Abstract
Distraction Osteogenesis (DO) is an orthopedic protocol which induces direct new bone formation as a result of the stimulating effects of mechanical distraction. Chronic ethanol (EtOH) exposure has been demonstrated to inhibit bone formation in rodent models of DO. Further it has been demonstrated that 1) Tumor Necrosis Factor-α (TNF) blockers are protective against EtOH exposure and 2) recombinant mouse TNF (rmTNF) inhibits direct bone formation in EtOH naïve mice through TNF receptor 1 (TNFR1). These results suggest that the inhibitory effects are significantly mediated by TNF signaling. Therefore, we hypothesized that direct new bone formation in TNFR1 knockout (KO) mice would be protected from EtOH exposure. We utilized a unique model of mouse DO combined with liquid/chow diets to compare the effects of EtOH on both a strain of TNFR1 knockout (TNFR1KO) mice and on mice of their C57BL/6 (B6) control strain. In the B6 study, and in concordance with previous work, both radiological and histological analyses of direct bone formation in the distraction gaps demonstrated significant osteoinhibition due to EtOH compared to chow or pair-fed mice. In the TNFR1KO study and in support of the hypothesis, both radiological and histological analyses of distraction gap bone formation demonstrated no significant differences between the EtOH, chow fed, or pair-fed. We conclude that exogenous rmTNF and EtOH-induced endogenous TNF act to inhibit new bone formation during DO by signaling primarily through TNFR1.
Keywords: ethanol, TNF, distraction osteogenesis, TNFR1 KO
Introduction
Distraction Osteogenesis (DO) is a term that relates to a collection of clinical and/or experimental protocols that can result in accelerated bone formation. DO, as modeled here, is induced by gradually separating the edges of a tibial bone fracture, using an external fixator, to permit formation of new bone in the slowly expanding gap. New bone formation (direct, intramembranous, appositional, regenerative osteoblastogenesis) during DO is well organized and during the early phases is spatially isolated from the subsequent process of bone resorption/remodeling.
Tumor necrosis factor-α (TNF) is an inflammatory cytokine that can play a role in modulating both osteoblasts and osteoclasts. Previous studies have demonstrated the ability of TNF to block multiple osteoblast functions in vitro, as well as, bone formation/repair in vivo (Nanes, 2003). Though high levels of TNF are known to inhibit osteoblastogenesis in culture and in vivo; nevertheless, low doses can enhance osteoblast proliferation in culture and impaired osteoblastogenesis has been demonstrated in TNFR1/R2 double knockout mice (Frost et al., Gerstenfeld et al., 2003). This suggests that normal expression of TNF is required for optimal bone formation, but that unregulated or excessive expression can contribute to skeletal pathology.
Previous studies have shown that aging, menopause, alcohol abuse, and arthritis can be correlated with osteoporosis, decreased bone mass, risk of fractures, and impaired fracture healing (Nanes, 2003, Aronson et al., 2001, Brown et al., 2002). Two characteristics of the above osteoporotic states are a relative impairment in osteoblastogenesis and an abnormal elevation in serum TNF levels. The DO model coupled with genetically modified mouse strains provides the platform to study the effects of modulation of the TNF signaling axis on direct bone formation (Aronson et al., 2002).
The direct effects of TNF on bone formation in rodents are hypothetically mediated through TNF receptor 1 and/or 2 (TNFR1/2) signaling. Recently, it has been demonstrated that rmTNF inhibits direct bone formation in EtOH naive mice through the TNFR1 receptor (Wahl et al., 2010). Therefore for these studies, we hypothesized that direct new bone formation in TNFR1 knockout (KO) mice would be protected from EtOH exposure when compared to their appropriate control strain.
Materials and Methods
Animals
Virus-free adult male C57BL/6 (control strain/WT, #0664), and TNFR1KO (#2818) mice were purchased from Jackson Industries (Bar Harbor, ME). They were housed in individual cages in temperature (22°C) and humidity (50%) controlled rooms having a 12 h light/12 h dark cycle. All mice were handled by animal care personnel for 5–7 days prior to surgery. In all studies, the mice were assigned to respective experimental groups with mean body weights equal to that of the control group (± 4 g) for the study, and the mice were weighed once a week there after. The following two studies were performed as replicates in overlapping time intervals. All research protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Arkansas for Medical Sciences.
Study Designs
Study 1: Effects of EtOH on DO in C57BL/6 (WT) mice
To replicate previous studies on the effects of EtOH on bone formation during DO in mice, n=36 two-three month old male C57BL/6 (WT) mice were purchased from JAX (Wahl et al., 2006, Wahl et al., 2007). Twelve of these mice were placed in a chow ad lib group. The remaining 24 mice were acclimated to the Lieber-DeCarli liquid control diet #710027 (Dyets Inc) over a four day period. The mice were acclimated to the diet by adding a feeding tube of the liquid diet (15–25 mls) to the cage along with the pellet chow for three days, and then taking out the pellet chow. Once acclimated to the diets, the liquid diet mice were separated into two groups: pair-fed control (n=12) and EtOH (n=12). The EtOH diet used was the Lieber-DeCarli liquid EtOH diet #710260 from Dyets. The control mice were pair-fed to the EtOH mice, so every day we measured how much diet each EtOH mouse consumed and then gave its “pair” mouse that amount of control diet. The EtOH group started on 1% EtOH on the fifth day of the study. The EtOH was increased to 2% on day 7, to 3% on day 9, to 4% on day 11, to 5% on day 13. The EtOH content stayed at 5% for the remainder of the study. After being on liquid diet for 51 days, all mice underwent placement of an external fixator and osteotomy on the left tibia. At this time the EtOH group had been receiving EtOH for 47 days. Following a three day latency period, distraction began at a rate of 0.075mm b.i.d. for 14 days. At harvest trunk blood was taken for serum, and both the distracted and contra-lateral tibiae were placed in formalin.
Study 2: Effects of EtOH on DO in TNFR1KO mice
As a replicate to the above study this experiment examined the effects of EtOH on a TNFR1KO mouse strain. The study was performed as above, but utilized n=48 male 3–4 month old TNFR1 KO mice, which were placed into three groups: chow-fed (n=12), pair-fed (n=18) and EtOH treated (n=18).
Distraction Protocol
Briefly as previously published, following acclimation and under Nembutal anesthesia, each mouse underwent placement of an external fixator and osteotomy to the left tibia (Wahl et al., 2006, Wahl et al., 2007). Four 27-gauge, 1.25 in needles were manually drilled through the tibia (two proximally, two distally). The titanium external fixator was then secured to the pins. A small incision was made in the skin distal to the tibia crest and the soft tissue was carefully retracted to visualize the bone. A single hole was manually drilled through both cortices of the mid-diaphysis, and surgical scissors were used to fracture the cortex on either side of the hole. The fibula was fractured by direct lateral pressure. The periosteum and dermal tissues were closed with a single suture. Finally, buprenex (1.0mg/kg) was given by intramuscular injection post surgery for analgesia. Distraction began three days after surgery (3 day latency) at a rate of 0.075 mm b.i.d. (0.15 mm/day) and continued for 14 days.
Radiographic and Histologic Analysis
After 48 hours of fixation in 10% neutral buffered formalin the left tibiae were removed from the fixators for high-resolution single beam radiography and subsequent histological processing. For initial radiography a Xerox Micro50 closed system radiography unit (Xerox, Pasadena, CA USA) was used at 40 kilovolts (3 mA) for 20 seconds using Kodak X-OMAT film. For quantification, the radiographs were video recorded under low power (2 X objective) microscopic magnification and the area of mineralized new bone in the distraction gaps were evaluated by NIH Image Analysis 1.62 software/Image J software 1.30 (rsb.info.nih.gov/ij/). The measured distraction gap was outlined from the outside corners of the two proximal and the two distal cortices forming a quadrilateral region of interest. The mineralized new bone area in the gap was determined by visually outlining the regions with radiodensity equivalent to or greater than an adjacent non-bone (background) area (excluding bone chips). The percentage of new mineralized bone area within the distraction gap (percent new bone) was calculated by dividing mineralized bone area by total gap area. Therefore, the percent new bone as measured by the radiograph analysis is an estimate of new “mineralized” bone in the entire gap.
After radiography, the distracted tibiae were decalcified in 5% formic acid, dehydrated, and embedded in paraffin. Experience in our laboratory has demonstrated that this achieves good morphology in murine orthopedic tissues and does not appear to significantly impair immunological detection of many epitopes (Skinner et al., 1997, Aronson et al., 1007). Five to seven micron longitudinal sections were cut on a microtome (Leitz 1512, Wetzlar, Germany) for hematoxylin and eosin staining (H&E). Sections were selected to represent a central or near central gap location. A quadrilateral region of interest was outlined and recorded as follows. Both the proximal and distal endocortical (measured from the inside corners of the cortices) and the intracortical (cortical wall included) new bone matrix was outlined together from the outside edges of the cortices, and the area was recorded as new gap bone formation. The percentage of new gap bone area within the DO gap (percent new bone) was calculated as above. The percent new bone as measured by the histological analysis is an estimate of new gap bone formation which would include non-mineralized osteoid columns, embedded new sinusoids, and maturing mineralized bone columns. To be included in both radiographic and histologic analyses the DO samples had to 1) be well aligned, 2) have no broken pin sites, 3) have few bone chips within the DO gap, 4) have an intact ankle, and 5) have had no significant weight loss or health problems during the distraction period.
Serum mouse TNFα Bead Array
Serum samples were run on the Luminex machine in the Pediatric Endocrinology Core Facility. The Linco mouse adipokine kits were used.
Serum ethanol concentrations
The serum ethanol concentration of each sample was determined using a GL5 Analox analyzer (Analox Instruments Limited, London, UK) according to the manufacturers directions as previously described (Wahl et al., 2006, Wahl et al., 2007). The sampling was taken only at sacrifice which was @ ~ 11:00 AM several hours after the most likely feeding time for the mice and thus do not represent peak blood alcohol levels. [In another study using this protocol the blood alcohol levels in the early light/late dark cycle varied between 40–300 mg/dl with a mean of 196 ± 10 mg/dl @ 8:00 AM (unpublished data)].
Statistics
For statistical analysis, differences between group means were determined by One Way ANOVA with the F values reported and including the post-hoc pair wise multiple comparison procedures (Student-Newman-Keuls Method). All data are reported as mean ± standard error of the mean (SEM). Differences were considered significant when P < 0.05.
Results
Study 1: EtOH Exposure Decreases Direct Bone Formation during DO
To replicate earlier studies on the effects of EtOH on DO, C57BL/6 mice were placed in three groups: EtOH-fed, pair-fed, and chow-fed. After dietary manipulations, all mice underwent the DO protocol. In agreement with previous studies, the average serum blood ethanol concentrations at harvest (~11:00 AM) confirmed administration of EtOH (EtOH: 73.2 ± 7.1 mg/dl; range = 21–152) which is in agreement with previous reported and unpublished termination values [see Methods] (Wahl et al., 2006, Wahl et al., 2007). The average alcohol diet consumption (5% EtOH; ~ 36% total calories) per week for both studies is presented in Table 1. The consumption was relatively constant until surgery after which a decrease is commonly noted. All mice were weighed weekly throughout the study. The average weight at the beginning of the study was 26.1 grams. The average weights over the course of the study were: chow 25.8g ± 0.1 g vs pair-fed 27.8 ± 0.3 vs EtOH treated 28.1 ± 0.4. No significant differences were noted in body weight gains or mean average weights between the groups (F value = 3.1, overall P=0.06). The average serum TNF concentrations at harvest confirmed EtOH induction of TNF (chow-fed: 4.8 ± 0.7 pg/ml vs pair-fed: 5.4 ± 0.5 pg/ml and EtOH treated: 14.4 ± 5.6 pg/ml, P< .05 for both comparisons).
Table 1. Weekly Average of Consumed EtOH Diet.
Average alcohol diet consumption (mls), once the 5% ethanol level was reached, per week for Studies 1 and 2. Surgery was performed during Week 7.
| Study 1 (mls) | Study 2 (mls) | |
|---|---|---|
| Week 1 | 12.3 | 13.0 |
| Week 2 | 12.1 | 13.7 |
| Week 3 | 11.8 | 13.2 |
| Week 4 | 11.9 | 13.1 |
| Week 5 | 11.2 | 13.3 |
| Week 6 | 12.1 | 13.2 |
| Week 7 | 11.7 | 11.3 |
| Week 8 | 12.3 | 14.0 |
| Week 9 | 12.7 | 14.3 |
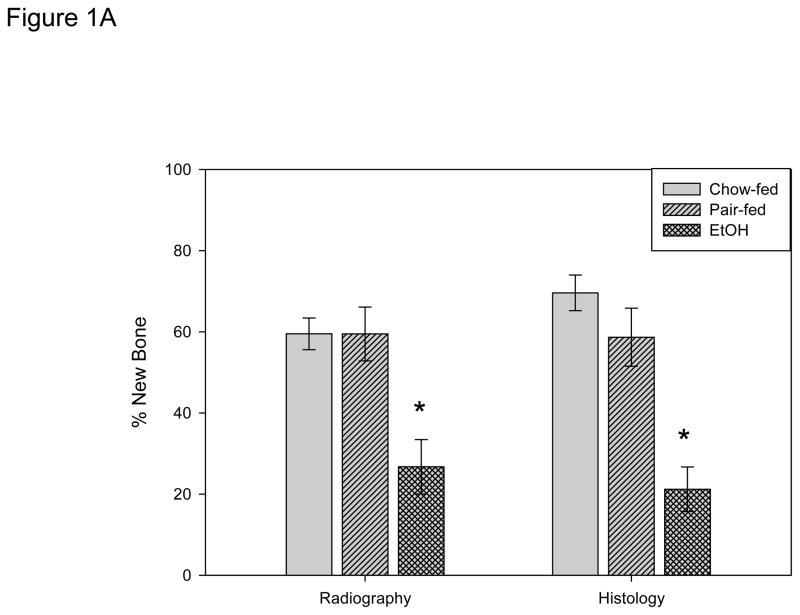
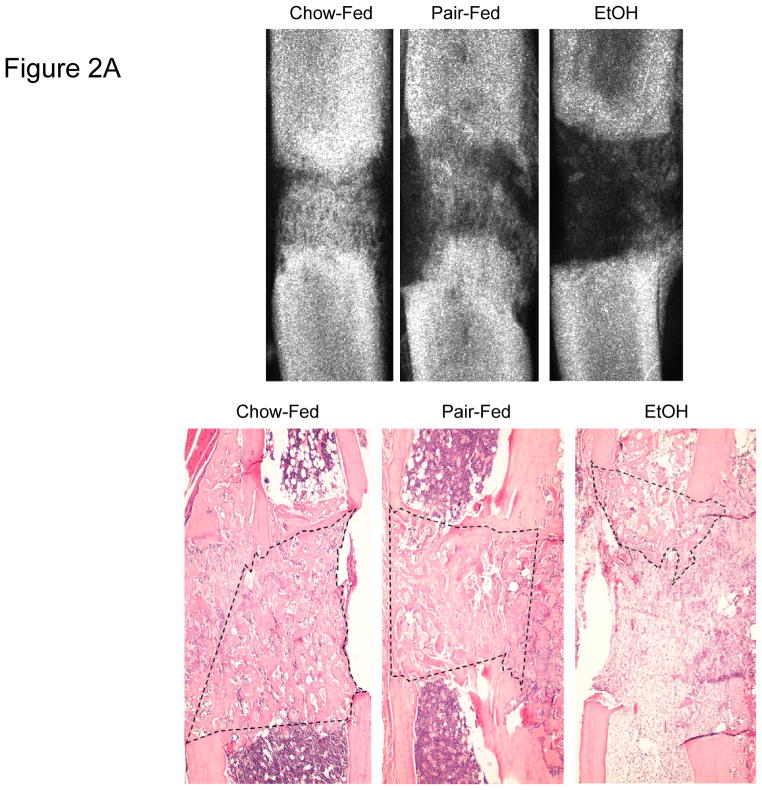
New gap bone formation after DO in chow-fed, pair-fed, and EtOH treated mice was assessed by single beam radiography and histology. Comparison of the distracted tibial radiographs by ANOVA demonstrated no significant differences between the pair-fed and chow-fed mice (chow-fed: 59.5 ± 3.9%, pair-fed: 59.5 ± 6.6%); however, as previously published the EtOH treated mice demonstrated significantly less mineralized new bone formation (EtOH: 26.7 ± 6.7% vs chow P=.004 vs pair-fed P<.001, F value = 9.1) (Figure 1A). Further, analysis of histological sections supported the radiological analyses by revealing significant differences in new gap bone formation between both chow-fed (69.6 ± 3.4.4%) vs EtOH treated (21.2 ± 5.5%) mice, p< 0.001, and pair-fed (58.6 ± 7.2%) vs EtOH treated mice, P<0.001, F value = 17.4 (Figure 1A). Representative radiographs and H&E stained histological sections of distracted tibial DO gaps from chow-fed, pair-fed, and EtOH treated mice are shown in Figure 2A.
Figure 1.
Figure 1A. New gap bone formation after DO in chow-fed, pair-fed and chronic EtOH fed B6 mice was assessed by single beam radiography and histology. Comparison of the distracted tibial radiographs demonstrated an EtOH–associated inhibition of % new bone mineralization (chow-fed: 59.5 ± 3.9% vs pair-fed: 59.5 ± 6.6% vs EtOH: 26.7 ± 6.7%, P= 0.004 & P<.001 respectively, F value=9.1 *). Further, analysis of histological sections supported the radiological analyses by revealing significant differences in new gap bone formation between both chow-fed (69.6 ± 3.4.4%) vs EtOH treated (21.2 ± 5.5%) mice, P< 0.001*, and pair-fed (58.6 ± 7.2%) vs EtOH treated mice, P<0.001, F value=17.4 *.
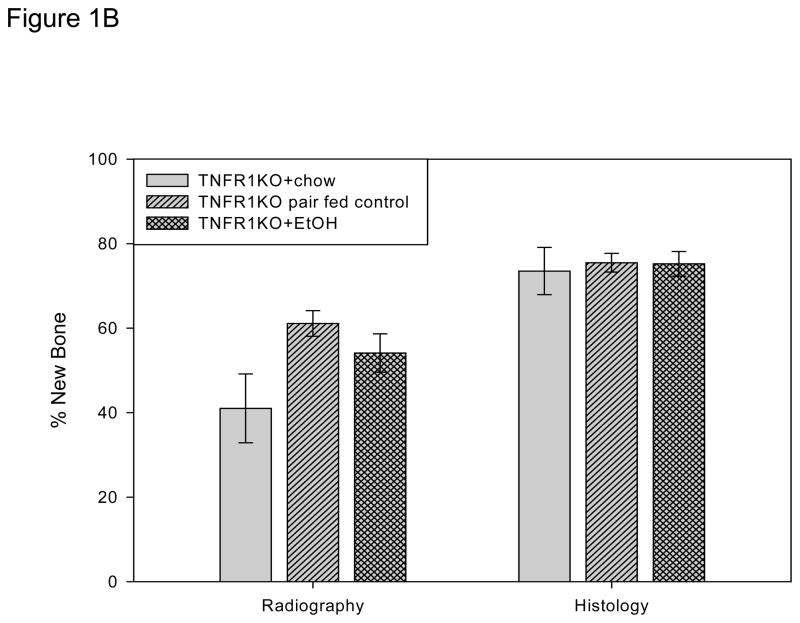
Figure 1B. New gap bone formation after DO in chow-fed, pair-fed, and chronic EtOH fed TNFR1 KO mice was assessed by single beam radiography and histology using One-Way ANOVA. Comparison of the distracted tibial radiographs demonstrated a trend for lower values in the chow fed control which reached significance vs the pair-fed (P<0.05) but not vs the EtOH treated mice (P=0.09). The EtOH vs the pair-fed values were not significant (P=.3). The values for the three groups were chow-fed: 41.0 ± 8.2% vs pair-fed: 61.1 ± 3.0% vs EtOH: 54.1 ± 6.7%. However, analysis of histological sections demonstrated no significant differences between the groups (chow-fed (73.5 ± 5.6%) vs pair-fed (75.5 ± 2.2%) vs EtOH treated: 75.2 ± 2.9%) (P=0.916, F value=.09).
Figure 2.
Figure 2A. Representative radiographs and H&E stained histological sections of distracted tibial DO gaps from chow-fed, pair-fed, and EtOH treated B6 mice are shown. The area of new gap bone formation in the histological sections is roughly outlined with dashed lines for clarification.
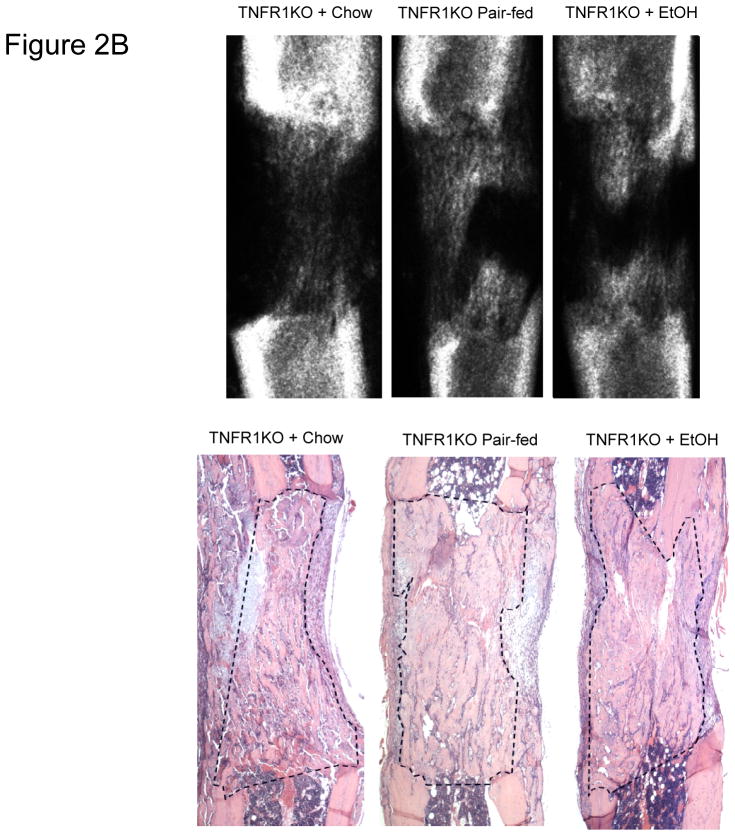
Figure 2B. Representative radiographs and H&E stained histological sections of distracted tibial DO gaps from chow-fed, pair-fed, and chronic EtOH fed TNFR1 KO mice are shown. The area of new gap bone formation in the histological sections is roughly outlined with dashed lines for clarification.
Study 2: TNFR1 Mediates the Osteoinhibitory Effects of Chronic Ethanol Exposure
Based on previous studies which suggest that TNFR1 mediates the negative effects of exogenous rmTNF on direct bone formation; we performed the following study to test the hypothesis that TNFR1 mediates the negative effects of chronic EtOH exposure by using TNFR1 KO mice in all groups. The study was designed as a replicate to Study 1 with the same three groups: chow-fed, pair-fed, and EtOH treated. As above, the average serum blood ethanol concentrations at harvest confirmed administration of EtOH (EtOH: 75.8 ± 2.4 mg/dl; range 19–160). The average weight of the mice at the beginning of the study was 28.3 grams. The average weights over the course of the study were: chow 29.1g ± 0.3 g vs pair-fed 31.7 ± 0.3 vs EtOH treated 28.7 ± 0.3. No significant difference was noted in body weight gains or mean average weights between the chow and the EtOH groups (P=0.7); however, the pair-fed weights were higher than those of the chow or EtOH groups (P<0.01 both comparisons). The average serum TNF concentrations at harvest showed only a trend of higher TNF levels in the EtOH treated R1 KO mice (EtOH: 8.1 ± 3.0 pg/ml vs pair-fed: 4.3 ± 0.4 pg/ml vs chow fed: 5.1 ± 0.4 pg/ml, P=0.1).
Comparison of the distracted tibial radiographs by One-way ANOVA demonstrated a trend for lower values in the chow fed control which reached significance vs the pair-fed (P<0.05) but not vs the EtOH (P=0.09). The EtOH vs the pair-fed was not significant (P=.3). The values for the three groups were chow-fed: 41.0 ± 8.2% vs pair-fed: 61.1 ± 3.0% vs EtOH: 54.1 ± 6.7%, F value=3.5 (Figure 1B). However, analysis of histological sections demonstrated equivalent and robust direct bone formation at the cellular level in all groups (chow-fed (73.5 ± 5.6%) vs pair-fed (75.5 ± 2.2%) vs EtOH treated: 75.2 ± 2.9%) (overall P=0.916, F value= .09) (Figure 1B). Representative radiographs and H&E stained histological sections of distracted tibial DO gaps from chow-fed, pair-fed, and EtOH treated mice are shown in Figure 2B.
Discussion
Previous studies have demonstrated that normal expression of TNF is required for optimal bone formation but that unregulated or excessive expression can contribute to skeletal pathology (Nanes, 2003; Frost et al.; Gerstenfeld et al., 2003). Studies using total enteral nutrition in the rat have demonstrated that EtOH exposure decreases tibial bending strength, and inhibits bone formation (osteoblastogenesis) during DO; while treatment with a TNF receptor antagonist normalizes bone formation during DO, all in the context of optimal nutrition (Brown et al., 2002). Further, treatment of non-ethanol exposed rats with recombinant rat TNF (rrTNF) inhibits bone formation during DO (Wahl et al., 2005). Taken together, these studies suggest a role for the TNF signaling axis in the inhibitory effects of EtOH exposure on direct bone formation, at least in this rat model.
Development of a mouse model for chronic ethanol studies on bone has proved helpful to further mechanistic studies in this area. The combination of EtOH delivery by liquid diet with the mouse DO model, utilized in this report, has demonstrated the osteoinhibition of bone formation during DO equivalent to that seen in the enteral rat model (Wahl et al., 2006; Wahl et al., 2007; Chakkalakal, 2005). Recently, studies employing the mouse DO/liquid diet model have demonstrated the positive effects of systemic delivery of a TNF receptor antagonist during DO to mice chronically exposed to EtOH, and the negative effects of systemic administration of recombinant mouse TNFα (rmTNF) on new bone formation in ethanol naive mice during DO (Wahl et al., 2007). Further the negative effects of rmTNF on DO have been demonstrated to signal through the TNF receptor 1 (TNFR1) (Wahl et al., 2010A). Collectively these results lead to the hypothesis that the negative effects of EtOH exposure on DO in the mouse are mediated through TNFR1. In this report we demonstrate data that supports a role for TNFR1 in the osteoinhibitive effects of EtOH exposure in C57BL/6 and TNFR1 KO mice.
In Study 1, where the effects of EtOH on DO in WT B6 male mice were determined, we confirm the previously reported significant inhibition of new bone formation along with the findings of no significant differences in the weight gains or weight recovery. This suggests the lack of confounding sub-optimal nutritional effects in the EtOH treated mouse DO model (Wahl et al., 2006; Wahl et al., 2007; Chakkalakal, 2005). These results are similar to those seen in a rat model of DO (Brown et al., 2002).
In Study 2, in comparison to the above study, we examined the effects of EtOH on a TNFR1 KO mouse strain. The average pair-fed weights over the course of the study were significantly higher than chow or EtOH. However, since the pair-fed, the chow, and EtOH bone repair values were equivalent; this suggests that any weight differences did not contribute to the bone formation during distraction. Comparison of the distracted tibial radiographs demonstrated a trend for lower values in the chow fed control which reached significance vs the pair-fed but not vs the EtOH treated (nor the chow fed B6 mice in Study 1). We caution that these radiographs only estimate the mineralized new bone in the gap and artifacts due to bone chips, fibrosis, and uneven osteotomy can be misleading. The histology; however, gives a much clearer picture of the actual bone formation as we can measure this at a cellular level and we routinely cut through of the entire gap to make sure the chosen slides are representative. As can be seen in the histology bar the new bone formation of the chow-fed group is equivalent to that of the other two groups. Therefore, the data from this study supports the hypothesis that TNFR1 mediates a significant proportion of the osteoinhibitory effects of EtOH presumably by signaling from EtOH–induced serum TNF.
These studies set the stage for using the ever widening array of genetically altered mouse strains to study the negative effects of EtOH exposure on bone formation during DO and fracture healing. The mouse DO model can also be used to study the effects of excess TNFα administration on bone regeneration, a situation common to other pathologies such as rheumatoid arthritis and aging (Wahl et al., 2010B). For future studies, we postulate that chronic high ethanol consumption results in local elevations of TNF activities, which inhibit osteoblastogenesis at multiple stages during DO. The goals of this research are to support pharmacological and/or nutritional interventions in orthopaedic procedures with the focus on non- and delayed unions in patients with alcohol/TNF associated bone loss.
Acknowledgments
Supported by NIH grant AA12223 (CKL), by NIH National Center for Research Resources Grant # 1CORR16517-01, and by Arkansas Biosciences Institute (CKL) funded by the Arkansas Tobacco Settlement Plan and administered by Arkansas Children’s Hospital Research Institute.
References
- Aronson J, Shen X, Gao GG, Miller F, Quattlebaum T, Skinner RA, Badger TM, Lumpkin CK., Jr Sustained proliferation accompanies distraction osteogenesis in the rat. J Ortho Res. 1997;15:563–569. doi: 10.1002/jor.1100150412. [DOI] [PubMed] [Google Scholar]
- Aronson J, Gao GG, Shen X, McLaren SG, Skinner RA, Badger TM, Lumpkin CK., Jr Effect of aging on distraction osteogenesis in the rat. J Ortho Res. 2001;19:421–427. doi: 10.1016/S0736-0266(00)90025-1. [DOI] [PubMed] [Google Scholar]
- Aronson J, Liu L, Liu Z, Gao GG, Perrien D, Brown E, Skinner RA, Thomas JR, Morris KD, Suva LJ, Badger TM, Lumpkin CK., Jr Decreased endosteal membranous bone formation accompanies aging in a mouse model of distraction osteogenesis. J Regenerative Med. 2002;3:7–16. [Google Scholar]
- Brown EC, Perrien DS, Fletcher TW, Irby DJ, Aronson J, Gao GG, Hogue WR, Skinner RA, Feige U, Suva LJ, Ronis MJJ, Badger TM, Lumpkin CK., Jr IL-1 and TNF antagonists attenuate ethanol-induced inhibition of bone formation in a rat model of distraction osteogenesis. J Pharmacol Exp Ther. 2002;303:904–908. doi: 10.1124/jpet.102.039636. [DOI] [PubMed] [Google Scholar]
- Chakkalakal DA. Alcohol-induced bone loss and deficient bone repair. Alcohol Clin Exp Res. 2005;29:2077–2090. doi: 10.1097/01.alc.0000192039.21305.55. [DOI] [PubMed] [Google Scholar]
- Frost A, Jonsson K, Nilsson O, Ljunggren O. Inflammatory cytokines regulate proliferation of cultured human osteoblasts. Acta Ortho Scand. 1997;68:91–96. doi: 10.3109/17453679709003987. [DOI] [PubMed] [Google Scholar]
- Gerstenfeld LC, Cho TJ, Kon T, Aizawa T, Tsay A, Fitch J, Barnes GL, Graves DT, Einhorn TA. Impaired fracture healing in the absence of TNF-alpha signaling: the role of TNF-alpha in endochondral cartilage resorption. J Bone Miner Res. 2003;18:1584–1592. doi: 10.1359/jbmr.2003.18.9.1584. [DOI] [PubMed] [Google Scholar]
- Nanes MS. Tumor necrosis factor-α: molecular and cellular mechanisms in skeletal pathology. Gene. 2003;321:1–15. doi: 10.1016/s0378-1119(03)00841-2. [DOI] [PubMed] [Google Scholar]
- Skinner RA, Hickmon SG, Lumpkin CK, Jr, Aronson J, Nicholas RW. Decalcified bone: twenty years of successful specimen management. J Histotech. 1997;20:267–277. [Google Scholar]
- Wahl EC, Perrien DS, Aronson J, Liu Z, Fletcher TW, Skinner RA, Feige U, Suva LJ, Badger TM, Lumpkin CK., Jr Ethanol-induced inhibition of bone formation in a rat model of distraction osteogenesis: A role for the tumor necrosis factor signaling axis. Alcohol Clin Exp Res. 2005;29:1466–1472. doi: 10.1097/01.alc.0000174695.09579.11. [DOI] [PubMed] [Google Scholar]
- Wahl EC, Liu L, Perrien DS, Aronson J, Hogue WR, Skinner RA, Hidestrand M, Ronis MJJ, Badger TM, Lumpkin CK., Jr A novel mouse model for the study of the inhibitory effects of chronic ethanol exposure on direct bone formation. Alcohol. 2006;39:159–167. doi: 10.1016/j.alcohol.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Wahl EC, Aronson J, Liu L, Liu Z, Perrien DS, Skinner RA, Badger TM, Ronis MJ, Lumpkin CK., Jr Chronic ethanol exposure inhibits distraction osteogenesis in a mouse model: role of the TNF signaling axis. Toxicol Appl Pharmacol. 2007;220:302–310. doi: 10.1016/j.taap.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl EC, Aronson J, Liu L, Skinner RA, Miller MJ, Bunn RC, Fowlkes JL, Thrailkill KM, Badger TM, Ronis MJJ, Lumpkin CK., Jr Direct bone formation during distraction osteogenesis does not require TNFα receptors and elevated serum TNFα fails to inhibit bone formation in TNFR1 deficient mice. Bone. 2010A;46:410–417. doi: 10.1016/j.bone.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl EC, Aronson J, Liu L, Skinner RA, Miller MJ, Fowlkes JL, Thrailkill KM, Badger TM, Ronis MJJ, Sims J, Lumpkin CK., Jr Restoration of regenerative osteoblastogenesis in aged mice: Modulation of TNF. J Bone Miner Res. 2010B;25:114–123. doi: 10.1359/jbmr.090708. [DOI] [PMC free article] [PubMed] [Google Scholar]