Also, cancer in these sites is frequently metastatic, and given our inability to perform complete review of the entire medical records of all subjects, this information could be inaccurate. Our database is limited to information obtained from family members who choose to participate and, because of inability to verify their complete medical record, we have relied on self-reported diagnoses of other cancers. Many family members chose not to participate, and differential participation due to chance or diminished survival in cancer sufferers could easily miss an association between FBE and other cancers. However, we speculate that another reason could be that FBE is caused specifically by genetic variants that predispose to an injury to the esophagus or the development of intestinal metaplasia rather than a more general cancer susceptibility gene.
This study should be interpreted within the limitations of the study design. We had tested the hypothesis that FBE is associated with an earlier age of cancer diagnosis in our previous study and had not found an effect.(7) By increasing our power with an expanded cohort, we were able to discern an effect in multiplex kindreds with the familial group definitions offered in this study. In this re-analysis of these data, the highly significant p-values showing marked differences between multiplex kindreds and other BE/EAC/EGJAC kindreds suggest that these differences are not artifactual. Due to the possibility of lead-time bias, this finding may warrant further replication. The database is missing information on several variables for a number of study subjects: missing data on deceased relatives with cancer could bias our results, especially since some age of cancer diagnosis information was collected from proxies. It is unlikely that proxy respondents would systematically misclassify a younger age of cancer diagnosis in multiplex FBE kindreds, as opposed to duplex or non-familial kindreds. As in previous studies,(6, 7) we used obesity 1 year prior to diagnosis for analysis, assuming that the majority of weight loss associated with cancer diagnosis was within the first year prior to diagnosis. Overweight individuals underreport weight, which could have lead to an underestimation of obesity.(22) As in our previous study, mulitvariable mixed linear models were used to adjust for familial correlations because they allow the specification of and accounting for family correlation structure in our univariable and multivariable models.(7) Although a statistically-significant difference in BMI was identified, the biological significance of <1 kg/m2 is unknown. It is likely that individuals in this study without a genetic susceptibility were misclassified as FBE, especially in the duplex kindreds and others with a genetic susceptibility were misclassified as non-familial because we had insufficient information on the family or the family size was small. However, these misclassifcations would only attenuate differences between multiplex FBE kindreds and non-familial cases. Of course, true differences in age of cancer diagnosis can only be measured once the genetic variants in multiplex FBE kindreds have been identified. Finally, the diagnosis of EAC in a family member might precipitate a lower threshold for evaluation of GERD symptoms, and perhaps an earlier diagnosis of EAC than might happen in sporadic cases. However, this bias seems less likely given that multiplex families with an EAC proband did not have a lower mean age of diagnosis than multiplex families with a BE proband, as might be expected if cancer fear precipitated earlier diagnosis. Also, as noted above, cancers diagnosed in multiplex families were not of earlier stage than those diagnosed duplex or non-FBE families.
To summarize, the results of this analysis indicated that EAC is diagnosed at an earlier age in multiplex FBE kindreds and might be more advanced at time of diagnosis. Along with the results of our prior segregation analysis,(8) which indicates that FBE is caused by an incompletely penetrant autosomally dominant genetic variant, the results of the present study argue strongly for investigations into the genetic basis of FBE. Such investigations should focus on FBE kindreds with 3 or more members affected with rigorously defined BE and EAC phenotypes, because these kindreds have the strongest epidemiological evidence for a genetic basis. The study results also suggest that screening practices may need to be modified for an earlier age in these multiplex FBE kindreds.
Acknowledgments
We are grateful to our research coordinators, Wendy Brock, Ellen Neumeister, Beth Bednarchcik, Mary Oldenburgh, Denise Buonocore-Sassano, Anna Haas, Kasey Orlowski, Elisabeth Gifford, Verna Scheeler, and Norma Dougherty in helping recruit patients. We sincerely thank Mike Warfe for creating and securely maintaining our database.
This project was supported by Grant Number R01DK070863 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), by Grant Number M01 RR00080 and Grant Number UL1 RR024989 from the National Center for Research Resources (NCRR), and Grant Number CA130901 from the National Cancer Institute, components of the National Institutes of Health (NIH). The contents are solely the responsibility of the authors and do not necessarily represent the official view of NIDDK, NCRR, NCI, or NIH. Amitabh Chak is also supported by a Midcareer Award in Patient Orient Research, K24DK002800 from the NIDDK.
Footnotes
Guarantor of the article: Amitabh Chak, MD
Specific author contributions: Amitabh Chak and Jill Barnholtz-Sloan planned the study, designed the protocol and contributed to the writing of the paper. Marcia Canto, Amitabh Chak, Gary Falk, William M. Grady, Margaret Kinnard, Sumeet Mittal, Ganapathy Prasad and Nicholas Shaheen performed clinical care and helped identify and recruit the patients. Sanford Markowitz and Kishore Guda have been performing genetic analyses on these families and suggested the idea of examining multiplex families. They helped in study design. Joseph Willis reviewed pathologic diagnoses and helped develop criteria for the FBE trait. Amitabh Chak supervised collection of all data into database and performed quality control on data. Jill Barnholtz-Sloan designed the primary study analysis with Yanwen Chen and Jaime Vengoechea who performed the analysis, and were major contributors to writing and revising the paper. All authors have contributed to writing the paper and approved the final draft of the paper.
Potential competing interests: No Conflicts of Interest exist for any of the authors
References
- 1.Wong A, Fitzgerald RC. Epidemiologic risk factors for Barrett’s esophagus and associated adenocarcinoma. Clin Gastroenterol Hepatol. 2005;3:1–10. doi: 10.1016/s1542-3565(04)00602-0. [DOI] [PubMed] [Google Scholar]
- 2.Falk GW. Risk factors for esophageal cancer development. Surg Oncol Clin N Am. 2009;18:469–85. doi: 10.1016/j.soc.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Wang KK, Sampliner RE Practice Parameters Committee of the American College of G. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett’s esophagus. Am J Gastroenterol. 2008;103:788–97. doi: 10.1111/j.1572-0241.2008.01835.x. [DOI] [PubMed] [Google Scholar]
- 4.Chak A, Lee T, Kinnard MF, Brock W, Faulx A, Willis J, et al. Familial aggregation of Barrett’s oesophagus, oesophageal adenocarcinoma, and oesophagogastric junctional adenocarcinoma in Caucasian adults. Gut. 2002;51:323–8. doi: 10.1136/gut.51.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drovdlic CM, Goddard KA, Chak A, Brock W, Chessler L, King JF, et al. Demographic and phenotypic features of 70 families segregating Barrett’s oesophagus and oesophageal adenocarcinoma. J Med Genet. 2003;40:651–6. doi: 10.1136/jmg.40.9.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chak A, Ochs-Balcom H, Falk G, Grady WM, Kinnard M, Willis JE, et al. Familiality in Barrett’s esophagus, adenocarcinoma of the esophagus, and adenocarcinoma of the gastroesophageal junction. Cancer Epidemiol Biomarkers Prev. 2006;15:1668–73. doi: 10.1158/1055-9965.EPI-06-0293. [DOI] [PubMed] [Google Scholar]
- 7.Chak A, Falk G, Grady WM, Kinnard M, Elston R, Mittal S, et al. Assessment of familiality, obesity, and other risk factors for early age of cancer diagnosis in adenocarcinomas of the esophagus and gastroesophageal junction. Am J Gastroenterol. 2009;104:1913–21. doi: 10.1038/ajg.2009.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun X, Elston R, Barnholtz-Sloan J, Falk G, Grady WM, Kinnard M, et al. A Segregation Analysis of Barrett’s Esophagus and Associated Adenocarcinomas. Cancer Epidemiol Biomarkers Prev. 2010;19(3):666–74. doi: 10.1158/1055-9965.EPI-09-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chak A, Faulx A, Kinnard M, Brock W, Willis J, Wiesner GL, et al. Identification of Barrett’s esophagus in relatives by endoscopic screening. Am J Gastroenterol. 2004;99:2107–14. doi: 10.1111/j.1572-0241.2004.40464.x. [DOI] [PubMed] [Google Scholar]
- 10.Ochs-Balcom HM, Falk G, Grady WM, Brock W, Willis J, Wiesner GL, et al. Consortium approach to identifying genes for Barrett’s esophagus and esophageal adenocarcinoma. Transl Res. 2007;150:3–17. doi: 10.1016/j.trsl.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Locke GR, Talley NJ, Weaver AL, Zinsmeister AR. A new questionnaire for gastroesophageal reflux disease. Mayo Clin Proc. 1994;69:539–47. doi: 10.1016/s0025-6196(12)62245-9. [DOI] [PubMed] [Google Scholar]
- 12.Haggitt RC, Tryzelaar J, Ellis FH, Colcher H. Adenocarcinoma complicating columnar epithelium-lined (Barrett’s) esophagus. Am J Clin Pathol. 1978;70:1–5. doi: 10.1093/ajcp/70.1.1. [DOI] [PubMed] [Google Scholar]
- 13.Cameron AJ, Lomboy CT, Pera M, Carpenter HA. Adenocarcinoma of the esophagogastric junction and Barrett’s esophagus. Gastroenterology. 1995;109:1541–6. doi: 10.1016/0016-5085(95)90642-8. [DOI] [PubMed] [Google Scholar]
- 14.Hameeteman W, Tytgat GN, Houthoff HJ, van den Tweel JG. Barrett’s esophagus: development of dysplasia and adenocarcinoma. Gastroenterology. 1989;96:1249–56. doi: 10.1016/s0016-5085(89)80011-3. [DOI] [PubMed] [Google Scholar]
- 15.Reid BJ, Blount PL, Rubin CE, Levine DS, Haggitt RC, Rabinovitch PS. Flow-cytometric and histological progression to malignancy in Barrett’s esophagus: prospective endoscopic surveillance of a cohort. Gastroenterology. 1992;102:1212–9. [PubMed] [Google Scholar]
- 16.Clark GW, Smyrk TC, Burdiles P, Hoeft SF, Peters JH, Kiyabu M, et al. Is Barrett’s metaplasia the source of adenocarcinomas of the cardia? Arch Surg. 1994;129:609–14. doi: 10.1001/archsurg.1994.01420300051007. [DOI] [PubMed] [Google Scholar]
- 17.MacDonald WC, MacDonald JB. Adenocarcinoma of the esophagus and/or gastric cardia. Cancer. 1987;60:1094–8. doi: 10.1002/1097-0142(19870901)60:5<1094::aid-cncr2820600529>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 18.Rice TW, Rusch VW, Ishwaran H, Blackstone EH Worldwide Esophageal Cancer C. Cancer of the esophagus and esophagogastric junction: data-driven staging for the seventh edition of the American Joint Committee on Cancer/International Union Against Cancer Cancer Staging Manuals. Cancer. 2010;116:3763–73. doi: 10.1002/cncr.25146. [DOI] [PubMed] [Google Scholar]
- 19.Grover S, Syngal S. Hereditary pancreatic cancer. Gastroenterology. 2010;139:1076–80. 1080, e1–2. doi: 10.1053/j.gastro.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilschut JA, Steyerberg EW, van Leerdam ME, Lansdorp-Vogelaar I, Habbema JD, van Ballegooijen M. How much colonoscopy screening should be recommended to individuals with various degrees of family history of colorectal cancer? Cancer. 2011;117:4166–74. doi: 10.1002/cncr.26009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johns LE, Houlston RS. A systematic review and meta-analysis of familial colorectal cancer risk. Am J Gastroenterol. 2001;96:2992–3003. doi: 10.1111/j.1572-0241.2001.04677.x. [DOI] [PubMed] [Google Scholar]
- 22.Rowland ML. Self-reported weight and height. Am J Clin Nutr. 1990;52:1125–33. doi: 10.1093/ajcn/52.6.1125. [DOI] [PubMed] [Google Scholar]


