Abstract
Hereditary diffuse leukoencephalopathy with spheroids (HDLS) was originally described in a large Swedish pedigree. Since then, 22 reports describing a total of 13 kindred's and 11 sporadic cases have been published. Inheritance is autosomal dominant, albeit the gene is unknown. Here we report on the clinical findings, genealogical data, brain MRI data, and autopsy/biopsy findings of four probands from three independently ascertained novel families from Norway, Germany and US.
We identified a 39-year-old female and her twin sister, a 52-year-old male and a 47-year-old male with progressive neurological illness characterized by personality changes, cognitive decline and motor impairments, such as gait problems, bradykinesia, tremor and rigidity. Brain MRI showed white matter abnormalities with frontal prominence. Brain biopsy/autopsies were consistent with HDLS.
HDLS is an under-recognized disease and in reporting these cases, we aim to increase the awareness of the disorder. Due to varied and wide phenotypic presentations, which may imitate several neurodegenerative diseases, HDLS can be difficult to diagnose. Definitive diagnosis can be established only by direct brain tissue examination. Familiarity with the clinical presentation and typical neuroimaging findings may be helpful in narrowing the diagnosis.
Keywords: HDLS, White matter disease, Autosomal dominant, Personality changes, Cognitive problems, Depression, Parkinsonism
Introduction
Hereditary diffuse leukoencephalopathy with axonal spheroids (HDLS) was first identified in a Western Swedish family reported in 1984 [1]. There have been 13 kindreds [2–5] and 11 sporadic cases [2, 6–9] since that first report. HDLS is probably closely related to familial pigmentary orthochromatic leukodystrophy (POLD) or is within the same disease spectrum [2]. The pathological hallmark of this disease is the presence of brain white matter changes with neuroaxonal spheroids present [1]. Inheritance is autosomal dominant with variable penetrance, but the causative genes are unknown. HDLS is characterized by a constellation of symptoms including personality changes, cognitive dysfunction and motor impairments such as gait dysfunction, tremor, bradykinesia and rigidity. Brain MRI shows white matter lesions in a frontal predominant distribution, spreading out from the periventricular and deep white matter into the subcortical areas with enlarged ventricles and often signal changes in corpus callosum. Van Gerpen et al. reported a HDLS case with serial MRI's. In the presymptomatic stage, MRI demonstrated subtle patchy abnormalities in the periventricular white matter, which later on subsequent MRI, performed when patient was in advance stage of disease, became widespread and confluent [3]. This important observation suggests that the disease process in the brain starts locally and then becomes more widespread with disease progression.
An international consortium on HDLS was established in 2005 by one of the authors (ZKW) after the first Mayo Clinic kindred with HDLS was reported [10]. Since then, 14 additional families have been collected at the Mayo Clinic and 4 have been reported [2, 3]. In our research study brains and brain biopsy specimens have been collected both retrospectively and prospectively. All have been examined by DWD, and only those demonstrating pathological features of HDLS were included in our study. Based on our collection we found that all of the 20 cases were misdiagnosed (unpublished data). Therefore, we describe here the HDLS cases from the smallest families as the evidence of familial clustering indicating a genetic disorder is not always obvious. Phenotypic variability may occur in the affected members of a given kindred, and can misleadingly be interpreted as two unrelated sporadic disorders. Diagnoses of HDLS in small families can therefore be challenging and particularly difficult since there is often not a convincing family history.
However, there is strong evidence that HDLS is a genetic disorder due to the larger families, both reported and unpublished, who show an autosomal dominant heredity [1, 3, 10]. Based on our Mayo Clinic HDLS collection and published reports, HDLS patients may be misdiagnosed with Alzheimer's disease (AD), frontotemporal dementia (FTD), atypical parkinsonism (AP), multiple sclerosis (MS) and/or small vessel diseases. Herein we report three new HDLS families to increase the awareness of this disorder and discuss the differential diagnoses from the clinical, imaging, and pathological perspective.
METHODS
Clinical and genealogic studies
We retrospectively reviewed the medical records of three kindred's that were previously collected from a world-wide collaboration on HDLS organized by the Mayo Clinic Florida by one of the authors (ZKW).
The probands of our families included in this report were identified and longitudinally followed in Norway, Germany, and the US. A written informed consent approved by the Mayo Clinic Institutional Review Board was obtained in order to perform the reviews of clinical, radiological and neuropathological material. Family members were assessed by neurologists and examinations included the standardized medical history, the Unified PD Rating Scale; and the Mini-Mental State Examination. Additional historical material, family documents, and medical records were also collected. The blood samples were collected from all cases for further genetic testing.
Neuroimaging
MRI scans were performed for diagnostic purposes locally. All studies used standard MRI techniques with 5 mm thickness and 5 mm spacing, and were performed on 1.5 Tesla scanners. All MRI studies were re-reviewed by two of the authors (DB and CS). One of the authors (DB) was not aware of the disease stage. Signal intensity (SI) was assessed visually; SI higher in the white matter than the grey matter was considered pathologic. Axial and sagittal T1- and T2- weighted images were used to detect the location of white matter foci, atrophy and structural changes in the brain in all cases. Contrast enhanced MRI examinations had been performed at least once on all patients during the course of the illness.
Pathology
Cases 1–3 had autopsies and Case 4 had brain biopsy preformed for diagnostic purposes locally. Paraffin blocks from the autopsies and glass slides from the biopsy of the probands were available for further study at the Mayo Clinic Florida. The neuropathologic examinations were conducted by one of the authors (DWD).
Sections of the neocortex, hippocampus, hypothalamus, basal ganglia, thalamus, midbrain, pons, cerebellum and spinal cord were examined. Selected sections were studied with H&E, thioflavin-S fluorescent microscopy, Luxol fast blue stains, immunohistochemistry for ubiquitin, amyloid precursor protein (APP, 22C11), ubiquitin, phosphorylated neurofilament (SMI-31), αB-crystallin, phospho-tau (CP13), α-synuclein (NACP), TDP-43 (TDP-25), HLA-DR (LN3) for microglia and glial fibrillary acidic protein (GFAP).
Results
Short case reports are provided below. Summary of the clinical characteristics and laboratory investigations of our probands are provided in Table 1. Pedigrees are presented in Figure 1.
Table 1.
Clinical characteristics of Case 1–4
| Case # | Sex | Onset Age (yrs) | Age at Death (yrs) | Years with HDLS | Initial Symptoms | Later Symptoms | Additional Features | Laboratory investigations | Differential Diagnosis |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 38 | 41 | 3 | -Depression -Personality changes -Cognitive problems |
-Apraxia -Bradykinesia, -Shuffling gait -Homonymous hemianopsia -Spasticity -Tetraparesis -Mutism -Dysphagia |
-Seizure -Alien limb sign |
-Routine blood and urine tests -Amino acids, VDRL, HIV serologies ArylsutfataseA, galactocerebrosidase, glucosidase, galactosidase, mannosidase, fucosidase, very long chain fatty acids -Anti-nuclear, anti-neutrophil cytoplasmic-/ anti-neuronal nuclear antibodies -Cerebrospinal fluid examination -CADASIL skin biopsy test and NOTCH 3 gene test -Mitochondrial muscle biopsies -All test were normal |
-Atypical MS |
| 2 | F | 36 | 40 | 4 | -Dizziness -Executive dysfunction -Depression -Personality changes -Cognitive problems |
-Apraxia -Bradykinesia -Spastic broad-based gait -Homonymous quadrantanopsia -Spasticity -Tetraparesis -Dysphasia -Dysphagia |
-Seizure -Dystonia |
-Routine blood and urine tests -Amino acids, VDRL, HIV serologies -ArylsulfataseA, galactocerebrosidase, glucosidase, galactosidase, mannosidase, fucosidase, very long chain fatty acids -Anti-nuclear, anti-neutrophil cytoplasmic-/ anti-neuronal nuclear antibodies -Cerebrospinal fluid examination -CADASIL skin biopsy test and NOTCH3 gene test -Mitochondrial muscle biopsies -All tests were normal |
-Atypical MS |
| 3 | M | 52 | 63 | 11 | -Personality changes -Cognitive problems -Memory problems |
-Reduced proprioceptions -Paresis in arm -Vertical gaze palsy -Ataxic and shuffling gait -Bradykinesia -Tetraparesis |
-Myoclouus finger movements -Palatal and arms tremor |
-Routine blood and unine tests -Amino acids, VDRL, HIV, syphilis serologies -Antinuclear-, antineutrophil cytoplasmic-/ antineuronal nuclear antibodies -Lead and copper -Cortisol -Unine organic acids and lactate -CSF -Nerve conduction study -CADASIL skin biopsy test -All tests were normal (except a slight protein elevation in CSF) |
-No specific final clinical diagnosis was made but:
|
| 4 | M | 48 | Patient still living | Patient still living | -Parkinsonism -Personality changes |
-Slow saccadic eye movements -Shuffling gait -Bradykinesia -Kinetic tremor -Rigidity -Dysarthria -Dysphagia |
-Occulomotor appraxia -Hypophonic speech |
-Routine blood and urine tests -Ceruloplasmin and lactate -Pyruvate -Aminoacids, VDRL, TPHA, Lyme,Brucella screens, HIV and NMO antigene -Antinuclear-, antineutrophil cytoplasmic-/ autineuronal nuclear antibodies -Urinary organic acids -ACE (serum and CSF) Arylsulfatase A, galactocerebrosidase, beta-galactosidase, hexosaminidase, sphingomyelinase, and very long chain fatty acids -CSF examination -CADASIL skin biopsy test and NOTCH3 gene test -Paraneoplastic panel -Malignancy screening with PET -Genetic analyses for Parkin, PINKI, LRRK2 -Nerve conduction study -EMG -All test were normal |
-No specific final clinical diagnosis was made but:
|
Abbreviations: ACE;Angiotensin Converting Enzyme (sarcoidosis),AD;Alzheimer Disease,CADASIL;Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy,CSF;Cerebrospinal fluid,HIV;Human immunodeficiency virus,MS;Multiple Sclerosis,NMO;NeuroMyelitis Optica,PD;Parkinson Disease,PET;Positron emission tomography,PSP;Progressive Supranuclear Palsy,TPHA;Treponema Pallidum Haemagglutination Test,VDRL;Venereal Disease Research Laboratory test (syphilis test)
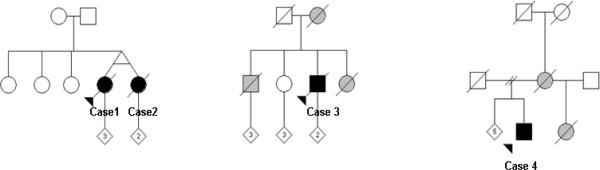
Fig. 1. Pedigrees.
Circles represent females and squares represent males. Black symbols indicate pathologically confirmed HDLS. Grey symbols indicate possible diagnosis. Arrow indicates the proband. Slash indicates deceased. Numbers inside the diagonal symbol indicates children. Cases 1 and 2: Norway; Case 3: Germany; Case 4: US (Polish and German descent).
Case 1. (Norwegian family)
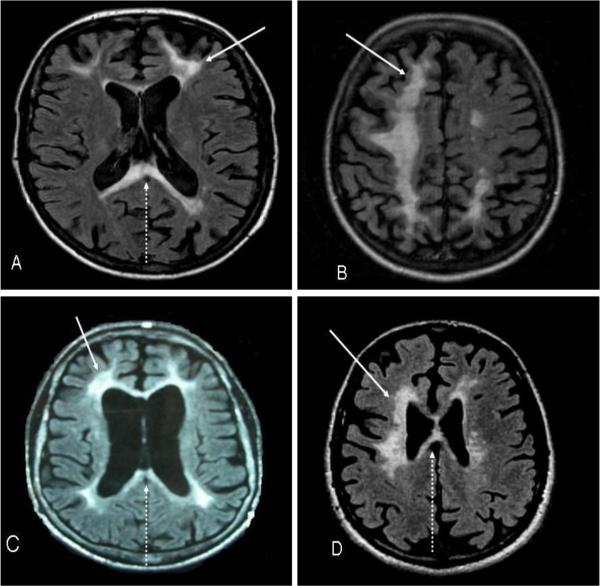
A 38-year-old female developed depression, difficulties with following directions and performing calculations, slowness of thoughts and fatigue. Due to progressive cognitive dysfunction, she had difficulties continuing her work as a mail carrier. During the first evaluation, approximately six months after symptom onset, she had slight bradykinesia in all extremities, which was more evident on her right side. She also had exaggerated deep tendon reflexes, but no extensor plantar responses. An axial T2-weighted brain MRI was performed 1.2 years after the onset of her symptoms and showed localized white matter T2 hyperintense foci in the bifrontal and biparietal white matter (more on the left) and corpus callosum, with associated atrophy (Figure 2A.). Clinical examination, at the time of the MRI, demonstrated increased bradykinesia in all extremities, slight apraxia, unsteady broad based gait and extensor plantar response on the right side. Over the next nine months, there was rapid deterioration of cognitive and motor functions with spasticity and multiple falls. She developed primitive reflexes, homonymous hemianopsia to the right side, alien limb sign in her right arm, mutism, somnolence and generalized tonic- clonic seizures. She became bedridden about six months before passing away at the age of 41, after a 3 year disease course. She was treated with intermittent regimes with intravenous (IV) methylprednisolone sodium succinate 1000 mg given in three consecutive days followed by oral prednisolone 60 mg daily with downward titration over 3 weeks. This therapy was repeated every two months three times over the course of her illness. No benefit was seen. Her clinical diagnosis was atypical MS.
Fig. 2. Magnetic resonance images (axial sections, T2-weighted) from the 4 patients.
(A)Case 1 (MRI performed 1.2 years after start of symptoms); localized white matter lesions (arrow) in both frontal and parietal hemispheres involving the corpus callosum (arrow dashed). (B)Case 2 (MRI performed 1.9 years after start of symptoms); confluent white matter lesions in both frontal and parietal hemispheres with cortical atrophy in the affected areas. (C)Case 3 (MRI performed 3.5 years after start of symptoms); localized periventricular lesions (arrow) with corresponding frontoparietal atrophy and involvement of the corpus callosum (arrow dashed). (D)Case 4 (MRI performed 2.5 years after start of symptoms); bilateral frontoparietal white matter changes (arrow) extending into the corpus callosum (arrow dashed).
Case 2. (Norwegian family)
The identical twin sister of Case 1 had similar symptoms and disease course. Her symptoms started insidiously with attacks of dizziness at age 36 and lasting intermittently for the following year. Subsequently, at the age of 37 year old, she developed executive dysfunction, depression, memory problems, reduced fine skill movements, clumsiness and stiffness in her left arm. Two years after the onset of her symptoms she was found to have left sided arm dystonia, left sided dysmetria, generalized bradykinesia, slight apraxia, exaggerated tendon reflexes and a positive bilateral extensor plantar responses. Her gait was spastic and she had left inferior homonymous quadrantanopsia. Over the next few months she developed generalized seizures, spasticity in all four extremities, tetraparesis, contractures of the large joints in both upper and lower extremities, dysphasia, dysphagia, and became totally bedridden. A brain MRI, axial T2-weighted, performed 1.9 years after the onset of symptoms demonstrated confluent bifrontal and biparietal white matter (more marked on the right) T2 hyperintensities with corresponding atrophy (Figure 2B.). She was treated with steroids followed the same protocol as for her sister. However, after completion of steroid therapy without any benefit, she was placed on subcutaneous interferon beta-1a (IFN-1a), 44 μg, three times weekly for six months. These provided no benefit. She died at 40 years of age. Her final clinical diagnosis was atypical MS.
Both sisters had negative routine blood and urine tests. Amino acids, VDRL and HIV serologies, arylsulfatase A, galactocerebrosidase, glucosidase, galactosidase, mannosidase, fucosidase, very long chain fatty acids, anti-nuclear, anti-neutrophil cytoplasmic, and anti-neuronal nuclear antibodies were all negative. Cerebrospinal fluid examination, CADASIL skin biopsy and NOTCH3 blood test and mitochondrial muscle biopsies were also normal.
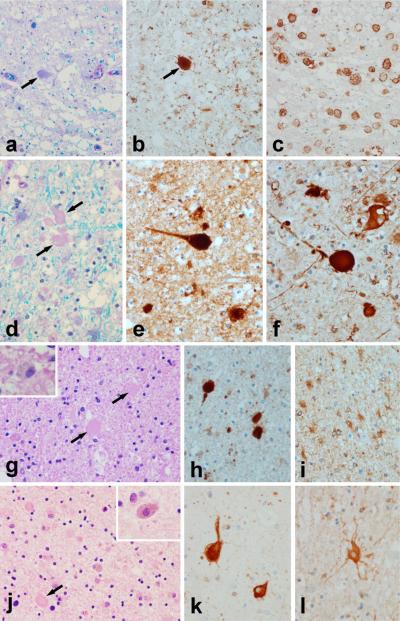
Autopsies demonstrated brain white matter abnormalities with axonal spheroids that were immunohistochemistry-positive for neurofilament and amyloid precursor protein, similarly to other reported cases of HDLS [10] (Figure 3.). Both patients also had corticospinal tract degeneration. Contrarily, their parents are alive and healthy and there are no other affected family members.
Fig. 3. Neuropathology.
Case 1: (a) Luxol fast blue stain for myelin shows myelin loss and tissue vacuolation with axonal spheroids [arrows in (a) and (b)], that are immunopositive for amyloid precursor protein (b). Affected white matter has many lipid-laden macrophages immunohistochemistry-positive for HLA-DR (c). Case 2: (d) Luxol fast blue stain for myelin shows myelin loss and tissue vacuolation with axonal spheroids [arrows in (d)] that are immunopositive for amyloid precursor protein (e) and for phosphorylated neurofilament (f). Astrocytes in affected white matter are hypertrophic and bizarre appearing with immunohistochemistry for alpha-B-crystallin (l).Case 3: (g) Hematoxylin & eosin stain shows myelin pallor with pigment-containing macrophages (inset) and axonal spheroids [arrows] that are immunopositive for amyloid precursor protein (h). Astrocytes in affected white matter are hypertrophic and bizarre appearing with immunohistochemistry for alpha-B-crystallin (i). The cortex overlying areas with white matter pathology frequently has ballooned / swollen neurons best appreciated with immunohistochemistry for alpha-B-crystallin (k). Case 4: (j) Hematoxylin & eosin stain of the brain biopsy shows myelin pallor with pigment-containing macrophages (inset) and axonal spheroids [arrow]. [All images are originally ×400]
Case 3. (German family)
A 52-year-old male developed personality and memory problems over a period of three months. He lost interest in daily activities, became withdrawn and aggressive. At neurological examination, three months after symptom start, no focal abnormalities were detected, with the exception of mini-mental state examination (MMSE) score of 24/30 points. Over the next 10 years, he developed insidious increase of symptoms with reduced vibration sensation in all extremities, finger myoclonus, vertical gaze palsy, brisk reflexes and upper motor neuron weakness in the right arm. Tremor was present in both arms and palate. The gait became ataxic with small steps; he also became doubly incontinent and became totally ADL dependent. In the last year of the disease he had reached a vegetative state; was totally bedridden and mute. He died 11 years after the onset of the first symptom.
Laboratory investigations including serum electrolytes; complete blood counts; liver and thyroid function tests; vitamin B12 and folate levels; vitamin E; cholesterol and triglycerides; amino acids (serum and urine); syphilis and HIV serologies; antinuclear, antineutrophil cytoplasmic, and antineuronal nuclear antibodies; erythrocyte sedimentation rate; lead and copper; and cortisol, as well as urine organic acids and lactate (blood and CSF), which did not revealed any abnormalities. CSF showed no abnormalities, except a slight protein elevation. Skin biopsy for granular osmiophilic material (GOM) pathology was absent in regard to Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL). MRI, axial T2-weighted, performed 3.5 years after symptom onset demonstrated localized bilateral frontoparietal periventricular white matter T2 hyperintensities with corresponding atrophy (Figure 2C.).
No final diagnosis was established, but the differential diagnoses included AD, MS and atypical CADASIL, even though skin biopsy was negative for GOM. Autopsy of Case 3 demonstrated white matter pathology; cortical atrophy and axonal spheroids consistent with HDLS (Figure 3gk.).
His brother had developed personality, memory and gait problems at age 58 and received the diagnosis of atypical CADASIL, even though he also had a negative skin biopsy test for GOM pathology. His sister developed neurological symptoms at age 18, and became wheelchair bound and bedridden. She died at age 49, diagnosed with MS. Their mother had developed dementia at age 54 and died 7 years after the onset of her symptoms (Figure 1B.). None of these family members had brain biopsy or autopsy.
Case 4. (US family)
A 48-year-old male of Polish and German descent experienced gradual progressive gait difficulty affecting the left leg, which gradually affected the right leg. Upon neurological examination six months after onset of symptoms, he was found to be oriented to date, time and place; his MMSE score was 27 of 30 points and he was reported to have a slight personality change, becoming withdrawn and having periods of sadness. He had mild apraxia, masked facies and slow saccadic eye movements (horizontal and vertical) with ocular motor apraxia. There was rigidity and dysdiadochokinesia in all extremities, more on the left side, dysarthria and dysphagia. Over the next six months, his gait became slow and shuffling and he developed neurogenic bladder. Investigational work up was extensive and included; routine complete blood count and chemistry, thyroid function, serum creatine phosphate, ceruloplasmin, lactate, pyruvate, amino acids, cerebrospinal fluid analysis with oligoclonal bands and IgG index, urinary organic acids, angiotensin conversion enzyme, arylsulfatase A, galactocerebrosidase, beta-galactosidase, hexosaminidase, sphingomyelinase, and very long chain fatty acids and all test results were normal. Skin biopsy and NOTCH3 blood test in regard to CADASIL were normal. VDRL, TPHA, Lyme and Brucella screens, HIV and NMO antigene were negative. Paraneoplastic panel, malignancy screening with PET and genetic analyses for Parkin, PINK1, LRRK2; all came out with negative results. EMG, nerve conduction studies and angiogram were both negative.
A brain MRI, axial T2-weighted, performed 2.5 years after disease onset showed patchy bilateral frontoparietal white matter T2 hyperintensities extending into the corpus callosum with correspondent atrophy (Figure 2D.). He received treatment with IV methylprednisolone sodium succinate (1000 mg for three consecutive days every other month for six months), IV plasmapheresis (every 3rd month for 9 months), subcutaneous interferon, 44 μg IFN-1a, (three times weekly for approximately a year) and L-dopa (up to 1000 mg/day over 5 months). These provided no benefit. Over the next three years, his symptoms progressed with increased tone in all extremities, could not ambulate secondary to bradykinesia, prominent bulbar signs with dysarthria and dysphagia and became total care dependent. He is today bedridden and almost mute. Prior to brain biopsy at the age of 50, he received multiple diagnoses including neuromyelitis optica (NMO), AP, atypical MS and progressive supranuclear palsy (PSP) superimposed on MS. His brain biopsy showed white matter abnormalities with axonal spheroids present consistent with HDLS (Figure 3).
His half-sister was diagnosed with MS and died, bedridden at age 46. His mother died at the age of 52 in a nursing home after a three-year course of an AP illness1 (Figure 1C). Neither individual had a brain biopsy or autopsy.
Discussion
HDLS is a devastating neurodegenerative disease with adult onset. The disease is inherited in an autosomal dominant fashion, but the genetic defect has not yet been identified. It is thought that HDLS is caused by primary disruption of the axon integrity, neuroaxonal damage, and focal axonal swelling (axonal spheroids) leading to secondary demyelination [10, 11]. However, demyelination may precede the axonal damage [12], triggering an autonomous neurodegenerative process.
Neuropathology of our 4 cases demonstrated white matter abnormalities confined to the cerebrum, sparing the optic nerve, and major fiber tracts in the diencephalon, and cerebellum. However, the corticospinal tract, posterior limb of the internal capsule and less affected anterior limb were involved in all cases. Most of the white matter tracts in the brainstem were unaffected, including the medial and lateral lemnisci, medial longitudinal fasciculus and the cerebellar peduncle. The exception was the involvement of the cerebral peduncle and frontopontine fibers in the pontine base. The white matter vacuolation and demyelination with axonal spheroids that are immunoreactive for neurofilament, APP and ubiquitin, the histopathologic hallmark in HDLS, was found in all our cases. Bizarre astrocytes and lipid-and myelin-laden macrophages are also found. There are no pathognomonic lesions that absolutely confirm the diagnosis, but the presence of white matter changes with neuroaxonal spheroids present, confirmed the diagnosis of HDLS in our cases. These findings are similar to those previously reported [1, 3, 10] (Figure 3.). In HDLS the basal ganglia, thalamus, hypothalamus, hippocampus, substantia nigra, raphe nucleus, reticular formation and cerebellar grey matter are unaffected. There is no significant evidence of amyloid angiopathy in parenchymal or leptomeningeal vessels.
In order to exclude common adult onset leukodystrophies, the Cases 1, 2 and 4 were tested for Arylsulfatase A, galactocerebrosidase, glucosidase, galactosidase, mannosidase, fucosidase and very long chain fatty acids. Metachromatic leukodystrophy (MLD), Krabbe disease and X-linked adrenoleukodystrophy (X-ADL) may start in adult age but the neuropathological findings in our cases are incompatible with these diagnoses. In MLD there is accumulation of metachromatic material in the white matter [13]. Krabbe disease is characterized by the presence of globoid cells derived from microglia which have multiple nuclei [14]. X-ALD has often significant inflammatory features in the white matter and lamellar cytoplasmic inclusions in the brain and other organs [15]. These histological features were clearly lacking in the brain in all of our patients, ruling out these diseases on pathologic grounds. Furthermore MLD often has a tigroid pattern of white matter lesion [16] and X-ALD has a predominant parieto-occipital white matter abnormality [17], neither of our cases had a tigroid pattern and all demonstrated frontal predominant white matter lesions. Vanishing white matter (VMW) disease can also present in adult age but it is neuropathologically characterized by increased white matter rarefaction and cystic degeneration, sparse dysmorphic astrocytes, scanty astrogliosis and the distinguishing increased macroglia around cavitated regions and in lesser affected areas. It has characteristic foamy oligodendrocytosis and apoptotic loss of oligodendrocytes [18]. These features were not present in our cases.
The rarer adult onset lysosomal storage diseases such as adult (Type 3) GM1 Gangliosidosis [19], Niemann –Pick (GM2 and GM 3 gangliosides) [20]; Fabrys disease [21] and the even more rare hexosaminidase A deficiency [22, 23] differ from HDLS both in clinical- and MRI presentations and neuropathologically.
White matter lesion with axonal spheroids can also be due to Nasu-Hakula disease or traumatic closed head injury [24, 25]. Naso-Hakula disease can present with a clinical syndrome similar to HDLS, but contrarily, affected patients complain of pain and tenderness of ankles/feet/wrist, and there are characteristic cystic bone lesions seen on plain radiological films [26]. There was no history of head trauma in any of our cases.
Adult onset autosomal dominant leukodystrophy (ADLD) often initially presents with autonomic symptoms followed by cerebellar and pyramidal signs. It has a characteristic MRI pattern and neuropathology with loss of myelin and rarefaction with vacuolated myelin in both the white matter of cerebrum and cerebellum; atrophy and signal changes in medulla oblongata and spinal cord; and normally usually no axonal spheroids, differentiating it from HDLS [27, 28]. All of our cases were post-mortem and were tested genetically for Naso-Hakula and ADLD with negative results.
Skin biopsies were obtained and the skin vessels were tested for accumulation of GOM by electron microscopy in all of our cases, but the NOTCH3 gene mutations were not performed in Case 3. GOM has been considered specifically diagnostic for CADASIL, but the reports on the sensitivity of detecting GOM in patients' skin biopsy have been contradictory. However, there was no characteristic white matter lesions in the temporal poles on MRI [24] and the neuropathology was incompatible with CADASIL; having no evidence for infarct or arteriolar pathology to support a vascular etiology [29].
The clinical characteristics of Case 1–4 are summarized in Table 1. The three unrelated families illustrate the clinical and neuroimaging similarities of HDLS. In these Cases, the disease commenced with changes in personality, memory and executive functions, depression and/or parkinsonism, and later progressed to devastation across multiple domains of neurological impairments.
There are several novel factors in relation to our cases. The first matter is that all cases we have identified so far are Caucasians. This may represent a possible risk allele in Caucasians. However, an ascertainment bias can not be excluded and more research is needed to confirm this observation. Secondly, we report the families from Norway and Germany. To our knowledge this is the first report of a Norwegian family with HDLS and first report of a familiar case with HDLS from Germany. However, Mayer et al. [8] have reported two German independent cases with sporadic leukoencephalopathy with axonal spheroids. Thirdly, our Case 3 had also a palatal tremor. Palatal tremor has not been reported in HDLS cases. Therefore, this observation expands the phenotypic presentation of HDLS. Finally, all of our cases have been neuropathological evaluated by the same pathologist confirming the diagnoses of HDLS. This is an important factor in studying rare neurodegenerative disorders.
The mean age of onset in our patients was 44 years (range, 36–52 years), disease duration was six years (range, 3–11 years) and the mean age of death was 48 years (range, 40–63 years). All patients had bilateral frontal and parietal white matter T2 hyperintense foci with frontal prominence; the foci often extended from the periventricular and deep regions to the subcortical tissues. Atrophy was associated with the regions of signal abnormality. All the cases demonstrated abnormal T2 signal in the corpus callosum with or without atrophy (Case 2 not shown). Cases 3 and 4 demonstrated a convincing family history, whereas it remains unclear whether the Norwegian family reflects reduced penetrance or a de novo mutation.
Leukoencephalopathies with adult onset are serious disorders and the combination of extensive white matter lesions in cases with progressive neuropsychiatric symptoms constitutes a common diagnostic dilemma. Many of these disorders, even a few hereditary disorders, are treatable, so it is of utmost importance to clarify the diagnostic spectrum.
Better understanding of leukoencephalopathies in neurological practice is crucial to differentiating among metabolic, toxic, inflammatory, vascular white matter diseases or heritable illness. Clinical differential diagnoses include dementia (e.g., FTD and AD) [30, 31]; atypical Parkinsonism (e.g., corticobasal degeneration, multisystem atrophy or PSP) [32]; progressive MS [33]; and leukodystrophies [34].
On MRI, HDLS could be mistaken for other leukoencephalopathies, usually early in the disease stage, like Nasu-Hakola disease, VWM, X-ALD, MLD, Krabbe disease and even a Susac's syndrome [34, 35]. In addition, a family history consistent with a dominant autosomal disease could suggest adult onset Alexander disease, ADLD and CADASIL [24, 28]. However, diagnostic MRI criteria may predict these latter diseases with high probability and most of the ones listed also have a known gene mutation [34, 36].
We conclude that HDLS imitates many neurodegenerative diseases. An accurate diagnosis of HDLS currently depends on a histopathologic evaluation, because the gene(s) causing HDLS remain unknown. Finding the gene for this condition is of paramount importance in understanding the neurodegeneration associated with white matter abnormalities. The identification of three new families with pathologically-confirmed HDLS in this study further suggest that HDLS may be frequently unrecognized and misdiagnosed. Alerting the clinicians to this disorder will feasibly increase identification of HDLS cases. Being familiar with the clinical presentation and neuroimaging will aid in the diagnostic evaluation.
Acknowledgements
Work was partially supported by the NIH/NINDS 1RC2NS070276, NS057567, P50NS072187, Mayo Clinic Florida (MCF) Research Committee CR program (MCF #90052030), Dystonia Medical Research Foundation, and a gift from Carl Edward Bolch, Jr., and Susan Bass Bolch (MCF #90052031/PAU #90052).
CS was sponsored by Anna-Lisa och Bror Björnssons-, Sven and Dagmar Saléns-, Signe och Olof Wallenius- and Gamla Tjänarinnor Foundations, Sweden. The Swedish Society of Medicine Gothenburg (GLS), Sweden, The Swedish Society of Medicine Sweden, The Swedish and Gothenburg Societies for the Neurologically Disabled and The Gothenburg Foundation for Neurological Research.
DWD was supported by P50NS072187 and a gift from Carl Edward Bolch, Jr., and Susan Bass Bolch. SR and HK were both funded by BMBF (Brain-Net-Germany (01GI0505).
We would also like to acknowledge the patients and their families for their participation. This research would not have been possible without their consistent support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Axelsson R, Roytta M, Sourander P, Akesson HO, Andersen O. Hereditary diffuse leucoencephalopathy with spheroids. Acta Psychiatr Scand Suppl. 1984;314:1–65. [PubMed] [Google Scholar]
- [2].Wider C, Van Gerpen JA, DeArmond S, Shuster EA, Dickson DW, Wszolek ZK. Leukoencephalopathy with spheroids (HDLS) and pigmentary leukodystrophy (POLD): a single entity? Neurology. 2009;72:1953–9. doi: 10.1212/WNL.0b013e3181a826c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Van Gerpen JA, Wider C, Broderick DF, Dickson DW, Brown LA, Wszolek ZK. Insights into the dynamics of hereditary diffuse leukoencephalopathy with axonal spheroids. Neurology. 2008;71:925–9. doi: 10.1212/01.wnl.0000325916.30701.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Swerdlow RH, Miller BB, Lopes MB, Mandell JW, Wooten GF, Damgaard P. Autosomal dominant subcortical gliosis presenting as frontotemporal dementia. Neurology. 2009;72:260–7. doi: 10.1212/01.wnl.0000339484.61490.a4. [DOI] [PubMed] [Google Scholar]
- [5].Mendes A, Pinto M, Vieira S, Castro L, Carpenter S. Adult-onset leukodystrophy with axonal spheroids. J Neurol Sci. 2010;297:40–5. doi: 10.1016/j.jns.2010.06.027. [DOI] [PubMed] [Google Scholar]
- [6].Mateen FJ, Keegan BM, Krecke K, Parisi JE, Trenerry MR, Pittock SJ. Sporadic leucodystrophy with neuroaxonal spheroids: persistence of DWI changes and neurocognitive profiles: a case study. J Neurol Neurosurg Psychiatry. 2010;81:619–22. doi: 10.1136/jnnp.2008.169243. [DOI] [PubMed] [Google Scholar]
- [7].Boisse L, Islam O, Woulfe J, Ludwin SK, Brunet DG. Neurological picture. Hereditary diffuse leukoencephalopathy with neuroaxonal spheroids: novel imaging findings. J Neurol Neurosurg Psychiatry. 2010;81:313–4. doi: 10.1136/jnnp.2009.180224. [DOI] [PubMed] [Google Scholar]
- [8].Mayer B, Oelschlaeger C, Keyvani K, Niederstadt T. Two cases of LENAS: diagnosis by MRI and biopsy. J Neurol. 2007;254:1453–4. doi: 10.1007/s00415-007-0541-8. [DOI] [PubMed] [Google Scholar]
- [9].Maillart E, Rousseau A, Galanaud D, Gray F, Polivka M, Labauge P. Rapid onset frontal leukodystrophy with decreased diffusion coefficient and neuroaxonal spheroids. J Neurol. 2009;256:1649–54. doi: 10.1007/s00415-009-5172-9. [DOI] [PubMed] [Google Scholar]
- [10].Baba Y, Ghetti B, Baker MC, Uitti RJ, Hutton ML, Yamaguchi K. Hereditary diffuse leukoencephalopathy with spheroids: clinical, pathologic and genetic studies of a new kindred. Acta Neuropathol (Berl) 2006;111:300–11. doi: 10.1007/s00401-006-0046-z. [DOI] [PubMed] [Google Scholar]
- [11].Lin WL, Wszolek ZK, Dickson DW. Hereditary diffuse leukoencephalopathy with spheroids: ultrastructural and immunoelectron microscopic studies. Int J Clin Exp Pathol. 2010;3:665–74. [PMC free article] [PubMed] [Google Scholar]
- [12].Trapp BD, Bo L, Mork S, Chang A. Pathogenesis of tissue injury in MS lesions. J Neuroimmunol. 1999;98:49–56. doi: 10.1016/s0165-5728(99)00081-8. [DOI] [PubMed] [Google Scholar]
- [13].Gieselmann V, Krageloh-Mann I. Metachromatic leukodystrophy--an update. Neuropediatrics. 2010;41:1–6. doi: 10.1055/s-0030-1253412. [DOI] [PubMed] [Google Scholar]
- [14].Sakai N. Pathogenesis of leukodystrophy for Krabbe disease: molecular mechanism and clinical treatment. Brain Dev. 2009;31:485–7. doi: 10.1016/j.braindev.2009.03.001. [DOI] [PubMed] [Google Scholar]
- [15].Berger J, Gartner J. X-linked adrenoleukodystrophy: clinical, biochemical and pathogenetic aspects. Biochim Biophys Acta. 2006;1763:1721–32. doi: 10.1016/j.bbamcr.2006.07.010. [DOI] [PubMed] [Google Scholar]
- [16].Eichler F, Grodd W, Grant E, Sessa M, Biffi A, Bley A. Metachromatic leukodystrophy: a scoring system for brain MR imaging observations. AJNR Am J Neuroradiol. 2009;30:1893–7. doi: 10.3174/ajnr.A1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Eichler F, Mahmood A, Loes D, Bezman L, Lin D, Moser HW. Magnetic resonance imaging detection of lesion progression in adult patients with X-linked adrenoleukodystrophy. Arch Neurol. 2007;64:659–64. doi: 10.1001/archneur.64.5.659. [DOI] [PubMed] [Google Scholar]
- [18].Bugiani M, Boor I, Powers JM, Scheper GC, van der Knaap MS. Leukoencephalopathy with vanishing white matter: a review. J Neuropathol Exp Neurol. 2010;69:987–96. doi: 10.1097/NEN.0b013e3181f2eafa. [DOI] [PubMed] [Google Scholar]
- [19].Brunetti-Pierri N, Scaglia F. GM1 gangliosidosis: review of clinical, molecular, and therapeutic aspects. Mol Genet Metab. 2008;94:391–6. doi: 10.1016/j.ymgme.2008.04.012. [DOI] [PubMed] [Google Scholar]
- [20].Vanier MT. Niemann-Pick disease type C. Orphanet J Rare Dis. 2010;5:16. doi: 10.1186/1750-1172-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Germain DP. Fabry disease. Orphanet J Rare Dis. 2010;5 doi: 10.1186/1750-1172-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Neudorfer O, Pastores GM, Zeng BJ, Gianutsos J, Zaroff CM, Kolodny EH. Late-onset Tay-Sachs disease: phenotypic characterization and genotypic correlations in 21 affected patients. Genet Med. 2005;7:119–23. doi: 10.1097/01.gim.0000154300.84107.75. [DOI] [PubMed] [Google Scholar]
- [23].Peters AS, Markovic K, Schramm A, Schwab S, Heuss D. Late onset hexosaminidase A deficiency in a young adult. Eur J Neurol. 2008;15:e70–1. e2–3. doi: 10.1111/j.1468-1331.2008.02170.x. author reply. [DOI] [PubMed] [Google Scholar]
- [24].Sundal C, Ekholm S, Andersen O. White matter disorders with autosomal dominant heredity: a review with personal clinical case studies and their MRI findings. Acta Neurol Scand. 2010;121:328–37. doi: 10.1111/j.1600-0404.2009.01219.x. [DOI] [PubMed] [Google Scholar]
- [25].Meythaler JM, Peduzzi JD, Eleftheriou E, Novack TA. Current concepts: diffuse axonal injury-associated traumatic brain injury. Arch Phys Med Rehabil. 2001;82:1461–71. doi: 10.1053/apmr.2001.25137. [DOI] [PubMed] [Google Scholar]
- [26].Kaneko M, Sano K, Nakayama J, Amano N. Neuropathology. 2010. Nasu-Hakola disease: The first case reported by Nasu and review. [DOI] [PubMed] [Google Scholar]
- [27].Melberg A, Hallberg L, Kalimo H, Raininko R. MR characteristics and neuropathology in adult-onset autosomal dominant leukodystrophy with autonomic symptoms. AJNR Am J Neuroradiol. 2006;27:904–11. [PMC free article] [PubMed] [Google Scholar]
- [28].Sundblom J, Melberg A, Kalimo H, Smits A, Raininko R. MR imaging characteristics and neuropathology of the spinal cord in adult-onset autosomal dominant leukodystrophy with autonomic symptoms. AJNR Am J Neuroradiol. 2009;30:328–35. doi: 10.3174/ajnr.A1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Tikka S, Mykkanen K, Ruchoux MM, Bergholm R, Junna M, Poyhonen M, et al. Congruence between NOTCH3 mutations and GOM in 131 CADASIL patients. Brain. 2009;132:933–9. doi: 10.1093/brain/awn364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Seelaar H, Rohrer JD, Pijnenburg YA, Fox NC, van Swieten JC. Clinical, genetic and pathological heterogeneity of frontotemporal dementia: a review. J Neurol Neurosurg Psychiatry. 2011;82:476–86. doi: 10.1136/jnnp.2010.212225. [DOI] [PubMed] [Google Scholar]
- [31].Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E. Alzheimer's disease. Lancet. 2011;377:1019–31. doi: 10.1016/S0140-6736(10)61349-9. [DOI] [PubMed] [Google Scholar]
- [32].Seppi K. MRI for the differential diagnosis of neurodegenerative parkinsonism in clinical practice. Parkinsonism Relat Disord. 2007;13(Suppl 3):S400–5. doi: 10.1016/S1353-8020(08)70038-5. [DOI] [PubMed] [Google Scholar]
- [33].Stadelmann C. Multiple sclerosis as a neurodegenerative disease: pathology, mechanisms and therapeutic implications. Curr Opin Neurol. 2011;24:224–9. doi: 10.1097/WCO.0b013e328346056f. [DOI] [PubMed] [Google Scholar]
- [34].Kohlschutter A, Bley A, Brockmann K, Gartner J, Krageloh-Mann I, Rolfs A, et al. Leukodystrophies and other genetic metabolic leukoencephalopathies in children and adults. Brain Dev. 2010;32:82–9. doi: 10.1016/j.braindev.2009.03.014. [DOI] [PubMed] [Google Scholar]
- [35].Susac JO, Murtagh FR, Egan RA, Berger JR, Bakshi R, Lincoff N, et al. MRI findings in Susac's syndrome. Neurology. 2003;61:1783–7. doi: 10.1212/01.wnl.0000103880.29693.48. [DOI] [PubMed] [Google Scholar]
- [36].van der Knaap M, Valk J, Barkhof F. 2005. Magnetic resonance of myelination and myelin disorders: Birkhäuser. [Google Scholar]