Abstract
Chagas disease, caused by the parasite Trypanosoma cruzi, is an important cause of cardiac disease in endemic areas of Latin America. It is now being diagnosed in non-endemic areas due to immigration. Typical cardiac manifestations of Chagas disease include dilated cardiomyopathy, congestive heart failure, arrhythmias, cardioembolism and stroke. Clinical and laboratory-based research to define the pathology resulting from T. cruzi infection has shed light on many of the cellular and molecular mechanisms leading to these manifestations. Antiparasitic treatment may not be appropriate for patients with advanced cardiac disease. Clinical management of Chagas heart disease is similar to that used for cardiomyopathies due to other processes. Cardiac transplantation has been successfully performed in a small number of patients with Chagas heart disease.
Keywords: Trypanosoma cruzi, Chagas disease, nifurtimox, benznidazole, heart transplantation, echocardiogram, cardiac magnetic resonance imaging
Chagas disease, a neglected tropical disease, has recently caught the attention of the medical community outside the endemic countries, particularly those involved in cardiovascular medicine and surgery. Carlos Chagas, the famous Brazilian physician-scientist, discovered the protozoan parasite Trypanosoma cruzi as well as the clinical manifestations it causes. Interestingly, it has been shown that Chagas disease was present in South America long before it was discovered by Chagas in 1909. Paleoparasitological studies revealed that T. cruzi was present in tissues from mummies in coastal northern Chile from the period 4000 BC to 1400 AD.1,2 The current paper is not meant to be an exhaustive review of Chagas disease since it has been the focus of several recent reviews;3–8 rather it is an overview of several topics that we believe will be of interest to cardiologists and cardiovascular surgeons.
BIOLOGY OF TRYPANOSOMA CRUZI
Most T. cruzi infections are acquired through vector-borne transmission. Other modes of transmission include transfusion of contaminated blood products, organ transplantation, congenital transmission, laboratory accidents, and the ingestion of contaminated food and drink. Small outbreaks of acute Chagas disease associated with the latter have been reported in South America, but the overall incidence of food-borne transmission is unknown. 9,10 In endemic areas of Mexico, Central and South America, infected vectors (triatomine bugs) often invade the primitive houses that are typical in rural areas and where the bugs feed on people as they sleep. Both wild and domestic mammals, including dogs living in and around dwellings, often are infected and act as reservoirs for the parasite.
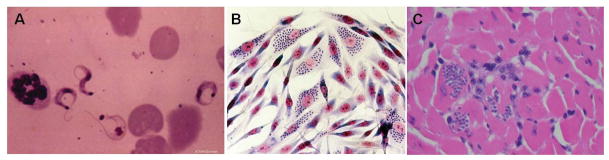
The parasite has a complex life-cycle consisting of four life stages (Fig. 1). Bloodform trypomastigotes are ingested by triatomine insects feeding on an infected mammalian host. Once in the insect vector, the trypomastigotes transform into epimastigotes that, after many rounds of multiplication by binary fission, become non-dividing but infectious metacyclic trypomastigotes in the hindgut. These forms are deposited with the feces of the vector during blood meals. Transmission to a new mammalian host occurs when the parasite-laden feces contaminate oral or nasal mucous membranes, the conjunctivae, or wounds in the skin, including vector bites. Once in the mammalian host, the trypomastigotes enter host cells and transform into amastigotes, which are the multiplying intracellular form (Figs. 2A,B). Amastigotes transform into bloodform trypomastigotes, which are released into the bloodstream as the host cell ruptures. Bloodform trypomastigotes (Fig. 2A) can infect adjacent cells or disseminate via the lymphatics and bloodstream to infect cells in distant sites. Any nucleated mammalian cell can be parasitized, including cardiac myocytes (Fig. 2C), peripheral muscle cells, endothelial and vascular smooth muscle cells, and cells of the central and peripheral nervous systems, the reticuloendothelial system and adipose tissue. Recent studies in mice and humans indicate that the adipose tissue is a target and a reservoir for this parasite.11,12
Figure 1.
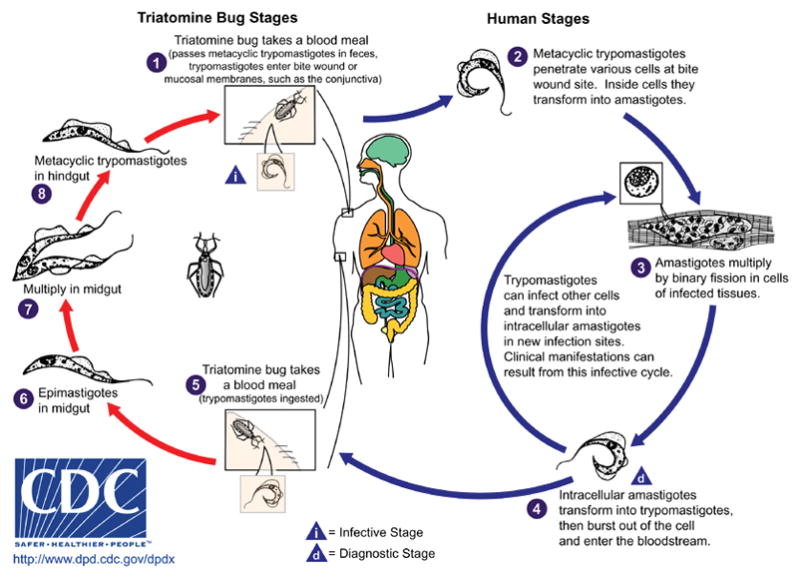
Life cycle of Trypanosoma cruzi (CDC Website www.dpd.cdc.gov/dpdx/html/trypanosom/asisAmerican.htm- open access)
Figure 2.
A Bloodform trypomastigotes B: Trypanosoma cruzi-infected myoblast culture. Note the intracellular amastigotes (arrow) C: Pseudocysts (arrow) in the myocardium of a Trypanosoma cruzi infected mouse. 2A from the collection of Herman Zaiman’s “A Presentation of Pictorial Parasites”, with permission from the American Society of Tropical Medicine and Hygiene. 2C from Tanowitz HB, Machado FS, Jelicks LA, et al: Perspectives on Trypanosoma cruzi-induced heart disease (Chagas disease). Prog Cardiovasc Dis 2009; 51: 524-39, with permission.
EPIDEMIOLOGY OF CHAGAS DISEASE IN THE UNITED STATES AND OTHER NON-ENDEMIC AREAS
Outside endemic areas, Chagas disease was long regarded as an exotic disease and given little attention in textbooks and medical school curricula. This disease is endemic in Mexico, Central and South America, where vector-borne transmission of T. cruzi typically occurs in persons living in rural areas. Since Chagas disease was thought to be confined to endemic areas of Latin America, it was not regarded as an important health concern elsewhere.
In recent decades patterns of emigration from Chagas-endemic areas have drastically altered the epidemiology of this disease in the United States and other non-endemic regions.13–21 For example, in 2009 Bern and Montgomery estimated that 300,000 persons living in the United States are chronically infected with T. Cruzi.22 Moreover, there are many Brazilian immigrants in Portugal, Bolivian immigrants in Spain and Latin American immigrants in France. The seroprevalence of Chagas disease among Bolivian women in Barcelona is 3.4%.14 Although the vast majority of infections in the United States are in immigrants from endemic areas, indigenous vector-borne T. cruzi infection was reported here as early as the 1950s and since then triatomine bugs and infected mammalian reservoirs have been reported from 18 states across the southern tier of the United States.
Serologically positive persons usually have the indeterminate, asymptomatic form of Chagas disease and are unaware of their infection, but remain potential sources of transmission. Congenital transmission is one such source, and it has been reported in Europe in several infants born to Latin American immigrant mothers with undiagnosed Chagas disease. 23–25 These observations and the background knowledge that congenital Chagas disease occurs in 2–10% of babies born to women with chronic T. cruzi infections suggest that in the non-endemic countries all pregnant women at risk for Chagas disease should be screened serologically for T. cruzi infection.
Chagas disease in immunosuppressed patients has become a consideration, particularly as it pertains to organ transplant recipients. Blood and organ donors unaware of their infection status may serve as sources of transfusion or transplant-transmitted infection, although blood donor screening programs in the United States, Canada, and the endemic countries essentially have eliminated transfusion transmission. Similarly, organ recipients who are chronically infected may experience severe disease related to reactivation of chronic infection. In non-endemic areas, the screening of donors and/or recipients for Chagas disease may not be performed routinely. In addition, persons with chronic Chagas disease and HIV/AIDS may have a recrudescence of the T. cruzi infection that can go unrecognized or misdiagnosed as other diseases such as Toxoplasma encephalitis.
The cardiac disease burden attributable to Chagas disease in immigrant populations is an emerging concern. For example, a recent paper by Roca et al18 examined the prevalence of Chagas disease among Latin American immigrants in a primary care setting in Barcelona. Of the 766 patients tested, 22 individuals were diagnosed with T. cruzi infection (prevalence of 2.8). A number of these patients had clinical Chagas disease including cardiac and gastrointestinal (GI) manifestations. Chagasic heart disease has been reported among Latin American immigrants in the United States and among Brazilian immigrants of Japanese origin in Japan.14 In the United States, as early as the 1980s, Kirchhoff et al described three Bolivian immigrants who presented to the National Institutes of Health in Bethesda, MD with clinical Chagas disease.26 In a study in Southern California, 42 cardiac disease patients with antibody to T. cruzi presented between 1975 and 1990.27,28 Despite these early and subsequent reports of serious cardiac disease associated with T. cruzi infection, awareness of Chagas disease among U.S. health care providers has remained limited.29,30
CLINICAL PATHOLOGICAL CORRELATIONS
As noted, individuals found to be serologically positive for Chagas disease as adults usually do not recall having had an illness attributable to acute T. cruzi infection and are unaware of their infection. This is because vector-borne acute Chagas disease is usually mild and most acute infections are subclinical. After an incubation period of 1 to 2 weeks, a newly-infected individual may develop non-specific signs and symptoms such as fever, chills, myalgias, tachycardia, rash, and meningeal irritation. A raised inflammatory lesion at the site of parasite entry (a chagoma), unilateral periorbital edema (Romaña sign) (Fig. 3), conjunctivitis, lymphadenopathy, and hepatosplenomegaly also may be observed in acute infection. Anemia, thrombocytopenia, and elevated liver enzymes may be present. In many patients bloodform trypomastigotes can be seen in wet preparations of blood (Fig. 2). Serologic tests for T. cruzi-specific IgM are inaccurate and tests for specific IgG are often negative during early acute infection. Myocarditis, cardiomegaly, vasculitis, or congestive heart failure (CHF) develops in a very small percentage of acutely infected patients. The presence of arrhythmias is usually a poor prognostic finding. One group has stated that the mortality rate of acutely infected patients, often children, is between 0.25 to 0.5% and the common cause of death is acute myocarditis and/or meningoencephalitis.5
Figure 3.
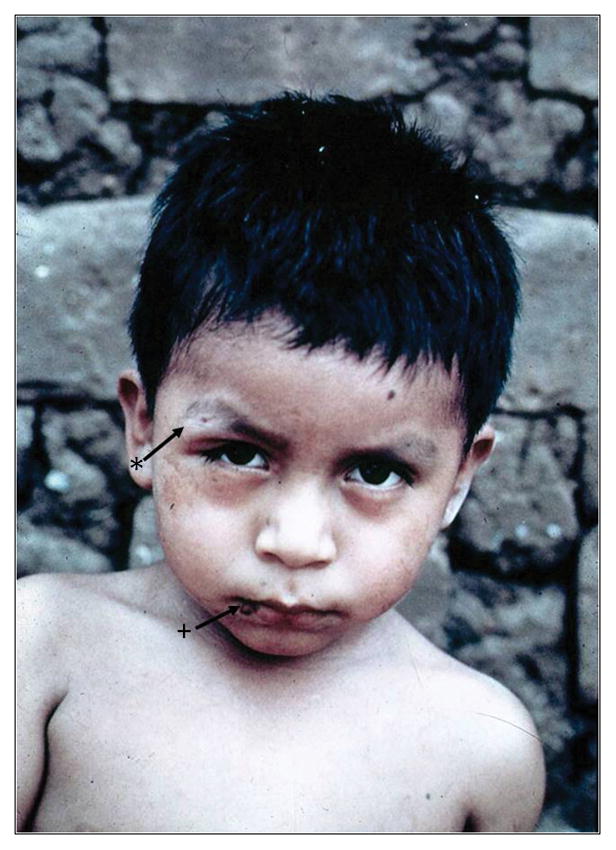
Child with acute Chagas disease with a chagoma (arrow with +) and Romaña’s sign (arrow with *). From the collection of Herman Zaiman’s “A Presentation of Pictorial Parasites”, with permission from the American Society of Tropical Medicine and Hygiene.
An immune response develops in immunocompetent patients, the parasitemia wanes, and signs and symptoms resolve completely in 1 to 2 months. These persons then enter the indeterminate form of chronic infection, which is characterized by positive serology but the absence of clinical manifestations. This form may last from months to an entire lifetime, and as noted, most chronically infected persons never develop clinical manifestations. Most experts believe that only 15% to 30% of infected individuals develop clinical chronic Chagas disease. Clinical chronic Chagas disease typically presents with cardiac or GI manifestations, or both. Cardiac manifestations usually predominate, although in some geographic areas GI alterations are also important. Chronic Chagasic heart disease represents extensive remodeling of the cardiovascular system, manifested insidiously as CHF or abruptly with arrhythmias and/or thromboembolic events. Stroke is recognized as an important manifestation of chronic Chagasic cardiomyopathy, and in some cases it is the initial manifestation in previously asymptomatic persons.7,31 While cardioembolism is the most common cause of stroke in these patients, other causes include microvascular disease, arteriosclerosis and atrial fibrillation.
Dilated congestive cardiomyopathy is also an important manifestation, and it usually occurs years or even decades after a person first becomes infected. Apical aneurysm of the left ventricle is one of the hallmarks of chronic Chagasic cardiomyopathy and is observed by cardiac imaging and at autopsy. Histology of cardiac tissue from patients with chronic Chagasic cardiomyopathy reveals cardiac myocyte hypertrophy, chronic inflammation and fibrosis (Fig. 4). The destruction of conduction tissue results in atrioventricular and intraventricular conduction abnormalities. A common ECG abnormality is right bundle branch block, which may also be associated with an anterior fascicular block, but any ECG alteration may be observed in patients with chronic Chagasic cardiomyopathy. Conduction defects may necessitate the placement of a pacemaker. Some have suggested that increases in levels of brain natriuretic peptide (BNP) may be important in the evaluation of patients with chronic Chagasic heart disease, 32,33 however, this has not attained universal acceptance. Echocardiography and cardiac magnetic resonance imaging (MRI) are often useful is assessing severity of disease.
Figure 4.
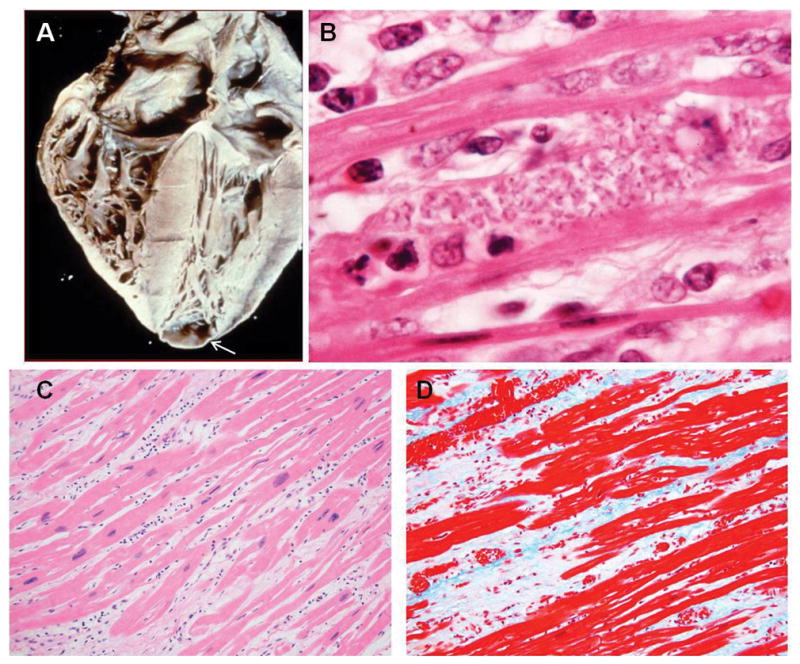
A: Heart of a patient with chronic Chagasic cardiomyopathy. There is four-chamber enlargement of the heart. Note the apical aneurysm (arrow). B: Rare pseudocyst in the myocardium of a patient with chronic Chagasic cardiomyopathy. C: H&E stained myocardium of a patient with chronic Chagasic cardiomyopathy showing bands of fibrous tissue. D: Myocardium of the same patient stained with Trichrome showing massive fibrosis. 4B from the collection of Herman Zaiman’s “A Presentation of Pictorial Parasites”, with permission from the American Society of Tropical Medicine and Hygiene.
The GI dysfunction that accompanies chronic Chagas disease may co-exist with heart disease and is likely the result of injury to the enteric nervous system. Megacolon and/or megaesophagus are most common.34–36 Other associated dysfunctions include achalasia and aspiration pneumonia, disturbances of gastric emptying, alterations in intestinal transit, and motility disorders of the colon and gallbladder. Imaging studies of the GI tract as well as pressure and motility studies are useful in assessing the extent of damage. Recently, the Jelicks and Tanowitz laboratories described intestinal dilation in an infected mouse model and the possibility that selenium could ameliorate this alteration.37,38 These observations may have therapeutic implications for GI and cardiac disease. 39
Pathology and Pathogenesis
Much of what we know regarding the pathology and pathogenesis of T. cruzi infection is derived from animal models and cell culture experiments. The outcome of a T. cruzi infection, as with other infections, is dependent on both parasite and host factors. The immunology of T. cruzi infection in experimental animals and humans has been the focus of several recent reviews and will only be discussed briefly here. 40–43 Suffice it to say that acute infection causes an intense inflammatory reaction which consists of leukocytes including macrophages and occasionally eosinophils. The expression of cytokines, chemokines, nitric oxide synthase and endothelin-144–46 in the cardiovascular system is elevated. CD4+ and CD8+ T cells are present in the inflammatory infiltrate of the myocardium, but in chronic Chagasic cardiomyopathy, CD8+ T cells predominate. 47–48 In the heart there are parasitic pseudocysts (Fig. 2C, 4B) associated with myonecrosis, myocytolysis and vasculitis. In that regard the involvement of the vasculature in the pathogenesis of Chagasic cardiovascular disease has only been appreciated in the past decade, although it had been described in earlier works. Mast cells have also been observed in inflamed tissues.49
Bloodform trypomastigotes gain access to the cardiac myocytes by first invading endothelial cells, vascular smooth muscle cells, and the interstitial areas of the vasculature and the myocardium. Cardiac myocytes are then invaded and destroyed as is the vasculature. The trypomastigote passes through two basal laminae areas and two layers of the extracellular matrix of the myocardium and the interstitial matrix between the basal laminae. Parasite-derived enzymes likely contribute to the degradation of the extracellular matrix and subsequent parasite invasion.50 In an experimental model, it was demonstrated that the expression and activity of myocardial zinc-dependent metalloproteinases are up-regulated (MMP-2, MMP-9) in infection, and that inhibition of these enzymes reduced the inflammation in the myocardium.51
In the heart, there are three layers of cardiac myocytes that are obliquely oriented to each other and meet at the apex. Because of ischemia or inflammation and necrosis, there is degradation of the extracellular matrix, which leads to slippage of the ventricular layers leading to mural thinning and apical aneurysm formation. As noted, damage to this area of the myocardium is common in chronic Chagasic heart disease. The remodeling process of the heart entails structural changes associated with inflammation, necrosis, hypertrophy, and ventricular dilation (Fig. 4). Myocytolysis, myonecrosis, and contraction band necrosis are frequently observed. Myocytolysis is likely the result of the differentiation of amastigotes into bloodform trypomastigotes and contraction band necrosis results from hypoperfusion followed by reperfusion such as that seen after local vasospasm of the branches of the coronary microvasculature. Bands of fibrous tissue replace cardiac myocytes and an important feature of Chagasic heart disease is the accumulation of extracellular collagen that encloses fibers or groups of fibers (Fig. 4C and D). Any part of the heart may be involved, including the conduction pathway. Microvascular involvement manifested by basement membrane thickening has been demonstrated. These changes are irreversible and lead to structural and functional alterations. Thus, cardiovascular remodeling processes result in damage to the extracellular matrix and fibrous tissue replacement of cardiac myocytes and cells of the vasculature.52 These pathological changes eventually result in thinning of the myocardium and cardiac hypertrophy.
In T. cruzi-infected mice and in cultured myoblasts there is increased expression of the components of the mitogen-activated protein kinase pathway specifically such as extracellular signal regulated kinase (ERK) and cell cycle regulatory proteins.53,54 The protein caveolin (Cav) is a negative regulator of extracellular signal regulated kinases (ERK) and cyclin D1. 55 A reduction in the expression of Cav-1 and Cav-3 usually results in the upregulation of ERK activity and an induction of cyclin D1 gene expression, which contribute to cardiac myocyte hypertrophy and ultimately cardiomyopathy. Cav-1 is expressed in non-cardiac myocytes and Cav-3 is expressed only in cardiac myocytes. Interestingly, Cav-1 and Cav-3 null mice, as well as the Cav-1/Cav-3 double null mice, display a cardiomyopathic phenotype associated with cardiac myocyte hypertrophy and interstitial fibrosis. 56–58 During acute T. cruzi infection a reduction in the expression of Cav-1, Cav-2 and Cav-3 was observed accompanied by activation of ERK, activator protein 1, nuclear factor kappa light chain enhancer of activated B cells (NFκB) and increased cyclin D1 expression.53,59,60 The Tanowitz and Lisanti laboratories have demonstrated that infection of the myocardium is associated with cell proliferation of non-cardiac myocytes.53,59
Infection may result in cells such as cardiac myocytes, which are terminally differentiated, reentering the cell cycle. Cyclins A and E, abundant in fetal/neonatal cardiac myocytes and not normally found in post-mitotic adult hearts, are believed to be involved in cardiac myocyte proliferation in the developing heart.45,61 The reappearance of cyclin E positive and cyclin A positive cells in the adult myocardium raises the possibility that infection causes a dedifferentiation of adult cardiac myocytes and enable their re-entry into the cell cycle. It is not known if this contributes to the cardiomyopathic phenotype.
The paucity of parasites in the myocardium has also led to several theories as to the etiology of chronic Chagasic cardiomyopathy including microvascular compromise,62 autoimmunity63–65 and neurogenic mechanisms.66,67 More recently, the group headed by Garg has presented evidence that T cruzi infection results in oxidative stress in the myocardium68–70 and targeted therapy can reverse these alterations in the mouse model.71,72 Also recently, Teixeira et al73 demonstrated that there may be an energetic defect as a result of a selective reduction in the creatine kinase and ATP synthase complex in the myocardium of humans with chronic Chagasic cardiomyopathy.
Vasculature and Chagas Disease
Since the vasculature comprises approximately 35% of the volume of the myocardium, it is reasonable to speculate that the nature of the interaction of the parasite and the endothelium would be important in the pathogenesis of this infection.62,74 The initial descriptions of a T cruzi–induced vasculitis were reported early in the discovery of this disease but popularized more recently. In the 1980s it was beginning to be appreciated that microvascular compromise was an important contributing factor in the pathogenesis of experimental and human cardiomyopathies of diverse etiologies and that treatment with verapamil improved the coronary blood flow and outcome.75 Factor et al,76 employing a mouse model of acute T cruzi infection, clearly demonstrated vasospasm and saccular aneurysms in the subendocardial microvasculature, similar to that described in other cardiomyopathies, and that these alterations might contribute to the development of the typical dilated cardiomyopathy observed in chronic Chagasic cardiomyopathy.45,76 Furthermore, Tanowitz et al77 demonstrated that T cruzi infection caused a reduction in blood flow in the microvascular bed, which was reversed by early and not late treatment with verapamil and an improvement in the myocardial pathology.77–79
Endothelin
Endothelin-1 (ET-1) is a 21 amino acid peptide (80) described as a powerful vasoconstrictor secreted by endothelial cells. T. cruzi-infection of cultured endothelial cells resulted in an increase in biologically active ET-1. It is now appreciated that other cell types can synthesize ET-1, such as cardiac myocytes, fibroblasts, astrocytes and macrophages.81 The synthesis of ET-1 is mediated by endothelin converting enzyme (ECE) which converts Big ET-1 (31 amino acids) to ET-1. The biological properties of ET-1 are mediated by the G-protein coupled endothelin receptors ETA and ETB. Although ET-1 is constitutively expressed in many cells, increased synthesis has been associated with many disease states such as malignant hypertension, primary pulmonary hypertension, CHF, sepsis, meningitis, eclampsia, subarachnoid hemorrhage and cerebral malaria. 82 Mice infected with T. cruzi display an increased expression of ET-1 protein and mRNA in the myocardium and an increase in plasma ET-1 levels83 and treatment of infected mice with phosphoramidon, an inhibitor of ECE, reduced T. cruzi-infection-induced right ventricular _____. 84 Importantly, when mice in which the gene for ET-1 was deleted either in cardiac myocytes or endothelial cells were infected with T. cruzi, there was an amelioration in cardiac remodeling. 85 Interestingly, elevated plasma levels of ET-1 have been demonstrated in patients with chronic Chagasic cardiomyopathy.86 However, it is unclear if this is a result of CHF in general or Chagasic cardiomyopathy in particular. Hassan et al87 found increased expression of ET-1 in the carotid arteries of T. cruzi-infected mice, suggesting the importance of ET-1 in the vasculature of infected mice, and by implication, in infected humans.
Eicosanoids
Eicosanoids are lipid mediators that participate in many biological activities including vascular tone, inflammation, ischemia, and tissue homeostasis.88 The mammalian biosynthetic pathways are dependent upon liberation of arachidonic acid from the inner leaflet of the plasma membrane. Thromboxane A2 (TXA2 ) is generated during arachidonic acid metabolism, and is the most potent vasoconstrictor known. It acts via its receptors TPα and its splice variant TPβ, both of which are expressed on human endothelial cells. Therefore, the observation in experimental animals and humans regarding vasospasm, platelet aggregation and thrombi in the coronary microcirculation was reminiscent of the actions of TXA2. As far back as 1990, Tanowitz and colleagues89 described an increase in platelet aggregation in infected mice accompanied by an increase in plasma TXA2. The increased levels of TXA2 could explain, in part, the vascular spasm and the platelet aggregation. Subsequently, we demonstrated that T. cruzi synthesized TXA2 and that the majority of TXA2 detected in the blood of infected mice is parasite derived.90 These observations suggest that TXA2 could contribute to the pathogenesis of chronic Chagasic cardiomyopathy and its clinical manifestations. More recently, on the basis of these observations, we administered aspirin to T. cruzi infected mice and observed a reduction in the plasma levels of TXA2. 91 Aspirin inhibits the mammalian cyclooxygenase-1 enzyme, thus reducing the levels of PGH2 available for the synthesis of TXA2. These data suggest that aspirin treatment of the infected host decreases the ability of the parasite to scavenge PGH2 from the host in order to synthesize TXA2. Despite the potential benefit of decreasing the T. cruzi-derived TXA2 production with aspirin, we also observed that aspirin-treated infected mice displayed a high parasitemia and mortality. These observations are likely a result of “off target” factors unrelated to TXA2.91 While these observations may suggest that caution should be used in the treatment of fever and pain with cyclooxygenase-1 inhibitors in acute infection, this has never been tested clinically.
LABORATORY DIAGNOSIS OF CHAGAS DISEASE
The diagnosis of acute T. cruzi infection in a patient is usually made by the detection of parasites in wet mounts of blood and in Giemsa-stained slides. Testing for anti–T. cruzi IgM antibodies is not useful because tests for this antibody isotype are not well-standardized. Inoculation of blood samples into a special medium or into mice may be required to demonstrate the parasite, but these culture methods are not clinically useful because parasites may not be seen for several weeks. Parasites may at times be observed at other sites, such as pericardial fluid, bone marrow, brain, skin, and lymph nodes. If acute Chagas disease is suspected in an immunocompromised patient, examination of other samples may be useful. Polymerase chain reaction (PCR) testing is thought to be the most sensitive method for detecting acute T. cruzi infection; PCR testing is available at the Centers for Disease Control and Prevention in the United States. PCR-based tests have been used to diagnose acute infection in congenitally-infected neonates and in immunosuppressed patients.
The diagnosis of chronic Chagas disease is usually based on detecting T. cruzi-specific antibodies. Several serologic assays are available commercially, including indirect immunofluorescence, enzyme-linked immunosorbent, and chemiluminescent assays. Serologic assays are used widely for clinical diagnosis and for screening of donated blood, as well as in epidemiologic studies. A radioimmunoprecipitation assay based on iodinated T. cruzi proteins is sensitive and specific and is currently being used for confirmatory testing of many U.S. blood donor samples that are positive in one of the two screening tests currently approved by the FDA for donor screening.
ANTI-PARASITIC TREATMENT
The treatment of Chagas disease involves both parasite-specific therapy and adjunctive therapy for the management of the clinical manifestations.92 The treatment of T. cruzi is not entirely satisfactory. There are two drugs available: nifurtimox (Lampit, Bayer 2502) and benznidazole (Rochagan, Roche 7–1051). Neither drug is FDA approved, but both are available under investigational use protocols. (Centers for Disease Control and Prevention (CDC) Drug Service (http://www.cdc.gov/laboratory/drugservice/index.html 404-639-3670). The drugs have variable efficacy, must be taken for extended periods, and patients may experience severe side effects. Common side effects associated with nifurtimox include anorexia, nausea and vomiting; less commonly, patients complain of mood changes, insomnia and myalgia. Benznidazole’s side effects include allergic dermatitis, paresthesia, and anorexia.92 These drugs are most effective for the treatment of acute and congenital infection. Parasitological cure is believed to occur in 60–85% of persons with acute infection who complete a full course of either drug, although there are no large-scale studies to support these data. The rate of parasitological cure in congenitally-infected infants has been shown to be greater than 90% if treatment is given during the first year of life. In general, the cure rate in patients with acute Chagas disease is thought to decrease as a function of the time patients have been infected; much lower proportions of individuals with longstanding chronic infection can be cured. There is growing evidence to suggest that treating patients with chronic infection may help to reduce progression of disease. 93 Patients with acute infections, including congenitally-acquired infections in neonates, and chronically-infected children should be treated. Most experts agree that treatment should be offered to eligible chronically-infected adults up to age 50 years. The benefit of treatment relative to risk of side effects must be considered in treatment decisions for older patients. A large clinical trial designed to address the efficacy of benznidazole treatment in patients with early manifestations of cardiac Chagas disease is underway. Allopurinol and several antifungal azoles have been shown to have anti–T. cruzi activity in vitro and in animal models. Most recently, posaconazole94,95 also has been shown to be efficacious in experimental animals, but its usefulness in T. cruzi-infected people has not been demonstrated.
MANAGEMENT OF CARDIAC DISEASE
The management of persons newly diagnosed with Chagas disease as a result of screening prompted by country of origin or blood donation was described in detail by Bern et al.96 The majority of persons have the indeterminate (asymptomatic) form of chronic Chagas disease, especially those identified by blood donor screening. Briefly, after infection is confirmed in a second serological test, a complete medical history and review of systems should be performed as well as a 12-lead ECG with a 30 second rhythm strip. If any clinical indication of cardiac disease is found, a complete cardiac evaluation is recommended including echocardiogram, ambulatory ECG monitoring, and other tests as indicated. If the initial evaluation reveals difficulty in swallowing or chronic constipation, evaluation of the GI tract, including imaging studies should be considered. If the patient fulfills the criteria for anti-parasitic treatment, either nifurtimox or benznidazole should be offered.92
Several general classification schemes similar to that of the New York Heart Association have been published for patients with cardiac manifestations of chronic Chagas disease.97,98 The usual therapeutic strategies for the management of CHF in patients with Chagas disease do not differ from those employed for the treatment of CHF caused by other processes. Thus, digoxin has been used to treat CHF in Chagas disease for many years with good success. However, digoxin should be used with caution because of the frequent extensive damage to the myocardium and the high likelihood of arrhythmias in chronically-infected individuals.3,99,100 Angiotensin-converting enzyme (ACE) inhibitors and β-blockers have become the cornerstone therapy for the management of patients with Chagasic cardiomyopathy in endemic areas.3,101,102 The anti-arrhythmic agent amiodarone has been used to manage arrhythmias in patients with Chagasic cardiomyopathy, but has not been demonstrated to reduce the incidence of sudden death.103 Interestingly, this drug has also been shown to have trypanocidal effects.104,105
Pacemakers and implantable cardioverter-defibrillators (ICDs) have also been used in the management of Chagasic cardiomyopathy with arrhythmias.3,103 Those individuals with ventricular premature contractions who are asymptomatic generally do not require specific anti-arrhythmic therapy. However, patients with sustained ventricular tachycardia and those who have been successfully resuscitated from a near sudden death episode in the setting of left ventricular dysfunction, or those who have recovered from cardiac arrest and have continued ventricular irritability, may benefit from ICD placement. Cardinalli-Neto et al106 have demonstrated that the number of shockd per patient per 30 days is an independent risk factor for death in patients with ICDs and Chagas cardiomyopathy. In addition, left ventricular assist devices have also been used in some patients, 107–109 usually in anticipation of cardiac transplantation.
As with other dilated cardiomyopathies, Chagasic cardiomyopathy is associated with stroke.7,31 Although some investigators have developed risk score schemes to assess the likelihood of cardioembolic or ischemic stroke110 in patients with Chagasic cardiomyopathy, there are insufficient data to make broad recommendations regarding anticoagulation in these patients. Some have recommended that in those patients living in poor conditions and in rural areas, anti-coagulation can be dangerous because of the lack of medical support.7
The issue of whether Chagas disease predisposes to myocardial infarction has been raised for many years. The concept that this might be the case is based, in part, on observations in experimentally-infected animals and in infected humans that the vasculature is damaged and that platelet thrombi are observed in coronary vessels. Although there are case reports and small series74,111–113 that suggest a link between Chagas disease, atherosclerosis, and myocardial infarction, there are no conclusive data. Furthermore, the increase in the prevalence of obesity and diabetes in Chagas–endemic areas and in the emigration of infected persons to North America and Europe, may lead to an increase in ischemic heart disease in Chagasic patients.
CARDIAC IMAGING
In humans
Cardiac imaging modalities provide investigators with non-invasive methods to evaluate cardiac anatomy and function. Echocardiography has been an integral part of the diagnosis, follow-up and prognostication in patients with chronic Chagas disease. Specific diagnostic and prognostic information is provided by echocardiography during the early acute phase and the late symptomatic advanced stage and is used as a guide for proper staging of the disease. During the acute stage of the infection, echocardiographic findings most commonly include pericardial effusion of variable sizes (42%), and segmental left ventricular wall motion abnormality involving the anterior, apical or basal inferoposterior regions.114 The overall left ventricular systolic function is typically preserved at this stage, although a small minority of those with cardiac involvement may present with symptomatic acute myocarditis. Pericardial effusion can be large and hemodynamically significant, leading to cardiac tamponade. The acute phase may be followed by a long indeterminate phase during which, by definition, symptoms and ECG or radiological abnormalities are absent. Routine transthoracic echocardiography is often normal at this stage. However, stress testing and more sensitive echocardiographic techniques may disclose latent myocardial abnormalities in some patients. Thus, abnormal myocardial relaxation indexes have been demonstrated by tissue Doppler imaging in some patients with otherwise normal echocardiograms.115 Barros et al. demonstrated that some patients thought to be in the indeterminate phase of Chagas disease exhibit cardiac wall motion abnormalities associated with functional and electrical defects that can be used to predict risk.116 Tissue Doppler echocardiography has also been used to evaluate right ventricular function (right ventricular peak annular systolic and diastolic velocities) in Chagasic patients during rest and exercise. 117 In addition, the advent of harmonic and contrast echocardiography has resulted in improved detection of subtle regional left ventricular wall motion abnormality.118 Using dobutamine stress echocardiography, blunted heart rate and left ventricular contractile reserve has been shown in some patients with normal resting echocardiograms. 119 Ischemic regional left ventricular wall motion abnormality may also be detected on dobutamine stress echocardiography or by MPI studies, and supports the notion of impaired coronary flow reserve due to abnormal myocardial microvasculature.66,67,119,120 Myocardial ischemia and scarring in the absence of epicardial coronary artery disease is also demonstrated by cardiac MRI.121 Recently, using tissue Doppler imaging, prolonged isovolumic relaxation time has been reported in some patients with the indeterminate form of Chagas disease despite otherwise normal echocardiograms and has been found to be associated with the presence of ventricular arrhythmia.116 A substantial proportion (20–30%) of patients eventually progress to the serious and potentially fatal chronic symptomatic phase of the disease, generally over several decades. Most early echocardiographic studies have focused on this latter phase of the disease.122 These studies identified typical apical left ventricular aneurysms, often with extension towards the apical and mid-portions of the inferolateral wall, in nearly one-half of such patients, whereas approximately 25 % of the patients had a dilated and diffusely hypokinetic left ventricle. Segmental wall motion abnormalities occurred in the absence of epicardial coronary artery disease. Apical thrombus, basal infero-posterior hypokinesis or akinesis, left atrial enlargement, and right ventricular dilation and systolic dysfunction were commonly detected as well. Some patients demonstrated four-chamber cardiac dilation and biventricular systolic dysfunction. Thus, advanced Chagasic heart disease phenotypically mimics either chronic ischemic or idiopathic dilated cardiomyopathy. Later studies have demonstrated that altered geometry is a significant predictor of progressive adverse left ventricular remodeling in this disease.123 Functional mitral and tricuspid regurgitation are often present in those with regional or global ventricular dysfunction. Left ventricular remodeling, as detected by echocardiography, has correlated directly with interleukin-6 concentrations as a marker of myocardial injury,124 and the presence of echocardiographic abnormalities is highly predictive of poor outcome in chronic Chagas disease.118 The significance of the right ventricular function117 and left atrial size125 in predicting overall cardiac function and functional capacity in patients with Chagas disease recently has been emphasized. The spectrum of cardiac abnormalities in Chagas disease is demonstrated in Fig. 5.
Figure 5.
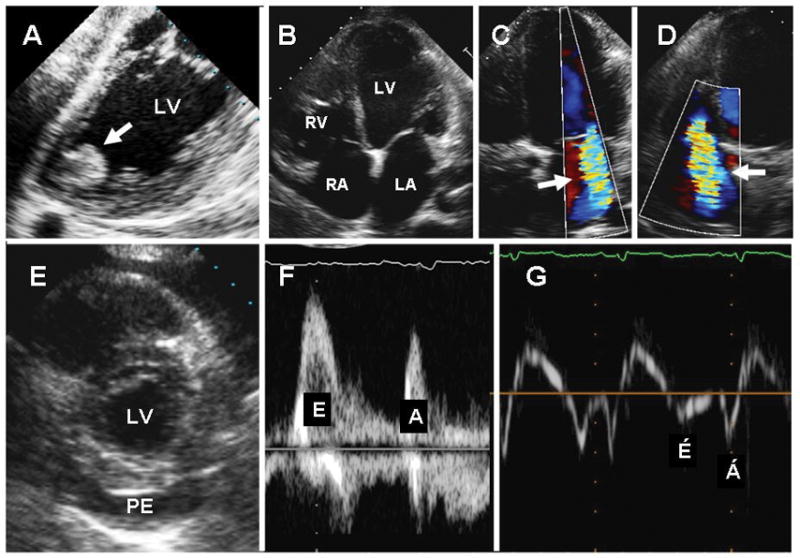
Echocardiographic findings in Chagas heart disease. A. Transesophageal echocardiography demonstrating an apical aneurysm containing a large, round, and protruding thrombus (arrow); B–D. Transthoracic apical 4-chamber views of the heart showing dilated cardiac chambers (B), and functional mitral (C) and tricuspid (D) regurgitation (arrows). E. Parasternal short-axis view of the heart showing a large pericardial effusion (PE). F–G. Trans-mitral pulsed-Doppler (F) and lateral annulus tissue Doppler (G) demonstrating apparently normal peak early (E) and late (A) transmitral velocities, E/A ratio, and E-wave deceleration time (F) but abnormal early (E′) and late (A′) velocities (G) consistent with advanced diastolic dysfunction. LA=left atrium; LV=left ventricle; PE=pericardial effusion; RA=right atrium; RV=right ventricle. From Tanowitz HB, Machado FS, Jelicks LA, et al: Perspectives on Trypanosoma cruzi-induced heart disease (Chagas disease). Prog Cardiovasc Dis 2009; 51: 524-39, with permission.
Cardiac gated MRI, having excellent soft tissue spatial resolution, has emerged as an exceptional technology for evaluating clinical and experimental Chagasic cardiomyopathy.121,126–132 In 1995, Ueno et al128 reported an MRI study of a 50-year-old Brazilian woman exhibiting dyspnea upon exertion. This was one of the first studies using cardiac MRI to evaluate a patient with Chagas disease and revealed localized thinning of the ventricular wall and a small apical left ventricular aneurysm. Subsequently, a number of reports have described the use of MRI in Chagasic cardiomyopathy.121,126,127,130,132 Using delayed gadolinium enhancement, MRI also allows detection of myocardial fibrosis as a determinant of myocardial electrical instability and ventricular arrhythmias. 121 Importantly, MRI with delayed gadolinium enhancement has been shown to detect cardiac involvement before the onset of symptoms and may be useful for predicting arrhythmogenesis. 131 Rochitte et al132 reported the first study quantifying myocardial fibrosis using delayed gadolinium enhanced MRI in patients with Chagasic cardiomyopathy. They demonstrated that the degree of fibrosis detected by delayed gadolinium enhancement increased progressively from mild to severe disease, suggesting that the technique could be used to evaluate the stage of the disease and to assess risk. Recently, Strauss et al133 have shown that 12-lead ECG QRS scoring is correlated with cardiac MRI with delayed gadolinium enhancement and may provide a more readily available tool to screen patients for risk stratification.
In Animals
Serial studies on patients are limited and researchers have relied on animal models, in particular the mouse, to evaluate the immunology, pathology, physiology and other aspects of the pathogenesis of Chagas disease. Non-invasive imaging technologies, permitting study of the same animal over an extended period of time, are ideal for investigating the transition from the acute to chronic stage of disease and therapeutic regimens. In small animal studies, MRI provides high resolution (~50–100 μm) and the same excellent soft tissue contrast as in humans. The Jelicks and Tanowitz laboratories reported the first MRI studies of mice infected with T. cruzi.85,134 Subsequently, the Tanowitz laboratory examined the cardiac structural and functional correlates of verapamil treatment in mice infected with T. cruzi using serial transthoracic echocardiography.78 Echocardiography imaging of small animals has high spatial resolution (~50 μm) and contrast in soft tissue and that study was the first demonstration of the utility of echocardiography to study the functional and structural abnormalities in chronic Chagasic heart disease in a mouse model. In other MRI studies it has been demonstrated that heart wall motion abnormalities and dilatation of the right ventricle are markers of disease severity in mice.135,136 In more recent studies, Souza et al,39 Goldenberg et al,137 and Jasmin et al138 used MRI to evaluate changes in the heart of T. cruzi-infected mice following administration of bone marrow derived cell therapy and selenium supplementation. In 2009 Prado et al139 performed multimodality longitudinal imaging study of T. cruzi infected mice. In that study MRI, echocardiography, and micro-positron emission tomography (PET) imaging were compared. PET is a highly sensitive (pM) molecular imaging technique that can be used to visualize a variety of in vivo biological processes. It is most commonly used to measure uptake of 18F-FDG for cancer diagnosis in patients. The resolution of microPET (1–2 mm) is not as high as MRI or echocardiography but is adequate for some small animal imaging. The study demonstrated that alterations in myocardial glucose uptake could be detected before any substantial structural or functional changes were observed by MRI or echocardiography (Fig. 6).
Figure 6.
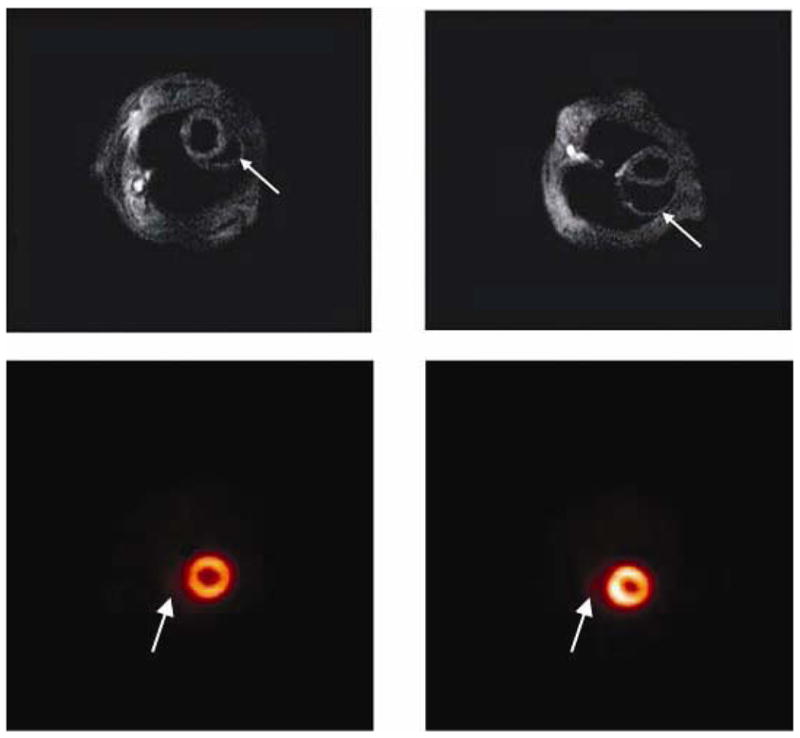
Cardiac imaging of Trypanosoma cruzi infected mice: A: Cardiac MRI of an uninfected mouse. The right ventricle is normal in size (arrow). B: Cardiac MRI of a Trypanosoma cruzi-infected mouse 100 days post infection. Note the enlarged right ventricle (arrow). C: Cardiac microPET of an uninfected mouse showing the right ventricle (arrow). D: Cardiac microPET showing an enlarged right ventricle (arrow) in heart of a mouse 60 days post infection. From Prado et al (Ref. 139) with permission.
CHAGAS DISEASE AND TRANSPLANT MEDICINE
Persons with serologically-proven severe chronic Chagasic heart disease with dilated cardiomyopathy and CHF may benefit from heart transplantation. In centers in Brazil, Chagas disease is the third most common reason for cardiac transplantation. It was recently reported that over a period of 25 years, 107 of 409 patients who underwent cardiac transplantation had Chagas disease.140 A review of published studies revealed that Chagas disease transplant recipients had a high perioperative mortality rate but that the rate among non-Chagas heart transplant recipients was comparable, and both rates likely were attributable to transplant conditions in developing countries. 141 Reported survival rates for Chagas heart transplant patients from one Brazilian study was 83% at one year, 76% at two years and 46% at ten years. 142 A major concern in the heart transplant recipient is the consequence of long-term immunosuppressive therapy after transplant that carries the risk of reactivation of T. cruzi infection. Reported rates of reactivation range from 27% to 90% among Chagas heart transplant recipients, and the mean number of reactivation episodes are 2.5±2.2 per patient.141
A frequently asked question is whether the recipient patient with Chagasic heart disease should receive pre-operative anti-parasitic treatment. The majority of experts do not support this, in part because the side effects may render the potential recipient ineligible for transplantation. In addition, it is unlikely that such treatment will eradicate the parasite in patients with longstanding infections. Following transplantation, chronically-infected recipients should be followed with periodic blood smears and PCR testing for evidence of reactivation disease. Patent parasitemia is an indication for treatment. In addition, if rejection is suspected and the myocardial biopsy is positive for T. cruzi, then the patient should be treated with anti-parasitic drugs.
Recommendations for the screening and treatment of Chagas disease in organ donors and transplant recipients who receive an organ(s) from an infected donor have recently been published in the American Journal of Transplantation. 143 The authors recommended targeted screening of organ donors based on assessment of risk and did not recommend transplanting the heart from any infected donor, although outcomes from transplantation of other organs, especially kidneys, can be successful. Any recipient who has received an organ(s) transplanted from an infected donor should be monitored post-transplant by serial blood smears, PCR testing, and examination of any biopsy samples for evidence of transplant transmitted infection. Similar to monitoring for reactivation in the chronically-infected organ recipient, monitoring of recipients of organs from infected donors should be intensive during the period immediately post-transplant and at any time that immunosuppression is increased or an illness or possible rejection event develops. Acute T. cruzi infection in the transplant recipient should be treated promptly.
STEM CELL-BASED THERAPY
Because of the risks of cardiac transplantation in a patient with Chagasic cardiomyopathy, stem cell transplantation currently is being evaluated in patients with severe CHF and Chagasic heart disease. In 2004, Soares et al144 demonstrated that bone marrow mononuclear cells from normal syngeneic donors significantly reduced cardiac inflammation and fibrosis in mice with chronic T. cruzi infections. Importantly, the improvement was long lasting, being observed up to six months after cell therapy. Cell dosing experiments demonstrated that a minimum of 105 cells were necessary for a significant reduction in the number of inflammatory cells and injection of 106 or 107 cells induced similar effects. In anticipation of attempts to validate this therapy in humans, since in clinical trials autologous bone marrow cells would be employed, bone marrow cells from chronically-infected mice were used and shown to also ameliorate the pathology of infected mice.144
In 2008, using MRI, Goldenberg et al137 demonstrated that 107 bone marrow mononuclear cells prevented and reversed the right ventricular dilatation induced in mice by T. cruzi- infection, illustrating that the histopathological improvement reported by Soares et al144 has a functional correlate. Later, Macambira et al145 determined that repeated injections of the granulocyte colony stimulating factor, which mobilizes stem cells from the bone marrow, decreases inflammation and fibrosis in the hearts of Chagasic mice. Furthermore, the combination of bone marrow mononuclear cells and granulocyte colony stimulating factor enhances the effect of the cell therapy in the reduction of the inflammatory infiltrate.
In a rat model of Chagasic cardiomyopathy Guarita-Souza et al146 reported that direct left ventricular injection of co-cultured skeletal myoblasts and mesenchymal bone marrow-derived cells improved heart function in chronically infected rats as measured by echocardiography. Injection of the co-cultured cells increased ejection fraction and decreased end-systolic and diastolic volumes. These findings demonstrate that local injection of stem cells is also effective and suggest that cells are able to diffuse out and reach other regions of the heart. This is an important observation, given the widespread involvement of the myocardium in Chagasic cardiomyopathy.
Based on the results in animal models, investigators in Brazil initiated a clinical trial to examine the feasibility and safety of autologous bone marrow cell transplantation in patients with CHF due to chronic Chagas disease. These patients generally have a poor prognosis, with mortality rates reaching 40% within two years of onset. At the most advanced stage of CHF the only therapy possible is heart transplantation and the trial was designed for such patients.147 Bone marrow cell aspiration was performed on the day of the injection and the bone marrow mononuclear fraction was obtained through Ficoll density gradient centrifugation and injected into the coronary arteries using an angioplasty catheter with the following distribution: 10 ml in the left descending coronary artery, 5 ml in the circumflex and 5 ml in the right coronary artery. Mean number of injected cells was 2.7 × 108. At the 25th day after cell injection patients received 5μg/kg of granulocyte colony stimulating factor for 5 days. Patients were followed for six months. Importantly there was no detectable increase in arrhythmias after cell therapy nor were troponin I levels increased during or after the procedure. Results indicated that cell therapy induced a small but significant increase in ejection fraction. Quality of life improved as determined by the Minnesota Questionnaire and by the New York Heart Association class. Six minute walking test also showed significant improvement. These results were observed 1 month after therapy and persisted for the 6 months follow-up period. The conclusion was that bone marrow mononuclear cell therapy by intracoronary delivery is feasible and safe in chronic Chagasic cardiomyopathy patients. Given the promising results of the phase I trial, a larger, multi-center, randomized, double-blind and placebo controlled trial was designed to test for efficacy of the intracoronary delivery of bone marrow-derived mononuclear cells in chronic Chagasic cardiomyopathy and is ongoing.
CONCLUSIONS
Chagas disease is one of the neglected tropical diseases now found in non-endemic areas of the world. In the past 100 years since the discovery of this disease by Carlos Chagas, our understanding of the pathology and pathogenesis of this disease has grown. However, physician awareness in non-endemic areas remains a problem.29,30 There are many developments that are exciting and require further investigation. Among these are the expanded use of left ventricular assist devices as a bridge to cardiac transplantation, and the development of immunosuppressive agents that do not result in reactivation of the infection. The further evaluation of stem cell therapy for the treatment of Chagasic cardiomyopathy requires increased efforts which are currently ongoing. As mentioned above there are no clear guidelines for the institution of anticoagulation therapy in either the treatment or the prevention of stroke in these patients. Other important areas of research are the evaluation of biomarkers for diagnosing infection and assessing parasitological cure, as well as the factors that contribute to the transition from acute to chronic disease. The development of efficacious non-toxic drugs is of utmost importance, as is vaccine development whose progress has been slow. Chagas disease remains an exciting area of clinical and basic research for many years to come.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Acknowledgments
This work was supported in part by NIH grants HL-73732 (ACC), AI-076248 (HBT), CA123334 and AI062730 (LAJ), the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (FSM [576200/2008-5, 473670/2008-9]), and the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) (FSM [14916]); CMP was supported by a Fogarty International Training Grant (HBT [D43TW007129]).
References
- 1.Aufderheide AC, Salo W, Madden M, et al. A 9,000-year record of Chagas’ disease. Proc Natl Acad Sci U S A. 2004;101:2034–2039. doi: 10.1073/pnas.0307312101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Araujo A, Jansen AM, Reinhard K, et al. Paleoparasitology of Chagas disease--a review. Mem Inst Oswaldo Cruz. 2009;104 (Suppl 1):9–16. doi: 10.1590/s0074-02762009000900004. [DOI] [PubMed] [Google Scholar]
- 3.Biolo A, Ribeiro AL, Clausell N. Chagas cardiomyopathy--where do we stand after a hundred years? Prog Cardiovas Dis. 2010;52:300–316. doi: 10.1016/j.pcad.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 4.Lescure FX, Le Loup G, Freilij H, et al. Chagas disease: changes in knowledge and management. Lancet Infect Dis. 2010;10:556–570. doi: 10.1016/S1473-3099(10)70098-0. [DOI] [PubMed] [Google Scholar]
- 5.Rassi A, Jr, Rassi A, Marin-Neto JA. Chagas disease. Lancet. 2010;375:1388–1402. doi: 10.1016/S0140-6736(10)60061-X. [DOI] [PubMed] [Google Scholar]
- 6.Parker ER, Sethi A. Chagas disease: coming to a place near you. Dermatol Clin. 2011;29:53–62. doi: 10.1016/j.det.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 7.Carod-Artal FJ, Gascon J. Chagas disease and stroke. Lancet Neurol. 2010;9:533–542. doi: 10.1016/S1474-4422(10)70042-9. [DOI] [PubMed] [Google Scholar]
- 8.Coura JR, Borges-Pereira J. Chagas disease: 100 years after its discovery. A systemic review. Acta Trop. 2010;115:5–13. doi: 10.1016/j.actatropica.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Bastos CJ, Aras R, Mota G, et al. Clinical outcomes of thirteen patients with acute chagas disease acquired through oral transmission from two urban outbreaks in northeastern Brazil. PLoS Neglect Trop Dis. 2010;4:e711. doi: 10.1371/journal.pntd.0000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alarcon de Noya B, Diaz-Bello Z, Colmenares C, et al. Large urban outbreak of orally acquired acute Chagas disease at a school in Caracas, Venezuela. J Infect Dis. 2010;201:1308–1315. doi: 10.1086/651608. [DOI] [PubMed] [Google Scholar]
- 11.Combs TP, Nagajyothi, Mukherjee S, et al. The adipocyte as an important target cell for Trypanosoma cruzi infection. J Biol Chem. 2005;280:24085–24094. doi: 10.1074/jbc.M412802200. [DOI] [PubMed] [Google Scholar]
- 12.Matos Ferreira AV, Segatto M, Menezes Z, et al. Evidence for Trypanosoma cruzi in adipose tissue in human chronic Chagas disease. Microbes and Infection. 2011;13:1002–05. doi: 10.1016/j.micinf.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmunis GA. Epidemiology of Chagas disease in non-endemic countries: the role of international migration. Mem Inst Oswaldo Cruz. 2007;102 (Suppl 1):75–85. doi: 10.1590/s0074-02762007005000093. [DOI] [PubMed] [Google Scholar]
- 14.Gascon J, Bern C, Pinazo MJ. Chagas disease in Spain, the United States and other non-endemic countries. Acta Trop. 2010;115:22–27. doi: 10.1016/j.actatropica.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 15.Develoux M, Lescure FX, Jaureguiberry S, et al. Emergence of Chagas’ disease in Europe: description of the first cases observed in Latin American immigrants in mainland France. Med Trop (Mars) 2010;70:38–42. [PubMed] [Google Scholar]
- 16.Lescure FX, Canestri A, Melliez H, et al. Chagas disease, France. Emerg Infect Dis. 2008;14:644–646. doi: 10.3201/eid1404.070489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanowitz HB, Weiss LM, Montgomery SP. Chagas disease has now gone global. PLoS Neglect Trop Dis. 2011;5:e1136. doi: 10.1371/journal.pntd.0001136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roca C, Pinazo MJ, Lopez-Chejade P, et al. Chagas disease among the Latin American adult population attending in a primary care center in Barcelona, Spain. PLoS Negl Trop Dis. 2011;5:e1135. doi: 10.1371/journal.pntd.0001135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yacoub S, Kotit S, Yacoub MH. Disease appearance and evolution against a background of climate change and reduced resources. Philos Transact A Math Phys Eng Sci. 2011;369:1719–1729. doi: 10.1098/rsta.2011.0013. [DOI] [PubMed] [Google Scholar]
- 20.Bern C, Kjos S, Yabsley MJ, et al. Trypanosoma cruzi and Chagas’ Disease in the United States. Clin Microbiol Rev. 2011;24:655–681. doi: 10.1128/CMR.00005-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirchhoff LV. Epidemiology of American trypanosomiasis (Chagas disease) Advances in parasitology. 2011;75:1–18. doi: 10.1016/B978-0-12-385863-4.00001-0. [DOI] [PubMed] [Google Scholar]
- 22.Bern C, Montgomery SP. An estimate of the burden of Chagas disease in the United States. Clin Infect Dis. 2009;49:e52–54. doi: 10.1086/605091. [DOI] [PubMed] [Google Scholar]
- 23.Munoz J, Portus M, Corachan M, et al. Congenital Trypanosoma cruzi infection in a non-endemic area. Trans R Soc Trop Med Hyg. 2007;101:1161–1162. doi: 10.1016/j.trstmh.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 24.Riera C, Guarro A, Kassab HE, et al. Congenital transmission of Trypanosoma cruzi in Europe (Spain): a case report. Am J Trop Med Hyg. 2006;75:1078–1081. [PubMed] [Google Scholar]
- 25.Jackson Y, Myers C, Diana A, et al. Congenital transmission of Chagas disease in Latin American immigrants in Switzerland. Emerg Infect Dis. 2009;15:601–603. doi: 10.3201/eid1504.080438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirchhoff LV, Neva FA. Chagas’ disease in Latin American immigrants. JAMA. 1985;254:3058–3060. [PubMed] [Google Scholar]
- 27.Hagar JM, Rahimtoola SH. Chagas’ heart disease. Curr Probl Cardiol. 1995;20:825–924. [PubMed] [Google Scholar]
- 28.Hagar JM, Rahimtoola SH. Chagas’ heart disease in the United States. New Eng J Med. 1991;325:763–768. doi: 10.1056/NEJM199109123251103. [DOI] [PubMed] [Google Scholar]
- 29.Stimpert KK, Montgomery SP. Physician awareness of Chagas disease, USA. Emerg Infect Dis. 2010;16:871–872. doi: 10.3201/eid1605.091440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verani JR, Montgomery SP, Schulkin J, et al. Survey of obstetrician-gynecologists in the United States about Chagas disease. Am J Trop Med Hyg. 2010;83:891–895. doi: 10.4269/ajtmh.2010.09-0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carod-Artal FJ, Vargas AP, Falcao T. Stroke in asymptomatic Trypanosoma cruzi-infected patients. Cerebrovas Dis. 2011;31:24–28. doi: 10.1159/000320248. [DOI] [PubMed] [Google Scholar]
- 32.Ribeiro AL, Teixeira MM, Reis AM, et al. Brain natriuretic peptide based strategy to detect left ventricular dysfunction in Chagas disease: a comparison with the conventional approach. Int J Cardiol. 2006;109:34–40. doi: 10.1016/j.ijcard.2005.05.048. [DOI] [PubMed] [Google Scholar]
- 33.Lima-Costa MF, Cesar CC, Peixoto SV, et al. Plasma B-type natriuretic peptide as a predictor of mortality in community-dwelling older adults with Chagas disease: 10-year follow-up of the Bambui Cohort Study of Aging. Am J Epidemiol. 2010;172:190–196. doi: 10.1093/aje/kwq106. [DOI] [PubMed] [Google Scholar]
- 34.da Silveira AB, Chaves AT, de Araujo FF, et al. Expression of caspase-3 in enteric cells is related to development of chagasic megacolon. Hum Pathol. 2009;40:605–606. doi: 10.1016/j.humpath.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 35.Garcia RL, Matos BM, Feres O, et al. Surgical treatment of Chagas megacolon. Critical analysis of outcome in operative methods. Acta Cir Bras. 2008;23(Suppl 1):83–92. doi: 10.1590/s0102-86502008000700015. discussion 92. [DOI] [PubMed] [Google Scholar]
- 36.Tanowitz HB, Kirchhoff LV, Simon D, et al. Chagas’ disease. Clin Microbiol Rev. 1992;5:400–419. doi: 10.1128/cmr.5.4.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ny L, Li H, Mukherjee S, et al. A magnetic resonance imaging study of intestinal dilation in Trypanosoma cruzi-infected mice deficient in nitric oxide synthase. Am J Trop Med Hyg. 2008;79:760–767. [PMC free article] [PubMed] [Google Scholar]
- 38.de Souza AP, Sieberg R, Li H, et al. The role of selenium in intestinal motility and morphology in a murine model of Typanosoma cruzi infection. Parasitol Res. 2010;106:1293–1298. doi: 10.1007/s00436-010-1794-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Souza AP, Jelicks LA, Tanowitz HB, et al. The benefits of using selenium in the treatment of Chagas disease: prevention of right ventricle chamber dilatation and reversion of Trypanosoma cruzi-induced acute and chronic cardiomyopathy in mice. Mem Inst Oswaldo Cruz. 2010;105:746–751. doi: 10.1590/s0074-02762010000600003. [DOI] [PubMed] [Google Scholar]
- 40.Junqueira C, Caetano B, Bartholomeu DC, et al. The endless race between Trypanosoma cruzi and host immunity: lessons for and beyond Chagas disease. Expert Rev Mol Med. 2010;12:e29. doi: 10.1017/S1462399410001560. [DOI] [PubMed] [Google Scholar]
- 41.Kayama H, Takeda K. The innate immune response to Trypanosoma cruzi infection. Microb Infect. 2010;12:511–517. doi: 10.1016/j.micinf.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 42.Boscardin SB, Torrecilhas AC, Manarin R, et al. Chagas’ disease: an update on immune mechanisms and therapeutic strategies. J Cell Mol Med. 2010;14:1373–1384. doi: 10.1111/j.1582-4934.2010.01007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Padilla AM, Bustamante JM, Tarleton RL. CD8+ T cells in Trypanosoma cruzi infection. Curr Opin Immunol. 2009;21:385–390. doi: 10.1016/j.coi.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Machado FS, Souto JT, Rossi MA, et al. Nitric oxide synthase-2 modulates chemokine production by Trypanosoma cruzi-infected cardiac myocytes. Microb Infect. 2008;10:1558–66. doi: 10.1016/j.micinf.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petkova SB, Huang H, Factor SM, et al. The role of endothelin in the pathogenesis of Chagas’ disease. Int J Parasitol. 2001;31:499–511. doi: 10.1016/s0020-7519(01)00168-0. [DOI] [PubMed] [Google Scholar]
- 46.Huang H, Chan J, Wittner M, et al. Expression of cardiac cytokines and inducible form of nitric oxide synthase (NOS2) in Trypanosoma cruzi-infected mice. J Mol Cell Cardiol. 1999;31:75–88. doi: 10.1006/jmcc.1998.0848. [DOI] [PubMed] [Google Scholar]
- 47.Martin DL, Tarleton RL. Antigen-specific T cells maintain an effector memory phenotype during persistent Trypanosoma cruzi infection. J Immunol. 2005;174:1594–1601. doi: 10.4049/jimmunol.174.3.1594. [DOI] [PubMed] [Google Scholar]
- 48.Martin DL, Weatherly DB, Laucella SA, et al. CD8+ T-Cell responses to Trypanosoma cruzi are highly focused on strain-variant trans-sialidase epitopes. PLoS pathogens. 2006;2:e77. doi: 10.1371/journal.ppat.0020077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meuser-Batista M, Correa JR, Soares MJ, et al. Isolation of cardiac mast cells in experimental Trypanosoma cruzi infection. Tissue Cell. 2008;40:309–316. doi: 10.1016/j.tice.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 50.Factor SM, Tanowitz H, Wittner M, et al. Interstitial connective tissue matrix alterations in acute murine Chagas’ disease. Clin Immunol Immunopathol. 1993;68:147–152. doi: 10.1006/clin.1993.1111. [DOI] [PubMed] [Google Scholar]
- 51.Gutierrez FR, Lalu MM, Mariano FS, et al. Increased activities of cardiac matrix metalloproteinases matrix metalloproteinase (MMP)-2 and MMP-9 are associated with mortality during the acute phase of experimental Trypanosoma cruzi infection. J Infect Dis. 2008;197:1468–1476. doi: 10.1086/587487. [DOI] [PubMed] [Google Scholar]
- 52.Higuchi ML, Fukasawa S, De Brito T, et al. Different microcirculatory and interstitial matrix patterns in idiopathic dilated cardiomyopathy and Chagas’ disease: a three dimensional confocal microscopy study. Heart. 1999;82:279–285. doi: 10.1136/hrt.82.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang H, Petkova SB, Cohen AW, et al. Activation of transcription factors AP-1 and NF-kappa B in murine Chagasic myocarditis. Infect Immun. 2003;71:2859–2867. doi: 10.1128/IAI.71.5.2859-2867.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bouzahzah B, Yurchenko V, Nagajyothi F, et al. Regulation of host cell cyclin D1 by Trypanosoma cruzi in myoblasts. Cell Cycle. 2008;7:500–503. doi: 10.4161/cc.7.4.5327. [DOI] [PubMed] [Google Scholar]
- 55.Hulit J, Bash T, Fu M, et al. The cyclin D1 gene is transcriptionally repressed by caveolin-1. J Biol Chem. 2000;275:21203–21209. doi: 10.1074/jbc.M000321200. [DOI] [PubMed] [Google Scholar]
- 56.Cohen AW, Park DS, Woodman SE, et al. Caveolin-1 null mice develop cardiac hypertrophy with hyperactivation of p42/44 MAP kinase in cardiac fibroblasts. Am J Physiol Cell Physiol. 2003;284:C457–474. doi: 10.1152/ajpcell.00380.2002. [DOI] [PubMed] [Google Scholar]
- 57.Park DS, Woodman SE, Schubert W, et al. Caveolin-1/3 double-knockout mice are viable, but lack both muscle and non-muscle caveolae, and develop a severe cardiomyopathic phenotype. Am J Pathol. 2002;160:2207–2217. doi: 10.1016/S0002-9440(10)61168-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Woodman SE, Park DS, Cohen AW, et al. Caveolin-3 knock-out mice develop a progressive cardiomyopathy and show hyperactivation of the p42/44 MAPK cascade. J Biol Chem. 2002;277:38988–38997. doi: 10.1074/jbc.M205511200. [DOI] [PubMed] [Google Scholar]
- 59.Nagajyothi F, Desruisseaux M, Bouzahzah B, et al. Cyclin and caveolin expression in an acute model of murine Chagasic myocarditis. Cell Cycle. 2006;5:107–112. doi: 10.4161/cc.5.1.2284. [DOI] [PubMed] [Google Scholar]
- 60.Adesse D, Lisanti MP, Spray DC, et al. Trypanosoma cruzi infection results in the reduced expression of caveolin-3 in the heart. Cell Cycle. 2010;9:1639–1646. doi: 10.4161/cc.9.8.11509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Petkova SB, Ashton A, Bouzahzah B, et al. Cell cycle molecules and diseases of the cardiovascular system. Front Biosci. 2000;5:D452–460. doi: 10.2741/petkova. [DOI] [PubMed] [Google Scholar]
- 62.Rossi MA, Tanowitz HB, Malvestio LM, et al. Coronary microvascular disease in chronic Chagas cardiomyopathy including an overview on history, pathology, and other proposed pathogenic mechanisms. PLoS Neglect Trop Dis. 2010:4. doi: 10.1371/journal.pntd.0000674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bonney KM, Engman DM. Chagas heart disease pathogenesis: one mechanism or many? Curr Mol Med. 2008;8:510–518. doi: 10.2174/156652408785748004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bonney KM, Taylor JM, Daniels MD, et al. Heat-killed Trypanosoma cruzi induces acute cardiac damage and polyantigenic autoimmunity. PLoS One. 2011;6:e14571. doi: 10.1371/journal.pone.0014571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Teixeira AR, Hecht MM, Guimaro MC, et al. Pathogenesis of chagas’ disease: parasite persistence and autoimmunity. Clin Microbiol Rev. 2011;24:592–630. doi: 10.1128/CMR.00063-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marin-Neto JA, Cunha-Neto E, Maciel BC, et al. Pathogenesis of chronic Chagas heart disease. Circulation. 2007;115:1109–1123. doi: 10.1161/CIRCULATIONAHA.106.624296. [DOI] [PubMed] [Google Scholar]
- 67.Simoes MV, Pintya AO, Bromberg-Marin G, et al. Relation of regional sympathetic denervation and myocardial perfusion disturbance to wall motion impairment in Chagas’ cardiomyopathy. Am J Cardiol. 2000;86:975–981. doi: 10.1016/s0002-9149(00)01133-4. [DOI] [PubMed] [Google Scholar]
- 68.Wen JJ, Vyatkina G, Garg N. Oxidative damage during chagasic cardiomyopathy development: role of mitochondrial oxidant release and inefficient antioxidant defense. Free Radic Biol Med. 2004;37:1821–1833. doi: 10.1016/j.freeradbiomed.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 69.Wen JJ, Yachelini PC, Sembaj A, et al. Increased oxidative stress is correlated with mitochondrial dysfunction in chagasic patients. Free Radic Biol Med. 2006;41:270–276. doi: 10.1016/j.freeradbiomed.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 70.Wen JJ, Dhiman M, Whorton EB, et al. Tissue-specific oxidative imbalance and mitochondrial dysfunction during Trypanosoma cruzi infection in mice. Microb Infect. 2008;10:1201–1209. doi: 10.1016/j.micinf.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wen JJ, Bhatia V, Popov VL, et al. Phenyl-alpha-tert-butyl nitrone reverses mitochondrial decay in acute Chagas’ disease. Am J Pathol. 2006;169:1953–1964. doi: 10.2353/ajpath.2006.060475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wen JJ, Gupta S, Guan Z, et al. Phenyl-alpha-tert-butyl-nitrone and benzonidazole treatment controlled the mitochondrial oxidative stress and evolution of cardiomyopathy in chronic chagasic rats. J Am Coll Cardiol. 2010;55:2499–2508. doi: 10.1016/j.jacc.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Teixeira PC, Santos RH, Fiorelli AI, et al. Selective decrease of components of the creatine kinase system and ATP synthase complex in chronic chagas disease cardiomyopathy. PLoS Negl Trop Dis. 2011;5:e1205. doi: 10.1371/journal.pntd.0001205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Prado CM, Jelicks LA, Weiss LM, et al. The vasculature in chagas disease. Advances in parasitology. 2011;76:83–99. doi: 10.1016/B978-0-12-385895-5.00004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sonnenblick EH, Fein F, Capasso JM, et al. Microvascular spasm as a cause of cardiomyopathies and the calcium-blocking agent verapamil as potential primary therapy. Am J Cardiol. 1985;55:179B–184B. doi: 10.1016/0002-9149(85)90629-0. [DOI] [PubMed] [Google Scholar]
- 76.Factor SM, Cho S, Wittner M, et al. Abnormalities of the coronary microcirculation in acute murine Chagas’ disease. Am J Trop Med Hyg. 1985;34:246–253. doi: 10.4269/ajtmh.1985.34.246. [DOI] [PubMed] [Google Scholar]
- 77.Tanowitz HB, Kaul DK, Chen B, et al. Compromised microcirculation in acute murine Trypanosoma cruzi infection. The Journal of Parasitology. 1996;82:124–130. [PubMed] [Google Scholar]
- 78.Chandra M, Shirani J, Shtutin V, et al. Cardioprotective effects of verapamil on myocardial structure and function in a murine model of chronic Trypanosoma cruzi infection (Brazil Strain): an echocardiographic study. Int J Parasitol. 2002;32:207–215. doi: 10.1016/s0020-7519(01)00320-4. [DOI] [PubMed] [Google Scholar]
- 79.De Souza AP, Tanowitz HB, Chandra M, et al. Effects of early and late verapamil administration on the development of cardiomyopathy in experimental chronic Trypanosoma cruzi (Brazil strain) infection. Parasitol Res. 2004;92:496–501. doi: 10.1007/s00436-004-1080-1. [DOI] [PubMed] [Google Scholar]
- 80.Yanagisawa M, Kurihara H, Kimura S, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 81.Kedzierski RM, Yanagisawa M. Endothelin system: the double-edged sword in health and disease. Ann Rev Pharmacol Toxicol. 2001;41:851–876. doi: 10.1146/annurev.pharmtox.41.1.851. [DOI] [PubMed] [Google Scholar]
- 82.Machado FS, Desruisseaux MS, Nagajyothi, et al. Endothelin in a murine model of cerebral malaria. Exper Biol Med. 2006;231:1176–1181. [PubMed] [Google Scholar]
- 83.Petkova SB, Tanowitz HB, Magazine HI, et al. Myocardial expression of endothelin-1 in murine Trypanosoma cruzi infection. Cardiovasc Pathol. 2000;9:257–265. doi: 10.1016/s1054-8807(00)00045-4. [DOI] [PubMed] [Google Scholar]
- 84.Jelicks LA, Chandra M, Shirani J, et al. Cardioprotective effects of phosphoramidon on myocardial structure and function in murine Chagas’ disease. Int J Parasitol. 2002;32:1497–1506. doi: 10.1016/s0020-7519(02)00136-4. [DOI] [PubMed] [Google Scholar]
- 85.Tanowitz HB, Huang H, Jelicks LA, et al. Role of endothelin 1 in the pathogenesis of chronic chagasic heart disease. Infect Immun. 2005;73:2496–2503. doi: 10.1128/IAI.73.4.2496-2503.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Salomone OA, Caeiro TF, Madoery RJ, et al. High plasma immunoreactive endothelin levels in patients with Chagas’ cardiomyopathy. Am J Cardiol. 2001;87:1217–1220. A1217. doi: 10.1016/s0002-9149(01)01502-8. [DOI] [PubMed] [Google Scholar]
- 87.Hassan GS, Mukherjee S, Nagajyothi F, et al. Trypanosoma cruzi infection induces proliferation of vascular smooth muscle cells. Infect Immun. 2006;74:152–159. doi: 10.1128/IAI.74.1.152-159.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Haeggstrom JZ, Rinaldo-Matthis A, Wheelock CE, et al. Advances in eicosanoid research, novel therapeutic implications. Bioch Biophys Res Comm. 2010;396:135–139. doi: 10.1016/j.bbrc.2010.03.140. [DOI] [PubMed] [Google Scholar]
- 89.Tanowitz HB, Burns ER, Sinha AK, et al. Enhanced platelet adherence and aggregation in Chagas’ disease: a potential pathogenic mechanism for cardiomyopathy. Am J Trop Med Hyg. 1990;43:274–281. doi: 10.4269/ajtmh.1990.43.274. [DOI] [PubMed] [Google Scholar]
- 90.Ashton AW, Mukherjee S, Nagajyothi FN, et al. Thromboxane A2 is a key regulator of pathogenesis during Trypanosoma cruzi infection. J Exper Med. 2007;204:929–940. doi: 10.1084/jem.20062432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mukherjee S, Machado FS, Huang H, et al. Aspirin treatment of mice infected with Trypanosoma cruzi and implications for the pathogenesis of Chagas disease. PLoS One. 2011;6:e16959. doi: 10.1371/journal.pone.0016959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bern C. Antitrypanosomal therapy for chronic Chagas’ disease. New Engl J Med. 2011;364:2527–2534. doi: 10.1056/NEJMct1014204. [DOI] [PubMed] [Google Scholar]
- 93.Viotti R, Vigliano C, Lococo B, et al. Long-term cardiac outcomes of treating chronic Chagas disease with benznidazole versus no treatment: a nonrandomized trial. Ann Intern Med. 2006;144:724–734. doi: 10.7326/0003-4819-144-10-200605160-00006. [DOI] [PubMed] [Google Scholar]
- 94.Urbina JA. Specific chemotherapy of Chagas disease: relevance, current limitations and new approaches. Acta Trop. 2010;115:55–68. doi: 10.1016/j.actatropica.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 95.Pinazo MJ, Espinosa G, Gallego M, et al. Successful treatment with posaconazole of a patient with chronic Chagas disease and systemic lupus erythematosus. Am J Trop Med Hyg. 2010;82:583–587. doi: 10.4269/ajtmh.2010.09-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bern C, Montgomery SP, Herwaldt BL, et al. Evaluation and treatment of chagas disease in the United States: a systematic review. JAMA. 2007;298:2171–2181. doi: 10.1001/jama.298.18.2171. [DOI] [PubMed] [Google Scholar]
- 97.Bocchi EA, Vilas-Boas F, Perrone S, et al. I Latin American Guidelines for the Assessment and Management of Decompensated Heart Failure. Arquivos brasileiros de cardiologia. 2005;85(Suppl 3):49–94. 41–48. [PubMed] [Google Scholar]
- 98.Rocha MO, Ribeiro AL, Teixeira MM. Clinical management of chronic Chagas cardiomyopathy. Front Biosci. 2003;8:e44–54. doi: 10.2741/926. [DOI] [PubMed] [Google Scholar]
- 99.Khoury AM, Davila DF, Bellabarba G, et al. Acute effects of digitalis and enalapril on the neurohormonal profile of chagasic patients with severe congestive heart failure. Int J Cardiol. 1996;57:21–29. doi: 10.1016/s0167-5273(96)02776-3. [DOI] [PubMed] [Google Scholar]
- 100.Bestetti RB, Rossi MA. A rationale approach for mortality risk stratification in Chagas’ heart disease. Int J Cardiol. 1997;58:199–209. doi: 10.1016/s0167-5273(96)02877-x. [DOI] [PubMed] [Google Scholar]
- 101.Mazzei de Davila CA, Davila DF, et al. Sympathetic nervous system activation, antivenin administration and cardiovascular manifestations of scorpion envenomation. Toxicon. 2002;40:1339–1346. doi: 10.1016/s0041-0101(02)00145-9. [DOI] [PubMed] [Google Scholar]
- 102.Botoni FA, Poole-Wilson PA, Ribeiro AL, et al. A randomized trial of carvedilol after renin-angiotensin system inhibition in chronic Chagas cardiomyopathy. Am Heart J. 2007;153:544.e1–8. doi: 10.1016/j.ahj.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 103.Bestetti RB, Cardinalli-Neto A. Sudden cardiac death in Chagas’ heart disease in the contemporary era. Int J Cardiol. 2008;131:9–17. doi: 10.1016/j.ijcard.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 104.Benaim G, Sanders JM, Garcia-Marchan Y, et al. Amiodarone has intrinsic anti-Trypanosoma cruzi activity and acts synergistically with posaconazole. J Med Chem. 2006;49:892–899. doi: 10.1021/jm050691f. [DOI] [PubMed] [Google Scholar]
- 105.Adesse D, Azzam EM, Meirelles Mde N, et al. Amiodarone inhibits Trypanosoma cruzi infection and promotes cardiac cell recovery with gap junction and cytoskeleton reassembly in vitro. Antimicrob Agents Chemother. 2011;55:203–210. doi: 10.1128/AAC.01129-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cardinalli-Neto A, Bestetti RB, Cordeiro JA, et al. Predictors of all-cause mortality for patients with chronic Chagas’ heart disease receiving implantable cardioverter defibrillator therapy. J Cardiovasc Electrophysiol. 2007;18:1236–1240. doi: 10.1111/j.1540-8167.2007.00954.x. [DOI] [PubMed] [Google Scholar]
- 107.Schmid C, Tjan TD, Etz C, et al. First clinical experience with the Incor left ventricular assist device. J Heart Lung Transplant. 2005;24:1188–1194. doi: 10.1016/j.healun.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 108.Moreira LF, Galantier J, Benicio A, et al. Left ventricular circulatory support as bridge to heart transplantation in Chagas’ disease cardiomyopathy. Artificial organs. 2007;31:253–258. doi: 10.1111/j.1525-1594.2007.00372.x. [DOI] [PubMed] [Google Scholar]
- 109.Galantier J, Moreira LF, Benicio A, et al. Hemodynamic performance and inflammatory response during the use of VAD-InCor as a bridge to transplant. Arquivos brasileiros de cardiologia. 2008;91:327–334. doi: 10.1590/s0066-782x2008001700008. [DOI] [PubMed] [Google Scholar]
- 110.Carod-Artal FJ. Trypanosomiasis, cardiomyopathy and the risk of ischemic stroke. Expert Rev Cardiovasc Ther. 2010;8:717–728. doi: 10.1586/erc.10.33. [DOI] [PubMed] [Google Scholar]
- 111.Lucchetti G, Granero AL, Caparei FC, et al. Bilateral papillary muscle infarction in a chagasic patient. Rev Port Cardiol. 2009;28:1441–1447. [PubMed] [Google Scholar]
- 112.Bestetti RB, Ariolli MT, do Carmo JL, et al. Clinical characteristics of acute myocardial infarction in patients with Chagas’ disease. Int J Cardiol. 1992;35:371–376. doi: 10.1016/0167-5273(92)90236-v. [DOI] [PubMed] [Google Scholar]
- 113.Lopes ER, de Mesquita PM, de Mesquita LF, et al. Coronary arteriosclerosis and myocardial infarction in chronic Chagas’ disease. Arq Brasil Cardiol. 1995;65:143–145. [PubMed] [Google Scholar]
- 114.Parada H, Carrasco HA, Anez N, et al. Cardiac involvement is a constant finding in acute Chagas’ disease: a clinical, parasitological and histopathological study. Int J Cardiol. 1997;60:49–54. doi: 10.1016/s0167-5273(97)02952-5. [DOI] [PubMed] [Google Scholar]
- 115.Barros MV, Rocha MO, Ribeiro AL, et al. Doppler tissue imaging to evaluate early myocardium damage in patients with undetermined form of Chagas’ disease and normal echocardiogram. Echocardiography. 2001;18:131–136. doi: 10.1046/j.1540-8175.2001.00131.x. [DOI] [PubMed] [Google Scholar]
- 116.Barros ML, Ribeiro A, Nunes MD, et al. Association between left ventricular wall motion abnormalities and ventricular arrhythmia in the indeterminate form of Chagas disease. Rev Soc Brasil Med Trop. 2011 doi: 10.1590/s0037-86822011005000020. [DOI] [PubMed] [Google Scholar]
- 117.Nunes Mdo C, Beloti FR, Lima MM, et al. Functional capacity and right ventricular function in patients with Chagas heart disease. Eur J echocardiog: the Journal of the Working Group on Echocardiography of the European Society of Cardiology. 2010;11:590–595. doi: 10.1093/ejechocard/jeq022. [DOI] [PubMed] [Google Scholar]
- 118.Viotti RJ, Vigliano C, Laucella S, et al. Value of echocardiography for diagnosis and prognosis of chronic Chagas disease cardiomyopathy without heart failure. Heart. 2004;90:655–660. doi: 10.1136/hrt.2003.018960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Acquatella H, Perez JE, Condado JA, et al. Limited myocardial contractile reserve and chronotropic incompetence in patients with chronic Chagas’ disease: assessment by dobutamine stress echocardiography. J Am Coll Cardiol. 1999;33:522–529. doi: 10.1016/s0735-1097(98)00569-5. [DOI] [PubMed] [Google Scholar]
- 120.Marin-Neto JA, Marzullo P, Marcassa C, et al. Myocardial perfusion abnormalities in chronic Chagas’ disease as detected by thallium-201 scintigraphy. Am J Cardiol. 1992;69:780–84. doi: 10.1016/0002-9149(92)90505-s. [DOI] [PubMed] [Google Scholar]
- 121.Rochitte CE, Nacif MS, de Oliveira Junior AC, et al. Cardiac magnetic resonance in Chagas’ disease. Artificial Organs. 2007;31:259–267. doi: 10.1111/j.1525-1594.2007.00373.x. [DOI] [PubMed] [Google Scholar]
- 122.Acquatella H, Schiller NB, Puigbo JJ, et al. M-mode and two-dimensional echocardiography in chronic Chages’ heart disease. A clinical and pathologic study. Circulation. 1980;62:787–799. doi: 10.1161/01.cir.62.4.787. [DOI] [PubMed] [Google Scholar]
- 123.Patel AR, Lima C, Parro A, et al. Echocardiographic analysis of regional and global left ventricular shape in Chagas’ cardiomyopathy. Am J Cardiol. 1998;82:197–202. doi: 10.1016/s0002-9149(98)00316-6. [DOI] [PubMed] [Google Scholar]
- 124.Lopez L, Arai K, Gimenez E, et al. C-reactive protein and interleukin-6 serum levels increase as Chagas disease progresses towards cardiac failure. Rev Esp Cardiol. 2006;59:50–56. [PubMed] [Google Scholar]
- 125.Nunes Mdo C, Barbosa Mde M, Rocha ES, et al. Function of the left atrium in Chagas’ cardiomyopathy. Arq BrasilCardiol. 2005;84:452–456. doi: 10.1590/s0066-782x2005000600004. [DOI] [PubMed] [Google Scholar]
- 126.Marcu CB, Beek AM, van Rossum AC. Chagas’ heart disease diagnosed on MRI: the importance of patient “geographic” history. Int J Cardiol. 2007;117:e58–60. doi: 10.1016/j.ijcard.2006.11.114. [DOI] [PubMed] [Google Scholar]
- 127.Sechtem U, Mahrholdt H, Vogelsberg H. Cardiac magnetic resonance in myocardial disease. Heart. 2007;93:1520–1527. doi: 10.1136/hrt.2005.067355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ueno Y, Nakamura Y, Takahashi M, et al. A highly suspected case of chronic Chagas’ heart disease diagnosed in Japan. Jap Circulat J. 1995;59:219–223. doi: 10.1253/jcj.59.219. [DOI] [PubMed] [Google Scholar]
- 129.Coelho-Filho OR, Nallamshetty L, Kwong RY. Risk stratification for therapeutic management and prognosis. Heart Failure Clinics. 2009;5:437–455. vii. doi: 10.1016/j.hfc.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 130.Mello RP, Szarf G, Nakano E, et al. Noncompaction of the myocardium, Chagas’ disease and dysfunction: a case report. Arq Brasil Cardiol. 2010;95:e4–6. doi: 10.1590/s0066-782x2010001100022. [DOI] [PubMed] [Google Scholar]
- 131.Strauss DG, Wu KC. Imaging myocardial scar and arrhythmic risk prediction--a role for the electrocardiogram? J Electrocardiol. 2009;42:138, e131–138. doi: 10.1016/j.jelectrocard.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 132.Rochitte CE, Oliveira PF, Andrade JM, et al. Myocardial delayed enhancement by magnetic resonance imaging in patients with Chagas’ disease: a marker of disease severity. J Am Coll Cardiol. 2005;46:1553–1558. doi: 10.1016/j.jacc.2005.06.067. [DOI] [PubMed] [Google Scholar]
- 133.Strauss DG, Cardoso S, Lima JA, et al. ECG scar quantification correlates with cardiac magnetic resonance scar size and prognostic factors in Chagas’ disease. Heart. 2011;97:357–361. doi: 10.1136/hrt.2010.210047. [DOI] [PubMed] [Google Scholar]
- 134.Jelicks LA, Shirani J, Wittner M, et al. Application of cardiac gated magnetic resonance imaging in murine Chagas’ disease. Am J Trop Med Hyg. 1999;61:207–214. doi: 10.4269/ajtmh.1999.61.207. [DOI] [PubMed] [Google Scholar]
- 135.Durand JL, Tang B, Gutstein DE, et al. Dyskinesis in Chagasic myocardium: centerline analysis of wall motion using cardiac-gated magnetic resonance images of mice. Magn Reson Imaging. 2006;24:1051–1057. doi: 10.1016/j.mri.2006.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.de Souza AP, Tang B, Tanowitz HB, et al. Magnetic resonance imaging in experimental Chagas disease: a brief review of the utility of the method for monitoring right ventricular chamber dilatation. Parasitol Res. 2005;97:87–90. doi: 10.1007/s00436-005-1409-4. [DOI] [PubMed] [Google Scholar]
- 137.Goldenberg RC, Jelicks LA, Fortes FS, et al. Bone marrow cell therapy ameliorates and reverses chagasic cardiomyopathy in a mouse model. J Infect Dis. 2008;197:544–547. doi: 10.1086/526793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jasmin, Torres AL, Nunes HM, et al. Optimized labeling of bone marrow mesenchymal cells with superparamagnetic iron oxide nanoparticles and in vivo visualization by magnetic resonance imaging. J Nanobiotechnology. 2011;9:4. doi: 10.1186/1477-3155-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Prado CM, Fine EJ, Koba W, et al. Micro-positron emission tomography in the evaluation of Trypanosoma cruzi-induced heart disease: Comparison with other modalities. Am J Trop Med Hyg. 2009;81:900–905. doi: 10.4269/ajtmh.2009.09-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fiorelli AI, Santos RH, Oliveira JL, Jr, et al. Heart transplantation in 107 cases of Chagas’ disease. Transplant Proc. 2011;43:220–224. doi: 10.1016/j.transproceed.2010.12.046. [DOI] [PubMed] [Google Scholar]
- 141.Bestetti RB, Theodoropoulos TA. A systematic review of studies on heart transplantation for patients with end-stage Chagas’ heart disease. J Card Fail. 2009;15:249–255. doi: 10.1016/j.cardfail.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 142.Bocchi EA, Fiorelli A. The paradox of survival results after heart transplantation for cardiomyopathy caused by Trypanosoma cruzi. First Guidelines Group for Heart Transplantation of the Brazilian Society of Cardiology. Ann Thorac Surg. 2001;71:1833–1838. doi: 10.1016/s0003-4975(01)02587-5. [DOI] [PubMed] [Google Scholar]
- 143.Chin-Hong PV, Schwartz BS, Bern C, et al. Screening and treatment of chagas disease in organ transplant recipients in the United States: recommendations from the Chagas in Transplant Working Group. Am J Transplant. 2011;11:672–680. doi: 10.1111/j.1600-6143.2011.03444.x. [DOI] [PubMed] [Google Scholar]
- 144.Soares MB, Lima RS, Rocha LL, et al. Transplanted bone marrow cells repair heart tissue and reduce myocarditis in chronic chagasic mice. Am J Pathol. 2004;164:441–447. doi: 10.1016/s0002-9440(10)63134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Macambira SG, Vasconcelos JF, Costa CR, et al. Granulocyte colony-stimulating factor treatment in chronic Chagas disease: preservation and improvement of cardiac structure and function. FASEB J. 2009;23:3843–3850. doi: 10.1096/fj.09-137869. [DOI] [PubMed] [Google Scholar]
- 146.Guarita-Souza LC, Carvalho KA, Woitowicz V, et al. Simultaneous autologous transplantation of cocultured mesenchymal stem cells and skeletal myoblasts improves ventricular function in a murine model of Chagas disease. Circulation. 2006;114:I120–124. doi: 10.1161/CIRCULATIONAHA.105.000646. [DOI] [PubMed] [Google Scholar]
- 147.Vilas-Boas F, Feitosa GS, Soares MB, et al. Bone marrow cell transplantation in chagas’ disease heart failure: report of the first human experience. Arq Brasil Cardiol. 2011;96:325–331. doi: 10.1590/s0066-782x2011005000028. [DOI] [PubMed] [Google Scholar]