Abstract
External support may improve task performance regardless of an individual’s ability to compensate for cognitive deficits through internally-generated mechanisms. We investigated if performance of a complex, familiar visual search task (the game of bingo) could be enhanced in groups with suboptimal vision by providing external support through manipulation of task stimuli. Participants were 19 younger adults, 14 individuals with probable Alzheimer’s disease (AD), 13 AD-matched healthy adults, 17 non-demented individuals with Parkinson’s disease (PD), and 20 PD-matched healthy adults. We varied stimulus contrast, size, and visual complexity during game play. The externally-supported performance interventions of increased stimulus size and decreased complexity resulted in improvements in performance by all groups. Performance improvement through increased stimulus size and decreased complexity was demonstrated by all groups. AD also obtained benefit from increasing contrast, presumably by compensating for their contrast sensitivity deficit. The general finding of improved performance across healthy and afflicted groups suggests the value of visual support as an easy-to-apply intervention to enhance cognitive performance.
Key terms: Externally-Supported Performance Intervention, Activities of Daily Living, Aging, Alzheimer’s Disease, Parkinson’s Disease, Vision, Contrast Sensitivity
Introduction
Visual perceptual functioning is reduced to varying degrees in normal aging and in individuals with the age-related neurodegenerative disorders of Alzheimer’s disease (AD) and Parkinson’s disease (PD), and may be related to cognitive difficulties. Reduced stimulus signal strength has been shown to interact with sensory and perceptual deficits, impairing cognition in these groups (Clay et al., 2009; Cronin-Golomb, 1995 & 2004; Cronin-Golomb, Corkin & Growdon, 1995; Cronin-Golomb, Gilmore, Neargarder, Morrison, & Laudate, 2007; Gilmore, Spinks, & Thomas, 2006; Mapstone, Steffenella, & Duffy, 2003; Mendez, Tomsak, & Remler, 1990; Rizzo, Anderson, Dawson, Myers, & Ball, 2000). A positive converse of this relation between vision and cognition is that visually-based interventions may enhance cognitive performance. For example, we have shown that the speed of letter identification by AD, PD and healthy older adults can be significantly improved by enhancement of stimulus contrast (Amick, Cronin-Golomb & Gilmore, 2003; Cronin-Golomb et al., 2007; Gilmore, Cronin-Golomb, Neargarder & Morrison, 2005; Gilmore, Thomas, Klitz, Persanyi & Tomsak, 1996).
External supports such as visual enhancement interventions may improve cognitive task performance regardless of an individual’s ability to counteract cognitive deficits through self-generated strategies. We refer to this class of interventions as Externally Supported Performance Interventions (ESPI), one end of a continuum of methods to enhance cognition, daily function, and independence in older adults or populations with sensory-cognitive disabilities. We define ESPI as interventions based on external support, meaning changes to the external environment of the individual. We were interested in whether a visual enhancement intervention could compensate for suboptimal visual abilities on a complex visual search task. Searching for objects is a necessary daily function, and the inability to quickly find sought-for items is a source of frustration for healthy adults and for those with vision-compromising disorders such as AD and PD. In the present study, we chose the game of bingo to investigate the possibility of an ESPI to enhance search performance.
Bingo is a leisure activity that is widely enjoyed and is available for play by adults in the community, in institutions, and online. It was found by one study to be the most popularly attended social activity at 50 surveyed senior citizen centers and institutions, representing nearly 7,000 active seniors (McNeilly & Burke, 2001). Bingo is not only a recreational activity for healthy older adults in the community, but it is also a familiar and often-played game for those in nursing homes, assisted living facilities, and assistive day centers that cater to individuals with neurodegenerative disorders such as AD and PD. Little consideration has been given, however, to the visual aspects of game play. Standard bingo cards that are used at community games are typically small in size and provide poor visual contrast. Moreover, bingo players often search multiple cards at once, adding a further cognitive load that could result in reduced processing speed and deficient visual search. Reduced visual acuity and decreased contrast sensitivity may interact with task complexity to negatively affect play. Accordingly, we focused on visual acuity, contrast sensitivity, and visual complexity as targets for experimental manipulation.
Visual Acuity
Changes of the visual system are associated with aging and with disorders such as AD and PD. A decline in visual acuity is correlated with increasing age in healthy adults who have no identifiable eye disease or condition, including those whose vision is corrected by optics (Jackson & Owsley, 2003; Owsley, 2010; Owsley, Sekuler, & Siemsen, 1983). Compared to healthy age-matched adults, patients with AD show reduced visual acuity (Cronin-Golomb et al., 2007; Neargarder, Stone, Cronin-Golomb, & Oross, 2003). While some studies report no conclusive acuity deficits in PD (Armstrong, 2008), other research suggests acuity may be reduced (Jones & Donaldson, 1995; Uc et al., 2005).
Contrast Sensitivity and Functional Tasks
Contrast sensitivity varies as a function of spatial frequency. Older adults show decreased sensitivity compared to younger adults, with greater deficits at higher spatial frequencies (Owsley et al., 1983; Owsley & Sloane, 1987; Nameda, Kawara, & Ohzu, 1989). In healthy adults across the lifespan, decreased contrast sensitivity has been found to predict a decreased ability to see pictures of real-world targets including road signs, faces of famous people, and everyday objects such as a lamp or coffee cup (Owsley & Sloane, 1987).
Reduced contrast sensitivity is noted in AD compared to healthy adults of similar age. This deficit can still be seen, typically at lower spatial frequencies or across spatial frequencies, when factoring in the effect of visual acuity on contrast sensitivity functions (Neargarder et al., 2003; Cronin-Golomb et al., 2007). In a visual intervention study, we were able to significantly increase food and liquid consumption in severely demented individuals with AD by replacing low-contrast white plates and cups with high-contrast tableware (Dunne, Neargarder, Cipolloni, & Cronin-Golomb, 2004), complementing earlier work by Koss and Gilmore (1998). These results provided strong evidence that contrast sensitivity deficits in severe cases of AD may deleteriously impact everyday visual functioning. The studies did not address to what extent patients in the earlier stages of the disease, presumably with relatively mild contrast sensitivity deficits, may be impaired in their daily tasks. In closely controlled laboratory tasks, we have found that those with milder AD showed benefit from increased contrast to help overcome contrast sensitivity deficits and enhance performance on tests using a variety of visual stimuli (Cronin-Golomb et al., 2007; Gilmore et al., 2005). Seeing stimuli at a higher contrast level allowed those with mild to moderate AD to identify briefly presented letters, words, and pictures at a level of proficiency that was similar to that of healthy age-matched adults who viewed the stimuli at lower contrast levels. AD performance on a more complex pattern-completion task did not benefit from a contrast increase, however, leaving questions about whether overcoming contrast sensitivity deficits in individuals with mild to moderate AD can attenuate difficulties on complex visually-based tasks.
In PD, abnormalities are found on measures of contrast sensitivity (e.g., Amick et al., 2003; Uc et al., 2005; Davidsdottir, Wagenaar, Young & Cronin-Golomb, 2008; Seichepine et al., in press), with sensitivity to middle and high spatial and temporal frequencies reduced in patients on dopamine precursor therapy (Bodis-Wollner et al., 1987). For PD, it has been suggested that “real world” functioning could be impacted under low-contrast conditions, such as driving in fog. Contrast sensitivity in PD has been found to be a univariate predictor of decreased driving control and crashes at intersections under foggy conditions in a driving simulator (Uc et al., 2009).
Visual Complexity
Bingo players often play multiple cards at once to increase their chances of winning. This adds to the size and complexity of the visual array to be searched in a set amount of time. Speed of visual processing declines in older adults and affects numerous functional activities, predicting such challenges to independence as driving cessation (Edwards, Bart, O’Connor & Cissell, 2010). Individuals with AD have shown particular impairment on a timed visual search task under complex visual conditions (Neargarder & Cronin-Golomb, 2005). For PD, it has been argued that deficits in visual processing, perception, and attention negatively affect search of complex scenes, such as while driving a car (Uc et al., 2006). Providing top-down information can help attenuate PD patients’ difficulties in locating a target in a complicated visual array (Horowitz, Choi, Horvitz, Côté & Mangels, 2006).
In light of the known deficiencies in visual acuity, contrast sensitivity, and performance on visually complex search tasks described above for normal aging, AD, and PD, we investigated their effects on bingo play. The hypothesis was that functional play could be enhanced by increasing the visual contrast and size of standard bingo cards to levels similar to commercially available “low-vision” cards. We refer to this type of manipulation as an Externally Supported Performance Intervention (ESPI). We predicted that the strongest facilitation of play would be seen in patients with AD, who typically have the most severe contrast and acuity deficits. We expected a lesser amount of facilitation in the patients with PD, and lesser still in healthy age-matched control participants, who experience visual deficits to a lesser degree. We also hypothesized that adding to the game complexity by increasing the number of cards played per game would reduce success the most for AD patients, then PD patients, then age-matched control participants. This is in accordance with the amount of deficit already experienced by the various groups due to decreased visual output, compounded by the added cognitive load of playing a more visually complex game.
Methods
Participants
Participants included non-demented individuals with idiopathic PD (n=17) and normal control participants (NC; n= 20) who were matched to the PD group for age (t [32.6] = .26, p = .80; range: PD = 53–76, NC = 47–81) and education (t [35] = .26, p = .80). Individuals with probable AD (n=14) and younger healthy control adults (YA; n=19) were compared to an older adult group (OA; n=13) that was age-matched to the AD group (t [20.2] = −1.881, p = .07; range: AD = 62–86, OA = 69–81), and education-matched to the YA group (t [30] = −.47, p = .64). The NC and OA were drawn from a larger group of healthy adults, matching age and education with comparison groups as described here. Nine of the 13 OA were also in the NC group. The AD and OA groups differed on education (t [25] = 3.16, p < .01; AD mean [SD] = 13.1 [2.6], range = 10–18; OA mean [SD] = 16.2 [2.4], range = 13–21). In analyses of AD-OA group differences, education was used as a covariate. The participants are described in Table 1.
Table 1.
Participant characteristics
| Age | Male: Female | Education, in years (SD) | MMSE, mean score (range) | Mean Acuity, logMAR (SD) [Snellen] | |
|---|---|---|---|---|---|
| YA (n=19) | 22 (2.1) | 8:11 | 15.8 (1.3) | N/A | −.097 (.000) [20/16] |
| OA (n=13) | 73 (4.5) | 4:9 | 16.2 (2.4) | 28.5 (27–30) | .093 (.144) [20/25] |
| AD (n=14) | 78 (8.4) | 5:9 | 13.1 (2.6) | 17.1 (11–26) | .232 (.201) [20/32] |
| NC (n=20) | 65 (8.7) | 11:9 | 17.0 (2.5) | 29.0 (27–30) | .061 (.127) [20/20] |
| PD (n=18) | 66 (5.4) | 10:8 | 16.8 (1.7) | 27.5 (24–30) | .107 (.216) [20/25] |
MMSE=Modified Mini-Mental State Examination scores, converted to standard Mini-Mental State Exam equivalent; YA=Younger adult control participants; OA=Older adults, matched to YA and AD; AD=Alzheimer’s disease participants; NC=Normal control participants, matched to PD; PD=Parkinson’s disease participants. SD = standard deviation. Acuity was measured at 16″, and Snellen values are approximations of mean logMAR values, for comparison.
Individuals in the AD group were recruited through day programs and hospitals in the Boston MA and Cleveland OH areas as part of a dual-site study, and all met NINCDS-ADRDA criteria for probable AD (McKhann et al., 1984). Dementia severity was estimated with the Modified Mini-Mental State Exam (mMMS; mean = 31.4, range = 19–49; Stern et al., 1987; equivalent to mean = 17.1, range = 11–26 on the standard Mini-Mental State Exam [MMSE]; Folstein, Folstein, & McHugh, 1975). We recruited non-demented participants with idiopathic PD from the Parkinson’s Disease and Movement Disorders Center of the Department of Neurology of Boston Medical Center and through local PD support groups. PD mMMS scores ranged from 49–57, with a mean of 52.9 (MMSE equivalent of 26–30, mean = 27.8).
NC participants were recruited from the Boston and Cleveland areas. All were free of signs of dementia (mMMS mean = 55.3, range 51–57; MMSE mean = 29, range 27–30; three NC were screened with the MMSE and not the mMMS). YA were undergraduates at Boston University or Case Western Reserve University who participated for course credit.
Other than for a PD or AD diagnosis for those respective groups, participants were free of major medical abnormalities as determined by health history screening. Exclusion criteria included co-existing serious chronic medical illnesses (including psychiatric or neurological), use of psychoactive medication besides antidepressants and anxiolytics in the PD or AD groups, use of psychoactive medications in the control groups, history of intracranial surgery, traumatic brain injury, alcoholism or other drug abuse, or eye disease or substantial abnormalities as noted on a neuro-ophthalmological examination. No participant met or exceeded pre-determined cutoff scores on measures of depression, including the Beck Depression Inventory II (cutoff = 14; Beck, Steer, & Brown, 1996) for YA, and the Geriatric Depression Scale (cutoff = 17; Yesavage, 1988) for the other groups.
Visual acuity (tested at 16 inches, logMAR values) was found to be worse in the older than younger adults (t [12] = 4.76, p < .001; YA mean [SD] = −.10 [.00]; OA mean [SD] = .09 [.14]), and for comparison, was also worse in the NC compared to YA group (t [19] = 5.54, p <.001; NC mean [SD] = .06 [.13]). Acuity did not differ between PD and NC groups (t [25] = .78, p = .44; PD mean [SD] = .11 [.26]; NC mean [SD] = .06 [.13]), nor between our AD and OA groups, though there was a trend toward worse acuity in the AD group (t [24] = 2.02, p = .055, with data missing from one AD; AD mean [SD] = .23 [.20]; OA mean [SD] = .09 [.14]). Contrast sensitivity reduction was noted (Pelli-Robson chart) for older compared to younger adults (t [30] = 5.80, p < .001; YA mean [SD] = 1.87 [.10]; OA mean [SD] = 1.62 [.16]), and for comparison, was also noted in the NC compared to YA group (t [37] = 5.40, p < .001; NC mean [SD] = 1.70 [.18]). Contrast sensitivity did not differ between the PD and NC groups (t [32.4] = .34, p = .74, with data missing from one PD; PD mean [SD] = 1.72 [.18]; NC mean [SD] = 1.70 [.18]). The AD group showed lower contrast sensitivity than the OA group (t [25] = 2.14, p < .05; AD mean [SD] = 1.36 [.40]; OA mean [SD] = 1.62 [1.6]). NC, OA, AD, and PD received a detailed neuro-ophthalmological examination to rule out visual disorders arising from dysfunction of the anterior pathways, including cataracts, glaucoma, and macular degeneration. One PD and two AD did not receive the exam because of scheduling issues. All YA reported that they had no history of significant abnormalities in vision or eye health.
Procedures: Externally Supported Performance Intervention
Study procedures were reviewed and approved by the Institutional Review Boards of Boston University, Case Western Reserve University, and University Hospitals Case Medical Center. All individuals gave written informed consent; for participants with AD, caregivers additionally gave written informed assent.
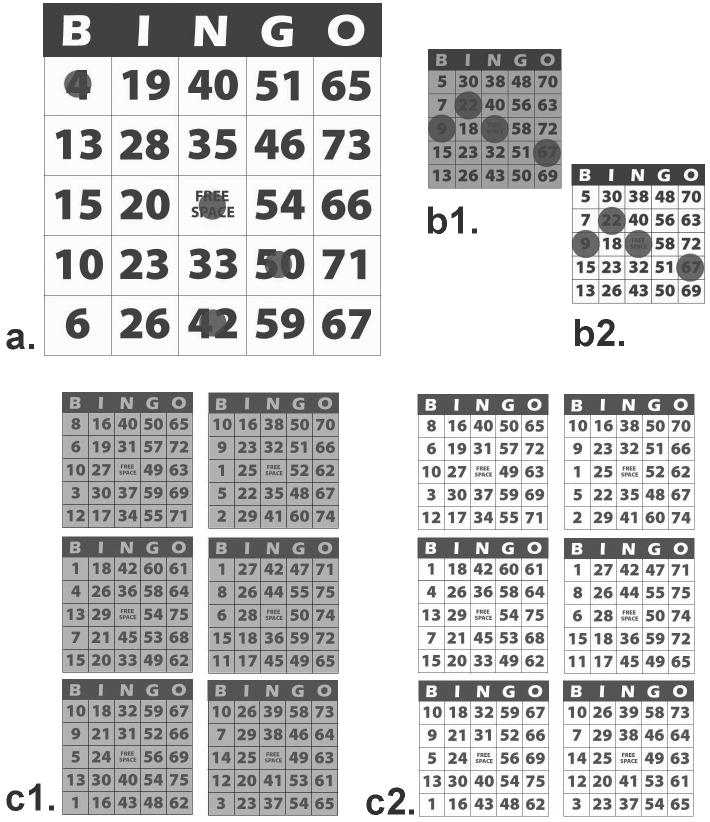
The bingo task was designed to simulate actual game play as closely as possible within the limits of a laboratory experiment. Bingo cards were based on those used at community bingo halls licensed by the Massachusetts State Lottery Commission, and on those commercially available for recreational use in the low-vision community. Black and white electronic versions of bingo cards were created to approximate the size, design, and visual contrast of standard bingo cards typically used in community bingo games in the Boston area. These served as our small-size normal-contrast cards (approximately 2.75 inches × 2.9 inches on the screen). For large-size cards, the electronic cards were scaled 222% to match the approximate font size of typical bingo cards commercially available for use by individuals with low vision (approximately 7.2 inches × 7.5 inches on the screen). For enhanced-contrast cards, the cards were adjusted in visual contrast to levels similar to those of low vision cards. The normal-contrast cards had an estimated Michelson contrast of 62% (approximately 7.2 cd/m2 black; 30.6 cd/m2 white), and the enhanced cards had an estimated Michelson contrast of 85% (approximately 7.2 cd/m2 black; 90.4 cd/m2 white). Task complexity was varied by presenting either one card or six concurrent cards per game. See Figure 1.
Figure 1.
Example bingo cards. a) large size, enhanced contrast, 1-card game (showing some electronic “ink daubs”); b) small size, normal (b1) and enhanced (b2) contrast, 1-card games (with “daubs”); c) small size, normal (c1) and enhanced (c2) contrast, 6-card games (no “daubs”). See text for description of actual card sizes.
Automated bingo games were presented by a Dell Latitude D820 laptop computer on a 17 inch cathode ray tube touch-screen monitor (ELO Touchsystems, ET1726C), using the stimulus presentation software SuperLab (Cedrus, version 4.0.4). The monitor was rotated 90 degrees to allow for bingo cards to be presented lengthwise, which is typical of how groups of cards are oriented in bingo hall play. The monitor was regularly calibrated (Colorvision Spyder2PRO, v. 2.2). Participants were positioned at eye level to the center of the monitor in a chinrest, at an eye-to-monitor distance of 16 inches, and were instructed to place their dominant hand on the handrest in front of them. The handrest was used to standardize the distance from the hand’s starting point to the monitor screen. They returned their hand to the handrest after they had made their choice on the touch screen. A rubber finger cot was worn on the pointing finger to maintain the integrity of the screen. After game playing instructions were given, confirmation that the participant understood the instructions was obtained by playing a sample game.
Game presentation was predetermined. For each game, a participant was shown a card or set of cards on the monitor. She or he was instructed to touch the “Free Space” to begin the game, after which a recorded voice called a letter-number combination (e.g., “B-5”). The laptop speakers played the voice recording at maximum volume, allowing all participants to hear it without difficulty. The experimenter simultaneously presented the letter-number combination on a paper flipbook located next to the monitor, simulating the current “called ball” board or television monitor present at many community bingo games. Participants were instructed to touch the number on their electronic card as soon as they heard it called. When the correct number was touched, a semi-transparent, colored electronic “ink daub” appeared over the number. A touch that registered anywhere on the screen other than within the box of the correct number did not produce an ink daub, was scored as an error, and caused the number to be called another time. Two seconds after a correct response, the next letter-number combination was called. Participants were instructed to say “Bingo!” when they completed a row, column, or diagonal as per the standard game, and were reminded between games to say “Bingo!” when applicable. Because “errors” were sometimes caused by over- or under-sensitivity of the touch screen to a particular individual’s touch, we confined analysis to reaction time, which is important to actual competitive game play.
Variables included size of card (small and large); visual contrast (normal, enhanced); and complexity of game (1 or 6 simultaneous cards). Participants received one game per combination of variables, except that it was not possible to present a large-size 6-card array due to size constraints of the computer monitor. Each participant played two unscored practice games prior to six scored games. Response time was recorded per bingo call and was averaged across conditions. For each game, target positions were distributed across the entire array. To reduce potential order effects, we used four different sets of games, varying presentation order of the conditions across participants.
Analyses were performed for within-participant and between-participant factors. Because 6-card arrays could not be presented in large size, only 1-card small and 1-card large games were used in analyses of card size, and comparisons were not made of size by complexity.
Participants were tested for dominant-hand motor speed using the Purdue Pegboard Test (Lafayette Instrument).
Results
Within-Participants Analyses
In the following analyses, planned t-tests were conducted in order to maximize the sensitivity of detecting differences within each group. All response times (RT) were measured in milliseconds. A mean RT and standard deviation were computed for each participant for each condition. Outliers were also examined.
Card Size
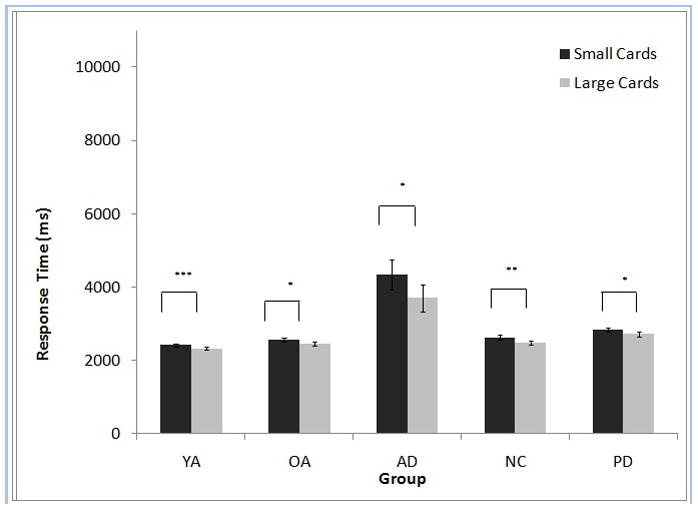
To determine the effect of card size on RT within each group, paired-sample t-tests were performed on 1-card small and 1-card large games, collapsed across contrast levels. For all groups, RTs were shorter when using large cards than when using small cards (YA: t [18] = 3.79, p < .001; NC: t [19] = 3.56, p < .01; OA: t [12] = 2.41, p < .05; AD: t [13] = 2.55, p < .05; PD: t [16] = 2.39, p < .05) (Figure 2).
Figure 2.
Example bingo cards. a) large size, enhanced contrast, 1-card game (showing some electronic “ink daubs”); b) small size, normal (b1) and enhanced (b2) contrast, 1-card games (with “daubs”); c) small size, normal (c1) and enhanced (c2) contrast, 6-card games (no “daubs”). See text for description of actual card sizes.
Game Complexity
To determine the effect of game complexity on RT within each group while controlling for effects of game size, we analyzed 1-card small and 6-card small games, collapsed across contrast levels. For all groups, RT was shorter for 1-card small games than for 6-card small games (YA: t [18] = 13.00, p <.001; NC: t [19] = 20.00, p < .001; OA: t [12] = −12.96, p < .001; AD: t [13] = 10.10, p < .001; PD: t [16] = 14.16, p < .001) (Figure 3).
Figure 3.
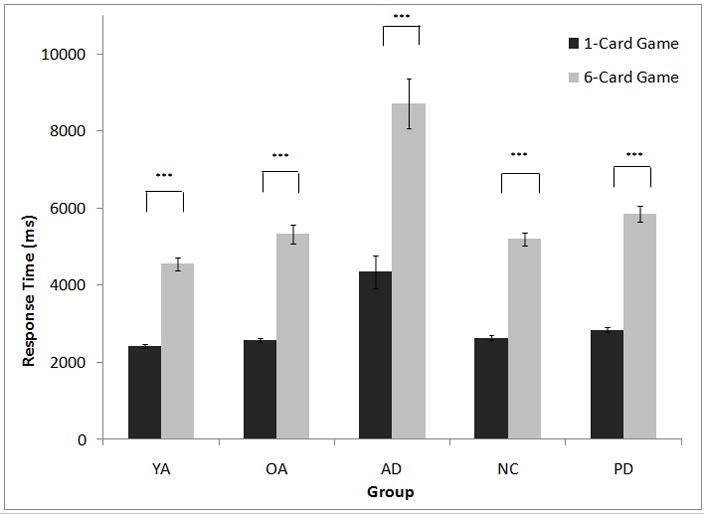
Effect of game complexity on response time for each participant group. YA=Younger adult control participants; OA=Older adults, matched to YA and AD; AD=Alzheimer’s disease participants; NC=Normal control participants, matched to PD; PD=Parkinson’s disease participants. ***p<0.001.
Visual Contrast
To determine the effect of visual contrast on RT within each group, normal-contrast games were compared to enhanced-contrast games, collapsed across size and game complexity. RTs were shorter for games with enhanced-contrast cards than with normal-contrast cards for AD (t [13] = 2.18, p < .05) and YA (t [18] = 2.49, p < .05). No effect of contrast was found for NC (t [19] =1.11, p = .28), OA (t [12] = .68, p = .51), or PD (t [16] = 1.09, p = .29) (Figure 4). For YAs, but not the other groups, performance on the Pelli-Robson contrast sensitivity test correlated with performance on the normal-contrast condition (Spearman’s rho = −.46, p < .048). When the two YAs with the lowest Pelli-Robson score (i.e, 1.65) were removed from the analysis, the previously noted contrast effect no longer held (p=.08).
Figure 4.
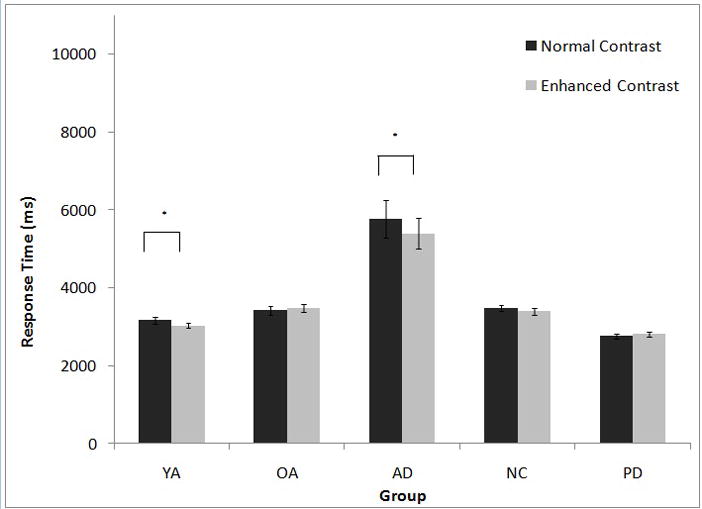
Effect of contrast on response time for each participant group. YA=Younger adult control participants; OA=Older adults, matched to YA and AD; AD=Alzheimer’s disease participants; NC=Normal control participants, matched to PD; PD=Parkinson’s disease participants. *p<0.05.
Between-Participants Analyses
Contrast by Size
We performed a series of 2×2×2 mixed design analyses of variance (ANOVAs) to examine variables of group (varies as shown below), contrast (normal, enhanced), and card size (small, large). Motor speed as measured by dominant-hand performance on the Purdue Pegboard was not a significant covariate, except where noted. Contrast sensitivity was measured by the Pelli-Robson chart and was not a significant covariate. Effect sizes for the combined effects of contrast and card size for group are reported in Table 2.
Table 2.
Effect Size of Group Comparisons
| Group Comparisons | Combined effects of card size and contrast: Mean RT in ms (SD) | Effect size | Combined effects of contrast and complexity: Mean RT in ms (SD) | Effect size |
|---|---|---|---|---|
| YA vs. OA | 2375 (41.5) vs. 2515 (50.2) | .13 | No effect | No effect |
| PD vs. NC | 2783 (57.4) vs. 2556 (52.9) | .19 | 4349 (111.3) vs. 3915 (102.6) | .19 |
| AD vs. OA | 4023 (272.1) vs. 2515 (282.4) | .48 | 6368 (362.6) vs. 3923 (376.3) | .38 |
RT = response time; SD = standard deviation
Note: The combined effects of card size and complexity could not be examined because the computer display could not physically accommodate the large-card, high-complexity array.
YA vs. OA
Because the average age of the OA group was higher than that of the NC group, we chose the OA group to compare to YA to investigate potential aging effects. When comparing YAs to OAs, results revealed a main effect of group (F [1, 30] = 4.64, p < .05), in which YAs (M [SE] = 2375 [42]) performed faster than OAs (M [SE] = 2515 [50]). There was a main effect of size (F (1, 30) = 17.89, p < .001), in which both groups performed faster on large cards (M [SE] = 2394 [32]) than on small cards (M [SE] = 2495 [36]). No other main effects or interactions were significant.
PD vs. NC
Results revealed a main effect of group (F (1, 35) = 8.45, p < .01) with PD participants (M [SE] = 2783 [57]) performing more slowly than NC participants (M [SE] = 2556 [53]). There was a main effect of size (F (1, 35) = 17.37, p < .001), in which both groups performed faster on large cards (M [SE] = 2603 [41]) than on small cards (M [SE] = 2736 [43]). No other main effects or interactions were significant.
AD vs. OA
Results revealed a main effect of group (F (1, 24) = 21.91, p < .001) with education as a covariate (F (1, 24) = 4.92, p < .05) in which OAs performed faster (M [SE] = 2515 [282]) than ADs (M [SE] = 4023 [272]). There was a main effect of size (F (1, 25) = 8.07, p < .001), in which both groups performed faster on large cards (M [SE] = 3082 [196]) than on small cards (M [SE] = 3456 [217]). No other main effects or interactions were significant.
Contrast by Complexity
We performed individual 2×2×2 mixed design ANOVAs to examine group (varies as shown below), contrast (normal, enhanced), and game complexity (1, 6 cards). Motor speed as measured by dominant-hand performance on the Purdue Pegboard was not a significant covariate, except where noted. Contrast sensitivity was measured by the Pelli-Robson chart and was not a significant covariate. Effect sizes for significant combined effects of contrast and complexity for group are reported in Table 2.
YA vs. OA
When comparing YAs to OAs, the main effect of group was not significant (F (1, 29) = .42, p = .52) when accounting for motor speed of the Purdue Pegboard (F (1, 29) = 4.62, p < .05). There was a main effect of complexity (F (1, 30) = 357.18, p < .001) for which performance was faster for the 1-card games (M [SE] = 2445 [33]) than for 6-card games (M [SE] = 4940 [143]). No other main effects or interactions were significant.
PD vs. NC
When comparing PDs to NCs, results revealed a main effect of group (F (1, 35) = 8.23, p < .01) with PD participants (M [SE] = 4349 [111]) performing more slowly than NC participants (M [SE] = 3915 [103]). A main effect of complexity was found (F (1, 35) = 538.88, p < .001), with faster performance on 1-card games (M [SE] = 2736 [43]) than on 6-card games (M [SE] = 5529 [130]). No other main effects or interactions were significant.
AD vs. OA
When comparing ADs to OAs, results revealed a main effect of group (F (1, 23) = 14.51, p < .001) with education (F (1, 23) = 5.01, p < .05) and motor speed (F (1, 25) = 5.08, p < .05) factored as covariates. AD participants (M [SE] = 4023 [272]) performed more slowly than OA (M [SE] = 2515 [282]). There was a main effect of complexity (F (1, 25) = 218.51, p < .001), with faster performance on 1-card games (M [SE] = 3269 [196]) than on 6-card games (M [SE] = 7022 [361]). There was also a group by complexity interaction (F (1, 25) = 13.62, p < .001); OA 1-card: M [SE] = 2515 [282]; 6-card: M [SE] = 5331 [520]; AD 1-card: M [SE] = 4023 [272]; 6-card: M [SE] = 8712 [501]. No other main effects or interactions were significant. There was a trend toward a group by contrast interaction (F (1,25) = 3.70, p=.07).
Summary of Results for the Externally Supported Performance Intervention
The effects of changing card size, complexity, and contrast were larger for AD than for PD or for healthy older adults, compared to their respective control groups. Using bingo cards that were larger than standard size increased the speed at which the correct target was found by healthy younger and older adults and by individuals with PD or AD. Playing with a reduced number of simultaneous cards also improved task performance in all tested groups. In addition, of the older groups, patients with AD benefited from using bingo cards that had greater visual contrast than is used for standard cards. An increase in contrast did not decrease response time for those with PD, or for the NC or OA control participants, which suggests that standard bingo cards provide sufficient contrast for optimal play by these groups. We did not observe differences in visual acuity between our patient and control groups, and found that increasing card size reduced response times for all groups.
Discussion
We found that introducing an externally supported performance intervention (ESPI), through the manipulation of visual aspects of game stimuli, improved performance in normal adults and in individuals with AD or PD on the complex visual search task used in this study. As hypothesized, response times were facilitated the most by strengthening visual stimulus characteristics for patients with AD, who typically have the largest contrast and acuity deficits. We found a lesser amount of facilitation for the PD patients, and the least for healthy age-matched control participants relative to their younger counterparts. We also found that increasing game complexity by adding to the number of cards played per game reduced success the most for AD patients, then PD patients, then age-matched healthy adults. It appears that visual and cognitive complexity adds to the cognitive load of game play, compounding the deficit already experienced by the various groups because of reduced visual input. Increasing visual contrast of game cards improved performance for both the YA and AD groups. For the YA, this effect was driven by the two individuals who had the poorest contrast sensitivity. When their data were removed from the analysis, the effect disappeared. Bingo performance was not similarly correlated with contrast sensitivity for those with AD, indicating a genuine effect for this group.
Bingo can be thought of as an ecologically valid visual search task. A strength of the current study is that it is a naturalistic representation of game play within an experimentally controlled environment. We were limited in our ability to collect accuracy data, not being able to distinguish between genuine errors and errors recorded as a result of over- or under-sensitivity of the touch screen for individual participants. We found, however, that a major problem experienced by many game players is insufficient speed of search. Our results speak directly to the ability to increase speed through manipulation of visual and cognitive variables associated with the game.
The implications of increasing the accessibility of leisure activities such as bingo for older adults are far from a trivial. There is growing evidence that being cognitively and socially engaged helps maintain the integrity of cognitive function as we grow older (reviewed in Bielak, 2010). Symptoms of depression (Rosenberg et al., 2010) and hypochondria (Krawczynski & Olszewski, 2000) can be reduced by combining cognitive and physical activity. It has also been shown that engaging in social and leisure activities helps maintain or even improve cognition over time (Bassuk, Glass, & Berkman, 1999), possibly reducing the risk of developing dementia (Hughes, Chang, Vander Bilt, & Ganguli, 2010; Wang, Karp, Winblad, & Fratiglioni, 2002). While these effects likely apply to a wide variety of leisure activities, longitudinal research that specifically includes bingo has shown that participating in this or another recreational gambling activity was predictive of greater self-reported social support (Vander Bilt, Dodge, Pandav, Shaffer, & Ganguli, 2004). For patients with moderate to severe AD (MMSE range of 8 to 24), participating in bingo for 20 minutes was reported to increase cognitive performance on picture naming and word list recognition, whereas participating in 20 minutes of physical activity did not (Sobel, 2001).
Though such findings advocate for patient engagement in games and other social activities, these benefits are not available to those who do not play. Difficulty in perceiving the component stimuli of a game such as bingo would likely decrease both the desirability and probability of participating. This is especially relevant for those residing in communal or long-term care facilities where bingo and related games represent a regular activity and where opportunities for social engagement may otherwise be limited in scope and frequency.
The willingness and ability to change the external environment in order to improve cognition and daily function in older adults and in those with neurodegenerative conditions has become more widespread in recent years. We reported a significant enhancement of the successful intake of food and liquids in severely demented AD patients in long-term care through strengthening the visual contrast between a nutritional object and its background, thereby directing the patients’ visual attention to the task (Dunne et al., 2004). Koss and Gilmore (1998) likewise found that modifications of the visual contrast environment improved nutritional intake in AD patients and also reduced agitated nighttime behavior (“sundowning”). General modification of the visual environment can enhance the success of spatial navigation as well as safety. Dunne (2004) has provided a practical guide for the lay reader that describes simple environmental modifications, room by room, that are based on empirical research on visual and visuospatial dysfunction in AD, with an emphasis on color discrimination and contrast sensitivity. “Design for aging” is a topic of great interest in the domains of architecture, interior design, acoustics, and occupational therapy, to name a few, with numerous books and websites devoted to its discussion.
ESPI is part of a continuum of methods to enhance cognition, daily function, and independence in older adults or populations with sensory-cognitive disabilities. We define ESPI as interventions based on external support, meaning changes to the external environment of the individual, in any sensory modality. Enhanced performance may be effected through use of corrective lenses, large-print books, and hearing aids as well as by use of modified stimulus presentation techniques such as in the present study in regard to vision. Toward the other end of the intervention continuum are techniques that require use of more internal resources, such as development of cognitive strategies (e.g., mnemonics) or reallocation of attentional resources, which may depend on motivation, feelings of self-efficacy, and control beliefs (see Wingfield and Tun, 2007, in regard to hearing and language). Between the external- and internal-based interventions are those that present stimuli using modified techniques and require training to enhance the speed or accuracy of performance. Examples include action video games (Dye, Green & Bavelier, 2009), training tasks in application to driving ability (Edwards et al., 2009) and techniques for auditory or communication training, known as aural rehabilitation (Sweetow & Sabes, 2006). Physical training programs also fall within this range of the continuum, with a great deal of research being devoted to understanding what types and aspects of physical exercise or other social-aerobic activity contribute to the enhancement or maintenance of cognitive ability, especially in older adults (e.g., Erikson et al., 2011; Kattenstroth, Kolankowska, Kalisch & Dinsel, 2010; Smith et al., 2010).
We have shown in the present ESPI study that increasing the size of stimuli, enhancing visual contrast, and limiting game complexity can improve performance on a familiar, complex visual search task. Of particular interest is the observed beneficial effect of increased stimulus contrast for the AD group. This finding provides a practical example of how decreased contrast sensitivity, observed in previous lab studies of AD (e.g., Neargarder et al., 2003; Cronin-Golomb et al., 2007) adversely impacts everyday activities. More importantly, it shows that increasing stimulus contrast can improve the successful performance of mildly to moderately demented patients on a real-world task. There is undoubtedly a need for improving successful performance. For instance, in interviews with 130 community-dwelling patients with mild-to-moderate AD, 65% of AD patients reported decreased initiation of engagement in leisure activities. Of those, 44% said the reason was a decreased interest and 34% said that they experienced impaired performance that dissuaded them from pursuing their interests (Cook, Fay, & Rockwood, 2008). By making leisure activities more visually and cognitively accessible with simple visual and cognitive interventions that are easy to apply, performance and likely participation in everyday leisure activities may be meaningfully enhanced. Benefits to health and cognition may follow from the promotion of more active social and cognitive engagement.
Acknowledgments
Funding
This work was supported by the National Institute of Neurological Disorders and Stroke (R01 NS052914) to ACG; and the National Institute on Aging (P30 AG13846) to the Boston University Alzheimer Disease Center.
We would like to thank all of the individuals who participated in this study and the patients’ caregivers. We are grateful for the extraordinary facilitation of this research on Alzheimer’s disease by the Community Family Buddy Coholan Center of Medford, MA and the Community Family Center of Lowell, MA. Special thanks are extended to Community Family’s Gina Hughes, R.N.C, Sheila Witkus, M.Ed., L.S.W., Barbara Keefe, and staff (Medford); to Maria Maskaluk, R.N., B.S.N., Sara Barrett, L.S.W., and staff (Lowell); and to Anne M. Marchetta, M.S.W., Executive Director, as well as to the University Memory and Aging Center of Case Medical Center in Cleveland and the Boston University Alzheimer’s Disease Center. Our recruitment of participants with Parkinson’s disease was supported, with our gratitude, by Marie Saint-Hilaire, M.D., F.R.C.P.C., Cathi Thomas, R.N., M.S., and Denyse Turpin, R.N., M.P.H. of the Department of Neurology, Boston Medical Center. Julie Belkin, M.D., Mark O’Donoghue, O.D., and Connie Lee, O.D. conducted the eye examinations. We thank Bruce Reese, M.A., Chelsea Toner, M.A., Jeffrey Syh, B.S., and Samantha Ekka-Duffy, B.A. for technical assistance, and Karin Schon, Ph.D., for informative discussions of the literature.
References
- Amick MM, Cronin-Golomb A, Gilmore GC. Visual processing of rapidly presented stimuli is normalized in Parkinson’s disease when proximal stimulus strength is enhanced. Vision Research. 2003;43:2827–2835. doi: 10.1016/s0042-6989(03)00476-0. [DOI] [PubMed] [Google Scholar]
- Armstrong RA. Visual signs and symptoms of Parkinson’s disease. Clinical and Experimental Optometry. 2008;91(2):129–138. doi: 10.1111/j.1444-0938.2007.00211.x. [DOI] [PubMed] [Google Scholar]
- Bassuk SS, Glass TA, Berkman LF. Social disengagement and incident cognitive decline in community-dwelling elderly persons. Annals of Internal Medicine. 1999;131:165–173. doi: 10.7326/0003-4819-131-3-199908030-00002. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory. 2. San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- Bielak AAM. How can we not ‘lose it’ if we still don’t understand how to ‘use it’? Unanswered questions about the influence of activity participation on cognitive performance in older age - A mini-review. Gerontology. 2010;56(5):507–519. doi: 10.1159/000264918. [DOI] [PubMed] [Google Scholar]
- Bodis-Wollner I, Marx MS, Mitra S, Bobak P, Mylin L, Yahr M. Visual dysfunction in Parkinson’s disease. Loss in spatiotemporal contrast sensitivity. Brain. 1987;110(6):1675–98. doi: 10.1093/brain/110.6.1675. [DOI] [PubMed] [Google Scholar]
- Clay OJ, Edwards JD, Ross LA, Okonkwo O, Wadley VG, Roth DL, Ball KK. Visual function and cognitive speed of processing mediate age-related decline in memory span and fluid intelligence. Journal of Aging and Health. 2009;21(4):547–566. doi: 10.1177/0898264309333326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook C, Fay S, Rockwood K. Decreased initiation of usual activities in people with mild-to-moderate Alzheimer’s disease: a descriptive analysis from the VISTA clinical trial. International Psychogeriatrics. 2008;20:952–963. doi: 10.1017/S1041610208007230. [DOI] [PubMed] [Google Scholar]
- Cronin-Golomb A. Vision in Alzheimer’s disease. Gerontologist. 1995;35(3):370–376. doi: 10.1093/geront/35.3.370. [DOI] [PubMed] [Google Scholar]
- Cronin-Golomb A. Heterogeneity of visual profile in Alzheimer’s disease. In: Cronin-Golomb A, Hof P, editors. Vision in Alzheimer’s Disease. Basel: Karger; 2004. pp. 96–111. [Google Scholar]
- Cronin-Golomb A, Gilmore GC, Neargarder SA, Morrison SR, Laudate TM. Enhanced stimulus strength improves visual cognition in aging and Alzheimer’s disease. Cortex. 2007;43:952–966. doi: 10.1016/s0010-9452(08)70693-2. [DOI] [PubMed] [Google Scholar]
- Davidsdottir S, Wagenaar R, Young D, Cronin-Golomb A. Impact of optic flow perception and egocentric coordinates on veering in Parkinson’s disease. Brain. 2008;131(11):2882–2893. doi: 10.1093/brain/awn237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunne T. Improved performance on activities of daily living in Alzheimer’s disease: Practical applications of vision research. In: Cronin-Golomb A, Hof PR, editors. Vision in Alzheimer’s Disease. Vol. 34. Basel: Karger; 2004. pp. 305–324. [Google Scholar]
- Dunne TE, Neargarder SA, Cipolloni PB, Cronin-Golomb A. Visual contrast enhances food and liquid intake in advanced Alzheimer’s disease. Clinical Nutrition. 2004;23(4):533–538. doi: 10.1016/j.clnu.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Dye MWG, Green CS, Bavelier D. Increasing speed of processing with action video games. Current Directions in Psychological Science. 2009;18:321–326. doi: 10.1111/j.1467-8721.2009.01660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards JD, Bart E, O’Connor ML, Cissell G. Ten years down the road: Predictors of driving cessation. Gerontologist. 2010;50(3):393–9. doi: 10.1093/geront/gnp127. Epub 2009 Sep 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards JD, Myers C, Ross LA, Roenker DL, Cissell GM, McLaughlin AM, Ball KK. The longitudinal impact of cognitive speed of processing training on driving mobility. Gerontologist. 2009;49:485–494. doi: 10.1093/geront/gnp042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, et al. Exercise training increases size of hippocampus and improves memory. Proceedings of the National Academy of Sciences USA. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical methodfor grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gilmore GC, Cronin-Golomb A, Neargarder SA, Morrison SR. Enhanced stimulus contrast normalizes visual processing of rapidly presented letters in Alzheimer’s disease. Vision Research. 2005;45(8):1013–1020. doi: 10.1016/j.visres.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Gilmore GC, Spinks RA, Thomas CW. Age effects in coding tasks: componential analysis and test of the sensory deficit hypothesis. Psychology of Aging. 2006;21(1):7–18. doi: 10.1037/0882-7974.21.1.7. [DOI] [PubMed] [Google Scholar]
- Horowitz TS, Choi WY, Horvitz JC, Côté LJ, Mangels JA. Visual search deficits in Parkinson’s disease are attenuated by bottom-up target salience and top-down information. Neuropsychologia. 2006;44(10):1962–1977. doi: 10.1016/j.neuropsychologia.2006.01.037. [DOI] [PubMed] [Google Scholar]
- Hughes TF, Chang CC, Vander Bilt J, Ganguli M. Engagement in reading and hobbies and risk of incident dementia: the MoVIES project. American Journal of Alzheimer’s Disease and Other Dementias. 2010;25(5):432–438. doi: 10.1177/1533317510368399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson GR, Owsley C. Visual dysfunction, neurodegenerative diseases, and aging. Neurologic Clinics. 2003;21(3):709–28. doi: 10.1016/s0733-8619(02)00107-x. [DOI] [PubMed] [Google Scholar]
- Jones RD, Donaldson IM. Fractionation of visuoperceptual dysfunction in Parkinson’s disease. Journal of the Neurological Sciences. 1995;131(1):43–50. doi: 10.1016/0022-510x(95)00043-2. [DOI] [PubMed] [Google Scholar]
- Kattenstroth J-C, Kolankowska I, Kalisch T, Dinsel HR. Superior sensory, motor, and cognitive performance in elderly individuals with multi-year dancing activities. Frontiers in Aging Neuroscience. 2010;2:31. doi: 10.3389/fnagi.2010.00031. Published 21 July 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koss E, Gilmore GC. Environmental interventions and functional ability of AD patients. In: Vellas B, Fitten J, Frisoni G, editors. Research and Practice in Alzheimer’s Disease. New York: Serdi Springer; 1998. pp. 185–99. [Google Scholar]
- Krawczynski M, Olszewski H. Psychological well-being associated with a physical activity programme for persons over 60 years old. Psychology of Sport and Exercise. 2000;1:57–63. [Google Scholar]
- Mendez MF, Tomsak RL, Remler B. Disorders of the visual system in Alzheimer’s disease. Journal of Clinical Neuroophthalmology. 1990;10(1):62–69. [PubMed] [Google Scholar]
- Mapstone M, Steffenella TM, Duffy CJ. A visuospatial variant of mild cognitive impairment: getting lost between aging and AD. Neurology. 2003;60(5):802–808. doi: 10.1212/01.wnl.0000049471.76799.de. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- McNeilly DP, Burke WJ. Gambling as a social activity of older adults. International Journal of Aging and Human Development. 2001;52(1):19–28. doi: 10.2190/A4U7-234X-B3XP-64AH. [DOI] [PubMed] [Google Scholar]
- Nameda N, Kawara T, Ohzu H. Human visual spatio-temporal frequency performance as a function of age. Optometry and Vision Science. 1989;66:760–765. doi: 10.1097/00006324-198911000-00007. [DOI] [PubMed] [Google Scholar]
- Neargarder SA, Cronin-Golomb A. Characteristics of visual target influence detection of change in naturalistic scenes in Alzheimer’s disease. Cognitive and Behavioral Neurology. 2005;18:151–158. doi: 10.1097/01.wnn.0000178229.39068.9b. [DOI] [PubMed] [Google Scholar]
- Neargarder SA, Stone ER, Cronin-Golomb A, Oross S., III The impact of acuity on performance of four clinical measures of contrast sensitivity in Alzheimer’s disease. The Journals of Gerontology: Psychological Sciences. 2003;58B(1):P54–P62. doi: 10.1093/geronb/58.1.p54. [DOI] [PubMed] [Google Scholar]
- Owsley C. Vision Research. 2010. Aging and vision. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owsley C, Sekuler R, Siemsen D. Contrast sensitivity throughout adulthood. Vision Research. 1983;23(7):689–699. doi: 10.1016/0042-6989(83)90210-9. [DOI] [PubMed] [Google Scholar]
- Owsley C, Sloane ME. Contrast sensitivity, acuity, and the perception of ‘real-world’ targets. British Journal of Ophthalmology. 1987;71:791–796. doi: 10.1136/bjo.71.10.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo M, Anderson SW, Dawson J, Myers R, Ball K. Visual attention impairments in Alzheimer’s disease. Neurology. 2000;54(10):1954–1959. doi: 10.1212/wnl.54.10.1954. [DOI] [PubMed] [Google Scholar]
- Rosenberg D, Depp CA, Vahia IV, Reichstadt J, Palmer BW, Kerr J, Norman G, Jeste DV. Exergames for subsyndromal depression in older adults: a pilot study of a novel intervention. The American Journal of Geriatric Psychiatry. 2010;18(3):221–226. doi: 10.1097/JGP.0b013e3181c534b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seichepine DR, Neargarder S, Miller IN, Riedel TM, Gilmore GC, Cronin-Golomb A. Relation of Parkinson’s disease subtypes to visual activities of daily living. Journal of the International Neuropsychological Society. doi: 10.1017/S1355617711000853. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PJ, Blumenthal JA, Hoffman BM, Cooper H, Strauman TA, Welsh-Bohmer K, et al. Aerobic exercise and neurocognitive performance: A meta-analytic review of randomized controlled trials. Psychosomatic Medicine. 2010;72:239–252. doi: 10.1097/PSY.0b013e3181d14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel BP. Bingo vs. physical intervention in stimulating short-term cognition in Alzheimer’s disease patients. American Journal of Alzheimers Disease and Other Dementias. 2001;16(2):115–120. doi: 10.1177/153331750101600214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, Sano M, Paulson J, Mayeux R. Modified Mini-Mental State Examination: Validity and reliability [abstract] Neurology. 1987;37(suppl 1):179. [Google Scholar]
- Sweetow RW, Sabes JH. The need for and development of an adaptive listening and communication enhancement (LACE) program. Journal of the American Academy of Audiology. 2006;17:538–558. doi: 10.3766/jaaa.17.8.2. [DOI] [PubMed] [Google Scholar]
- Uc EY, Rizzo M, Anderson SW, Dastrup E, Sparks JD, Dawson JD. Driving under low-contrast visibility conditions in Parkinson disease. Neurology. 2009;73(14):1103–1110. doi: 10.1212/WNL.0b013e3181bacf6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uc EY, Rizzo M, Anderson SW, Qian S, Rodnitzky RL, Dawson JD. Visual dysfunction in Parkinson disease without dementia. Neurology. 2005;65(12):1907–1913. doi: 10.1212/01.wnl.0000191565.11065.11. [DOI] [PubMed] [Google Scholar]
- Uc EY, Rizzo M, Anderson SW, Sparks J, Rodnitzky RL, Dawson JD. Impaired visual search in drivers with Parkinson’s disease. Annals of Neurology. 2006;60(4):407–13. doi: 10.1002/ana.20958. [DOI] [PubMed] [Google Scholar]
- Vander Bilt J, Dodge HH, Pandav R, Shaffer HJ, Ganguli M. Gambling participation and social support among older adults: a longitudinal community study. Journal of Gambling Studies. 2004;20(4):373–389. doi: 10.1007/s10899-004-4580-0. [DOI] [PubMed] [Google Scholar]
- Wang HX, Karp A, Winblad B, Fratiglioni L. Late-life engagement in social and leisure activities is associated with a decreased risk of dementia: a longitudinal study from the Kungsholmen project. American Journal of Epidemiology. 2002;155(12):1081–1087. doi: 10.1093/aje/155.12.1081. [DOI] [PubMed] [Google Scholar]
- Wingfield A, Tun PA. Cognitive supports and cognitive constraints on comprehension of spoken language. Journal of the American Academy of Audiology. 2007;18:548–558. doi: 10.3766/jaaa.18.7.3. [DOI] [PubMed] [Google Scholar]
- Yesavage JA. Geriatric Depression Scale. Psychopharmacology Bulletin. 1988;24(4):709–711. [PubMed] [Google Scholar]