Abstract
Background
N-myc downstream-regulated gene 2 (NDRG2), a member of a newly described family of differentiation-related genes, has been characterized as a regulator of dendritic cells. However, the role of NDRG2 on the expression and activation of transcription factors in blood cells remains poorly understood. In this study, we investigated the effects of NDRG2 overexpression on GATA-1 expression in PMA-stimulated U937 cells.
Methods
We generated NDRG2-overexpressing U937 cell line (U937-NDRG2) and treated the cells with PMA to investigate the role of NDRG2 on GATA-1 expression.
Results
NDRG2 overexpression in U937 cells significantly induced GATA-1 expression in response to PMA stimulation. Interestingly, JAK2/STAT and BMP-4/Smad pathways associated with the induction of GATA-1 were activated in PMA-stimulated U937-NDRG2 cells. We found that the inhibition of JAK2 activation, but not of BMP-4/Smad signaling, can elicit a decrease of PMA-induced GATA-1 expression in U937-NDRG2 cells.
Conclusion
The results reveal that NDRG2 promotes the expression of GATA-1 through activation of the JAK2/STAT pathway, but not through the regulation of the BMP-4/Smad pathway in U937 cells. Our findings further suggest that NDRG2 may play a role as a regulator of erythrocyte and megakaryocyte differentiation during hematopoiesis.
Keywords: GATA-1, NDRG2, U937, JAK2/STAT pathway
INTRODUCTION
Hematopoiesis is controlled by a number of cell type-specific transcription factors that induce differentiation of cells to fulfill particular functions (1). These factors give rise to a specific lineage commitment via a coordinated regulation of expression and activation. However, little is known about how transcription factors regulate hematopoiesis. To this end, it is important to gain an understanding of how transcription factors are modulated during the differentiation of hematopoietic cells.
Globin transcription factor 1 (GATA binding protein 1, GATA-1) is a transcription factor known to be involved in the development of various hematopoietic lineages (2). GATA-1 is a C4 zinc finger transcription factor that recognizes WGATAR DNA motifs and is essential for erythrocyte, megakaryocyte, mast cell, and eosinophil differentiation. GATA-1 has a reciprocal interaction with PU.1, another transcription factor that promotes macrophage and dendritic cell development (3). It has been reported that there are various genes associated with the modulation of GATA-1 expression. A gain of function JAK2(JAK2V617F) mutation leads to enhanced GATA-1 expression, whereas morpholino knock-down of jak2a or a specific JAK2 inhibitor (TG101209) significantly suppresses GATA-1 expression in zebrafish embryos (4,5). Rab7b-induced IL-6 production and STAT3 activation promote GATA-1 activity in K562 cells (6). GATA-1 expression is also increased by treatment with recombinant BMP-4, but is reduced by Smad5 knockdown in the embryoid body (EB) (7,8). Moreover, dorsomorphin, a selective inhibitor of BMP-induced Smad activation, decreases expression of GATA-1 during embryonic stem (ES) cell differentiation (9,10). Ectopic expression of erythroid differentiation-associated gene (EDAG) induces GATA-1 expression in 32D cells (11), whereas HSP27 promotes ubiquitination of GATA-1 in K562 cells (12).
N-myc downstream-regulated gene 2 (NDRG2) belongs to the NDRG family, a new family of differentiation-related genes composed of four members which share 57~65% amino acid identity (13). NDRG proteins have common structural features, including an NDR-domain and an α/β hydrolase fold, which show high homology among NDRG members (13). Of the NDRG family members, NDRG2 is highly expressed in the adult brain, salivary glands, and skeletal muscle (13). It has been characterized as a regulator of dendritic cell differentiation from monocytes, CD34+ progenitor cells, and myelomonocytic leukemic cell line (14,15). NDRG2 has also been shown to regulate cell growth, apoptosis, and neurodegeneration (16-19). Recently, it has been proposed to be a novel intrinsic factor for the modulation of IL-10 production in myeloid cells (20). However, the role of NDRG2 in the expression and activation of transcription factors in blood cells has remained poorly understood.
Interestingly, NDRG2 overexpression induces a significant decrease of PU.1 expression in U937 cells. We previously showed that NDRG2 overexpression activates the STAT3 pathway in PMA-treated U937 cells (21) and also induces BMP-4 production in MDA-MB-231 cells (17). Given that STAT3 and BMP-4 are involved in GATA-1 expression, and that NDRG2 inhibits PU.1 expression, we hypothesized that NDRG2 increases GATA-1 expression through regulation of either the JAK2/STAT or BMP-4/Smad pathway. To test this hypothesis, we investigated whether NDRG2 promotes expression of GATA-1 in PMA-stimulated U937 cells. GATA-1 expression was substantially increased in NDRG2-overexpressing U937 cells in response to PMA. In addition, NDRG2 expression induced activation of the JAK2/STAT and BMP-4/Smad pathway in PMA-stimulated U937 cells. Inhibition of JAK2 decreased PMA-induced GATA-1 expression in U937-NDRG2 cells. However, inhibition of the BMP-4/Smad pathway did not suppress PMA-induced GATA-1 expression in U937-NDRG2 cells. Taken together, these data indicate that NDRG2 expression promotes the GATA-1 expression through regulation of the JAK2/STAT pathway and not through the BMP4/Smad pathway in U937 cells. Additionally, we speculate that NDRG2 can serve as a regulator of erythrocyte and megakaryocyte differentiation during hematopoiesis.
MATERIALS AND METHODS
Cell lines and reagents
U937 cells were cultured in RPMI 1640 (Gibco/Invitrogen, Carlsbad, CA) supplemented with 10% heat-inactivated FBS (Gibco). Phorbol 12-myristate 13-acetate (PMA), AG490, and dorsomorphin (6-[4-(2-piperidin-1-yl-ethoxy) phenyl]-3-pyridin-4-yl-pyrazolo [1,5-a] pyrimidine) were purchased from Sigma-Aldrich (St. Louis, MO). Recombinant human noggin was obtained from Peprotech, Inc. (Rocky Hill, NJ).
Construction and transfection of U937 cells
Cells expressing NDRG2 were previously generated in our laboratory (20). Among the established clones, clone #61 was used as a stable NDRG2-expressing U937 cell line.
RT-PCR
Total RNA from harvested cells was isolated using the TRI reagent (Molecular Research Center, Cincinnati, OH). cDNA was synthesized from 1µg of total RNA and amplified by PCR for 35~40 cycles in a 20µl reaction mixture containing 1µl cDNA, 10 pmol of each primer, 10 mM dNTP, and 0.5 U Taq DNA polymerase (Bioneer, Daejeon, Republic of Korea). β-actin was amplified for 22 cycles and used as a loading control. PCR products (10µl) were electrophoresed on a 1% agarose gel and visualized by ethidium bromide staining. The primer sequences used for RT-PCR are summarized in Table I.
Table I.
PCR primers
§Number indicates the reference.
Western blot analysis
The cells were lysed in a protein extraction solution (iNtRON Biotechnology, Seongnam, Korea). The proteins were separated on an SDS-polyacrylamide gel and transferred onto a PVDF membrane (Amersham Biosciences, Burkes, UK), which was then blocked with Tris-buffered saline and 0.05% Tween-20 (TBST) containing 5% skim milk. The membranes were incubated with specific antibodies and washed with TBST. The antibodies for phospho-STAT1, phospho-STAT3, phospho-STAT5, phospho-Smad1/5/8, and Smad5 were purchased from Cell Signaling Technology Inc. (Beverly, MA). Antibodies against GATA-1, SOCS1, SOCS2, SOCS3, NDRG2, Lamin A/C, actin, and α-actinin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The phospho-JAK2 antibody was purchased from Millipore (Billerica, MA), and the antibody against BMP-4 was purchased from R&D Systems (Minneapolis, MN). After incubating the membrane with diluted secondary antibody coupled to horseradish peroxidase, the antigen-antibody complexes were detected by enhanced chemiluminescence and exposed using LAS 3000 imaging system (FUJIFILM Corporation, Tokyo, Japan).
Isolation of cytoplasmic and nuclear fraction
The cells were suspended with 100µl cold buffer A (25 mM Tris, pH 8.0, 10 mM KCl, 1 mM DTT, 0.5 mM PMSF) and incubated on ice for 15 min. Then, 25µl 10% NP-40 was added to the sample and mixed by vortexing for 10 sec. After centrifugation for 2 min at 4℃, supernatants were collected as the cytoplasmic fraction. The pellet was suspended in 50µl buffer C (50 mM Tris, pH 8.0, 400 mM NaCl, 1 mM DTT, 1 mM PMSF) and incubated for 10 min at 4℃ on a shaking platform. After centrifugation for 5 min at 4℃, supernatants were collected as the nuclear fraction.
Trypan blue exclusion assay
The cells were cultured in a 24-well plate in RPMI 1640 medium containing 10% FBS and treated with 0.1µg/ml PMA for 2 days. The cells were harvested every 1 day, stained with 0.4% trypan blue, and live cells were counted using a hematocytometer.
CCK-8 assay
The cells were seeded at a density of 1×104 cells/150µl in 96-well plates in RPMI-1640 medium containing 10% FBS and treated with 0.1µg/ml PMA for 2 days. Then, 10µl of CCK-8 solution (Dojindo, Gaithersburg, MD) was added to each well. After incubation for 2~3 h at 37℃, the absorbance at 450 nm was recorded using a VICTOR3™ plate reader (PerkinElmer, Waltham, MA).
Statistical analysis
All the experiments were performed more than three times. The Student's t-test was used for statistical analysis. The values are represented as the mean±SD. A value of p<0.05 was considered significant.
RESULTS
NDRG2 overexpression prevents cell growth arrest in response to PMA
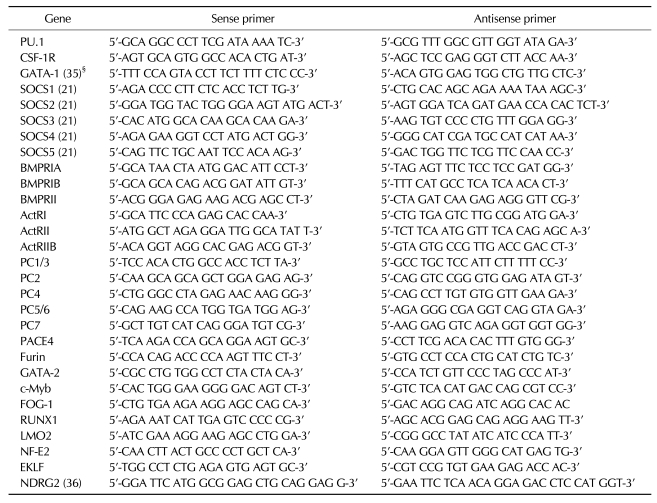
U937 cells differentiate into monocyte-like cells upon treatment with phorbol 12-myristate 13-acetate (PMA), and their growth is arrested by enhanced expression of PU.1-dependent genes (22). To identify the effect of NDRG2 expression during PMA-induced differentiation in U937 cells, U937-mock and U937-NDRG2 cells were treated with PMA for 48 h. Ectopic expression of NDRG2 was detected only in the cytoplasm of U937 cells (Fig. 1A). After treatment with PMA for 48 h, U937-mock cells attached to culture plate, but U937-NDRG2 cells showed comparatively reduced attachment (data not shown). A trypan blue exclusion assay revealed that both U937-mock and U937-NDRG2 cells showed no significant differences in cell proliferation. However, U937-mock cells did not proliferate more in response to PMA. Interestingly, U937-NDRG2 cells continued to proliferate during PMA-induced differentiation into macrophages (Fig. 1B). In addition, it was also confirmed using a CCK-8 assay that U937-NDRG2 cells maintained a higher level of proliferation than U937-mock cells during PMA-induced differentiation (Fig. 1C). Interestingly, NDRG2 expression significantly inhibited PU.1 expression in U937 cells although PU.1 levels were also weakly increased in U937-NDRG2 cells after treatment with PMA (Fig. 1D). Consistent with these results, CSF-1R expression, which is regulated by PU.1, was not observed in U937-NDRG2 cells after treatment with PMA. Accordingly, these data demonstrate that NDRG2 hinders the differentiation of U937 premonocytes into macrophages by PMA stimulation and promotes continued cell growth through the suppression of PU.1 expression.
Figure 1.
Overexpression of NDRG2 in U937 cells. (A) Cell lysates were fractionated into cytoplasmic and nuclear components. Western blotting using an anti-NDRG2 antibody was performed for each fraction. Lamin A/C and α-actinin were used as control for protein loading as well as marker proteins for nuclear and cytoplasmic fraction, respectively. (B, C) U937-mock and U937-NDRG2 cells were treated with 0.1 µg/ml PMA for 48 h. Cell viability was determined using either the trypan blue exclusion assay (B) or the CCK-8 assay (C). The results represent the means±SD of duplicates. *p<0.05, **p<0.01. (D) PU.1, CSF-1R, and NDRG2 mRNA levels were measured by RT-PCR. β-actin was used as a loading control.
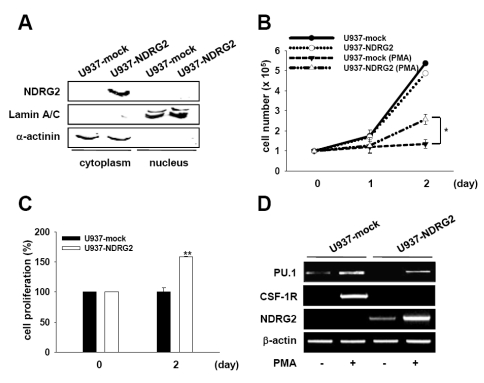
GATA-1 expression is induced by NDRG2 overexpression
PU.1, which is suppressed by NDRG2 expression in U937 cells, has a reciprocal interaction with GATA-1 during hematopoiesis. Thus, we examined whether NDRG2 induces GATA-1 expression in U937 cells. As shown in Fig. 2A and B, when U937-mock and U937-NDRG2 cells were treated with PMA for 48 h, GATA-1 expression at both the mRNA and the protein levels was only detected in PMA-treated U937-NDRG2 cells. These data suggest that although PU.1 levels in U937-NDRG2 cells are weakly induced after stimulation with PMA, GATA-1 expression is remarkably enhanced by NDRG2 expression.
Figure 2.
NDRG2 induces GATA-1 expression. (A) U937-mock and U937-NDRG2 cells were treated with 0.1µg/ml PMA for 48 h. mRNA levels for GATA-1 and NDRG2 were measured by RT-PCR. β-actin was used as a loading control. (B) Equal amounts of whole protein lysates were subjected to electrophoresis on SDS-PAGE gels, and Western blot analysis was performed using specific antibodies. Actin was used as an internal loading control. (C) Expression of genes promoting the differentiation of erythrocyte and megakaryocyte. U937-mock and U937-NDRG2 cells were treated with 0.1 µg/ml PMA for 48 h prior to RT-PCR analysis.
GATA-1 induction by NDRG2 correlates with the gene expression patterns marking differentiation into erythrocytes/megakaryocytes
Because GATA-1, which is increased by NDRG2 overexpression in response to PMA, is essential for differentiation of erythrocytes and megakaryocytes, we explored whether NDRG2 controls the expression levels of other genes that regulate erythroid/megakaryocyte differentiation processes. It is well known that a number of factors help to promote the differentiation of erythrocytes and megakaryocytes, including FOG-1, NF-E2, RUNX1, SCL/tal, LMO2, EKLF, and c-Myb (23,24). U937-mock and U937-NDRG2 cells were treated with 0.1µg/ml PMA and gene expression was analyzed after 48 h using RT-PCR. mRNA expression of NF-E2, RUNX1, LMO2, c-Myb, and FOG-1 was detected in both U937-mock and U937-NDRG2 cells in the absence of PMA stimulation (Fig. 2C). Interestingly, the expression of these factors was strongly inhibited by treatment with PMA in U937-mock cells but was maintained in U937-NDRG2 cells. Moreover, EKLF expression was newly induced in U937-NDRG2 cells. Additionally, GATA-2, which is often replaced by GATA-1 during hematopoiesis, disappeared in U937-NDRG2 cells. Collectively, these results indicate that NDRG2 can help to maintain the expression of other genes involved in promoting the differentiation of erythrocytes and megakaryocytes.
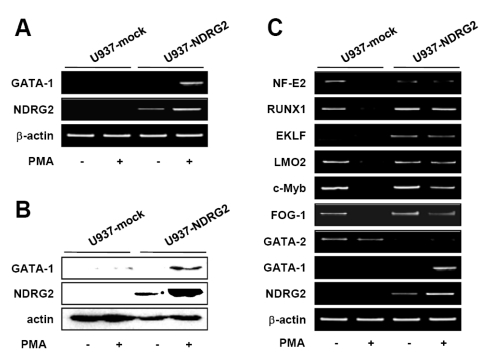
JAK2/STAT signaling is activated by NDRG2 expression
To better define the mechanism that regulates GATA-1 expression by NDRG2, we investigated whether NDRG2 can control the activation of the JAK2/STAT pathway, which is involved in GATA-1 expression. After a 48 h treatment with 0.1µg/ml PMA, protein production and mRNA expression of related genes were analyzed in U937-mock and U937-NDRG2 cells. As shown in Fig. 3A, phosphorylation of JAK2 and STAT1 was markedly increased by NDRG2 expression and PMA stimulation. The phosphorylation of STAT3 and STAT5 was severely impaired by PMA treatment in U937-mock cells, whereas phosphorylation was maintained in U937-NDRG2 cells. SOCS family proteins serve as negative regulators of the JAK/STAT pathway. U937-mock cells showed an increased expression of SOCS3 after treatment with PMA, whereas U937-NDRG2 cells did not (Fig. 3B and C). Other SOCS family proteins did not show any significant alterations by NDRG2 expression. Together, our findings suggest that NDRG2 activates JAK2/STAT signaling, which can mediate GATA-1 expression in U937 cells in response to PMA.
Figure 3.
JAK2/STAT pathway is activated by NDRG2 expression. U937-mock and U937-NDRG2 cells were treated with 0.1µg/ml PMA for 48 h. Western blot analysis (A, B) and RT-PCR (C) were performed. α-actinin and β-actin were used as an internal loading control.
BMP-4/Smad signaling is activated by NDRG2
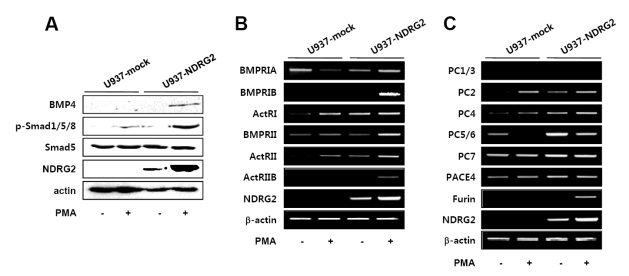
To evaluate other mechanisms by which NDRG2-induced GATA-1 expression is regulated, we further examined whether NDRG2 can control the activation of the BMP-4/Smad pathway that is associated with the induction of GATA-1 expression. After 48 h of treatment with 0.1µg/ml PMA, Western blot analysis was performed. NDRG2 expression promoted the production of active BMP-4 (37 kDa) in PMA-treated U937 cells (Fig. 4A). Consistent with BMP-4 production, phosphorylation of Smad1/5/8, which can be induced by active BMP-4, was also detected only in PMA-treated U937-NDRG2 cells. These results demonstrate that NDRG2 can activate the BMP-4/Smad pathway when cells are stimulated with PMA.
Figure 4.
BMP-4/Smad signaling pathway is activated by NDRG2 expression. U937-mock and U937-NDRG2 cells were treated with 0.1µg/ml PMA for 48 h. (A) Equal amounts of whole cell lysates were subjected to electrophoresis on SDS-PAGE gels, and Western blot analysis was performed using specific antibodies. Actin was used as an internal loading control. (B, C) mRNA levels were measured by RT-PCR.
NDRG2 regulates the expression of PC and BMPR to modulate the BMP-4/Smad signaling pathway
BMP-4, similar to other TGF-β family members, binds to type I and type II serine/threonine kinase receptors. Type I receptors are activated by phosphorylated type II receptors to initiate intracellular signals through the activation of Smad proteins (25-27). We therefore evaluated the effects of NDRG2 on BMP receptor (BMPR) expression in response to PMA. Type I receptors include BMPRIA, BMPRIB, and ActRI. Type II receptors include BMPRII, ActRII, ActRIIB. As shown in Fig. 4B, BMPRIA expression was inhibited by treatment with PMA in U937-mock cells but increased in U937-NDRG2 cells. The expression of ActRI and ActRII was enhanced by NDRG2 expression. Although the expression of ActRI and ActRII was detected in U937-mock cells in response to PMA, it was still higher in U937-NDRG2 cells. Expression of BMPRIB, BMPRII, and ActRIIB was highly induced in PMA-treated U937-NDRG2 cells. These data show that NDRG2 expression generally increases expression of BMPR in U937 cells and also suggest that phosphorylation of Smad1/5/8 may be induced by the increased BMPR activation in PMA-treated U937-NDRG2 cells.
The activity of BMP-4 is regulated by pro-protein convertase (PC) family members. Nine members of the PC family, including PC1/3, PC2, PC4, PC5/6, PC7, PACE4, Furin, SKI-1/S1P, and PCSK9, have been identified (28). The seven PCs, except for SKI-1/S1P and PCSK9, cleave pro-proteins at single or pairs of basic residues in the Golgi, secretory granules, cell surface, or endosomes. In particular, PC5/6, PC7, PACE4, and Furin are known to cleave pro-BMP-4 (29-31). As shown in Fig. 4C, expression of PC2, PC4, and PC5/6 increased with NDRG2 expression. Although the expression of PC2 and PC4 was detected in U937-mock cells in response to PMA, their expression was still higher in U937-NDRG2 cells. Furin was expressed only in PMA-treated U937-NDRG2 cells. These data suggest that NDRG2 expression increases the expression of PC to promote production of active BMP-4.
NDRG2 modulates GATA-1 expression through regulation of the JAK2/STAT pathway but not through regulation of the BMP-4/Smad pathway
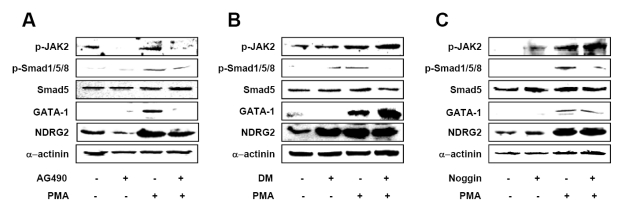
To identify which pathway regulates NDRG2-mediated GATA-1 expression, U937-NDRG2 cells were pre-treated with a JAK2 inhibitor (AG490), a BMP-4 antagonist (Noggin), or a Smad1/5/8 inhibitor (dorsomorphin). As shown in Fig. 5A, phosphorylation of JAK2 was suppressed by AG490 treatment in U937-NDRG2 cells. Phosphorylation of Smad1/5/8 was not significantly altered by JAK2 inhibition in U937-NDRG2 cells. Importantly, PMA-induced GATA-1 expression was inhibited by pre-treatment with AG490 in U937-NDRG2 cells. PMA-induced Smad1/5/8 phosphorylation was decreased by pre-treatment with dorsomorphin (DM) or noggin (Fig. 5B and C). Phosphorylation of JAK2 was not regulated by treatment with dorsomorphin or noggin. Interestingly, PMA-induced GATA-1 expression was not inhibited by pre-treatment with DM or noggin in U937-NDRG2 cells. Together, these data suggest that NDRG2 increases GATA-1 expression through the regulation of the JAK2/STAT pathway, but not through regulation of the BMP-4/Smad1/5/8 pathway, in response to PMA.
Figure 5.
NDRG2 increases GATA-1 expression through regulation of JAK2/STAT pathway. U937-NDRG2 cells were pre-treated with 5µg/ml AG490 (A), 1µM dorsomorphin (DM) (B), or 5 ng/ml noggin (C) for 3 h and then, 0.1µg/ml PMA was added. After 48 h, Western blot analysis was performed.
DISCUSSION
NDRG2 belongs to the NDRG family, a new characterized group of differentiation-related genes composed of four family members (13). NDRG2 has been characterized as a regulator of dendritic cell differentiation from monocytes, CD34+ progenitor cells, and myelomonocytic leukemic cell line (14,15). Recently, it was suggested to be a novel intrinsic factor for the modulation of IL-10 production in myeloid cells (20). However, the role of NDRG2 in the expression and activation of transcription factors in hematopoietic cells remains poorly understood.
Since Friend et al.(32) first demonstrated an induction of murine virus-induced erythroleukemic cell (MEL) differentiation in vitro in the early 1970s, several groups have used malignant hematopoietic cell lines, including HL-60, K-562, HEL, U937, TF-1, ML-1, and KG-1, to study hematopoiesis. We used U937 myeloid leukemic cells to investigate the effect of NDRG2 on GATA-1 expression. Myeloid leukemic cells differentiate in response to PMA, and their growth is arrested by increased expression of PU.1-dependent genes (22). In our study, PMA-treated U937 cells showed suppression of proliferation or differentiation into monocyte-like cells, whereas U937-NDRG2 cells continued their growth in response to PMA (Fig. 1B and C). In addition, NDRG2 impaired PU.1 expression in U937 cells (Fig. 1D).
In a previous report, it was shown that NDRG2 activates the STAT3 pathway in PMA-treated U937 cells (21). More over, NDRG2 induced BMP-4 production in MDA-MB-231 breast cancer cells (17). Because STAT3 and BMP-4 are known regulators of GATA-1 expression, and PU.1 suppression by NDRG2 is regulated by GATA-1 in an antagonistic manner, we hypothesized that NDRG2 increases GATA-1 expression through the regulation of the JAK2/STAT or BMP-4/Smad signaling pathway. While U937-mock cells did not express GATA-1, U937-NDRG2 cells showed an increased GATA-1 expression in response to PMA after 48 h (Fig. 2). NDRG2 did not appear to form a complex with GATA-1, which was induced by NDRG2 overexpression in response to PMA (data not shown). It has previously been reported that NDRG2 is downregulated by myc (13). GATA-1 is able to promote repression of the c-myc proto-oncogene during erythroid maturation (33). Thus, it is conceivable that a positive feedback loop exists between GATA-1 and NDRG2.
There are many proteins capable of controlling the differentiation of erythroid and megakaryocytic cells. Most of these genes, including NF-E2, RUNX1, LMO2, c-Myb, and FOG-1, were expressed in both U937-mock and U937-NDRG2 cells. However, their expression was inhibited by treatment with PMA in U937-mock cells but maintained in U937-NDRG2 cells. Moreover, the EKLF gene, which is specific for erythroid lineages, was only expressed in U937-NDRG2 cells, and RUNX1, which is specific for megakaryocyte lineages, was stably expressed even after treatment with PMA. Because both EKLF and RUNX1 were modulated by NDRG2 in U937 cells, the type of lineage specifically regulated by NDRG2 still remains unclear. GATA-2, which promotes proliferation of progenitor cells during early hematopoiesis and is suppressed by GATA-1 directly during hematopoiesis (34), was detected in U937-mock cells but not in U937-NDRG2 cells (Fig. 3). Based on these results, we speculate that NDRG2 expression can regulate the expression of other genes that promote the differentiation of erythrocytes and megakaryocytes. Therefore, further studies of how NDRG2 controls gene expression involved in hematopoiesis dependent or independent of GATA-1 are warranted.
It has been suggested that various genes are involved in GATA-1 expression during embryonic developmental processes and hematopoiesis. We previously reported that NDRG2 expression activates the STAT3 pathway upon treatment with PMA for up to 60 min in U937 cells (21). Furthermore, NDRG2 increased BMP-4 production in MDA-MB-231 breast tumor cells (17). Thus, we hypothesized that NDRG2 induced GATA-1 expression through regulation of the JAK2/STAT or BMP-4/Smad pathway. As shown in Fig. 3, NDRG2 promoted or maintained the phosphorylation of the JAK2/STAT pathway, whereas NDRG2 expression inhibited the expression of SOCS3, a negative regulator of the JAK/STAT pathway, in response to PMA for 48 h in U937 cells. Moreover, NDRG2 expression induced protein expression of active BMP-4 and activated Smad1/5/8 in response to PMA for 48 h in U937 cells (Fig. 4A). BMP-4 binds to type I and type II serine/threonine kinase receptors and activates intracellular signals through the phosphorylation of Smad proteins (25-27). As shown in Fig. 4B, expression of various BMP receptors was enhanced by NDRG2 when cells were treated with PMA. Furthermore, the expression of PCs, which regulate cleavage of pro-BMP-4 to active BMP-4, increased due to NDRG2 expression (Fig. 4C). Together, these findings suggest that NDRG2 expression can induce PC expression, produce active BMP-4, and phosphorylates Smad1/5/8 through increased BMPR activity in U937 cells.
To identify the signal transduction pathways by which NDRG2 induces GATA-1 expression, we analyzed JAK-STAT and BMP4-SMAD pathways using specific inhibitors. PMA-induced GATA-1 expression was suppressed by pre-treatment with AG490, a JAK2 inhibitor, in U937-NDRG2 cells (Fig. 5A). However, dorsomorphin (DM), a Smad1/5/8 inhibitor, and noggin, a BMP-4 antagonist, did not have any inhibitory effect on PMA-induced GATA-1 expression in U937-NDRG2 cells (Fig. 5B and C). Recently, the cross-talk between the BMP and JAK/STAT signaling pathways has been a focus of research to better understand hematopoiesis, embryonic development, and leukemia development (5). When the relationship between the BMP-4/Smad and JAK/STAT pathways was evaluated using specific inhibitors in PMA-treated U937-NDRG2 cells, there was no significant effect on activation of either pathway (Fig. 5), raising the possibility that these systems act independently.
In summary, we report in this study that NDRG2 induces GATA-1 expression through activation of the JAK2/STAT signaling pathway, but not through BMP-4/Smad signaling, in PMA-treated U937 cells. These results suggest that NDRG2 may serve as a regulator in erythrocyte and megakaryocyte differentiation during hematopoiesis. Further study is required to identify the effects of NDRG2 expression on the differentiation of erythroid/megakaryocytic lineages in primary hematopoietic stem cells and in vivo. Moreover, study of the effects of NDRG2 expression on myeloid cell differentiation through the activation of the BMP-4/Smad pathway also requires further elucidation.
Footnotes
The authors have no financial conflict of interest.
References
- 1.Cantor AB, Orkin SH. Hematopoietic development: a balancing act. Curr Opin Genet Dev. 2001;11:513–519. doi: 10.1016/s0959-437x(00)00226-4. [DOI] [PubMed] [Google Scholar]
- 2.Arinobu Y, Mizuno S, Chong Y, Shigematsu H, Iino T, Iwasaki H, Graf T, Mayfield R, Chan S, Kastner P, Akashi K. Reciprocal activation of GATA-1 and PU.1 marks initial specification of hematopoietic stem cells into myeloerythroid and myelolymphoid lineages. Cell Stem Cell. 2007;1:416–427. doi: 10.1016/j.stem.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Nerlov C, Graf T. PU.1 induces myeloid lineage commitment in multipotent hematopoietic progenitors. Genes Dev. 1998;12:2403–2412. doi: 10.1101/gad.12.15.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma AC, Fan A, Ward AC, Liongue C, Lewis RS, Cheng SH, Chan PK, Yip SF, Liang R, Leung AY. A novel zebrafish jak2a(V581F) model shared features of human JAK2(V617F) polycythemia vera. Exp Hematol. 2009;37:1379–1386. doi: 10.1016/j.exphem.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 5.Ma AC, Ward AC, Liang R, Leung AY. The role of jak2a in zebrafish hematopoiesis. Blood. 2007;110:1824–1830. doi: 10.1182/blood-2007-03-078287. [DOI] [PubMed] [Google Scholar]
- 6.He D, Chen T, Yang M, Zhu X, Wang C, Cao X, Cai Z. Small Rab GTPase Rab7b promotes megakaryocytic differentiation by enhancing IL-6 production and STAT3-GATA-1 association. J Mol Med (Berl) 2011;89:137–150. doi: 10.1007/s00109-010-0689-z. [DOI] [PubMed] [Google Scholar]
- 7.Adelman CA, Chattopadhyay S, Bieker JJ. The BMP/BMPR/Smad pathway directs expression of the erythroid-specific EKLF and GATA1 transcription factors during embryoid body differentiation in serum-free media. Development. 2002;129:539–549. doi: 10.1242/dev.129.2.539. [DOI] [PubMed] [Google Scholar]
- 8.Lohmann F, Bieker JJ. Activation of Eklf expression during hematopoiesis by Gata2 and Smad5 prior to erythroid commitment. Development. 2008;135:2071–2082. doi: 10.1242/dev.018200. [DOI] [PubMed] [Google Scholar]
- 9.Anderson GJ, Darshan D. Small-molecule dissection of BMP signaling. Nat Chem Biol. 2008;4:15–16. doi: 10.1038/nchembio0108-15. [DOI] [PubMed] [Google Scholar]
- 10.Hao J, Daleo MA, Murphy CK, Yu PB, Ho JN, Hu J, Peterson RT, Hatzopoulos AK, Hong CC. Dorsomorphin, a selective small molecule inhibitor of BMP signaling, promotes cardiomyogenesis in embryonic stem cells. PLoS One. 2008;3:e2904. doi: 10.1371/journal.pone.0002904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ding YL, Xu CW, Wang ZD, Zhan YQ, Li W, Xu WX, Yu M, Ge CH, Li CY, Yang XM. Over-expression of EDAG in the myeloid cell line 32D: induction of GATA-1 expression and erythroid/megakaryocytic phenotype. J Cell Biochem. 2010;110:866–874. doi: 10.1002/jcb.22597. [DOI] [PubMed] [Google Scholar]
- 12.de Thonel A, Vandekerckhove J, Lanneau D, Selvakumar S, Courtois G, Hazoume A, Brunet M, Maurel S, Hammann A, Ribeil JA, Zermati Y, Gabet AS, Boyes J, Solary E, Hermine O, Garrido C. HSP27 controls GATA-1 protein level during erythroid cell differentiation. Blood. 2010;116:85–96. doi: 10.1182/blood-2009-09-241778. [DOI] [PubMed] [Google Scholar]
- 13.Melotte V, Qu X, Ongenaert M, van Criekinge W, de Bruïne AP, Baldwin HS, van Engeland M. The N-myc downstream regulated gene (NDRG) family: diverse functions, multiple applications. FASEB J. 2010;24:4153–4166. doi: 10.1096/fj.09-151464. [DOI] [PubMed] [Google Scholar]
- 14.Choi SC, Kim KD, Kim JT, Kim JW, Yoon DY, Choe YK, Chang YS, Paik SG, Lim JS. Expression and regulation of NDRG2 (N-myc downstream regulated gene 2) during the differentiation of dendritic cells. FEBS Lett. 2003;553:413–418. doi: 10.1016/s0014-5793(03)01030-5. [DOI] [PubMed] [Google Scholar]
- 15.Choi SC, Kim KD, Kim JT, Kim JW, Lee HG, Kim JM, Jang YS, Yoon DY, Kim KI, Yang Y, Cho DH, Lim JS. Expression of human NDRG2 by myeloid dendritic cells inhibits down-regulation of activated leukocyte cell adhesion molecule (ALCAM) and contributes to maintenance of T cell stimulatory activity. J Leukoc Biol. 2008;83:89–98. doi: 10.1189/jlb.0507300. [DOI] [PubMed] [Google Scholar]
- 16.Deng Y, Yao L, Chau L, Ng SS, Peng Y, Liu X, Au WS, Wang J, Li F, Ji S, Han H, Nie X, Li Q, Kung HF, Leung SY, Lin MC. N-Myc downstream-regulated gene 2 (NDRG2) inhibits glioblastoma cell proliferation. Int J Cancer. 2003;106:342–347. doi: 10.1002/ijc.11228. [DOI] [PubMed] [Google Scholar]
- 17.Shon SK, Kim A, Kim JY, Kim KI, Yang Y, Lim JS. Bone morphogenetic protein-4 induced by NDRG2 expression inhibits MMP-9 activity in breast cancer cells. Biochem Biophys Res Commun. 2009;385:198–203. doi: 10.1016/j.bbrc.2009.05.038. [DOI] [PubMed] [Google Scholar]
- 18.Mitchelmore C, Büchmann-Møller S, Rask L, West MJ, Troncoso JC, Jensen NA. NDRG2: a novel Alzheimer's disease associated protein. Neurobiol Dis. 2004;16:48–58. doi: 10.1016/j.nbd.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi K, Yamada M, Ohata H, Honda K, Yamada M. Ndrg2 promotes neurite outgrowth of NGF-differentiated PC12 cells. Neurosci Lett. 2005;388:157–162. doi: 10.1016/j.neulet.2005.06.055. [DOI] [PubMed] [Google Scholar]
- 20.Choi SC, Kim KD, Kim JT, Oh SS, Yoon SY, Song EY, Lee HG, Choe YK, Choi I, Lim JS, Kim JW. NDRG2 is one of novel intrinsic factors for regulation of IL-10 production in human myeloid cell. Biochem Biophys Res Commun. 2010;396:684–690. doi: 10.1016/j.bbrc.2010.04.162. [DOI] [PubMed] [Google Scholar]
- 21.Lee EB, Kim A, Kang K, Kim H, Lim JS. NDRG2-mediated Modulation of SOCS3 and STAT3 Activity Inhibits IL-10 Production. Immune Netw. 2010;10:219–229. doi: 10.4110/in.2010.10.6.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carey JO, Posekany KJ, de Vente JE, Pettit GR, Ways DK. Phorbol ester-stimulated phosphorylation of PU.1: association with leukemic cell growth inhibition. Blood. 1996;87:4316, 4324. [PubMed] [Google Scholar]
- 23.Goldfarb AN. Megakaryocytic programming by a transcriptional regulatory loop: a circle connecting RUNX1, GATA-1, and P-TEFb. J Cell Biochem. 2009;107:377–382. doi: 10.1002/jcb.22142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wickrema A, Crispino JD. Erythroid and megakaryocytic transformation. Oncogene. 2007;26:6803–6815. doi: 10.1038/sj.onc.1210763. [DOI] [PubMed] [Google Scholar]
- 25.Harrison CA, Wiater E, Gray PC, Greenwald J, Choe S, Vale W. Modulation of activin and BMP signaling. Mol Cell Endocrinol. 2004;225:19–24. doi: 10.1016/j.mce.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 26.Heldin CH, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 27.Feng XH, Derynck R. Specificity and versatility in tgf-beta signaling through Smads. Annu Rev Cell Dev Biol. 2005;21:659–693. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- 28.Seidah NG. What lies ahead for the proprotein convertases? Ann N Y Acad Sci. 2011;1220:149–161. doi: 10.1111/j.1749-6632.2010.05883.x. [DOI] [PubMed] [Google Scholar]
- 29.Thomas G. Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nat Rev Mol Cell Biol. 2002;3:753–766. doi: 10.1038/nrm934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nelsen SM, Christian JL. Site-specific cleavage of BMP4 by furin, PC6, and PC7. J Biol Chem. 2009;284:27157–27166. doi: 10.1074/jbc.M109.028506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cui Y, Jean F, Thomas G, Christian JL. BMP-4 is proteolytically activated by furin and/or PC6 during vertebrate embryonic development. EMBO J. 1998;17:4735–4743. doi: 10.1093/emboj/17.16.4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Friend C, Scher W, Holland JG, Sato T. Hemoglobin synthesis in murine virus-induced leukemic cells in vitro: stimulation of erythroid differentiation by dimethyl sulfoxide. Proc Natl Acad Sci U S A. 1971;68:378–382. doi: 10.1073/pnas.68.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rylski M, Welch JJ, Chen YY, Letting DL, Diehl JA, Chodosh LA, Blobel GA, Weiss MJ. GATA-1-mediated proliferation arrest during erythroid maturation. Mol Cell Biol. 2003;23:5031–5042. doi: 10.1128/MCB.23.14.5031-5042.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bresnick EH, Lee HY, Fujiwara T, Johnson KD, Keles S. GATA switches as developmental drivers. J Biol Chem. 2010;285:31087–31093. doi: 10.1074/jbc.R110.159079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fan C, Ouyang P, Timur AA, He P, You SA, Hu Y, Ke T, Driscoll DJ, Chen Q, Wang QK. Novel roles of GATA1 in regulation of angiogenic factor AGGF1 and endothelial cell function. J Biol Chem. 2009;284:23331–23343. doi: 10.1074/jbc.M109.036079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park Y, Shon SK, Kim A, Kim KI, Yang Y, Cho DH, Lee MS, Lim JS. SOCS1 induced by NDRG2 expression negatively regulates STAT3 activation in breast cancer cells. Biochem Biophys Res Commun. 2007;363:361–367. doi: 10.1016/j.bbrc.2007.08.195. [DOI] [PubMed] [Google Scholar]